Abstract
Implementation of comprehensive rehabilitation therapy in postoperative care of patients with cholangiocarcinoma was studied to explore its impact on patients' quality of life. Two hundred and nineteen patients with cholangiocarcinoma who underwent surgery in Yidu Central Hospital of Weifang from April 2014 to June 2017 were selected as study subjects. Of these patients, 116 received comprehensive rehabilitation therapy, in addition to chemotherapy, after surgery and were assigned to the experimental group. The remaining 103 patients received routine treatment after surgery and were assigned to the control group. Under the guidance of experts, patients in the experimental group carried out multiple comprehensive rehabilitation activities, such as exercises on general physical function, adjustment of psychological state and recovery of social family function. The outcome after 1 month of treatment was evaluated for the two groups according to the RECIST 1.1 guidelines. Nutritional status of patients before surgery, at 1 day and at 1 month after surgery was measured according to the Nutritional Risk Screening endorsed by the European Society for Clinical Nutrition and Metabolism (ESPEN). The quality of life at 1 month after surgery was assessed based on the QLQ-C30 quality of life questionnaire. The negative emotions that patients experienced at 1 month after surgery were assessed using the self-rating anxiety scale (SAS) and the self-rating depression scale (SDS). The response rate in the experimental group was 76.72%, which was significantly higher than 46.60% in the control group (P<0.05). At 1 month after surgery, the nutritional status and quality of life, as well as SAS/SDS scores, were significantly better in the experimental group than in the control group, and the differences were statistically significant (P<0.05). In clinical anticancer treatment, synergistic implementation of comprehensive rehabilitation therapy can improve patients' psychological health status, nutritional status and the overall quality of life, and reduce the impact of negative emotions on the physical state.
Keywords: cholangiocarcinoma, comprehensive rehabilitation therapy, quality of life, efficacy, nutritional status, negative emotions
Introduction
Cholangiocarcinoma is a common malignant tumor of the digestive system, occurring in the hepatic duct and extrahepatic bile duct. The main clinical manifestations include serious progressive obstructive jaundice, impaired liver function, slim body and underweight (1,2). Patients with cholangiocarcinoma usually present a long history of bile duct stones, which has a certain impact on patients' psychological state and quality of life. These patients usually have poor prognoses (3,4). As a malignant tumor originating from the bile duct epithelial cells, cholangiocarcinoma is difficult to detect in the early stage and has strong invasiveness. Therefore, most patients reach the advanced stage at the time of diagnosis (5). As far as the treatment of cholangiocarcinoma is concerned, relief of progressive obstruction is the first and necessary step, which is achieved by surgery in order to restore the normal excretion of bile and eliminate jaundice. The surgery is then followed by chemotherapy and other treatments (6). Surgical treatment is traumatic and can incur a profound stress response, influencing patients' postoperative recovery and quality of life (7). Most patients with cholangiocarcinoma are middle-aged or elderly people, who may experience difficulties in their postoperative recovery due to age (8). In addition, patients' adverse reactions to chemotherapy impose further burden to postoperative recovery. Therefore, implementation of comprehensive rehabilitation therapy is necessary to promote patients' recovery and improve quality of life after surgical treatment of cholangiocarcinoma.
Comprehensive rehabilitation therapy for cancer refers to a combination of medical treatment, psychological counseling, nutritional support and physical exercise, aiming at improving the quality of life, promoting patients' recovery and prolonging survival (9). According to studies, comprehensive rehabilitation therapy has already been implemented in breast cancer (10) and cervical cancer (11) with high response rates. However, to the best of our knowledge, there is no relevant report on comprehensive rehabilitation therapy implemented in postoperative care of patients with cholangiocarcinoma. Studies need to be conducted to determine if comprehensive rehabilitation therapy is effective in a specific cancer in spite of successful implementations in other cancers. This study aims to provide an optimal solution to postoperative treatment of cholangiocarcinoma by implementing comprehensive rehabilitation therapy in postoperative patients with cholangiocarcinoma and exploring its impact on patients' quality of life.
