Seasonal influenza virus causes significant morbidity and mortality each year. Point-of-care (POC) testing using rapid influenza diagnostic tests (RIDTs), immunoassays that detect viral antigens, are often used for diagnosis by physician offices and urgent care centers.
KEYWORDS: clinical impact, influenza, PCR, point-of-care testing, rapid diagnosis
ABSTRACT
Seasonal influenza virus causes significant morbidity and mortality each year. Point-of-care (POC) testing using rapid influenza diagnostic tests (RIDTs), immunoassays that detect viral antigens, are often used for diagnosis by physician offices and urgent care centers. These tests are rapid but lack sensitivity, which is estimated to be 50 to 70%. Testing by PCR is highly sensitive and specific, but historically these assays have been performed in centralized clinical laboratories necessitating specimen transport and increasing the time to result. Recently, Clinical Laboratory Improvement Amendments (CLIA)-waived, POC PCR influenza assays have been developed with >95% sensitivity and specificity compared to centralized PCR assays. To determine the clinical impact of a POC PCR test for influenza, we compared antimicrobial prescribing patterns of one urgent care location using the Cobas LIAT Influenza A/B assay (LIAT assay; Roche Diagnostics, Indianapolis, IN) to other urgent care centers in our health system using traditional RIDT, with negative specimens being reflexed to PCR. Antiviral prescribing was lower in patients with a negative LIAT PCR result (2.3%) than in patients with a negative RIDT result (25.3%; P < 0.005). Antivirals were prescribed more often in patients that tested positive by LIAT PCR (82.4%) than in those testing positive by either RIDT or reflex PCR (69.9%; P < 0.05). Antibacterial prescriptions for patients testing negative by LIAT PCR were higher (44.5%) than for those testing negative by RIDT (37.7%), although the difference was not statistically significant. In conclusion, having results from a PCR POC test during the clinic visit improved antiviral prescribing practices compared to having rapid results from an RIDT.
INTRODUCTION
Influenza is an important cause of mortality and morbidity worldwide with up to 500,000 deaths annually (1). Clinically, influenza is often diagnosed by combining presenting symptoms, physical examination, and local patterns of influenza-like illness (ILI), but such diagnoses are often inaccurate (2–4). A laboratory confirmation of infection aids in the initiation of early and appropriate antiviral therapy, reduces unnecessary antibacterial use, and impacts social stability during seasonal outbreaks and pandemics of influenza (5, 6).
Rapid influenza diagnostic tests (RIDTs) are the mainstay of point-of-care (POC) testing due to their ease of use and prompt availability of results in less than 20 min. Unfortunately, these assays have demonstrated poor performance. A recent meta-analysis of 159 studies evaluating 26 RIDTs yielded a pooled sensitivity of only 62.3% (95% confidence interval [CI], 57.9 to 66.6%) (7). In addition, a negative test result is often unreliable with negative likelihood ratios as low as 0.38 (CI, 0.34 to 0.43) (7). Reverse transcription PCR (RT-PCR; here by referred to as PCR) assays have become the gold standard for diagnosis of respiratory viral infections with improved sensitivity and reduced turnaround time compared to viral culture; their performance characteristics have also been found to be significantly better than RIDTs, which are preferred as POC tests (8). Traditionally, PCR assays have been performed at a centralized laboratory due to the high level of technical skills and sophisticated instrumentation required for testing. Specimens are often processed in batches and, along with transportation time, this leads to a delay in the availability of results which can exceed 24 h. Current guidelines recommend that antivirals should be initiated within 48 h of onset of symptoms, and within this window period early initiation increases the medication’s efficacy (9, 10). Therefore, in the urgent care setting, influenza PCR testing in centralized laboratories has very little impact on the management of patients, contributing to the continued use of RIDTs despite their suboptimal performance.
Recently the U.S. Food and Drug Administration has cleared several Clinical Laboratory Improvement Amendments (CLIA)-waived, POC PCR assays for the detection of influenza A and B viruses. One such assay, the Cobas LIAT Influenza A/B assay (LIAT assay; Roche Diagnostics, Indianapolis, IN), is simple to perform, requiring 2 min of hands-on time and a sample-to-answer turnaround time (TAT) of approximately 20 min. Each instrument can run one test at a time, and the platform has the ability to be interfaced to the laboratory information system for direct result reporting. The Cobas LIAT Influenza A/B assay has been evaluated in multiple studies with demonstrated sensitivity and specificity of >95% compared to moderate complexity PCR assays (11–13).
