Abstract
Correlation of cardiomyocyte apoptosis with duration of hypertension, severity of hypertension and caspase-3 expression in hypertensive rats was analyzed. Sixty male Sprague-Dawley (SD) rats were selected and randomly divided into the observation group (n=30) and control group (n=30), and the rat models of hypertension were established by virtue of transverse aortic constriction (TAC). The rats in the two groups were further divided into the 7-day subgroup (n=10), 14-day subgroup (n=10) and 28-day subgroup (n=10), respectively according to their survival time after TAC. The blood pressure values of the rats in each group were measured through intubation of carotid artery to calculate the mean arterial pressure (MAP). The conditions of cardiomyocyte apoptosis were detected using terminal dexynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay. Enzyme-linked immunosorbent assay (ELISA) was applied to measure the expression of caspase-3 in the myocardial tissues, and correlation analysis was performed. The MAPs in 7-, 14- and 28-day subgroups of the observation group were significantly higher than those in the corresponding subgroups of the control group (P<0.05). The 7-, 14- and 28-day subgroups of the observation group had remarkably elevated myocardial caspase-3 expression levels compared with the subgroups of the control group (P<0.05). The apoptosis rates of myocardial cells in the three subgroups of the observation group were obviously higher than those in the corresponding subgroups of the control group (P<0.05). Pearson's correlation analysis indicated that the cardiomyocyte apoptosis rate of hypertensive rats was positively correlated with the duration of hypertension, severity of hypertension and caspase-3 expression (P<0.05). Hypertension can induce apoptosis of myocardial cells, and the apoptosis becomes more serious with the constantly elevated level and prolonged duration of hypertension. In addition, the activity of caspase-3 has a close correlation with cardiomyocyte apoptosis.
Keywords: hypertension, myocardial cells, apoptosis, caspase-3
Introduction
Hypertension extensively participates in the damage to the body, with a ‘three-high’ characteristics, namely, high incidence rate, high disability rate and high death rate (1). The incidence rate of hypertension is increasing, and patients with the disease are getting younger along with the constant development of global economy, extension of life expectancy and the aging society, as well as the accelerated pace of people's life and work and the changed dietary patterns. Therefore, how to prevent and treat hypertension in a better way has become one of the important medical problems at present (2,3). Hypertension can cause damage to various target organs in the body (including heart, brain and kidneys) (4). Long-term and sustained hypertension can lead to increased cardiac load, easily resulting in myocardial morphological and structural changes such as cardiac hypertrophy, interstitial fibrosis, ventricular dilatation and cardiomyocyte apoptosis (5). A study has demonstrated that cardiomyocyte apoptosis, as a cytological basis and initiator of left ventricular remodeling of hypertension, plays a regulatory role in the whole ventricular remodeling (6). Caspases are initiators and executioners of cell apoptosis, and in particular, caspase-3 is essential for the apoptosis of myocardial cells (7). Reducing or eliminating the hypertension-induced damage to the myocardial cells has great clinical significance in preventing and treating pathological changes in the heart with high blood pressure. Currently, there are few studies or reports on the association of cardiomyocyte apoptosis with the duration and severity of hypertension. As one of the important model organisms, the rat is recognized as the closest to human in essential hypertension. This study analyzed the cardiomyocyte apoptosis of rats with hypertension and explored the correlation between cardiomyocyte apoptosis and duration of hypertension, severity of hypertension as well as caspase-3 expression, which provides a theoretical basis for the prevention and treatment of cardiac pathological changes of patients with hypertension.
Materials and methods
Experimental materials
Sixty normal adult Sprague-Dawley (SD) rats, weighing ~200 g, were purchased from Beijing Huafukang Bioscience Co., Ltd. (Beijing, China) and were selected and raised at room temperature, with humidity of 50–60% and free access to food and water. The rats were maintained in a 12:12-h light/dark cycle. Major experimental instruments and reagents included centrifuge [Eppendorf (Shanghai) International Trade Co., Ltd., Shanghai, China], microtome (Leica Microsystems, Wetzlar, Germany), visible spectrophotometer [Eppendorf (Shanghai) International Trade Co., Ltd.], microscope (JEOL Ltd., Tokyo, Japan), terminal dexynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay kit (Roche, Basel, Switzerland) and caspase-3 enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Yuanye Bio-Technology Co., Ltd., Shangai, China).
The study was approved by the Ethics Committee of Affiliated Hospital of Jining Medical University (Jining, China).
