A real-time quantitative reverse transcriptase PCR assay with single-copy sensitivity targeting the integrase region of HIV-1 (integrase single-copy assay [iSCA] v1.0) has been widely used to quantify plasma viremia in individuals on antiretroviral therapy (ART). iSCA v1.0 requires the use of an ultracentrifuge, and only about half of the nucleic acid extracted from plasma is assayed for HIV-1 RNA.
KEYWORDS: HIV-1, antiretroviral therapy, single-copy assay
ABSTRACT
A real-time quantitative reverse transcriptase PCR assay with single-copy sensitivity targeting the integrase region of HIV-1 (integrase single-copy assay [iSCA] v1.0) has been widely used to quantify plasma viremia in individuals on antiretroviral therapy (ART). iSCA v1.0 requires the use of an ultracentrifuge, and only about half of the nucleic acid extracted from plasma is assayed for HIV-1 RNA. We sought to simplify sample processing using microcentrifugation and improve assay sensitivity by testing more than 75% of the total extracted nucleic acid for HIV-1 RNA (iSCA v2.0). We evaluated the limit of detection (LoD) of iSCA v2.0 by testing replicates of low-copy plasma HIV-1 RNA standards. By probit analysis, the 95% LoD was 1 copy of HIV-1 RNA per milliliter for a 5-ml plasma sample. To compare the sensitivity of iSCA v1.0 and v2.0, we tested plasma samples with both assays from 60 participants on ART with HIV-1 RNA below 20 cps/ml. Of the 31 samples that had no detectable HIV-1 RNA by iSCA v1.0, 17 (55%) were detectable by v2.0 with an HIV-1 RNA mean value of 3.5 cps/ml. Twenty-nine samples were detectable with both assay versions, but average values of HIV-1 RNA cps/ml were 2.7-fold higher for v2.0 than v1.0. These results support the adoption of a new, more sensitive and simpler single-copy HIV-1 RNA assay (iSCA v2.0) to quantify residual viremia on ART and to assess the impact of experimental interventions designed to decrease HIV-1 reservoirs.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) infection leads to high levels of plasma viremia (HIV-1 RNA) within days of infection (1). Without treatment, plasma viremia almost always persists, and its level is a strong predictor of the speed of HIV-1 disease progression to AIDS and death (2–4). Treatment with potent, combination antiretroviral therapy (ART) suppresses plasma viremia below the limit of detection of available commercial HIV-1 RNA assays, but ART does not eliminate viremia or cure HIV-1 infection (5–8). Prior work has shown that the majority of individuals receiving ART with HIV-1 RNA plasma concentrations below the detection limit of commercial assays have persistent plasma viremia when tested with a real-time quantitative reverse transcriptase PCR (qRT-PCR) assay with single-copy HIV-1 RNA sensitivity (9–11).
The first-generation two-step qRT-PCR assay with single-copy sensitivity targeted HIV-1 gag (gSCA) (12). Data obtained using the gSCA assay showed that plasma viremia persists in most suppressed participants and that three progressively longer phases of plasma HIV-1 RNA decay occur after initiation of ART, followed by a fourth phase of decay with a half-life of 11.2 years (9, 10, 13, 14). The next generation of the single-copy qRT-PCR assay improved the detection of HIV-1 RNA by targeting a highly conserved region of integrase in HIV-1 pol (iSCA) and by enhancing nucleic acid recovery from plasma (15).
Despite its successful implementation in many clinical studies, the current version of the iSCA assay has limitations. First, the method requires the use of an ultracentrifuge that presents a financial barrier and limits throughput. Ultracentrifugation may also make recovery of HIV-1 RNA from pellets more difficult due to high g-force (170,000 × g) and unwieldy ultracentrifuge tubes. Second, only about half (54%) of the extracted nucleic acid from a sample is assayed for HIV-1 RNA, which limits detection; the remaining extract is used to assess the spiked internal control for viral RNA recovery and HIV-1 DNA contamination. Third, despite the greater sensitivity of iSCA than the original gSCA, just over half (57%) of individuals on long-term ART have no detectable HIV-1 RNA in 3 to 4 ml of plasma by iSCA (16). We sought to address these shortcomings of the first version of integrase SCA (iSCA v1.0) by replacing the ultracentrifugation with simpler centrifugation in a microcentrifuge and by testing a greater fraction (more than three-fourths) of the extracted total nucleic acid for HIV-1 RNA, while maintaining the other controls. As the duration of HIV-1 RNA suppression on ART increases and the level of persistent viremia declines, more sensitive assays for plasma HIV-1 RNA are needed in a variety of research settings, especially those assessing the impact of curative strategies on viremia, such as latency reversal agents and immune effectors targeting virus-expressing cells. Here, we report on the performance assessment of a new, improved version of iSCA (iSCA v2.0).
MATERIALS AND METHODS
Clinical specimens.
All participants who donated plasma were volunteers at the University of Pittsburgh AIDS Clinical Trials Unit and provided written informed consent. Sample collection was approved by the University of Pittsburgh’s Institutional Review Board. Plasma specimens were collected from individuals on effective ART (plasma HIV-1 RNA, <20 copies/ml by Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 test) for at least 4 years. Participants underwent phlebotomy (100 ml to 180 ml), and blood was processed within 4 hours of collection. Plasma was separated by centrifugation of whole blood at 400 × g for 10 minutes, and then plasma was centrifuged at 1,350 × g for 15 minutes. Both centrifugations used a Thermo Scientific Sorvall Legend X1 centrifuge accommodating 50-ml tubes. The cell-free plasma was then harvested and stored at −80°C in 1.5-ml aliquots. All plasma samples were collected between 2012 and 2015.
Low copy number HIV-1 RNA plasma standards.
Plasma from a viremic HIV-1-positive individual with an HIV-1 RNA value of 139,845 cps/ml and an exact integrase sequence match to iSCA primers and probe (15) was collected and stored in aliquots at −80°C. To generate low copy number HIV-1 RNA plasma standards of 20, 5, 4, 3, 2, 1, 0.3, and 0.1 cps/ml, the viremic plasma was diluted with SeraCon Matribase negative Diluent (catalog number 1800-0005, SeraCare) and filtered with an EMD Millipore Stericup sterile vacuum filter unit (0.45-μm HV Durapore membrane). Low copy number HIV-1 RNA plasma standards were stored at −80°C in 1.8-ml aliquots.