We implemented the LIAT assay at one urgent care center and compared antimicrobial prescribing for respiratory disease to the other five urgent care centers in our system that continued to use POC RIDT for the detection of influenza A and B. We hypothesized that using a highly sensitive POC PCR assay for detection of influenza with results available within the time frame of the patient visit would result in more appropriate antimicrobial prescribing practices.
MATERIALS AND METHODS
Study setting and design.
This retrospective study from January through June 2017 included patients that visited urgent care centers that are a part of NorthShore University HealthSystem and were tested for influenza during this period. This four-hospital health care organization in suburban Chicago has more than 100 physician offices and six urgent care centers. All of the urgent care centers have a common medical and administrative leadership. Each urgent care is located in a medical office building that houses not only the urgent care office but other family medicine and specialty care offices. The urgent care facilities have between three and four exam rooms and are usually staffed by one physician at a time with a nurse practitioner or physician’s assistant providing additional support at peak times. All sites have a laboratory/phlebotomy area in the building where specimen collections and POC testing are performed by phlebotomists, and these laboratory sites are under the supervision of the Department of Pathology and Laboratory Medicine. Patients receive POC testing at the urgent care labs only when it has been ordered by a provider. These sites currently do not perform testing for any symptoms prior to being admitted to an exam room.
The study was approved by the Institutional Review Board at NorthShore University HealthSystem as a waived study.
Point-of-care testing algorithm.
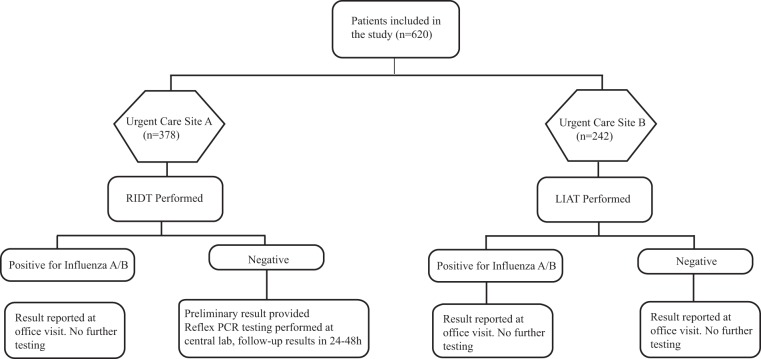
The testing algorithm is shown in Fig. 1. At urgent care site B, patients were tested using the LIAT assay at the phlebotomy service center located on site, and this was performed by phlebotomists that had been trained to use POC tests. The results were reported immediately to the clinician, and no confirmatory testing was performed.
FIG 1.
Influenza testing protocols.
At all of the other five urgent care sites (referred to collectively as urgent care site A) patients were tested using the Quidel QuickVue Influenza A+B assay (RIDT assay; Quidel Corp., San Diego, CA). Negative specimens were further evaluated by batched reflex confirmatory testing by PCR using the Simplexa Flu A/B RSV assay (Diasorin Molecular LLC, Cypress, CA) performed at our centralized clinical laboratory.
Both RIDT and LIAT assays were performed on nasopharyngeal swabs collected from patients presenting to the urgent care center. The swabs were placed in universal transport media (UTM; Becton Dickinson, Sparks, MD) after collection and, in the event of a negative RIDT result, the UTM was transported to the centralized laboratory for confirmatory PCR testing.
Data collection and analysis.
A retrospective chart review was performed for all patients tested by the LIAT assay, and all patients tested using RIDT were also reflex tested by PCR. Demographic data, influenza testing results, and antimicrobial prescription records were reviewed and recorded.
Antimicrobial prescribing data were collected for (i) patients that received oseltamivir and (ii) patients that received an antibacterial agent recommended for upper respiratory tract infections as per a physician note in the chart. Prescriptions of antibacterial agents for reasons other than a respiratory tract infection, such as for a wound infection, were excluded from the analysis.
The antimicrobial prescription, the clinical diagnosis, and the result of the POC testing in patients visiting urgent care site A were compared to those for patients visiting the other urgent care sites. Statistical significance was assessed using a Pearson’s χ2 test. All data processing and statistical analysis were performed in Excel or R version 3.4.4 (14).
