The Accelerate Pheno system (AXDX) is a rapid phenotypic bacterial identification and susceptibility testing system which is approved for use with positive blood cultures. Acinetobacter baumannii is a nosocomial pathogen for which the limited treatment options include minocycline in the case of multidrug resistance.
KEYWORDS: fluorescence in situ hybridization, morphokinetic cellular analysis, rapid diagnostics, susceptibility testing
ABSTRACT
The Accelerate Pheno system (AXDX) is a rapid phenotypic bacterial identification and susceptibility testing system which is approved for use with positive blood cultures. Acinetobacter baumannii is a nosocomial pathogen for which the limited treatment options include minocycline in the case of multidrug resistance. Here, we studied the performance of A. baumannii identification and minocycline susceptibility testing by AXDX using 101 contemporary Acinetobacter sp. clinical isolates. Overall, the sensitivity for A. baumannii and A. baumannii complex identification was 100% (73/73) and 97.6% (82/84), respectively. Specificity for A. baumannii complex identification was 86.6% (13/15). The essential agreement of minocycline susceptibility results (±1 log2 MIC agreement) of AXDX MICs with reference broth microdilution was 98.0% (96/98). There were no very major errors or major errors. Overall, 24.5% (24/98) of results yielded minor errors. AXDX reliably identified A. baumannii and predicted minocycline susceptibility results, which should help guide treatment choices in a timely manner for infections where options are limited.
INTRODUCTION
Acinetobacter baumannii is a major causative organism of bacteremia, pneumonia, and wound infection. Healthcare-associated A. baumannii infections are associated with high mortality rates, due to multidrug resistance (1). A. baumannii is one of four major genomospecies which constitute the A. baumannii complex, which also includes Acinetobacter nosocomialis, Acinetobacter pittii, and Acinetobacter calcoaceticus. The latter three genomospecies are collectively less pathogenic in humans than A. baumannii.
Minocycline is one of the few agents that retains activity against multidrug-resistant A. baumannii, including against carbapenem-resistant strains (2), and may have clinical utility in the treatment of infections caused by such strains (3). Early identification and antimicrobial susceptibility testing (AST) of A. baumannii in positive blood and respiratory cultures is key to early administration of appropriate therapy and may improve the clinical outcome (4). The Accelerate Pheno system (AXDX) is a rapid bacterial identification and AST device which is currently cleared for use with positive blood cultures by the U.S. Food and Drug Administration (FDA) and under development for bronchoalveolar lavage (BAL) and mini-BAL fluid specimens (5). AXDX minocycline results are currently available as research use only (RUO), due to an insufficient number of Acinetobacter spp. encountered in the original clinical trial to allow FDA clearance (6). The aims of this study were to (i) determine the spectra of Acinetobacter species identified by the A. baumannii probe used in the Accelerate Pheno system and (ii) test the performance of the Accelerate Pheno system minocycline AST compared to reference broth microdilution (BMD) for Acinetobacter species.
MATERIALS AND METHODS
Bacterial isolates and identification.
A total of 101 Acinetobacter clinical isolates recovered from unique patients at the University of Pittsburgh Medical Center between 2013 and 2017 were evaluated in this study. Based on the initial species identification at the clinical microbiology laboratory using a Bruker matrix-assisted laser desorption ionization (MALDI) Biotyper (Billerica, MA), these included 71 A. baumannii isolates and 30 belonging to other Acinetobacter genomospecies. For A. baumannii, strains that were nonsusceptible to minocycline were preferentially included for the purpose of AST performance evaluation. Among the isolates evaluated, 60 met the critia for multidrug resistance (i.e., not susceptible to at least 1 agent in ≥3 antimicrobial categories appropriate for Acinetobacter spp.) and 42 met criteria for extensive drug resistance (i.e., not susceptible to at least 1 agent in all but ≤2 antimicrobial categories appropriate for Acinetobacter spp.) as defined by Magiorakos and colleages (7).