RCAS internal control for viral RNA recovery.
For this and previous studies, a known quantity of replication-competent avian leukosis virus (ALV) long terminal repeat (LTR) with a splice adaptor (RCAS) (12, 15, 17–19) virions (1.2 × 106) was spiked into each plasma sample and measured as an internal control for viral RNA recovery and amplification. The RCAS internal control was obtained from the HIV Dynamics and Replication Program (HIV DRP) at the National Cancer Institute courtesy of Stephen H. Hughes (https://home.ncifcrf.gov/hivdrp/rcas/contact.html). Each batch of cell culture supernatant is tested by our laboratory by iSCA v2.0 to confirm the amount of virus in the RCAS spikes. The number of virions in the RCAS spike was determined by performing qRT-PCR on serial dilutions of culture supernatant from RCAS plasmid-transfected DF-1 cells (18, 19). Only plasma samples with greater than 10% of the average RCAS recovery in the within-run plasma standards (5 and 20 cps/ml) were considered to have adequate RNA recovery.
Integrase single copy assay v1.0.
iSCA v1.0 was performed as reported without modification (17).
Integrase single copy assay v2.0.
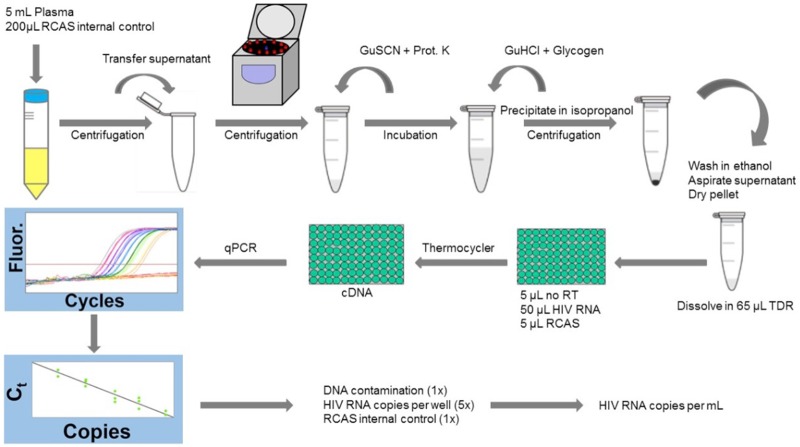
Isolation of nucleic acid. Total nucleic acid was isolated from plasma samples by modifying previously reported methods (12, 15) (Table 1; Fig. 1). Plasma aliquots from the same donor or HIV-1 RNA standard were thawed, pooled, and spiked with RCAS as described above. The samples were centrifuged at 2,700 × g for 15 minutes at 4°C to pellet debris, such as fibrin, lipid, and other insoluble complexes. Prior studies showed that this initial round of centrifugation improved HIV-1 and RCAS RNA recovery (15). Plasma supernatants were then transferred to 5-ml Eppendorf PCR-clean safe-lock tubes. Virions were pelleted by centrifugation at 21,000 × g for 2 hours at 4°C in a refrigerated Eppendorf 5427R centrifuge with a 5-ml rotor. Pilot experiments comparing centrifugation for 60, 90, and 120 minutes revealed that recovery of HIV-1 and RCAS RNA were greatest at 120 minutes (data not shown). Pelleted virions were then lysed with 3 M guanidinium hydrochloride (GuHCl) supplemented with 50 mM Tris-HCl, 1 mM CaCl2, and 1 mg/ml proteinase K for 1 hour at 42°C, followed by the addition of 6 M guanidinium thiocyanate (GuSCN) supplemented with 50 mM Tris-HCl, 1 mM EDTA, and 600 μg/ml of glycogen, and then total nucleic acids were precipitated by the addition of 100% isopropanol and centrifuged at 21,000 × g for 20 minutes at room temperature. Pellets were washed with 70% ethanol, air-dried, and resuspended in 65 μl of 5 mM Tris-HCl supplemented with 1 mM dithiothreitol (DTT) and 1,000 units/ml of RNasin.
TABLE 1.
Protocol differences between iSCA v1.0 and v2.0a
| iSCA v1.0 | iSCA v2.0 |
|---|---|
| Extract ≤7 ml plasma | Extract ≤5 ml plasma |
| Pellet virions at 170,000 × g for 30 minutes at 4°C in ultracentrifuge | Pellet virions at 21,000 × g for 2 hours at 4°C in tabletop centrifuge |
| 18 wells for HIV-1 RNA standard curve | 14 wells for HIV-1 RNA standard curve |
| 2 RCAS internal standard wells for each sample | 1 RCAS internal standard well for each sample |
| Extracted RNA pellet resuspended in 50 μl TDRb | Extracted RNA pellet resuspended in 65 μl TDRb |
| 3 wells of 10 μl each tested for HIV-1 RNA | 5 wells of 10 μl each tested for HIV-1 RNA |
| 54.5% extracted nucleic acid tested for HIV-1 RNA | 76.9% extracted nucleic acid tested for HIV-1 RNA |
| 5- and 20-cps/ml standards obtained from VQAc | HIV-1 standards of 5- and 20-cps/ml diluted from exact primer match patient plasma |
Bolded items represent major changes.
TDR, 5 mM Tris-HCl, 1 mM DTT, and 1,000 units/ml RNasin.
Virology Quality Assessment.