RESULTS
A total of 620 patients were enrolled in the study with 378 in site A and 242 in site B. Patient demographics were comparable as outlined in Table 1 . The median turnaround times for specimen collection to result verification for the POC tests were 16 min (95% CI, 5 to 29 min) for the RIDT and 29 min (95% CI, 21 to 59 min) for the LIAT. The median turnaround time for the reflex PCR performed at our centralized clinical laboratory was 21 h (95% CI, 5 to 28 h).
TABLE 1.
Patient characteristics, prescriptions and testing results using POC testing and reflex PCRa
| Characteristic | RIDT |
LIAT |
P | ||||
|---|---|---|---|---|---|---|---|
| Flu A (n = 81) | Flu B (n = 52) | Negative (n = 245)b | Flu A (n = 63) | Flu B (n = 51) | Negative (n = 128) | ||
| Age, mean (range) | 36.0 (0.5–81.0) | 29.2 (1.7–90.4) | 45.9 (2.8–94.5) | 41.0 (5.1–88.3) | 32.8 (2.6–93.7) | 41.3 (0.7–86.3) | NS |
| Male (%) | 40.7 | 42.3 | 40.0 | 39.7 | 47.1 | 37.5 | NS |
| Antivirals prescribed (n) | 67 | 32 | 42 | 47 | 38 | 3 | |
| Antibiotics prescribed (n) | 0 | 1 | 70 | 2 | 3 | 57 | |
| Both prescribed (n) | 0 | 4 | 20 | 5 | 4 | 0 | |
| None prescribed (n) | 14 | 15 | 113 | 9 | 6 | 68 | |
| Reflex PCR positive (n) | NA | NA | 70 | NA | NA | NA | |
n, number of patients. NS, not significant; NA, not applicable.
Reflexed to PCR. Median TAT to PCR results: 20.2 h (1.8–64.2 h).
At urgent care site A, 378 patients were tested by RIDT and 133 (35.2%) tested positive for influenza. Upon reflex testing, however, an additional 70 patients were determined to be positive by PCR (40 positive for influenza A and 30 positive for influenza B), yielding an overall positivity rate for influenza at site A of 53.7% and a negative predictive value for RIDT of 71.4% (95% CI, 65.2 to 76.9%) (Table 1). At urgent care site B, 242 patients were tested by LIAT, and 114 (47.3%) tested positive for influenza (Table 1). The positivity rate of influenza in the urgent care centers A and B were similar when total number of positive patients (RIDT and PCR) was considered (P = 0.1); however, if RIDT alone was considered, the positivity rate for influenza was significantly higher at site B compared to site A (P = 0.003).
Antimicrobials (including antivirals and antibiotics) were prescribed in 395 (63.7%) patients that visited the urgent care centers for ILI, with 166 (26.7%) patients receiving antibiotics either alone or in combination with antiviral agents. When a result was available at the time of visit, patients that were positive for influenza by LIAT assay were prescribed antiviral medications 82.4% of the time compared to 77.4% of patients prescribed antivirals following positive RIDT testing (not significant [NS]) (Table 2). Patients testing positive by either RIDT or PCR were prescribed antivirals 69.9% of the time, which is significantly less than the LIAT group (P < 0.05). Only 2.3% of patients that tested negative for influenza received antivirals following LIAT testing compared to 13.1% of patients testing negative for influenza by RIDT and reflex PCR (P < 0.005) (Table 2).
TABLE 2.
Comparison of antimicrobial prescriptions based on POC and batched PCR test resultsa
| Patient group | Negative results: |
Positive results: |
||||||
|---|---|---|---|---|---|---|---|---|
| At visit (RIDT) | After visit (RIDT+PCR) | At visit (LIAT) | P | At visit (RIDT) | After visit (RIDT+PCR) | At visit (LIAT) | P | |
| % with antiviral prescription | 25.3 (62/245) | 13.1 (23/175) | 2.3 (3/128) | <0.005† | 77.4 (103/133)* | 69.9 (142/203)A | 82.4 (94/114)B* | <0.05(A vs B) |
| % with antibiotic prescription | 36.7 (90/245)* | 37.7 (66/175)* | 44.5 (57/128)* | 3.8 (5/133)C | 14.3 (29/203)* | 12.3 (14/114)D* | <0.05(C vs D) | |
*, The difference between these groups was not statistically significant; †, the P value shows the comparison of both groups (RIDT and RIDT+PCR) to LIAT.