Patients and methods
Subjects
Retrospective analysis was performed on 219 patients with cholangiocarcinoma who underwent surgery in Yidu Central Hospital of Weifang (Weifang, China) from April 2014 to June 2017. There were 138 males and 81 females with an average age of 63.5±6.4 years. Of these patients, 116 received comprehensive rehabilitation therapy in addition to routine treatment after surgery and were assigned to the experimental group. The remaining 103 patients received only routine treatment after surgery and were assigned to the control group. As shown in Table I, there were no significant differences in sex, age, and pathological staging between the two groups (P>0.05).
Table I.
Patients' general medical records [n (%)].
| Variables | Experimental group (n=116) | Control group (n=103) | χ2 value | P-value |
|---|---|---|---|---|
| Sex | 0.064 | 0.800 | ||
| Male | 74 (63.79) | 64 (62.14) | ||
| Female | 42 (36.21) | 39 (37.86) | ||
| Age (years) | 0.225 | 0.635 | ||
| ≤63 | 69 (59.48) | 58 (56.31) | ||
| >63 | 47 (40.52) | 45 (43.69) | ||
| BMI (kg/m2) | 0.061 | 0.805 | ||
| ≤21 | 65 (56.03) | 56 (54.37) | ||
| >21 | 51 (43.97) | 47 (45.63) | ||
| Alcohol consumption | 0.019 | 0.891 | ||
| Yes | 72 (62.07) | 63 (61.17) | ||
| No | 44 (37.93) | 40 (38.83) | ||
| Tumor location | 0.029 | 0.986 | ||
| Intrahepatic cholangiocarcinoma | 35 (30.17) | 30 (29.13) | ||
| Hilar cholangiocarcinoma | 42 (36.21) | 38 (36.89) | ||
| Distal cholangiocarcinoma | 39 (33.62) | 35 (33.98) | ||
| Pathological type | 0.197 | 0.978 | ||
| Papillary carcinoma | 41 (35.34) | 36 (34.95) | ||
| Sclerosing cancer | 22 (18.97) | 19 (18.45) | ||
| Nodular carcinoma | 32 (27.59) | 27 (26.21) | ||
| Diffuse invasive carcinoma | 21 (18.10) | 21 (20.39) |
The study was approved by the Ethics Committee of Yidu Central Hospital of Weifang. Patients who participated in this research had complete clinical data. Signed informed consents were obtained from the patients and/or guardians.
Inclusion and exclusion criteria
Patients who were diagnosed with cholangiocarcinoma by pathological test were eligible for this study. Patients who met the following criteria were excluded from this study: i) patients who had already received chemotherapy and/or radiotherapy; ii) patients who had other serious organ disorders; iii) patients who were unable to undergo surgery; iv) patients who had cognitive-communication disorders; and v) patients who were not cooperative with the examination.
