Borrelia miyamotoi disease (BMD) is a newly recognized borreliosis that is cotransmitted by ticks wherever Lyme disease is zoonotic. Unlike Borrelia burgdorferi sensu lato, the agent of Lyme disease, B. miyamotoi is closely related to relapsing fever spirochetes, such as Borrelia hermsii.
KEYWORDS: blood smears, Borrelia miyamotoi, borreliosis, diagnosis, microscopy
ABSTRACT
Borrelia miyamotoi disease (BMD) is a newly recognized borreliosis that is cotransmitted by ticks wherever Lyme disease is zoonotic. Unlike Borrelia burgdorferi sensu lato, the agent of Lyme disease, B. miyamotoi is closely related to relapsing fever spirochetes, such as Borrelia hermsii. Some authors have suggested that the disease caused by B. miyamotoi should be considered a hard-tick-transmitted relapsing fever, and thus, the main mode of confirming a diagnosis for that infection, microscopy to analyze a blood smear, may have clinical utility. To determine whether blood smears may detect B. miyamotoi in the blood of acute BMD patients, we made standard malariological thick smears from anticoagulated blood samples that were previously determined to contain this agent (by PCR) and analyzed them for morphological evidence of spirochetes. Spirochetes were not detected in the blood smears from 20 PCR positive patient blood samples after examination of 100 thick smear fields and only 2 of 20 demonstrated spirochetes when the examination was extended to 300 thick smear fields. Inoculation of severe combined immunodeficient (SCID) mice yielded isolates from 5 of 5 samples, but 0 of 3 BALB/c mice became infected. We conclude that in strong contrast to the diagnosis of typical relapsing fever, microscopy of blood smears is not sensitive enough for confirming a diagnosis of BMD but that SCID mouse inoculation could be a useful complement to PCR.
INTRODUCTION
Borrelia miyamotoi is a tick-transmitted spirochete that was first incriminated as a human etiologic agent in Russia in 2011 (1). The index case of B. miyamotoi disease (BMD) in the United States comprised meningoencephalitis in an elderly immunocompromised patient (2). Additional American cases demonstrated a presentation that was similar to that for human granulocytic ehrlichiosis/anaplasmosis (3), and a recently reported case series from New England (4) demonstrates that BMD is a common zoonosis wherever Lyme disease is reported Although the agent is closely related to that of relapsing fever (5), BMD is distinguished from classical relapsing fever by the absence of sudden onset high fevers terminating with rigors, hyperpyrexia, hypertension, and diaphoresis (6). Nonetheless, there is a suggestion that microscopic examination of blood smears, a definitive method of confirming a relapsing fever diagnosis, might be used to help diagnose BMD (7). Although nucleic acid amplification tests (NAAT; mainly PCR) are now routine for many infectious diseases, blood smears remain a critical “at the bedside” method for some bloodborne infections (e.g., malaria and babesiosis) because a smear could be stained and examined more rapidly than performing PCR within a hospital laboratory. Accordingly, we determined whether the microscopic analysis of Giemsa-stained blood smears might confirm human infection by B. miyamotoi. In addition, we confirmed that inoculation of SCID mice with patient blood sensitively confirms the diagnosis of BMD.
MATERIALS AND METHODS
Aliquots of a series of 20 consecutive acute BMD patient samples submitted to Imugen, Inc. for diagnostic testing were forwarded to the Tufts laboratory. The samples were submitted during July and August 2015 by physicians from clinical practices in southern New England, New Jersey, or New York. The EDTA-anticoagulated blood samples were deidentified but were determined at Imugen by real-time PCR to contain DNA specific for the glpQ gene of B. miyamotoi, as previously described (3, 4). Cycle threshold (CT) values were provided for each sample but no other information was available. At Tufts, standard malariological blood smears (thin and thick) were immediately prepared by a parasitologist experienced in the method. Thick smears were allowed to dry overnight at 37°C and dehemoglobinized in tap water prior to staining. Thin smears were fixed in absolute methanol, and all slides were stained in 2.5% Giemsa (Harleco 619-71; EMD Chemicals, Gibbstown NJ) at pH 7.0 (Sorenson phosphate buffer) for 45 minutes. To ensure that the staining conditions were optimal for detecting borreliae, positive-control blood smears were prepared from blood samples taken from CB17-SCID mice (Taconic, Inc., Germantown, NY) that were chronically infected by the Ipswich strain of B. miyamotoi.