Antibiotic prescribing was not statistically different between the LIAT and RIDT with reflex PCR groups. For patients testing influenza negative by LIAT, 57 of 128 (44.5%) patients received an antibiotic as opposed to 66 of 175 (37.7%) in the RIDT with reflex PCR group (NS; P = 0.2) (Table 2). Antibiotic prescribing was not significantly different between patients testing positive for influenza by LIAT and RIDT with reflex PCR group, with 12.3 and 14.3%, respectively, receiving antibiotics (Table 2). Antibiotics prescribed in the two groups were similar, with >80% being azithromycin or a combination of amoxicillin and amoxicillin/clavulanate. The specific antibiotics prescribed were azithromycin (49.5%), amoxicillin (22%), amoxicillin/clavulanate (16%), doxycycline (10.5%), and penicillin (1%) in the RIDT group and azithromycin (47%), amoxicillin (14%), amoxicillin/clavulanate (21%), doxycycline (4%), levofloxacin (13%), sulfamethoxazole/trimethoprim (6%), and cefdinir (1%) in the LIAT group (three patients in LIAT group received more than one antibiotic).
DISCUSSION
Our study was designed to measure the effect of POC PCR testing for influenza on antimicrobial prescribing practices compared to that of traditional RIDTs in an urgent care setting. Specifically, we measured the impact of the LIAT assay, a CLIA-waived, PCR assay that requires only 2 min of hands-on time, with a total run time of <20 min, and which is designed for POC use. The assay has >95% sensitivity and specificity compared to traditional influenza PCR assays, which negates the need for PCR confirmation of negative results. Because our LIAT assay results were available within a median time of 29 min, clinicians had laboratory results available during the patient visit which they could use to determine whether an antiviral or antibacterial prescription was warranted.
LIAT testing resulted in a 12.5% increase in antiviral prescriptions for patients with positive results, from 69.9% with RIDT and reflex PCR to 82.4% with LIAT. On the other hand, only 2.3% of patients that tested negative by LIAT were prescribed antiviral medication compared to 13.1% using the RIDT with the reflex PCR method. Both were statistically significant improvements in prescribing patterns. Our results suggest that the higher sensitivity and negative predictive value provide confidence in the test results provided during the patient encounter, thus positively impacting antimicrobial stewardship. Antibacterial use was similar in patients that tested influenza positive by LIAT and RIDT with reflex PCR yielding 12.3 and 14.3%, respectively. These data are in agreement with a recent study evaluating the use of POC RT-PCR testing and its impact in the care of patients in the emergency department that found the rapid assays impacted patient management in 61% of cases, with the greatest impact being in antimicrobial stewardship (15).
Of patients testing negative for influenza in our study, those tested by LIAT received antibacterials more frequently than those tested by RIDT (44.5% versus 37.7%). While the difference was not statistically significant, this observation is different than a recent randomized controlled trial that measured the impact of a multiplexed molecular POC PCR for a broad range of respiratory viruses. This study performed in hospitalized adults found that while the assay provided a faster TAT for results, the numbers of patients placed on antibacterials were similar, regardless of the results of the assay (83% versus 84%). This study was performed in the hospital setting where antibiotics might have been administered to patients after they were admitted and were likely more ill, as opposed to our study where patients were sent home with prescriptions for oral antibiotics. However, the study did find that an increased proportion of patients in the positive group (i.e., patients with a respiratory viral infection) received shorter antibacterial courses (16). Since our study was performed in an outpatient setting, we were unable to extract data regarding discontinuation of antibiotics. Future studies in the outpatient setting should focus on antibacterial use to determine whether the nonsignificance observed in our study is due to the limited cohort size or whether there was truly no difference.