Species identification.
The species were identified by a Bruker MALDI Biotyper system (MALDI-TOF) Biotyper in the clinical microbiology laboratory at the time of isolation, which was then repeated with the same instrument at the research laboratory. In addition, 14 non-baumannii A. baumannii complex isolates and 5 non-A. baumannii complex isolates that did not yield a consensus identification between the two MALDI-TOF identification runs were subjected to rpoB sequencing for definitive species identification (8).
Antimicrobial susceptibility testing.
AST was performed on each isolate by Clinical and Laboratory Standards Institute (CLSI) reference broth microdilution (BMD), using panels prepared by Accelerate Diagnostics, Inc. For BMD, the minocycline concentrations tested ranged from 0.125 to 32 μg/ml, and minocycline was obtained from Sigma. Mueller-Hinton broth (Difco, BD, Sparks, MD) was cation adjusted in-house. BMD for minocycline was performed in triplicates using the same inoculum of bacteria, and the modal MIC was used for evaluation (6, 9). If no modal MIC was obtained, BMD testing was repeated in triplicates for the isolate, and a modal MIC was obtained from the results of the six replicate MICs. Samples that did not produce a modal MIC after six replicate BMD tests were excluded from analysis.
Species identification and AST on AXDX.
Using the same source plate used to select colonies for BMD, isolates were subcultured into human blood at a concentration of 10 to 100 CFU/ml, which was then inoculated into a Bactec Plus aerobic blood culture bottle and incubated on a Bactec FX blood culture system (BD, Sparks, MD). Species identification and AST were performed using the Accelerate PhenoTest BC kit run on the Accelerate Pheno system according to the manufacturer’s instructions (10). The range of minocycline MICs available on AXDX is 1 μg/ml to 32 μg/ml.
Statistics.
Sensitivity and specificity were calculated for each identification (ID) target using MALDI-TOF mass spectrometry (MS; or rpoB sequencing, if performed) as the comparator. Essential and categorical agreement values were calculated for minocycline for Accelerate Pheno system-based MICs using the BMD modal MIC as the gold standard. Susceptibility results were interpreted using current CLSI breakpoints (11).
RESULTS AND DISCUSSION
Identification of A. baumannii complex.
Among the 101 isolates evaluated, 30 were initially identified as non-baumannii species by the clinical microbiology laboratory and were examined further by rpoB sequencing. These were identified as A. pittii (n = 5 isolates), Acinetobacter ursingii (n = 5), A. calcoaceticus (n = 4), A. nosocomialis (n = 3), Acinetobacter radioresistens (n = 3), A. baumannii (n = 3), Acinetobacter lwoffii (n = 2), Acinetobacter johnsonii (n = 2), Acinetobacter baylyi (n = 1), and Acinetobacter bereziniae (n = 1). The species of one isolate, initially identified as Acinetobacter haemolyticus, could not be identified definitively by rpoB sequencing. In summary, there were a total of 74 A. baumannii isolates and 27 non-baumannii Acinetobacter isolates.
Accelerate pheno system-based species identification.
Two isolates, one A. baumannii and one A. ursingii, could not be identified due to an insufficient number of cells upon testing; this result was repeated when the isolates were tested a second time. For these two isolates, AXDX yielded an error code, signaling the laboratory would need to test by an alternative method. Of the remaining 73 A. baumannii isolates, all were identified as A. baumannii by AXDX. Among the 11 non-baumannii A. baumannii complex isolates, 5 of 5 A. pittii, 3 of 3 A. nosocomialis, and 2 of 4 A. calcoaceticus isolates resulted in a positive identification by the A. baumannii probe in the Accelerate Pheno system. The 2 A. calcoaceticus isolates that were not identified by AXDX yielded nonidentification upon repeat testing. Among the 15 non-A. baumannii complex isolates, 2 (1 A. radioresistens and 1 A. baylyi) resulted in a positive identification by the A. baumannii probe in the Accelerate Pheno system, while 13 resulted in nonidentification by the A. baumannii target probe, with 11/13 producing an “off panel” result via signal from the universal bacterial fluorescence in situ hybridization (FISH) probe and 2/13 producing no result due to too few cells.