Because some of the patient blood samples had been held for a minimum of 48 hours, and for as long as 7 days at 4°C before they were forwarded to Tufts, we determined whether the lysis of neutrophils in such samples might interfere with the microscopic detection of borreliae. Blood from chronically infected SCID mice was collected into disodium EDTA and held at 4°C, with thin and thick smears made on days 0, 2, 4, and 7. Slides were processed in the same manner as those from the human blood samples.
Blood smears were examined under brightfield (Kohler) illumination using a Zeiss standard 16 microscope (Carl Zeiss, Oberkochen, Germany) using a 63× Plan-apochromat oil immersion objective and 12.5× ocular, resulting in ×788 magnification; a Zeiss Optovar variable magnification insert allowed for rapid inspection of suspicious objects at magnifications up to ×1500. A subcondensor didymium filter was used to enhance the contrast of stained objects. All blood smears were read by the same investigator, who was blind to the DNA copy number estimated by quantitative real-time PCR. A minimum of 100 random oil immersion thick smear fields were examined for each slide, as suggested for malariological studies (8). All slides were read a second time by the same individual for an additional 200 thick smear fields. Finally, slides from those samples containing DNA measuring in the top quartile of genome equivalents (CT values) were read in a blind manner by a second microscopist. We focused solely on analyzing the thick blood smears because they are considered 25 times more sensitive than the standard thin smear for malarial diagnosis (9).
To determine whether mouse inoculation might serve as a useful complement to PCR and blood smear in confirming a diagnosis of BMD, we intraperitoneally inoculated 0.1-ml aliquots of the first 8 blood samples into CB-17 SCID (n = 5) and BALB/c (n = 3) mice (male, 3 to 4 week old, specific pathogen free for both strains; Taconic Laboratories, Germantown, NY) for a total of 8 unique patient samples. Mice were examined for evidence of infection by blood smear every 7 days 7 to 24 days after inoculation. All animal research was conducted under protocols approved by the Tufts University IACUC.
RESULTS AND DISCUSSION
Spirochetes in SCID mouse blood were readily visualized in thick blood smears at ×400 magnification with or without didymium filter enhancement (Fig. 1). Prolonged storage of EDTA-anticoagulated blood from such mice did not alter the staining or morphology of the spirochetes (Fig. 2).
FIG 1.
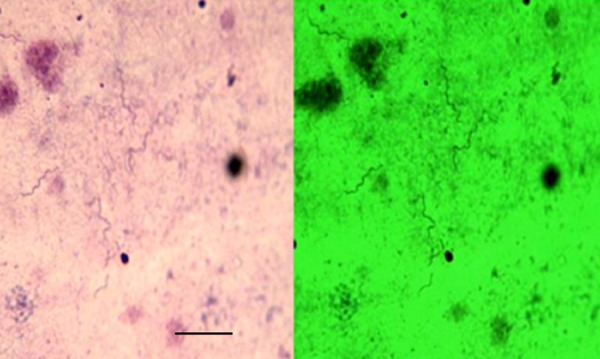
Giemsa-stained thick blood smear. CB-17 SCID mouse model Borrelia miyamotoi infection (×630 magnification). (Left) Unenhanced field. (Right) Didymium filter contrast enhancement.
FIG 2.