FIG 1.
iSCA v2.0 work flow. Plasma samples are spiked with a known quantity of RCAS and centrifuged for 15 minutes at 2,700 × g and 4°C to pellet debris. The supernatant is transferred to a 5-ml snap-cap tube and centrifuged at 21,000 × g at 4°C for 2 hours, pelleting the virions. After aspirating the supernatant, the pellet is lysed with 3 M GuHCl supplemented with 50 mM Tris-HCl, 1 mM CaCl2, and 1 mg/ml proteinase K for 1 hour at 42°C. Next, 6 M GuSCN supplemented with 50 mM Tris-HCl, 1 mM EDTA, and 600 μg/ml glycogen is added, and the entire volume is transferred to a 2-ml snap-cap tube. To precipitate the nucleic acid, 100% isopropanol is added and the tube is centrifuged at 21,000 × g for 20 minutes at room temperature. The supernatant is aspirated and the pellet washed with 70% ethanol and centrifuged at 21,000 × g at room temperature for 15 minutes. After the 70% ethanol is aspirated, the pelleted nucleic acid is resuspended in 65 μl of TDR. The resuspended nucleic acid extract is divided into 7 reactions, including 5 HIV-1 RNA reactions, 1 RCAS reaction, and 1 no reverse transcriptase reaction. cDNA is synthesized by reverse transcriptase. Each cDNA reaction contains 30 μl total, including 20 μl of cDNA reaction mix (with or without RT), 5 or 10 μl of nucleic acid extract, and 5 μl of molecular biology grade water as needed. For real-time PCR, 20 μl of ready-made master mix with forward and reverse primers and probe for HIV-1 integrase or RCAS gag brings the final reaction volume up to 50 μl. See Materials and Methods for further details.
Reverse transcriptase quantitative PCR. A two-step qRT-PCR was used to quantify HIV-1 RNA. cDNA was produced in a reverse transcription reaction as previously described (12, 15). Each cDNA reaction contained a final concentration of 5 mM MgCl2, 0.5 mM deoxynucleoside triphosphate (dNTP), 1 mM DTT, 0.15 μg random hexamers, 1× in-house buffer A (100 mM Tris-HCl, 500 mM M KCl, 0.2% Tween, and molecular grade water), 20 U RNAsin, and 20 U AffinityScript multiple temperature reverse transcriptase (catalog number 600107, Agilent). A total of 50 μl of nucleic acid extract was tested for HIV-1 RNA (5 separate 10-μl PCRs), representing 77% of the extract assayed for HIV-1 RNA. Reverse transcriptase (RT) reaction mixtures were incubated at 25°C for 15 minutes, 42°C for 40 minutes, 85°C for 10 minutes, and 25°C for 30 minutes. Of the remaining nucleic acid extract, 5 μl was tested for RCAS RNA and 5 μl was tested for HIV-1 DNA contamination by excluding the reverse transcriptase from the reaction mix. Single “no template” control reaction mixtures containing no HIV-1 RNA or RCAS transcripts were included in each run to screen for contaminating HIV-1 or RCAS nucleic acids. All no template control and no “RT control” reactions were negative throughout the course of this study.
To quantify HIV-1 RNA, a standard curve was generated for each run by a serial 3-fold dilution of HIV-1 RNA transcripts that had been characterized by optical density at 260 nm and serial endpoint dilution to 1 copy per qRT-PCR (15). The diluted HIV-1 RNA transcript reactions contained 10 μl of transcripts in a 30-μl total RT reaction.
For real-time PCR, a total volume of 50 μl containing 1.0× ready-made LightCycler 480 probes master mix (catalog number 4887301001, Roche) was used with either 400 nM forward (5′-TTTGGAAAGGACCAGCAAA-3′) and reverse primers (5′-CCTGCCATCTGTTTTCCA-3′) and 200 nM probe (5′-AAAGGTGAAGGGGCAGTAGTAATACA-3′) for HIV-1 integrase in the pol gene or 600 nM forward (5′-GTCAATAGAGAGAGGGATGGACAAA-3′) and reverse primers (5′-TCCACAAGTGTAGCAGAGCCC-3′) and 100 nM probe (5′-TGGGTCGGGTGGTCGTGCC-3′) for the RCAS gag gene. Cycling parameters for real-time PCR were 95°C for 10 minutes, followed by 95°C for 15 seconds and 60°C for 1 minute, for 50 cycles of amplification.
Donor plasma samples were always processed in parallel with HIV-negative plasma (Rush University, Virology Quality Assurance Lab) and low copy number HIV-1 RNA plasma standards containing 5 cps/ml and 20 cps/ml of HIV-1 RNA (described above in “Low copy number HIV-1 RNA plasma standards”). HIV-1 RNA or DNA was not detected in the HIV-negative plasma samples throughout the study.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 7 and SAS 9.3, and all tests for significance were performed using a two-sided α value of 0.05 as significant. To assess the limit of detection (LoD) of iSCA v2.0, samples with detectable HIV-1 RNA were designated a “hit” and samples with nondetected HIV-1 RNA were designated a “nonhit.” The LoD was calculated using the LoD_Est SAS macro (17).
RESULTS
Performance of iSCA v2.0.
Each iSCA v2.0 run included processing and testing of an HIV-1-negative plasma sample for HIV-1 RNA and DNA (no RT control) contamination. A total of 42 HIV-1-negative plasma samples and 431 no RT reactions did not detect HIV-1 RNA or DNA. Serial 3-fold dilutions of HIV-1 RNA transcripts were used to generate a standard curve for each assay run; a statistical evaluation of the dilution series for all assay runs reported here showed minimal variation between runs and maintained a strong correlation (R2 = 0.9935) with expected values (see Fig. S1 in the supplemental material).
RCAS recovery from plasma standards averaged 76% (range, 34% to 100%) of the RCAS spike and 33% (range, 12% to 100%) from donor plasma samples. The lower RCAS recovery in donor plasma than that of the filtered plasma standards is not surprising and likely results from insoluble macromolecular complexes precipitated from unfiltered plasma.
iSCA v2.0 LoD.
Prior studies evaluated the single-copy detection limit by diluting HIV-1 RNA transcripts to endpoint and observing a detection frequency adhering to a Poisson distribution (12, 15). Testing a dilution series of virions in plasma provides a better assessment of the LoD since it includes the crucial variable of extraction efficacy of HIV-1 viral RNA from plasma. To determine the LoD, multiple 5-ml replicates (between 10 and 20) of a serial dilution series of low copy number HIV-1 RNA plasma standards ranging from 4.0 cps/ml to 0.1 cps/ml were tested by iSCA v2.0. HIV-1 RNA was detected in all 10 replicates of HIV-1 RNA plasma standards containing 4, 3, and 2 cps/ml (100% detection). Twenty replicates were tested of the 1, 0.3, and 0.1 cps/ml HIV-1 RNA standards to define the 95% LoD (Table 2). Using the LoD_Est SAS macro (17) the estimated 95% LoD was 0.994 cps/ml (95% confidence intervals (CI), 0.62 to 2.55 cps/ml) for a 5-ml sample (Fig. 2). Table 2 also shows that the observed and expected values for the total HIV-1 RNA copies detected from each standard are remarkably similar, with the detected total copies being ≥100% of expected.