Methods
Model preparation and grouping
The rats were fasted for 12 h before transverse aortic constriction (TAC), but they were allowed to drink water freely. Then 4% chloral hydrate (300 mg/kg) was injected intraperitoneally, and no signs of peritonitis were observed. A longitudinal incision was made at the lower left costal margin and 0.5 cm away from the middle line of abdomen after anesthesia, and the abdominal aorta was bluntly dissected after entry into the abdominal cavity. In the observation group, a 7-gauge injection needle (with a blunt tip) was placed along the vessel course of the rat, and the abdominal aorta was ligated. After that, the needle was withdrawn to form stenosis of the abdominal aorta. In the control group, however, only the abdominal aorta of the rat was dissected, and TAC was not performed. Then the incision was sutured and dressed, and the rats were administered with penicillin after operation to prevent infection and keep them warm. After the rats were fully awake, they were sent back to the cage for breeding, and the experiment was conducted at 7, 14 and 28 days after operation, respectively.
Measurement of blood pressure of the rats
A non-invasive blood pressure measurement and analysis system (Shanghai Alcott Biotech Co., Ltd., Shanghai, China) was utilized to measure the caudal arterial blood pressure values of each group of rats for 3 consecutive times at 7, 14 and 28 days after TAC, respectively, and the average values were calculated. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) of the ventricles were recorded, and the mean arterial pressure (MAP) was calculated using the following formula: MAP = DBP + [1/3 (SBP - DBP)].
Collection of myocardium specimens
The rats were sacrificed at 7, 14 and 28 days after TAC, respectively. The rats were anesthetized with 3% pentobarbital sodium (35 mg/kg) by intraperitoneal injection. After thoracotomy, the heart was taken out after being rinsed with 0.9% sodium chloride solution, and the maximum transverse diameter of the coronal plane was cut off. Next, a portion of the heart was fixed in paraformaldehyde overnight, sliced to 3-mm-thick sections with a microtome, embedded in paraffin and stored at 4°C for TUNEL assay and analysis. The other portion of the heart was placed in liquid nitrogen for 1 h and then preserved in a refrigerator at −80°C for ELISA.
Measurement of caspase-3
The preserved myocardial tissues (~60 mg) of different groups of rats were fetched separately and mashed in a mortar containing an appropriate amount of 0.9% sodium chloride solution. Then the tissues were centrifuged at 2,650 × g/min for 15 min, and the supernatant (100 µl) was extracted. The ELISA was performed to detect the content of caspase-3 in the myocardial tissues in strict accordance with the manufacturer's instructions. Next, the optical density (OD) was measured at the wavelength of 450 nm using the spectrophotometer, and the concentration of caspase-3 was calculated.
TUNEL staining
The tissues embedded in paraffin in different groups were sliced to sections (with a thickness of 3 µm) via the microtome. After routine deparaffinization, 50 µl 3% hydrogen peroxide solution was added and incubated at 20°C for 10 min, so as to block the activity of endogenous peroxidase, followed by rinsing with phosphate-buffered saline (PBS) 3 times. The reaction mixture of TUNEL was prepared at 4°C away from light: 20 µl Reagent A (components: terminal deoxynucleotidyl transferase of Escherichia coli recombined with bovine thymosin) + 180 µl Reagent B (components: mixture of nucleotides). Then the sections were added into 50 µl reaction mixture of TUNEL and incubated at 37°C for 60 min, followed by washing with PBS 3 times. Next, 50 µl converter-peroxidase (POD) was added and incubated at 37°C for 30 min, followed by rinsing with PBS 3 times. Reagents A, B and C in the Dolichos biflorus agglutinin (DBA) kit were added into the sections for color development for 10 min. After that, the sections were washed with PBS 3 times, then counterstained with hematoxylin for 10 sec and mounted in neutral balsam. The apoptotic cells stained yellowish brown were observed and counted under the microscope.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 19.0 software (IBM Corp., Armonk, NY, USA) was utilized to process data. Measurement data were presented as mean ± standard deviation (mean ± SD), and t-test was performed. Pearson's correlation coefficients were applied to analyze the correlations. P<0.05 indicates that the difference was statistically significant.
Results
Comparison of MAP changes in hypertensive rats at different time-points after TAC
The MAPs in subgroups 7, 14 and 28 days of the observation group (148.15±7.73, 149.07±7.84 and 151.36±7.25) were significantly higher than those in the corresponding subgroups of the control group (121.76±6.42, 119.35±6.63 and 123.74±6.54) (P<0.05) (Table I).