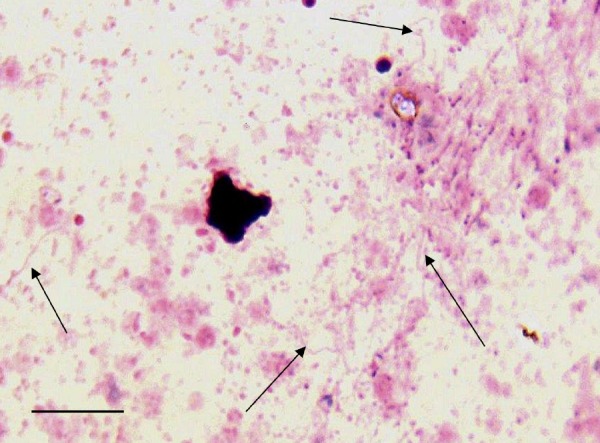
Giemsa-stained thick smear. B. miyamotoi-infected SCID mouse blood refrigerated for 14 days (×788 magnification). A degenerating leukocyte can be seen on the left.
To determine whether there was variability in the amount of blood represented among thick smears, we counted leukocyte nuclei as an index of the amount of blood within a field. A median of 33 white blood cell nuclei (range, 14 to 61) was present on the first thick smear field of each slide, suggesting that at least 0.5 µl of whole blood was represented within 100 fields (assuming a leukocyte count of about 5,000). Of 20 PCR-positive blood samples, none (0%) contained spirochetes demonstrable within 100 thick smear fields. When another 200 fields were examined at an independent time, 2 samples (10%) demonstrated rare spirochetes (Fig. 3). The median CT value for these 20 samples was 32.0 (range, 27.0 to 37.1), similar to what has been previously described (4). The 2 samples that were confirmed by thick blood smear to contain spirochetes had CT values of 29.9 and 28.1. A more targeted sample of slides (from the first quartile of lowest CT value samples excluding the 2 previously determined to contain spirochetes) was selected and reanalyzed for 300 fields by an independent microscopist. Spirochetes were not detected in the thick blood smears of these 5 samples. We conclude that although a BMD diagnosis might be confirmed by examination of thick blood smears, the sensitivity is poor and requires an extended duration of microscopy.
FIG 3.
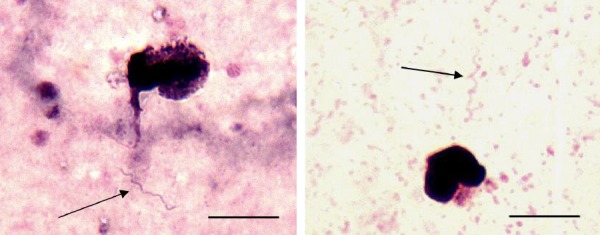
Giemsa-stained thick blood smear, patient samples (×788 magnification).
Subinoculation of SCID mice, but not BALB/c mice, consistently yielded isolates of B. miyamotoi from refrigerated anticoagulated blood containing variable numbers of spirochetes. These mice have been routinely used in the Tufts laboratory to serially propagate B. miyamotoi and maintain good spirochetemias (about 4,000/microliter of blood) for the duration of their life.
Although some authors have suggested that BMD is a hard-tick-borne relapsing fever (7) because B. miyamotoi is grouped by molecular phylogenetic analyses within the relapsing fever spirochete clade (5), the major features of BMD significantly differ (6). Prominent febrile relapses, with a typical crisis terminating severe fever episodes, have not been generally described with the two large case series that have been reported (1, 4). Blood smears sensitively detect peripheral blood spirochetes in acute relapsing fever cases (10, 11), particularly when there are >105 spirochetes per milliliter (12), but the utility of blood smears for confirming a BMD diagnosis has remained speculative. We tested the hypothesis that thick blood smears might detect peripheral blood spirochetes by analyzing samples from 20 consecutive BMD patients for whom infection had been confirmed by PCR. Thick smears were chosen because of their known theoretical and empirical sensitivity over the more commonly performed thin smears, even though the latter are easier to interpret. An experienced parasitologist with malariological expertise failed to find spirochetes in any sample when only 100 fields were analyzed. In retrospect, it is not surprising that microscopy generally failed to confirm infection; the sensitivity of the thick blood smear method for confirming a malarial diagnosis has been estimated to be 10 to 20 parasitized erythrocytes per microliter of blood (8, 13) when a minimum of 100 oil immersion thick blood smear fields are scanned and the microscopist is experienced. Blood smears only detected 27% of samples identified as positive by PCR in a study of endemic East African relapsing fever (14), with estimated median spirochetemias of 800/ml; however, in American relapsing fever cases, febrile episodes are characterized by spirochetemias of >105 per ml. The range (95% confidence interval) for BMD spirochetemia, as estimated by calculations from quantitative real-time PCR, is 4,000 to 12,000/ml (4), which is the threshold for detection (10,000 organisms, by extrapolation from malariology) by thick smears. It may be that increasing the number of examined oil immersion