TABLE 2.
Results of low-copy plasma standards used for calculating the 95% LoD of iSCA v2.0
| HIV-1 RNA plasma standard (cps/ml) | No. of replicates tested | No. of replicates detected | Replicates detected (%) | Mean HIV-1 RNA cps expected | Mean HIV-1 RNA cps detected | Mean HIV-1 RNA (cps/ml) |
|---|---|---|---|---|---|---|
| 4 | 10 | 10 | 100 | 20 | 23.7 | 4.7 |
| 3 | 10 | 10 | 100 | 15 | 19.0 | 3.8 |
| 2 | 10 | 10 | 100 | 10 | 11.6 | 2.3 |
| 1 | 20 | 19 | 95 | 5 | 6.4 | 1.3 |
| 0.3 | 20 | 12 | 60 | 1.5 | 3.8 | 1.2 |
| 0.1 | 20 | 4 | 20 | 0.5 | 0.52 | 0.5 |
FIG 2.
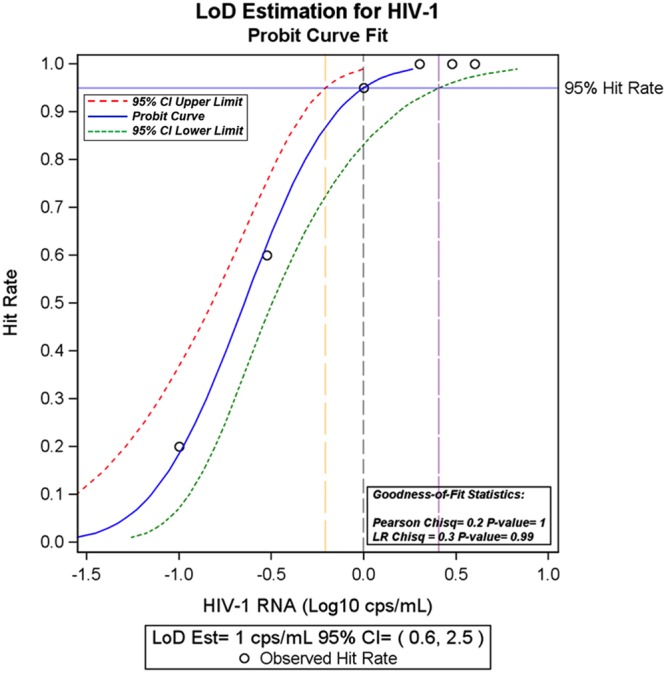
Probit analysis of LoD for iSCA v2.0. Multiple replicates of low-copy HIV-1 plasma RNA standards were tested using iSCA v2.0 (Table 2). To calculate the LoD, samples with detectable HIV-1 RNA were designated a hit and samples without detectable HIV-1 RNA were classified as a nonhit. The LoD-Est SAS macro was used to analyze the data in SAS. LoD is conventionally defined as the concentration where 95% of samples are detectable (17). For a 5-ml plasma sample, the 95% LoD is 1 copy per ml (95% CI, 0.6 to 2.5 copies/ml).
Intraassay and interassay variation.
We evaluated the intraassay and interassay variability of the iSCA v2.0 assay by testing 11 replicates of low copy number HIV-1 RNA plasma standards (5 cps/ml and 20 cps/ml) in each of three runs (Fig. 3). Measurement variation was higher for the 5-copy/ml than the 20-copy/ml standard. Specifically, for the 5-copy/ml standard, intraassay mean values (standard deviation) were 5.5 (2.8), 5.3 (2.5), and 3.6 (1.5) cps/ml with coefficients of variation (%CV) of 50.9%, 47.7%, and 43.1%, respectively (see Table S1 in the supplemental material). For the 20-copy/ml standard, mean values (standard deviation) were 23.4 (4.9), 21.6 (2.2), and 34.8 (7.9) copies/ml with %CV of 20.4%, 10.4%, and 22.9%, respectively (Table S1). As expected, interrun %CV was higher for both standards, but not substantially so, being 51% for the 5-copy/ml standard, and 30% for the 20-copy/ml standard.
FIG 3.
Intraassay and interassay variation of iSCA v2.0. Each detected value was log10 transformed and subtracted from the log10 expected value (log10 5 or log10 20 copies per ml). The results were plotted as a histogram illustrating the difference between the detected and expected values for HIV-1 RNA in the 5-cps/ml standard (A) and 20-cps/ml standard (B). The dashed lines indicate no difference between expected and detected values.
Comparison of iSCA v1.0 and iSCA v2.0 results on donor plasma samples.
To determine whether iSCA v2.0 enhanced detection of HIV-1 RNA, we tested a panel of 60 plasma samples from individuals on suppressive ART with both versions of the assay. For clinical samples, an identical volume of plasma was tested by iSCA v1.0 and iSCA v2.0 with a mean volume of 3.5-ml plasma and a range of 1.0-ml to 5.0-ml plasma. Of the 60 samples, 31 had no detectable HIV-1 RNA by iSCA v1.0. Of these 31 samples, 17 (55%) had HIV-1 RNA detected by iSCA v2.0. The 17 newly detectable samples had an HIV-1 RNA mean of 3.5 cps/ml, with a range of 0.4 cps/ml to 18.9 cps/ml (Table 3). Twenty-nine samples had HIV-1 RNA detected by iSCA v1.0. Of these 29 samples, 17 (59%) had higher levels of HIV-1 RNA measured by iSCA v2.0, averaging 2.7-fold higher than the value obtained by iSCA v1.0 (Table 4). Figure 4 shows the Bland-Altman plot comparing the values obtained with the two versions of the assays and the bias toward higher levels with v2.0, especially at higher HIV-1 RNA concentrations.
TABLE 3.