Table I.
Changes in MAP in hypertensive rats at different time-points (mean ± SD, mmHg).
| Days | Control group | Observation group | t | P-value |
|---|---|---|---|---|
| 7 | 121.76±6.42 | 148.15±7.73 | 8.305 | <0.001 |
| 14 | 119.35±6.63 | 149.07±7.84 | 9.153 | <0.001 |
| 28 | 123.74±6.54 | 151.36±7.25 | 8.945 | <0.001 |
Comparison of myocardial caspase-3 expression in hypertensive rats at different time-points
The 7-, 14- and 28-day subgroup of the observation group had remarkably elevated myocardial caspase-3 expression levels (5.15±0.73, 10.36±1.18 and 15.67±1.43) compared with the subgroups of the control group (3.76±0.42, 6.24±1.03 and 9.38±1.29) (P<0.05) (Table II).
Table II.
Myocardial caspase-3 levels in hypertensive rats at different time-points (mean ± SD, pmol/l).
| Days | Control group | Observation group | t | P-value |
|---|---|---|---|---|
| 7 | 3.76±0.42 | 5.15±0.73 | 5.219 | <0.001 |
| 14 | 6.24±1.03 | 10.36±1.18 | 8.318 | <0.001 |
| 28 | 9.38±1.29 | 15.67±1.43 | 10.328 | <0.001 |
Comparison of cardiomyocyte apoptosis in hypertensive rats
There was a small quantity of apoptotic cells observed in the 7-day subgroups of both the observation and control groups, and a certain number of apoptotic cells were visible in the remaining subgroups of the observation and control groups. Moreover, apoptosis rates of myocardial cells in the three subgroups of the observation group were obviously higher than those of the control group (P<0.05) (Table III).
Table III.
Apoptosis rates of myocardial cells in hypertensive rats at different time-points (mean ± SD, %).
| Days | Control group | Observation group | t | P-value |
|---|---|---|---|---|
| 7 | 4.15±0.85 | 7.76±0.72 | 10.248 | <0.001 |
| 14 | 12.34±1.38 | 16.75±1.54 | 6.744 | <0.001 |
| 28 | 27.27±1.45 | 31.53±1.64 | 6.154 | <0.001 |
Analysis of correlation of cardiomyocyte apoptosis with duration of hypertension, severity of hypertension and caspase-3
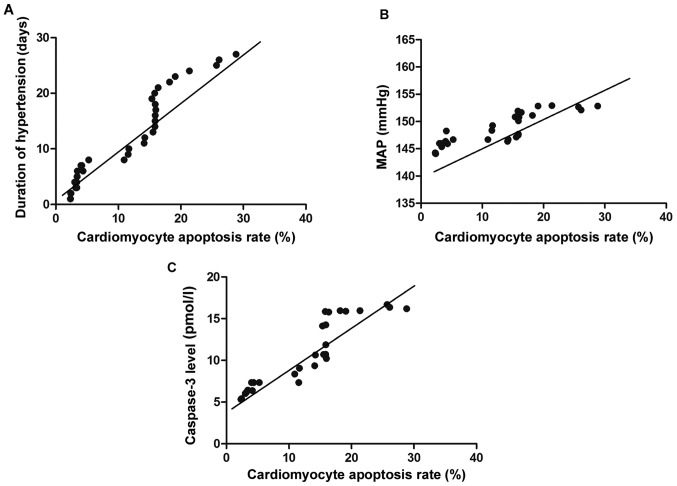
Pearson's correlation coefficient analyses indicated that the cardiomyocyte apoptosis rate of hypertensive rats was positively correlated with the duration of hypertension, severity of hypertension and caspase-3 expression (P<0.05) (Table IV and Fig. 1).
Table IV.
Analysis of correlation of cardiomyocyte apoptosis with duration and severity of hypertension and caspase-3.
| Correlation with SBP | ||
|---|---|---|
| Items | r | P-value |
| Duration of hypertension | 0.413 | 0.014 |
| Severity of hypertension | 0.407 | 0.008 |
| Caspase-3 expression | 0.426 | 0.013 |
Figure 1.
Correlation analysis. (A) Correlation between cardiomyocyte apoptosis and the duration of hypertension. (B) Correlation between cardiomyocyte apoptosis and the severity of hypertension. (C) Correlation between cardiomyocyte apoptosis and caspase-3 expression.