There are several studies that have measured the impact of performing influenza testing compared to diagnosis by symptoms alone on the management of patients with influenza during emergency department visits. The results of these studies are variable. In children, the use of POC antigen testing has been shown to reduce the number of additional tests performed (including chest X-rays, blood cultures, procalcitonin tests, and respiratory viral panel PCRs, among others), as well as reduce antibiotic prescriptions and length of hospital stay. In adults, the discontinuation of antibiotics was increased for patients that tested positive for influenza by RIDT, but the hospital length of stay was not significantly different regardless of the influenza testing result (17, 18). McCulloh et al. investigated antiviral prescribing practices in children with influenza-like illness using a conventional PCR assay (19). These researchers found that patients that were positive for influenza by PCR were prescribed more oseltamivir than those that tested negative (76.9% versus 18%), but discontinuation of empirically prescribed oseltamivir was not associated with a negative influenza test, which was attributed to a 20-h TAT for the respiratory viral panel testing used in their study. Finally, RIDTs for influenza have been shown to reduce the ordering of additional tests and reduce the number of antibiotic prescriptions (20, 21).
Outside patient age and gender, as outlined in Table 1, multiple study characteristics were analyzed to ensure comparability between our two urgent care sites. Ordering provider training was compiled and found to be similar in both groups, with >75% of patients in both groups being seen by a physician board certified in either family medicine, emergency medicine, or internal medicine. The only apparent difference is that significantly more patients were seen by a physician board certified in pediatrics in the LIAT group and, upon further analysis, this was attributable to one active physician that only practices at that site. In addition, to help control for differences in provider practice, we analyzed how frequently patients were seen by a physician that provided care at both sites. This showed that of the 620 patients in the study, 445 or 72% were seen by a provider that saw patients at both locations. Finally, all sites showed a similar distribution for insurance payers, with 21% having either Medicare or Medicaid, and 83% of all patients had previously been seen at our health system in some capacity (data in this paragraph are not shown in the tables).
Future studies measuring the economic impact of POC molecular testing in outpatient setting are warranted. Although LIAT testing is more expensive than RIDTs alone, the cost to the patient is comparable to that of RIDT plus a confirmatory PCR test. In addition, in our study a majority of these patients (>90%) with positive results on reflex testing received calls from their physician’s office to inform them of their test results and often received their antiviral prescription during this follow-up call. Therefore, in patients that need a reflex PCR testing additional logistic and clinical expenses such as transport to the lab, management of orders and specimens, and administrative tasks pose a significant economic impact on laboratory testing and clinical care. For our RIDT site, 65% of the specimens were negative and required confirmatory PCR testing at our centralized clinical laboratory.
Our study is limited in that this was a retrospective study and the LIAT assay was used at a single urgent care center during a single influenza season. While we attempted to select similar urgent care facilities for LIAT and RIDT testing sites, we could not completely control for differences in physician practices between the centers. Testing was only performed for influenza, so it is unknown whether patient symptoms were due to other respiratory viruses. In addition, patients with positive RIDTs were not confirmed by PCR, so the false-positivity rate for influenza in our study is unknown. Several other studies, however, have evaluated the Quidel QuickVue A+B assay and found it to have specificities greater than 97% for influenza A and B compared to RT-PCR (22–24). Based on this, we would estimate around seven false positives in our patient cohort. This number of false positives was modeled in our data set, and we found that it did not affect the statistical significance of our study conclusions. Future, prospective studies, including larger patient cohorts, would be useful in studying the clinical and economic impact of POC testing in the management of influenza, especially with regard to antimicrobial stewardship.
We do not have follow-up data on patients after their urgent care visit, including prescription discontinuation, patient outcomes, hospital admissions, or emergency department visits, for either the LIAT or the RIDT group. Patients with positive RIDTs were not confirmed by PCR, so the false-positivity rate for influenza in our study is unknown. Future prospective studies that include larger patient cohorts would be useful in studying the clinical and economic impact of POC testing in the management of influenza especially with regard to antimicrobial stewardship.
Conclusion.
To the best of our knowledge, there are no studies that have investigated the impact of POC molecular assays in the management of ILI in urgent care settings. We found that the use of a highly sensitive and specific POC PCR test for influenza detection led to higher antiviral prescribing in positive patients (82.4% versus 69.9%) and decreased use in negative patients (2.3% versus 13.1%) compared to the RIDT with reflex PCR. For patients who tested negative for influenza, we saw a slight, but nonsignificant increase in antibiotic use. The availability of rapid tests with high levels of sensitivity and specificity enables clinicians to have greater confidence in these timely results, in turn facilitating more appropriate prescribing patterns and overall improved antimicrobial stewardship. The potential impact on patient care may justify the added cost of performing PCR assays on site. Future studies are needed to investigate additional clinical and economic impacts of POC PCR for influenza in this and other patient care settings.