Summary of results of donor samples tested with iSCA v2.0 that were negative for HIV-1 RNA by v1.0
| Plasma sample | iSCA v1.0 |
iSCA v2.0 |
||||
|---|---|---|---|---|---|---|
| Vol (ml) | HIV-1 RNA (copies) | HIV-1 RNA (cps/ml) | Vol (ml) | HIV-1 RNA (copies) | HIV-1 RNA (cps/ml) | |
| 1 | 4.4 | 0.0 | <0.4 | 4.3 | 0.0 | <0.3 |
| 2 | 4.2 | 0.0 | <0.6 | 4.2 | 36.4 | 8.7 |
| 3 | 2.9 | 0.0 | <0.6 | 2.9 | 15.6 | 5.4 |
| 4 | 5.0 | 0.0 | <0.4 | 5.0 | 0.0 | <0.3 |
| 5 | 3.0 | 0.0 | <0.6 | 2.8 | 1.3 | 0.5 |
| 6 | 2.9 | 0.0 | <0.6 | 2.9 | 0.0 | <0.4 |
| 7 | 4.3 | 0.0 | <0.4 | 4.2 | 2.6 | 0.6 |
| 8 | 2.9 | 0.0 | <0.6 | 2.9 | 7.8 | 2.7 |
| 9 | 2.6 | 0.0 | <0.7 | 2.6 | 0.0 | <0.5 |
| 10 | 3.0 | 0.0 | <0.6 | 2.6 | 0.0 | <0.5 |
| 11 | 4.4 | 0.0 | <0.4 | 4.4 | 0.0 | <0.3 |
| 12 | 4.5 | 0.0 | <0.4 | 4.5 | 5.2 | 1.2 |
| 13 | 4.4 | 0.0 | <0.6 | 4.2 | 0.0 | <0.3 |
| 14 | 2.9 | 0.0 | <0.6 | 2.9 | 1.3 | 0.4 |
| 15 | 4.4 | 0.0 | <0.4 | 4.4 | 3.9 | 0.9 |
| 16 | 2.8 | 0.0 | <0.7 | 2.8 | 15.6 | 5.6 |
| 17 | 4.2 | 0.0 | <0.4 | 4.2 | 2.6 | 0.6 |
| 18 | 4.4 | 0.0 | <0.4 | 4.4 | 0.0 | <0.3 |
| 19 | 4.4 | 0.0 | <0.4 | 4.4 | 0.0 | <0.3 |
| 20 | 4.3 | 0.0 | <0.4 | 4.3 | 0.0 | <0.3 |
| 21 | 4.4 | 0.0 | <0.4 | 4.4 | 0.0 | <0.3 |
| 22 | 3.2 | 0.0 | <0.6 | 2.9 | 15.6 | 5.4 |
| 23 | 4.5 | 0.0 | <0.4 | 4.2 | 15.6 | 3.7 |
| 24 | 2.7 | 0.0 | <0.7 | 2.7 | 0.0 | <0.5 |
| 25 | 2.9 | 0.0 | <0.6 | 2.9 | 5.2 | 1.8 |
| 26 | 4.4 | 0.0 | <0.4 | 4.4 | 0.0 | <0.3 |
| 27 | 4.3 | 0.0 | <0.4 | 4.3 | 6.5 | 1.5 |
| 28 | 4.2 | 0.0 | <0.4 | 4.2 | 79.3 | 18.9 |
| 29 | 2.9 | 0.0 | <0.6 | 2.9 | 0.0 | <0.4 |
| 30 | 4.4 | 0.0 | <0.4 | 4.3 | 5.2 | 1.2 |
| 31 | 4.4 | 0.0 | <0.4 | 4.4 | 3.9 | 0.9 |
TABLE 4.
Summary of results of donor samples with HIV-1 RNA detected by iSCA v1.0 and tested by iSCA v2.0
| Plasma sample | iSCA v1.0 |
iSCA v2.0 |
|||||
|---|---|---|---|---|---|---|---|
| Vol (ml) | HIV-1 RNA (copies) | HIV-1 RNA (cps/ml) | Vol (ml) | HIV-1 RNA (copies) | HIV-1 RNA (cps/ml) | Fold change from v1.0a | |
| 1 | 4.2 | 7.1 | 1.7 | 4.3 | 75.4 | 17.5 | 10.3 |
| 2 | 2.9 | 1.7 | 0.6 | 2.8 | 28.6 | 10.2 | 17.0 |
| 3 | 2.7 | 5.4 | 2.0 | 2.7 | 15.6 | 5.8 | 2.9 |
| 4 | 2.9 | 18.3 | 6.3 | 2.9 | 16.9 | 5.8 | 0.9 |
| 5 | 4.4 | 18.5 | 4.2 | 4.4 | 26.0 | 5.9 | 1.4 |
| 6 | 4.3 | 36.6 | 8.5 | 4.3 | 37.7 | 8.8 | 1.0 |
| 7 | 2.9 | 18.3 | 6.3 | 2.9 | 19.5 | 6.7 | 1.1 |
| 8 | 2.9 | 5.5 | 1.9 | 2.7 | 1.3 | 0.5 | 0.3 |
| 9 | 4.3 | 1.7 | 0.4 | 4.2 | 5.2 | 1.3 | 3.3 |
| 10 | 1.0 | 1.8 | 1.8 | 1.0 | 10.4 | 10.4 | 5.8 |
| 11 | 2.9 | 7.3 | 2.5 | 2.9 | 1.3 | 0.4 | 0.2 |
| 12 | 2.8 | 3.6 | 1.3 | 2.8 | 10.4 | 3.7 | 2.8 |
| 13 | 2.9 | 16.5 | 5.7 | 2.9 | 3.9 | 1.3 | 0.2 |
| 14 | 4.3 | 14.6 | 3.4 | 4.2 | 0.0 | <0.3b | N/Ac |
| 15 | 4.3 | 9.0 | 2.1 | 4.3 | 5.2 | 1.2 | 0.6 |
| 16 | 4.2 | 9.2 | 2.2 | 4.2 | 2.6 | 0.6 | 0.3 |
| 17 | 3.0 | 20.1 | 6.7 | 2.9 | 27.3 | 9.4 | 1.4 |
| 18 | 2.9 | 11.0 | 3.8 | 2.8 | 31.2 | 11.3 | 3.0 |
| 19 | 3.0 | 3.6 | 1.2 | 2.8 | 2.6 | 0.9 | 0.8 |
| 20 | 2.9 | 11.0 | 3.8 | 2.9 | 23.4 | 8.1 | 2.1 |
| 21 | 3.2 | 27.5 | 8.6 | 3.2 | 83.2 | 26.0 | 3.0 |
| 22 | 4.4 | 7.5 | 1.7 | 4.3 | 6.5 | 1.5 | 0.9 |
| 23 | 3.0 | 12.9 | 4.3 | 2.9 | 22.1 | 7.6 | 1.8 |
| 24 | 3.0 | 5.4 | 1.8 | 3.0 | 22.1 | 7.6 | 4.2 |
| 25 | 3.0 | 23.7 | 7.9 | 2.8 | 67.6 | 24.1 | 3.1 |
| 26 | 4.3 | 5.6 | 1.3 | 4.3 | 0.0 | <0.3b | N/A |
| 27 | 2.8 | 27.4 | 9.8 | 2.8 | 3.9 | 1.4 | 0.1 |
| 28 | 2.9 | 5.5 | 1.9 | 2.9 | 28.6 | 9.9 | 5.2 |
| 29 | 4.1 | 36.5 | 8.9 | 4.1 | 16.9 | 4.1 | 0.5 |
The average fold difference was 2.7.