Discussion
Hypertension is a kind of progressive cardiovascular syndrome, which is generally divided into essential type and secondary type (8). In developed countries, the incidence rate of the disease is as high as 20%, and it may be triggered by various causes which are mainly environmental and social factors, including infection, toxin effect, medicine, diet, psychological pressure, urbanization and socioeconomic status (9). Hypertension can damage multiple target organs, especially different levels of the heart (cardiac tissues and cells), leading to functional and structural changes in the heart and blood vessels (10,11). In the state of hypertension, the neuro-endocrine abnormality occurs. The cardiac cavity is enlarged and the left ventricle is thickened when the cardiac load is increased, which is known as ventricular remodeling (12). The ventricular remodeling can induce a variety of cardiovascular diseases (such as heart failure, acute myocardial infarction, sudden cardiac death and arrhythmia) (13), and the mechanism is complex and diversified. In related studies, it is stated that ventricular remodeling of hypertension occurs and develops on the basis of cardiomyocyte apoptosis which is associated with various factors (including ischemia and hypoxia, activation of neurohormonal factors, mechanical strain and oxidative stress) (14).
Cell apoptosis refers to the proactive process of programmed cell death triggered by the combined actions of extracellular environment and cellular factors under certain circumstances where the body maintains the stability of internal environment following its own procedures (15). There are usually three pathways for cell apoptosis, namely, death receptor pathway, mitochondrial pathway and endoplasmic reticulum pathway (16). Imbalance of endoplasmic reticulum stability occurs due to the influence of some factors, which causes reactions at the cellular level, i.e., endoplasmic reticulum stress (ERS), thereby leading to physiological disorders in body (17). Endowed with double functions, ERS not only mediates resistance to apoptosis but also promotes apoptotic response, thus participating in the apoptosis of myocardial cells (18). The TUNEL staining in this study manifested that a small number of apoptotic cells existed in the 7-day subgroups of both the observation and control groups, and the quantities of apoptotic cells in the 14- and 28-day subgroups of the observation and control groups were gradually increased. Furthermore, the apoptosis rates of myocardial cells in the 7-, 14- and 28-day subgroups of the observation group were remarkably higher than those in the control group (P<0.05). It was revealed in the analysis through Pearson's correlation coefficients that the cardiomyocyte apoptosis rate of hypertensive rats had a positive correlation with the duration of hypertension, severity of hypertension and caspase-3 expression. It could demonstrate that ERS becomes excessively long and strong as the duration of hypertension is extended and the degree of hypertension is exacerbated. Therefore, the homeostasis of ERS in the myocardial tissues is broken, the ERS-mediated protective response is weakened, and the pro-survival effect of the myocardial cells converts into a pro-apoptotic effect. Accordingly, when the severity and duration of hypertension increase constantly and exceed certain limits, the apoptotic proteases are activated in the endoplasmic reticulum, and the apoptosis rate of myocardial cells is elevated and increasingly severe.
Caspases, as initiators and executioners of cell apoptosis, are generally divided into two major categories: promoter caspase (such as caspase-2, −8 and −9) and effector caspase (including caspase-3, −6 and −7), of which caspase-3 is the most critical apoptotic protease playing a key executive role downstream of cascade connection and has a decisive role in the process of cell death (19,20). The results of this research revealed that the 7-, 14- and 28-day subgroup of the observation group had remarkably higher myocardial caspase-3 levels than the corresponding subgroups of the control group. Pearson's correlation coefficient analysis manifested that the cardiomyocyte apoptosis rate of hypertensive rats had a positive correlation with caspase-3 level (P<0.05). It is likely because the caspase-3 is inactive in normal state, but the ERS is induced as the blood pressure is rising. In consequence, caspase in the cytoplasm and caspase-9 are activated, and then caspase-3 on the membrane of endoplasmic reticulum is activated, leading to degradation of deoxyribonucleic acid (DNA) repair enzyme, destruction of nuclear protein and skeleton protein as well as cell apoptosis. With the upregulation of caspase-3 expression, the number of TUNEL positive cells is increasing, and the apoptosis rate of myocardial cells is on the rise. This is consistent with the study results of Morishima et al (21).
In conclusion, the constantly increased level and extended duration of hypertension aggravates the cardiomyocyte apoptosis in hypertensive rats, and the activation of caspase-3 is an important factor for apoptosis of myocardial cells, whose upregulated expression can promote apoptosis.
Acknowledgements
We would like to thank the National Natural Science Foundation of China for supporting this study.