Table 1 summarizes the results. Overall, the sensitivity of identifying A. baumannii complex strains was 97.6% (82 of 84), and the specificity was 86.6% (13 of 15).
TABLE 1.
Number of isolates of each species identified by the AXDX A. baumannii probe
| Species | No. detected/no. tested (%) |
|---|---|
| A. baumannii complexa | 82/84 (97.6) |
| A. baumannii | 73/73 (100) |
| A. nosocomialis | 5/5 (100) |
| A. pittii | 3/3 (100) |
| A. calcoaceticus | 2/4 (50) |
| Non-baumannii complex | 2/15 (13.3) |
| A. ursingiia | 1/5 (20) |
| A. radioresistens | 0/3 (0) |
| A. lwoffii | 0/2 (0) |
| A. johnsonii | 0/2 (0) |
| A. baylyi | 1/1 (100) |
| A. bereziniae | 0/1 (0) |
One isolate yielded a “no identification” result due to an insufficient number of cells in the system.
Few studies have assessed the performance of FDA-cleared rapid ID systems for testing blood cultures for their ability to correctly identify Acinetobacter spp. In the multicenter trial to evaluate AXDX, 69 of 70 isolates of A. baumannii complex were correctly identified (98.6% sensitive), and 3 specimens negative for A. baumannii among a total of 1,857 tested yielded a false-positive A. baumannii complex result (99.8% specificity) (8). The BioFire BCID panel (bioMérieux, Durham, NC), which was designed to identify A. baumannii alone, was shown in a multicenter evaluation to falsely identify 5 non-baumannii isolates among 23 (21.7%) tested as A. baumannii. These included all 4 A. pittii isolates and the 1 A. junii isolate evaluated in the study. In this study, all 51 A. baumannii isolates were correctly identified (12). The Verigene BC-GN test kit (Luminex, Austin, TX), in contrast, identified 62 isolates of Acinetobacter spp. in clinical trials, of which 2 were false positives (not Acinetobater spp.). Additionally, during this study, one isolate of A. baumannii was not detected by the Verigene BC-GN test kit. Unfortunately, in this study, the species of Acinetobacter were not elucidated (13). As such, laboratorians and physicians should be aware that a result of A. baumannii in blood from these rapid identification systems may in fact be A. baumannii, A. baumannii complex, or in some cases, non-baumannii complex species. Importantly, for A. baumannii complex species which are considered to be of clinical significance, AXDX positively identified all strains of A. baumannii, A. pittii, and A. nosocomialis and two of four isolates of A. calcoaceticus.
Accelerate Pheno system-based AST of minocycline.
Minocycline is active against the majority of A. baumannii strains and considered one of the few treatment options available for infections caused by extensively drug-resistant A. baumannii (1). The susceptibility breakpoint currently approved by the CLSI is an MIC of ≤4 μg/ml, with an MIC of 8 μg/ml considered intermediate and ≥16 μg/ml resistant. By the standard BMD, MICs were reliably determined for 100 of 101 tested isolates. One isolate did not grow sufficiently in the BMD panels to accurately determine the MIC. Of the 100 isolates, 85 were susceptible, 14 intermediate, and 1 resistant by BMD. By AXDX, MICs were determined for 98 isolates, where 69 were susceptible, 24 were intermediate, and 5 were resistant (Fig. 1). The isolate that did not grow in BMD panels also did not yield an MIC on AXDX.
FIG 1.