Samples 14 and 26 were initially undetected by iSCA v2.0 with HIV-1 RNA values of <0.3 cps/ml. However, upon subsequent iSCA v2.0 testing, the samples both were positive with HIV-1 RNA values of 0.3 and 3.9 cps/ml, respectively.
N/A, not applicable.
FIG 4.
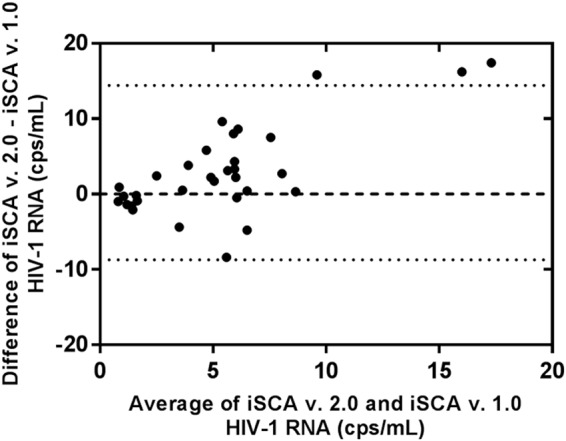
Bland-Altman plot comparing results of donor samples with detectable HIV-1 RNA by both iSCA v1.0 and v2.0. Dotted lines indicate 95% confidence interval; the dashed line indicates no difference in the values for HIV-1 RNA (cps/ml) generated by the two methods.
DISCUSSION
We report here a new version of the iSCA assay (v2.0) that simultaneously simplifies sample processing and increases sensitivity for HIV-1 RNA detection in clinical samples. The previous version of the single-copy assay targeting HIV-1 integrase region of pol (iSCA v1.0) has been used to gain insight into the persistence of viremia on ART and to evaluate the efficacy of clinical interventions aimed at altering the level of persistent viremia on ART (9, 10, 13, 14, 16, 18–21). An important improvement in v2.0 was the replacement of ultracentrifugation with centrifugation in a refrigerated microcentrifuge. Switching to a microcentrifuge simplifies processing of samples and reduces expense, making the assay more feasible for other laboratories. In addition, widely available 5-ml snap-cap tubes are used instead of cumbersome tubes with narrow openings required for ultracentrifugation.
Compared with the original version, iSCA v2.0 saves about an hour during the HIV-1 RNA extraction step. The primary workflow advantage of iSCA v2.0 is scalability; purchasing additional microcentrifuges allows a laboratory to increase throughput. iSCA v2.0 should be immediately accessible to most laboratories with qRT-PCR experience. Establishing designated clean and dirty spaces would be one of the greater limiting factors for a laboratory interested in using iSCA v2.0. Furthermore, it is significantly faster and easier to train an individual on iSCA v2.0 because ultracentrifugation and the use of awkward ultracentrifuge tubes with narrow openings is not required.
To improve the sensitivity of the assay for HIV-1 RNA, a greater proportion (77%) of the extract was tested for HIV-1 RNA. We noted in iSCA v1.0 that HIV-1 RNA was not homogeneously distributed through the plasma extract because not all of the three PCRs were positive for HIV-1, suggesting that testing a greater fraction of the extraction would reduce false-negative results. The rarity of HIV-1 DNA contamination in properly collected and processed plasma and the consistent recovery of the RCAS internal standard motivated us to reduce the volume of extract used for the no RT control (from 10 μl to 5 μl) and for quantification of RCAS recovery (also from 10 μl to 5 μl) and increase the volume of extract assayed for HIV-1 RNA. Indeed, testing a greater fraction of the extract for HIV-1 RNA improved the detection of HIV-1 RNA both qualitatively (higher proportion of samples positive for HIV-1 RNA) and quantitatively (higher levels detected). About half (55%) of the plasma samples from participants on suppressive ART that were undetectable by iSCA v1.0 had HIV-1 RNA detected by iSCA v2.0, with HIV-1 RNA values ranging from 0.4 to 18.9 cps/ml. The greater frequency of detection in v2.0 was not attributable to higher volumes of plasma because equal volumes were tested by both versions of the assay. However, a greater proportion of the total nucleic acid was tested by iSCA v2.0 (76.9%) than iSCA v1.0 (54.5%) by increasing the number of RT-PCRs for HIV-1 RNA. The higher sensitivity is, thus, due to more nucleic acid extract being assayed from a greater proportion of the plasma sample. Each RT-PCR for HIV-1 RNA did not contain more extract (10 μl) in iSCA v2.0; hence, we did not expect a difference in interfering substances. In addition, for samples with detectable HIV-1 RNA by both assays, iSCA v2.0 detected higher values in 17 of 29 samples, with an average of 2.7-fold higher levels of HIV-1 RNA detected across all samples. Lower centrifugation speed in the user-friendly snap-cap centrifuge tubes may have also contributed to greater HIV-1 RNA recovery than ultracentrifugation in larger tubes with narrow and difficult-to-access openings.