Methods
After surgery, patients in the control group received only chemotherapy, while patients in the experimental group received comprehensive rehabilitation therapy under expert guidance in addition to chemotherapy. The detailed protocols are given below. i) Nutritional support: patients were weak after surgery, and the body was in a state of high metabolism. Their diets were designed and prepared by professional nutritionists to provide effective enteral and parenteral nutrition support. The goal was to promote patients' physical recovery by improving their nutritional status. After referring to The American Society for Parenteral and Enteral Nutrition (12), a jejunal nutrition tube was indwelled in each patient during operation, and at 12 h after surgery the patient was fed with 5% glucose sodium chloride solution. When the patient stayed in bed after surgery, the daily calorie intake of the patient was 104.3 kJ, which could be increased to 125.7 kJ after the patient got out of bed. If the patient had no discomfort, enteral nutrition combined with parenteral nutrition was gradually replaced by parenteral nutrition according to their condition and gastrointestinal function. ii) Physical exercise: when the patient woke up after surgery, stayed in bed to rest for 1 week. During that time, the patient rested in a semi-reclining position to better control drainage and breathing. The patient was later instructed to carry out appropriate exercises by correctly changing his body position in the bed. Early ambulation was always encouraged. During ambulation, the patient was instructed to take aerobic exercises, such as walking or Tai Chi, to promote recovery. iii) Psychological counseling: after surgery patients may experience postoperative irritability and anxiety associated with pain. Therefore, in comprehensive rehabilitation therapy patients received psychological counseling. Briefly, medical professionals made more conversations with each individual patient on a daily basis, and a group psychological counseling was run twice a week. The goal was to relieve the patient's psychological stress, in order to participate more actively in the overall rehabilitation. iv) Health knowledge expansion: cancer knowledge was imparted to patients and their families once or twice every week, especially knowledge about cholangiocarcinoma. v) Miscellaneous: during chemotherapy, patients could choose to listen to some soft and light music. Music therapy could reduce distress associated with chemotherapy by shifting patients' attention from chemotherapy to something pleasant.
The clinical outcome, physical function, psychological health status, and the nutritional status of spatients in the two groups were assessed after the rehabilitation treatment.
Observation indicators
Clinical outcome after 1 month of treatment was evaluated and defined as complete remission, partial remission, stable disease and progressive disease, according to the RECIST 1.1 guidelines (13). The response rate was defined as the proportion of patients achieving complete remission and partial remission. Nutritional status of patients before surgery, at 1 day and at 1 month after surgery was measured according to the Nutritional Risk Screening endorsed by the European Society for Clinical Nutrition and Metabolism (ESPEN) (14). The quality of life at 1 month after surgery was assessed based on the QLQ-C30 quality of life questionnaire (15). The questionnaire included 30 items in 5 categories: role function, physical function, emotional function, cognitive function and social function. Higher score indicated higher quality of life. The negative emotions that patients experienced at 1 month after surgery were assessed using the self-rating anxiety scale (SAS) and the self-rating depression scale (SDS) (16).
Statistical analysis
The data acquired in this study were analyzed using SPSS 19.0 statistics software from Asia Analytics (formerly SPSS China, Beijing, China). χ2 test was used for the comparison of enumeration data. t-test was used for the comparison of measurement data. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical outcomes in the two groups
As shown in Table II, after surgery and chemotherapy, the clinical outcome in the experimental group was: 10 cases with complete remission, 79 cases with partial remission, 17 cases with stable disease, and 10 cases with progressive disease. The response rate was 76.72%. The clinical outcome in the control group was: 3 cases with complete remission, 45 cases with partial remission, 40 cases with stable disease, and 15 cases with progressive disease. The response rate was 46.60%. Thus, the response rate in the experimental group was significantly higher than that in the control group (P<0.05).
Table II.
Clinical outcomes in the two groups [n (%)].
| Outcome | Experimental group (n=116) | Control group (n=103) | χ2 value | P-value |
|---|---|---|---|---|
| Complete remission | 10 (8.62) | 3 (2.91) | − | − |
| Partial remission | 79 (68.10) | 45 (43.69) | − | − |
| Stable disease | 17 (14.66) | 40 (38.83) | − | − |
| Progressive disease | 10 (8.62) | 15 (14.56) | − | − |
| Response rate | 89 (76.72) | 48 (46.60) | 21.13 | <0.001 |
Nutritional status scores in the two groups
As shown in Table III, the nutritional status scores before surgery, at 1 day and at 1 month after surgery were 22.31±3.26, 20.17±2.12 and 28.52±2.73 points, respectively, in the experimental group, and 23.01±3.18, 20.56±2.23 and 24.16±2.09 points, respectively, in the control group. There were no significant differences in nutritional status scores between the two groups before surgery and at 1 day after surgery. However, at 1 month after surgery, the nutritional status score in the experimental group was significantly higher than that in the control group (P<0.05).