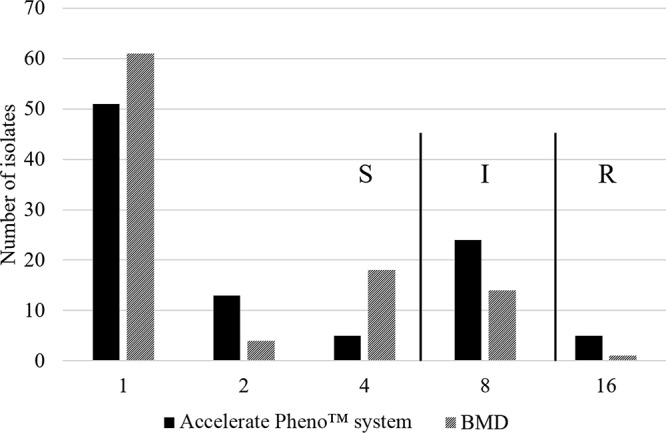
Minocycline MIC (μg/ml) distributions of Acinetobacter isolates by AXDX. S, susceptible; I, intermediate; R, resistant.
The essential agreement (±1 log 2 MIC agreement) of AXDX-based MICs with BMD was 98.0% (96/98). The categorical agreement was 75.5% (74/98); all errors were minor errors, i.e., cases in which one AST method yielded an intermediate interpretation and the other yielded either a susceptible or resistant interpretation. There were no very major errors (i.e., susceptible by AXDX, resistant by BMD) or major errors (resistant by AXDX, susceptible by BMD). The majority of minor errors (20/24) involved AXDX reporting an MIC one dilution higher than the modal BMD MIC (16 μg/ml versus 8 μg/ml in 5 isolates, 8 μg/ml versus 4 μg/ml in 15 isolates). As seen in Fig. 1, the relatively high rate of minor errors was caused by the MIC distribution for minocycline, which bisected the susceptibility breakpoint. Nonetheless, the bimodal distribution of the MICs and the excellent essential agreement suggests that AXDX will rapidly identify the majority of Acinetobacter isolates with low MICs, for which minocycline may be considered a treatment option. A previous study demonstrated similar performance for minocycline MIC determination for Acinetobacter spp. by AXDX. Among 224 isolates evaluated, essential agreement was 97.4% and categorical agreement was 92.1%, and 17 minor errors and 1 major error were observed (8). Previous studies have demonstrated that other commercial AST instruments have similarly yielded a high rate of minor errors when testing A. baumannii against minocycline. In one study, which evaluated the performance of Spanish laboratories to accurately determine A. baumannii AST results, minor errors of 88.6% were observed across all systems evaluated (14). A second study demonstrated minor errors of 15% to 40% across different disk diffusion, Sensititre, and Etest methods (15).
Conclusion.
AXDX was able to identify A. baumannii complex species of clinical significance, including A. baumannii, A. pittii, and A. nosocomialis, with 100% sensitivity. Two non-A. baumannii complex strains were identified as A. baumannii (1 A. radioresistens and 1 A. baylyi). Minocycline MIC AXDX results showed excellent essential agreement of 98.0% with BMD. One limitation of this study was that the majority of isolates tested were susceptible or intermediate to minocycline. Overall, the findings support the ability of AXDX to reliably predict the susceptibility of A. baumannii complex to minocycline, which should help guide treatment choices for infections where options are limited.
ACKNOWLEDGMENTS
This study was funded by Accelerate Diagnostics, Inc. The effort of Y.D. was supported by NIH grants R01AI104895, R21AI123747, and R21AI135522.
J.W.T. and R.M.H. are employees of Accelerate Diagnostics, Inc. Y.D. has consulted for Roche, Tetraphase, Geom, and Recida and received speaking fees from Merck and Astellas.