Although there was an overall increase in the concentration of HIV RNA detected in donor plasma using iSCA v2.0, in 10 of 29, samples the values generated from iSCA v2.0 were somewhat lower than the original values generated by iSCA v1.0. In addition, 2 samples that were detectable by iSCA v1.0 were not initially detectable by iSCA v2.0; both were detectable upon repeat testing with iSCA v2.0. The variability observed could be explained, in part, by high %CV at low copy numbers due to Poisson distribution. In addition, donor plasma contains more lipids and proteinaceous complexes than the HIV RNA standards that were generated with the filtered plasma, which could also contribute to higher variation of results from the same plasma donation. Finally, a nonhomogeneous distribution of virions in plasma aliquots and of extracted RNA in solution could contribute to the variation in results.
The 95% LoD for HIV-1 RNA of FDA-cleared automated assays ranges from 40 to 20 cps/ml (22–24). By comparison, the 95% LoD for iSCA v2.0, determined by probit analysis of 10 to 20 replicates of plasma HIV-1 RNA standards, is 1 copy/ml for a 5-ml plasma sample. This result is the first formal evaluation using probit analysis of the LoD for a single-copy assay. Furthermore, the observed HIV-1 RNA recovery from the HIV-1 standards approximates 100% of the expected recovery (Table 2). These observations suggest that the improvements in v2.0 of the assay have increased HIV-1 RNA recovery to near maximal. The limit of blank (LoB) is defined as the highest possible concentration that results when testing a blank. Because none of our negative samples had any detectable HIV-1 RNA, we could not calculate an LoB. We did not calculate a limit of quantitation (LoQ) due to variation in definitions of LoQ. We, therefore, focused on defining the LoD rather than the LoQ.
By testing plasma standards with known low concentrations of HIV-1 RNA (20 cps/ml and 5 cps/ml) in 3 runs with 11 replicates each, we were able to determine interassay and intraassay variation as measured by %CV. As expected, assay variation was higher at lower nominal HIV-1 RNA concentrations due to the decreased probability of detecting a copy of HIV-1 RNA in any given reaction. The interrun variation was somewhat greater than the intrarun variation for both 5-copy/ml and 20-copy/ml plasma standards. In the case of clinical interventions, the difference between a few copies of HIV-1 RNA per milliliter is not likely to be meaningful. For example, if the HIV-1 RNA value detected by iSCA v2.0 decreases from 3 to 2 cps/ml after an intervention, it would not be considered significant. In contrast, a decrease of several fold of the HIV-1 RNA concentration or a change from a detectable value to a consistently nondetectable value could be considered therapeutic responses. In the latter instance, the high %CV at 5 copies/ml would be less important. As for many manual assays, greater interrun than intrarun variation is likely due to operator, procedural, and reagent differences between runs. Consequently, to minimize the impact of assay variation on results, it is recommended that longitudinal samples from the same individual be assayed together in one iSCA v2.0 run. A total of 11 samples along with standards can be included in each run. In a circumstance where it would be necessary to decrease %CV, it would be possible to implement up to 11 5-mL replicates in a single run. Prior studies with iSCA v1.0 have demonstrated that running multiple replicates will decrease %CV and the LoD (15). The limitations of this approach are obtaining the required plasma volumes and sacrificing sample throughput.
The limitations of the current study include the relatively small number of participant samples evaluated by iSCA v1.0 and iSCA v2.0 and that only HIV-1 subtype B was used. The number of plasma samples with adequate volume was limited because it was necessary to test an equal volume of plasma by both methods.
The improved version of iSCA v2.0 has important implications for assessing persistent viremia. Compared with v1.0, v2.0 had fewer false-negative samples. Censoring below the limit of detection is the major limitation of single copy assays because the level of persistent viremia on ART is generally less than a few copies/ml and declines with time on ART (14). In addition, the greater sensitivity of iSCA v2.0 did not generate more frequent false-positive results, as none of the 0-copy/ml controls or no template controls included in each run detected HIV-1 DNA or RNA.
Recent technological advances have resulted in increasingly accurate and low-cost automated droplet digital PCR (ddPCR) platforms which can be used to quantify HIV-1 nucleic acid in cells and plasma at the single-copy level (25, 26). However, multiple studies have reported difficulties in differentiating positive from negative droplets as well as encountering positives in negative controls (25–27). Minimizing false positives and further validation would be required before using ddPCR as a single-copy assay to quantify HIV-1 nucleic acid in a diagnostic setting. iSCA v2.0 could be used as a comparison for the performance validation of ddPCR platforms for single-copy HIV-1 RNA detection. Furthermore, iSCA v2.0 could also be used to validate the results of automated platforms that report values below the limit of quantification as either “detectable but not quantifiable” or “target not detected.” Indeed, a recent comparison of iSCA v1.0 with an automated HIV-1 RNA platform showed strong concordance between single-copy assay results and those from the automated platform below the limit of quantification (28).
In summary, we have improved and validated an HIV-1 RNA single-copy assay (iSCA v2.0) with a lower LoD and the potential for more widespread use. The growing interest in assessing a broad range of immunological and pharmacological interventions to lower plasma viremia and reduce HIV-1 reservoirs requires the continued improvement of measures of residual viremia. The lower LoD of iSCA v2.0, combined with less startup cost and simpler sample processing, makes iSCA v2.0 a useful tool for evaluating persistent viremia before and after experimental interventions.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number 5UM1AI126603. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract number HHSN261200800001E. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
J.W.M. is a consultant to Gilead Sciences and Merck and has received grants from Gilead Sciences and Janssen Pharmaceuticals, Inc. He owns shares in Co-Crystal Pharma, Inc. M.A.T., J.L.J., K.A.S., and J.C.C. have no conflicts to report.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01714-18.