Table III.
Nutritional status scores in the two groups.
| Time point | Experimental group (n=116) | Control group (n=103) | t value | P-value |
|---|---|---|---|---|
| Before surgery | 22.31±3.26 | 23.01±3.18 | 1.604 | 0.110 |
| 1 day after surgery | 20.17±2.12 | 20.56±2.23 | 1.326 | 0.186 |
| 1 month after surgery | 28.52±2.73 | 24.16±2.09 | 13.14 | <0.001 |
Quality of life scores at 1 month after surgery in the two groups
As shown in Table IV, the scores for the role function, physical function, emotional function, cognitive function and social function in the experimental group were all higher than those in the control group, and the differences were statistically significant (P<0.05).
Table IV.
Quality of life scores at 1 month after surgery in the two groups.
| Category | Experimental group (n=116) | Control group (n=103) | t value | P-value |
|---|---|---|---|---|
| Role function | 76.76±3.18 | 58.55±2.79 | 44.79 | <0.001 |
| Physical function | 73.16±2.93 | 59.12±2.78 | 36.25 | <0.001 |
| Emotional function | 74.12±2.89 | 60.11±3.09 | 34.66 | <0.001 |
| Cognitive function | 72.69±3.11 | 59.54±2.91 | 32.19 | <0.001 |
| Social function | 73.87±2.77 | 58.69±2.90 | 39.59 | <0.001 |
SAS and SDS scores at 1 month after surgery in the two groups
As shown in Table V and Fig. 1, the SAS and SDS scores were 40.12±2.13 and 39.66±2.79 points, respectively, in the experimental group, and 48.17±2.56 and 47.91±3.03 points; respectively; in the control group. Apparently, both scores in the experimental group were significantly lower than those in the control group, and the differences were statistically significant (P<0.05).
Table V.
SAS and SDS scores at 1 month after surgery in the two groups.
| Score | Experimental group (n=116) | Control group (n=103) | t value | P-value |
|---|---|---|---|---|
| SAS | 40.12±2.13 | 48.17±2.56 | 25.39 | <0.001 |
| SDS | 39.66±2.79 | 47.91±3.03 | 20.97 | <0.001 |
SAS, self-rating anxiety scale; SDS, self-rating depression scale
Figure 1.
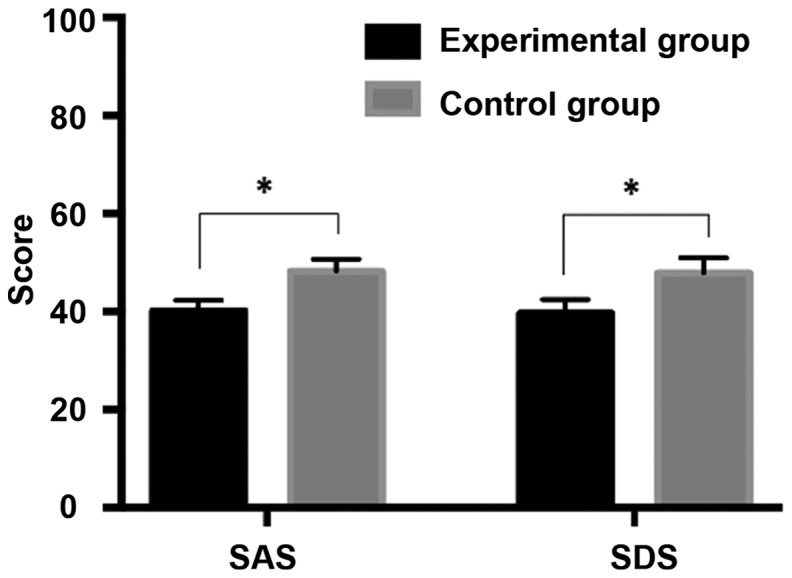
SAS and SDS scores at 1 month after surgery in the two groups. The SAS and SDS scores in the experimental group were significantly lower than those in the control group, and the differences were statistically significant (*P<0.05). SAS, self-rating anxiety scale; SDS, self-rating depression scale.