fields to as many as 1,000 would increase the sensitivity of this method inasmuch as it does so by a third for malarial diagnosis (13). However, it seems unlikely that any clinical laboratory would be able to expend such an amount of time on microscopic analysis of a blood smear. It should be noted that as with malarial diagnosis, a negative blood smear does not prove that a patient is not parasitized, and in fact, standard malaria clinical recommendations are to repeat blood smears at intervals and to time the sampling to just before the paroxysm. Whether repeated blood smears would improve the sensitivity of blood smears for confirming a diagnosis of BMD remains undescribed. Our blood samples were suboptimal because they had been stored a minimum of 48 hours since they were drawn from the acutely ill patient; in practice, blood smears would be immediately made. We do not know whether smears of fresh blood might enhance the detection of B. miyamotoi. Thus, although our study suggests that blood smears do not provide any information to rule out BMD, we do note that examination of a blood smear from an acutely febrile tick-exposed patient in the eastern United States where Lyme disease is endemic might identify infection by Babesia microti or Anaplasma phagocytophilum, which are often cotransmitted in the same exposure sites (15). Accordingly, examination of a blood smear should always be considered in the clinical workup of a tick-exposed patient but is unlikely to serve as the primary mode of confirming a BMD diagnosis.
Until recently with the routine use of NAAT, the gold standard for confirming a diagnosis of babesiosis due to Babesia microti was hamster inoculation, and in fact, smear-negative suspected relapsing fever was often resolved by mouse inoculation (12). Inoculation of SCID mice appears to sensitively confirm infection by B. miyamotoi but is clearly now to be reserved for research use, particularly given the expense of purchasing and maintaining the mice. B. miyamotoi may now be directly cultivated in vitro from human blood (16), but at the time when we attempted to culture several of the patient-derived blood samples (using Barbour-Stoenner-Kelly medium supplemented with 50% fetal bovine serum under 5% carbon dioxide atmosphere), we failed to obtain any propagation. This is likely due to the initial inoculum being no more than 5,000 to 10,000 spirochetes. Neither mouse inoculation or cultivation is likely to be used for routinely confirming a BMD diagnosis, given the practical and logistic considerations. We caution that a positive PCR, unless targeting specific RNA, does not necessarily imply a viable organism because DNA from dead microbes would amplify. Mouse inoculation or cultivation would provide information about viability if needed.
NAAT (PCR) is now recommended as the primary test for acute BMD (15). Similar to all other PCR assays, the failure to detect specific DNA or RNA in the blood does not exclude the possibility of BMD. Serology is a useful complementary method to PCR for confirming a BMD diagnosis, although the recommended two-tiered serologic protocol (17) for confirming a B. burgdorferi infection (enzyme immunoassay [EIA] followed by immunoblot of reactive samples) is not useful for diagnosis of BMD. However, the recombinant glycerophosphate glycerophosphodiesterase (rGlpQ) EIA (18) appears to be sensitive and specific for confirming BMD (4), with 86% of PCR-confirmed acute cases seroconverting; and adding recombinant variable major proteins of B. miyamotoi to the rGlpQ assay greatly enhances EIA sensitivity and specificity (19). A diagnosis of BMD, thus, appears to be best confirmed by PCR analysis of acute blood samples and complemented by a demonstration of a specific antibody.
ACKNOWLEDGMENTS
S.R.T. and H.K.G. are supported, in part, by grants from the National Institutes of Health (U01AI109656 and R41AI078631), the Evelyn Lilly Lutz Foundation, the Dorothy Harrison Egan Foundation, and by a gift from Catherine C. Lastavica.
At the time of this research, all authors were affiliated with Imugen, Inc. (now a division of Oxford Immunotec), which serves as a clinical diagnostic laboratory focusing on tick-borne disease.