REFERENCES
- 1.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17:1871–1879. doi: 10.1097/01.aids.0000076308.76477.b8. [DOI] [PubMed] [Google Scholar]
- 2.Mellors JW, Rinaldo CR Jr, Gupta P, White RM, Todd JA, Kingsley LA. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167–1170. [DOI] [PubMed] [Google Scholar]
- 3.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR Jr, 1997. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 126:946–954. [DOI] [PubMed] [Google Scholar]
- 4.Mellors JW, Kingsley LA, Rinaldo CR Jr, Todd JA, Hoo BS, Kokka RP, Gupta P. 1995. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med 122:573–579. [DOI] [PubMed] [Google Scholar]
- 5.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 6.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582–1586. [DOI] [PubMed] [Google Scholar]
- 7.Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 8.Dornadula G, Zhang H, VanUitert B, Stern J, Livornese JL, Ingerman MJ, Witek J, Kedanis RJ, Natkin J, DeSimone J, Pomerantz RJ. 1999. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 9.Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, Kovacs JA, Davey RT, Rock-Kress D, Dewar R, Liu S, Metcalf JA, Rehm C, Brun SC, Hanna GJ, Kempf DJ, Coffin JM, Mellors JW. 2007. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog 3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS. 2008. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng L, Bosch RJ, Chan ES, Read S, Kearney M, Margolis DM, Mellors JW, Eron JJ, Gandhi RT, Team DSCTGA. 2013. Predictors of residual viraemia in patients on long-term suppressive antiretroviral therapy. Antivir Ther 18:39–43. doi: 10.3851/IMP2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, Dewar RL, Planta A, Liu S, Metcalf JA, Mellors JW, Coffin JM. 2003. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol 41:4531–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrade A, Rosenkranz SL, Cillo AR, Lu D, Daar ES, Jacobson JM, Lederman M, Acosta EP, Campbell T, Feinberg J, Flexner C, Mellors JW, Kuritzkes DR, Team ACTGA. 2013. Three distinct phases of HIV-1 RNA decay in treatment-naive patients receiving raltegravir-based antiretroviral therapy: ACTG A5248. J Infect Dis 208:884–891. doi: 10.1093/infdis/jit272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riddler SA, Aga E, Bosch RJ, Bastow B, Bedison M, Vagratian D, Vaida F, Eron JJ, Gandhi RT, Mellors JW, Team AAP. 2016. Continued slow decay of the residual plasma viremia level in HIV-1-infected adults receiving long-term antiretroviral therapy. J Infect Dis 213:556–560. doi: 10.1093/infdis/jiv433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cillo AR, Vagratian D, Bedison MA, Anderson EM, Kearney MF, Fyne E, Koontz D, Coffin JM, Piatak M Jr, Mellors JW. 2014. Improved single-copy assays for quantification of persistent HIV-1 viremia in patients on suppressive antiretroviral therapy. J Clin Microbiol 52:3944–3951. doi: 10.1128/JCM.02060-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi RT, McMahon DK, Bosch RJ, Lalama CM, Cyktor JC, Macatangay BJ, Rinaldo CR, Riddler SA, Hogg E, Godfrey C, Collier AC, Eron JJ, Mellors JW, Team AA. 2017. Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 13:e1006285. doi: 10.1371/journal.ppat.1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canchola JA, Hemyari P. 2016. Limit of detection (LoD) estimation using parametric curve fitting to (hit) rate data: the LoD_Est SAS macro, abstr 1720-2016. SAS Global Forum 2016, Las Vegas, NV, April 2016 http://support.sas.com/resources/papers/proceedings16/1720-2016.pdf. [Google Scholar]
- 18.Gandhi M, Gandhi RT, Stefanescu A, Bosch RJ, Cyktor JC, Horng H, Louie A, Phung N, Eron JJ, Hogg E, Macatangay BJC, Hensel C, Fletcher CV, Mellors JW, McMahon DK, Team A. 2018. Cumulative antiretroviral exposure measured in hair is not associated with measures of HIV persistence or inflammation among individuals on suppressive ART. J Infect Dis 218:234–238. doi: 10.1093/infdis/jiy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N, Cyktor JC, Coffin JM, Acosta EP, Koup RA, Mellors JW, Eron JJ, Team ACTS. 2017. Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J Infect Dis 215:1725–1733. doi: 10.1093/infdis/jix191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291–1295. [DOI] [PubMed] [Google Scholar]
- 21.Simonetti FR, Spindler J, Wu X, Hill S, Shao W, Mellors JW, Hughes SH, Kearney MF, Maldarelli F, Coffin JM. 2016. Analysis of HIV proviruses in clonally expanded cells in vivo, abstr 337. Conference on Retroviruses and Opportunistic Infection (CROI), Boston, MA, February 2016. [Google Scholar]
- 22.Hologic, Inc. 2017. Aptima HIV-1 Quant assay package insert. AW-13242, Rev. 001. Hologic, Inc., San Diego, CA: https://www.hologic.com/sites/default/files/package-insert/AW-13242_001_02.pdf. [Google Scholar]
- 23.Roche Molecular Systems, Inc. 2007. COBAS AmpliPrep/COBAS TaqMan HIV-1 test FDA premarket approval application. Roche Molecular Systems, Inc, Pleasanton, CA: https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/ucm092879.pdf. [Google Scholar]
- 24.Abbott Molecular, Inc. 2011. Abbott RealTime HIV-1 assay package insert. Abbott Molecular, Inc., Des Plaines, IL: https://www.molecular.abbott/sal/en-us/staticAssets/realtime-hiv-1-package-insert.pdf. [Google Scholar]
- 25.Kiselinova M, Pasternak AO, de Spiegelaere W, Vogelaers D, Berkhout B, Vandekerckhove L. 2014. Comparison of droplet digital PCR and seminested real-time PCR for quantification of cell-associated HIV-1 RNA. PLoS One 9:e85999. doi: 10.1371/journal.pone.0085999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trypsteen W, Kiselinova M, Vandekerckhove L, De Spiegelaere W. 2016. Diagnostic utility of droplet digital PCR for HIV reservoir quantification. J Virus Erad 2:162–169. [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson EM, Wiegand A, Boltz VF, Spindler J, Dueppen P, Troppman R, Maldarelli F, Mellors JW, Kearney MF, Coffin J. 2012. Single-copy detection of plasma HIV-1 RNA using droplet digital PCR technology, abstr V-1002. Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA, March 2012. [Google Scholar]
- 28.Margot N, Koontz D, McCallister S, Mellors JW, Callebaut C. 2018. Measurement of plasma HIV-1 RNA below the limit of quantification (<20 copies/mL) of commercial assays with the integrase HIV RNA single-copy assay. J Clin Virol 108:50–52. doi: 10.1016/j.jcv.2018.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.