Discussion
Currently, the main treatment methods for cholangiocarcinoma are surgery, radiotherapy and chemotherapy. In terms of surgical treatment, both operation and intraoperative anesthesia can cause tissue trauma and mental distress to patients (17). In addition, postoperative pain can lead to stress reactions as well as negative emotions, while radiotherapy and chemotherapy may cause adverse reactions, thereby extending hospital stay (18). Seifter has reported that multiple problems, such as functional disorders, psychological disorders, physical disability, and social disorders exist at different levels in most cancer patients (19). Therefore, the implementation of comprehensive rehabilitation therapy for cancer patients is becoming increasingly urgent. Although it has been proposed to implement psychological counseling, nutritional support and physical exercise in postoperative care of cancer patients to improve their quality of life (20), there is still little research on comprehensive rehabilitation therapy for cancer patients. In this study, comprehensive rehabilitation therapy was implemented in postoperative care of patients with cholangiocarcinoma, and its impact on patients' quality of life was explored, aiming at finding a better solution for postoperative recovery of patients with cholangiocarcinoma.
Comparing the clinical outcomes at 1 month after surgery between the two groups, it was found that the response rate in the experimental group (76.72%) was significantly higher than that in the control group (46.60%), and the difference was statistically significant (P<0.05). This finding suggests that implementing comprehensive rehabilitation therapy enhances the therapeutic efficacy of surgery and chemotherapy in patients. The underlying reason of this effect is that the improvement of patients' mood and quality of life due to comprehensive rehabilitation therapy made patients more cooperative in the treatment. As a result, more favorable clinical outcome was observed. Comparing the nutritional status scores, quality of life scores, and SAS and SDS scores at 1 month after surgery between the two groups, it was found that all the scores in the experimental group were significantly better than those in the control group (P<0.05). These findings indicate that implementing comprehensive rehabilitation therapy improves patients' quality of life and promotes their acceptance of and integration into the normal social life; plus, the dietary guidance for patients can effectively improve the nutritional status of patients. Good health and psychological counseling reduced the negative emotions of patients. Patients were more cooperative in postoperative chemotherapy and their overall recovery when they were in a healthy psychological and physiological state. Our results are consistent with a literature report, in which Saggini et al concluded that comprehensive rehabilitation therapy in cancer patients significantly improves patients' quality of life (21). In the present study, we did not however develop different comprehensive rehabilitation treatment plans for patients at different stages of cholangiocarcinoma, which is the limitation of the current research, and still needs to be addressed.
In summary, in clinical anticancer treatment, synergistic implementation of comprehensive rehabilitation therapy can improve cholangiocarcinoma patients' psychological health status, nutritional status and overall quality of life, and reduce the impact of negative emotions on patients' physical state.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SL and LZ were responsible for the surgery and chemotherapy treatment; LA assisted with observation indicators. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Yidu Central Hospital of Weifang (Weifang, China). Patients who participated in this research had complete clinical data. Signed informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jonas S, Benckert C, Thelen A, Lopez-Hänninen E, Rösch T, Neuhaus P. Radical surgery for hilar cholangiocarcinoma. Eur J Surg Oncol. 2008;34:263–271. doi: 10.1016/j.ejso.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 3.Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120–125. doi: 10.1016/j.amjsurg.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, Chung JB. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: Association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Fang M, Zhu J, Feng H, Warner E, Yi C, Ji J, Gu X, Gao C. Serum N-glycans outperform CA19-9 in diagnosis of extrahepatic cholangiocarcinoma. Electrophoresis. 2017;38:2749–2756. doi: 10.1002/elps.201700084. [DOI] [PubMed] [Google Scholar]