ACKNOWLEDGMENTS
We thank Tammy Taylor and Adam Matsil for their assistance with data retrieval.
REFERENCES
- 1.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, Krishnan A, Ope M, Lindblade KA, Carosone-Link P, Lucero M, Ochieng W, Kamimoto L, Dueger E, Bhat N, Vong S, Theodoratou E, Chittaganpitch M, Chimah O, Balmaseda A, Buchy P, Harris E, Evans V, Katayose M, Gaur B, O’Callaghan-Gordo C, Goswami D, Arvelo W, Venter M, Briese T, Tokarz R, Widdowson M-A, Mounts AW, Breiman RF, Feikin DR, Klugman KP, Olsen SJ, Gessner BD, Wright PF, Rudan I, Broor S, Simões EAF, Campbell H. 2011. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 378:1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 2.Babcock HM, Merz LR, Dubberke ER, Fraser VJ. 2008. Case-control study of clinical features of influenza in hospitalized patients. Infect Control Hosp Epidemiol 29:921–926. doi: 10.1086/590663. [DOI] [PubMed] [Google Scholar]
- 3.Babcock HM, Merz LR, Fraser VJ. 2006. Is influenza an influenza-like illness? Clinical presentation of influenza in hospitalized patients. Infect Control Hosp Epidemiol 27:266–270. doi: 10.1086/501539. [DOI] [PubMed] [Google Scholar]
- 4.van den Dool C, Hak E, Wallinga J, van Loon AM, Lammers JW, Bonten MJ. 2008. Symptoms of influenza virus infection in hospitalized patients. Infect Control Hosp Epidemiol 29:314–319. doi: 10.1086/529211. [DOI] [PubMed] [Google Scholar]
- 5.Rolfes MA, Yousey-Hindes KM, Meek JI, Fry AM, Chaves SS. 2016. Respiratory viral testing and influenza antiviral prescriptions during hospitalization for acute respiratory illnesses. Open Forum Infect Dis 3:ofv216. doi: 10.1093/ofid/ofv216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juretschko S, Mahony J, Buller RS, Manji R, Dunbar S, Walker K, Rao A. 2017. Multicenter clinical evaluation of the Luminex Aries Flu A/B & RSV Assay for pediatric and adult respiratory tract specimens. J Clin Microbiol 55:2431–2438. doi: 10.1128/JCM.00318-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chartrand C, Leeflang MM, Minion J, Brewer T, Pai M. 2012. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med 156:500–511. doi: 10.7326/0003-4819-156-7-201204030-00403. [DOI] [PubMed] [Google Scholar]
- 8.Merckx J, Wali R, Schiller I, Caya C, Gore GC, Chartrand C, Dendukuri N, Papenburg J. 2017. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: a systematic review and meta-analysis. Ann Intern Med 167:394–409. doi: 10.7326/M17-0848. [DOI] [PubMed] [Google Scholar]
- 9.Andrews D, Chetty Y, Cooper BS, Virk M, Glass SK, Letters A, Kelly PA, Sudhanva M, Jeyaratnam D. 2017. Multiplex PCR point of care testing versus routine, laboratory-based testing in the treatment of adults with respiratory tract infections: a quasi-randomized study assessing impact on length of stay and antimicrobial use. BMC Infect Dis 17:671. doi: 10.1186/s12879-017-2784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthuri SG, Venkatesan S, Myles PR, Leonardi-Bee J, Al Khuwaitir TSA, Al Mamun A, Anovadiya AP, Azziz-Baumgartner E, Báez C, Bassetti M, Beovic B, Bertisch B, Bonmarin I, Booy R, Borja-Aburto VH, Burgmann H, Cao B, Carratala J, Denholm JT, Dominguez SR, Duarte PAD, Dubnov-Raz G, Echavarria M, Fanella S, Gao Z, Gérardin P, Giannella M, Gubbels S, Herberg J, Iglesias ALH, Hoger PH, Hu X, Islam QT, Jiménez MF, Kandeel A, Keijzers G, Khalili H, Knight M, Kudo K, Kusznierz G, Kuzman I, Kwan AMC, Amine IL, Langenegger E, Lankarani KB, Leo Y-S, Linko R, Liu P, Madanat F, Mayo-Montero E, McGeer A, Memish Z, Metan G, Mickiene A, Mikić D, Mohn KGI, Moradi A, Nymadawa P, Oliva ME, Ozkan M, Parekh D, Paul M, Polack FP, Rath BA, Rodríguez AH, Sarrouf EB, Seale AC, Sertogullarindan B, Siqueira MM, Skręt-Magierło J, Stephan F, Talarek E, Tang JW, To KKW, Torres A, Törün SH, Tran D, Uyeki TM, Van Zwol A, Vaudry W, Vidmar T, Yokota RTC, Zarogoulidis P, Nguyen-Van-Tam JS. 2014. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2:395–404. doi: 10.1016/S2213-2600(14)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binnicker MJ, Espy MJ, Irish CL, Vetter EA. 2015. Direct detection of influenza A and B viruses in less than 20 minutes using a commercially available rapid PCR assay. J Clin Microbiol 53:2353–2354. doi: 10.1128/JCM.00791-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Tian Y, Chen S, Liesenfeld O. 2015. Performance of the Cobas® Influenza A/B assay for rapid PCR-based detection of influenza compared to Prodesse ProFlu+ and viral culture. Eur J Microbiol Immunol 5:236–245. doi: 10.1556/1886.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolte FS, Gauld L, Barrett SB. 2016. Direct comparison of Alere I and COBAS Liat influenza A and B tests for rapid detection of influenza virus infection. J Clin Microbiol 54:2763–2766. doi: 10.1128/JCM.01586-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R Core Team. 2018. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 15.Hansen GT, Moore J, Herding E, Gooch T, Hirigoyen D, Hanson K, Deike M. 2018. Clinical decision making in the emergency department setting using rapid PCR: results of the CLADE study group. J Clin Virol 102:42–49. doi: 10.1016/j.jcv.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brendish NJ, Malachira AK, Armstrong L, Houghton R, Aitken S, Nyimbili E, Ewings S, Lillie PJ, Clark TW. 2017. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomized controlled trial. Lancet Respir Med 5:401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonner AB, Monroe KW, Talley LI, Klasner AE, Kimberlin DW. 2003. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics 112:363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- 18.Falsey AR, Murata Y, Walsh EE. 2007. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med 167:354–360. doi: 10.1001/archinte.167.4.ioi60207. [DOI] [PubMed] [Google Scholar]
- 19.McCulloh RJ, Andrea S, Reinert S, Chapin K. 2014. Potential utility of multiplex amplification respiratory viral panel testing in the management of acute respiratory infection in children: a retrospective analysis. J Pediatr Infect Dis Soc 3:146–153. doi: 10.1093/jpids/pit073. [DOI] [PubMed] [Google Scholar]
- 20.Blaschke AJ, Shapiro DJ, Pavia AT, Byington CL, Ampofo K, Stockmann C, Hersh AL. 2014. A national study of the impact of rapid influenza testing on clinical care in the emergency department. J Pediatr Infect Dis Soc 3:112–118. doi: 10.1093/jpids/pit071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hojat K, Duppenthaler A, Aebi C. 2013. Impact of the availability of an influenza virus rapid antigen test on diagnostic decision making in a pediatric emergency department. Pediatr Emerg Care 29:696–698. doi: 10.1097/PEC.0b013e3182948f11. [DOI] [PubMed] [Google Scholar]
- 22.Koul PA, Mir H, Bhat MA, Khan UH, Khan MM, Chadha MS, Lal RB. 2015. Performance of rapid influenza diagnostic tests (QuickVue) for influenza A and B Infection in India. Indian J Med Microbiol 33:26–31. doi: 10.4103/0255-0857.148831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehlmann M, Bonner AB, Williams JV, Dankbar DM, Moore CL, Kuchta RD, Podsiad AB, Tamerius JD, Dawson ED, Rowlen KL. 2007. Comparison of the MChip to viral culture, reverse transcription-PCR, and the QuickVue influenza A+B test for rapid diagnosis of influenza. J Clin Microbiol 45:1234–1237. doi: 10.1128/JCM.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouleau I, Charest H, Douville-Fradet M, Skowronski DM, De Serres G. 2009. Field performance of a rapid diagnostic test for influenza in an ambulatory setting. J Clin Microbiol 47:2699–2703. doi: 10.1128/JCM.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]