REFERENCES
- 1.Doi Y, Murray GL, Peleg AY. 2015. Acinetobacter baumannii: evolution of antimicrobial resistance-treatment options. Semin Respir Crit Care Med 36:85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flamm RK, Castanheira M, Streit JM, Jones RN. 2016. Minocycline activity tested against Acinetobacter baumannii complex, Stenotrophomonas maltophilia, and Burkholderia cepacia species complex isolates from a global surveillance program (2013). Diagn Microbiol Infect Dis 85:352–355. doi: 10.1016/j.diagmicrobio.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie DJ, Garavaglia-Wilson A. 2014. A review of intravenous minocycline for treatment of multidrug-resistant Acinetobacter infections. Clin Infect Dis 59 Suppl 6:S374–S380. doi: 10.1093/cid/ciu613. [DOI] [PubMed] [Google Scholar]
- 4.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. 2010. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 54:4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marschal M, Bachmaier J, Autenrieth I, Oberhettinger P, Willmann M, Peter S. 2017. Evaluation of the Accelerate Pheno system for fast identification and antimicrobial susceptibility testing from positive blood cultures in bloodstream infections caused by Gram-negative pathogens. J Clin Microbiol 55:2116–2126. doi: 10.1128/JCM.00181-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pancholi P, Carroll KC, Buchan BW, Chan RC, Dhiman N, Ford B, Granato PA, Harrington AT, Hernandez DR, Humphries RM, Jindra MR, Ledeboer NA, Miller SA, Mochon AB, Morgan MA, Patel R, Schreckenberger PC, Stamper PD, Simner PJ, Tucci NE, Zimmerman C, Wolk DM. 2018. Multicenter evaluation of the Accelerate Phenotest BC kit for rapid identification and phenotypic antimicrobial susceptibility testing using morphokinetic cellular analysis. J Clin Microbiol 56:e01329-17. doi: 10.1128/JCM.01329-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 8.La Scola B, Gundi VA, Khamis A, Raoult D. 2006. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol 44:827–832. doi: 10.1128/JCM.44.3.827-832.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed (M07-A10) Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Accelerate Diagnostics. 2018. Accelerate PhenoTest BC kit instructions for use. Accelerate Diagnostics, Tucson, AZ. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed (M100-S28) Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Salimnia H, Fairfax MR, Lephart PR, Schreckenberger P, DesJarlais SM, Johnson JK, Robinson G, Carroll KC, Greer A, Morgan M, Chan R, Loeffelholz M, Valencia-Shelton F, Jenkins S, Schuetz AN, Daly JA, Barney T, Hemmert A, Kanack KJ. 2016. Evaluation of the FilmArray blood culture identification panel: results of a multicenter controlled trial. J Clin Microbiol 54:687–698. doi: 10.1128/JCM.01679-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledeboer NA, Lopansri BK, Dhiman N, Cavagnolo R, Carroll KC, Granato P, Thomson R Jr, Butler-Wu SM, Berger H, Samuel L, Pancholi P, Swyers L, Hansen GT, Tran NK, Polage CR, Thomson KS, Hanson ND, Winegar R, Buchan BW. 2015. Identification of Gram-negative bacteria and genetic resistance determinants from positive blood culture broths by use of the Verigene Gram-negative blood culture multiplex microarray-based molecular assay. J Clin Microbiol 53:2460–2472. doi: 10.1128/JCM.00581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Cuenca F, Tomas M, Caballero-Moyano FJ, Bou G, Pascual A, Spanish Society of Clinical Microbiology and Infectious Diseses (SEIMC). 2017. Reporting antimicrobial susceptibilities and resistance phenotypes in Acinetobacter spp: a nationwide proficiency study. J Antimicrob Chemother 73:692–697. doi: 10.1093/jac/dkx464. [DOI] [PubMed] [Google Scholar]
- 15.Wang P, Bowler SL, Kantz SF, Mettus RT, Guo Y, McElheny CL, Doi Y. 2016. Comparison of minocycline susceptibility testing methods for carbapenem-resistant Acinetobacter baumannii. J Clin Microbiol 54:2937–2941. doi: 10.1128/JCM.01810-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