REFERENCES
- 1.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. 2011. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis 17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gugliotta JL, Goethert HK, Berardi VP, Telford SR. 2013. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowdri HR, Gugliotta JL, Berardi VP, Goethert HK, Molloy PJ, Sterling SL, Telford SR. 2013. Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann Intern Med 159:21–27. doi: 10.7326/0003-4819-159-1-201307020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Molloy PJ, Telford SR, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. 2015. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med 163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 5.Barbour AG. 2014. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Infect Genet Evol 27:551–558. doi: 10.1016/j.meegid.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Telford SR, Goethert HK, Molloy PJ, Berardi VP, Chowdri HR, Gugliotta JL, Lepore TJ. 2015. Borrelia miyamotoi disease: neither Lyme disease nor relapsing fever. Clin Lab Med 35:867–882. doi: 10.1016/j.cll.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause PJ, Fish D, Narasimhan S, Barbour AG. 2015. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect 21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce Chwatt LJ. 1985. Essential malariology, 2nd ed, p 452 John Wiley and Sons, New York, NY. [Google Scholar]
- 9.Ross R. 1903. The improved method of microscopical diagnosis of intermittent fever. Lancet 164:86. [Google Scholar]
- 10.Barbour AG. 1987. Immunobiology of relapsing fever. Contrib Microbiol Immunol 8:125–127. [PubMed] [Google Scholar]
- 11.Southern P, Sanford J. 1969. Relapsing fever: a clinical and microbiological review. Medicine 48:129–149. doi: 10.1097/00005792-196903000-00002. [DOI] [Google Scholar]
- 12.Barbour AG. 2018. Clinical features, diagnosis, and management of relapsing fever. In Sexton DJ, Kaplan MD (ed), UpToDate. UpToDate, Inc., Waltham, MA: https://www.uptodate.com/contents/clinical-features-diagnosis-and-management-of-relapsing-fever. Accessed 11 January 2019. [Google Scholar]
- 13.Wilcox A. 1960. Manual for the microscopic diagnosis of malaria in man. Public Health Service Publication No. 796 Division of Infectious Diseases, National Institutes of Health, Washington, DC. [Google Scholar]
- 14.Reller ME, Clemens EG, Schachterle SE, Mtove GA, Sullivan DJ, Dumler JS. 2011. Multiplex 5′ nuclease-quantitative PCR for diagnosis of relapsing fever in a large Tanzanian cohort. J Clin Microbiol 49:3245–3249. doi: 10.1128/JCM.00940-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, Gonzalez MD, Jerris RC, Kehl SC, Patel R, Pritt BS, Richter SS, Robinson-Dunn B, Schwartzman JD, Snyder JW, Telford S, Theel ES, Thomson RB, Weinstein MP, Yao JD. 2018. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 67:e1–e94. doi: 10.1093/cid/ciy381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koetsveld J, Kolyasnikova NM, Wagemakers A, Toporkova MG, Sarksyan DS, Oei A, Platonov AE, Hovius JW. 2017. Development and optimization of an in vitro cultivation protocol allows for isolation of Borrelia miyamotoi from patients with hard tick-borne relapsing fever. Clin Microbiol Infect 23:480–484. doi: 10.1016/j.cmi.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1995. Recommendations for test performance from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 44:590–591. [PubMed] [Google Scholar]
- 18.Schwan TG, Schrumpf ME, Hinnebusch BJ, Anderson DE, Konkel ME. 1996. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol 34:2483–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koetsveld J, Kolyasnikova NM, Wagemakers A, Stukolova OA, Hoornstra D, Sarksyan DS, Toporkova MG, Henningsson AJ, Hvidsten D, Ang W, Dessau R, Platonov AE, Hovius JW. 2018. Serodiagnosis of Borrelia miyamotoi disease by measuring antibodies against GlpQ and variable major proteins. Clin Microbiol Infect 24:1338.e1–1338.e7. doi: 10.1016/j.cmi.2018.03.009. [DOI] [PubMed] [Google Scholar]