- 6.Morise Z, Sugioka A, Tokoro T, Tanahashi Y, Okabe Y, Kagawa T, Takeura C. Surgery and chemotherapy for intrahepatic cholangiocarcinoma. World J Hepatol. 2010;2:58–64. doi: 10.4254/wjh.v2.i2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SG, Song GW, Hwang S, Ha TY, Moon DB, Jung DH, Kim KH, Ahn CS, Kim MH, Lee SK, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: The Asan experience. J Hepatobiliary Pancreat Sci. 2010;17:476–489. doi: 10.1007/s00534-009-0204-5. [DOI] [PubMed] [Google Scholar]
- 8.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 9.Huether A, Höpfner M, Baradari V, Schuppan D, Scherübl H. Sorafenib alone or as combination therapy for growth control of cholangiocarcinoma. Biochem Pharmacol. 2007;73:1308–1317. doi: 10.1016/j.bcp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Cho OH, Yoo YS, Kim NC. Efficacy of comprehensive group rehabilitation for women with early breast cancer in South Korea. Nurs Health Sci. 2006;8:140–146. doi: 10.1111/j.1442-2018.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- 11.Tilley DM, Hristov S, Templeton DJ, Sharp NC, O'Connor CC. Cervical cancer screening and abnormalities among women in a residential drug-rehabilitation program. Aust J Prim Health. 2012;18:266–267. doi: 10.1071/PY12067. [DOI] [PubMed] [Google Scholar]
- 12.Compher C, Jain AK, Nichol PF, Blackmer A, Earthman C, Evans DC, McCarthy MS, Taylor B, Mehta N. Research Agenda 2018: The American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2018;42:838–844. doi: 10.1002/jpen.1312. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): New guidelines. Med Pediatr Oncol. 2001;37:1–3. doi: 10.1002/mpo.1154. [DOI] [PubMed] [Google Scholar]
- 14.Donini LM, Poggiogalle E, Molfino A, Rosano A, Lenzi A, Rossi Fanelli F, Muscaritoli M. Mini-nutritional assessment, malnutrition universal screening tool, and nutrition risk screening tool for the nutritional evaluation of older nursing home residents. J Am Med Dir Assoc. 2016;17:959.e11–959.e18. doi: 10.1016/j.jamda.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Derogar M, van der Schaaf M, Lagergren P. Reference values for the EORTC QLQ-C30 quality of life questionnaire in a random sample of the Swedish population. Acta Oncol. 2012;51:10–16. doi: 10.3109/0284186X.2011.614636. [DOI] [PubMed] [Google Scholar]
- 16.Dunstan DA, Scott N, Todd AK. Screening for anxiety and depression: Reassessing the utility of the Zung scales. BMC Psychiatry. 2017;17:329. doi: 10.1186/s12888-017-1489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty B, Nambudiri VE, Palmer WC. Update on the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2017;19:2. doi: 10.1007/s11894-017-0542-4. [DOI] [PubMed] [Google Scholar]
- 18.Xue F, Huang F. The effect of psychological intervention on nutrient status of perioperative patients with lung cancer. Iran J Public Health. 2018;47:531–537. [PMC free article] [PubMed] [Google Scholar]
- 19.Seifter EJ. Clinical Oncology, Second Edition. J Natl Cancer Inst. 2001;93:63. doi: 10.1093/jnci/93.1.63. [DOI] [PubMed] [Google Scholar]
- 20.Saotome T, Klein L, Faux S. Cancer rehabilitation: A barometer for survival? Support Care Cancer. 2015;23:3033–3041. doi: 10.1007/s00520-015-2673-1. [DOI] [PubMed] [Google Scholar]
- 21.Saggini R, Bellomo RG, Carmignano SM, Saggini A. Cancer pain - the role of an integrated, comprehensive rehabilitation program in its management. In: Updates on Cancer Treatment. In: Rangel LBA, Silva IV, editors. InTech. 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