Funding
This study was supported by National Natural Science Foundation of China (Dysfunctional autophagic-lysosomal system in degenerative aortic stenosis 81400291).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
QW drafted the manuscript and was responsible for model preparation and grouping. YC collected myocardium specimens. NL and SP were mainly devoted to TUNEL staining. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Affiliated Hospital of Jining Medical University (Jining, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nkeh-Chungag BN, Sekokotla AM, Sewani-Rusike C, Namugowa A, Iputo JE. Prevalence of hypertension and pre-hypertension in 13–17 year old adolescents living in Mthatha - South Africa: A cross-sectional study. Cent Eur J Public Health. 2015;23:59–64. doi: 10.21101/cejph.a3922. [DOI] [PubMed] [Google Scholar]
- 2.Baszczuk A, Kopczynski Z, Thielemann A. Endothelial dysfunction in patients with primary hypertension and hyperhomocysteinemia. Postepy Hig Med Dosw. 2014;68:91–100. doi: 10.5604/17322693.1087521. (In Polish) [DOI] [PubMed] [Google Scholar]
- 3.Lin BM, Curhan SG, Wang M, Eavey R, Stankovic KM, Curhan GC. Hypertension, diuretic use, and risk of hearing loss. Am J Med. 2016;129:416–422. doi: 10.1016/j.amjmed.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumura K, Arima H, Tominaga M, Ohtsubo T, Sasaguri T, Fujii K, Fukuhara M, Uezono K, Morinaga Y, Ohta Y, et al. COMFORT Investigators: Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37:192–196. doi: 10.3109/10641963.2014.933968. [DOI] [PubMed] [Google Scholar]
- 5.Ozturk N, Olgar Y, Aslan M, Ozdemir S. Effects of magnesium supplementation on electrophysiological remodeling of cardiac myocytes in L-NAME induced hypertensive rats. J Bioenerg Biomembr. 2016;48:425–436. doi: 10.1007/s10863-016-9666-8. [DOI] [PubMed] [Google Scholar]
- 6.Peters EL, Offringa C, Kos D, Van der Laarse WJ, Jaspers RT. Regulation of myoglobin in hypertrophied rat cardiomyocytes in experimental pulmonary hypertension. Pflugers Arch. 2016;468:1697–1707. doi: 10.1007/s00424-016-1865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu M, Sun L, Jiang B, Tan S, Liu K, Xiao X. [Effect of nucleolin on cardiac cell apoptosis in Type 2 diabetic cardiomyopathy mice] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42:241–245. doi: 10.11817/j.issn.1672-7347.2017.03.001. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 8.Missault LH, Duprez DA, Brandt AA, de Buyzere ML, Adang LT, Clement DL. Exercise performance and diastolic filling in essential hypertension. Blood Press. 1993;2:284–288. doi: 10.3109/08037059309077169. [DOI] [PubMed] [Google Scholar]
- 9.Gradman AH, Basile JN, Carter BL, Bakris GL; American Society of Hypertension Writing Group: Combination therapy in hypertension. J Clin Hypertens (Greenwich) 2011;13:146–154. doi: 10.1111/j.1751-7176.2010.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davey R, Raina A. Hemodynamic monitoring in heart failure and pulmonary hypertension: From analog tracings to the digital age. World J Transplant. 2016;6:542–547. doi: 10.5500/wjt.v6.i3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao C, Kang C, Xue J, Shi K, Lv H, Li Z. Effects of blood pressure and sex on heart-vessel coupling in essential hypertension. Turk J Med Sci. 2016;46:680–685. doi: 10.3906/sag-1406-25. [DOI] [PubMed] [Google Scholar]
- 12.Cavasin MA, Stenmark KR, McKinsey TA. Emerging roles for histone deacetylases in pulmonary hypertension and right ventricular remodeling (2013 Grover Conference series) Pulm Circ. 2015;5:63–72. doi: 10.1086/679700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamirani YS, Kundu BK, Zhong M, McBride A, Li Y, Davogustto GE, Taegtmeyer H, Bourque JM. Noninvasive detection of early metabolic left ventricular remodeling in systemic hypertension. Cardiology. 2016;133:157–162. doi: 10.1159/000441276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iglarz M, Landskroner K, Bauer Y, Vercauteren M, Rey M, Renault B, Studer R, Vezzali E, Freti D, Hadana H, et al. Comparison of macitentan and bosentan on right ventricular remodeling in a rat model of non-vasoreactive pulmonary hypertension. J Cardiovasc Pharmacol. 2015;66:457–467. doi: 10.1097/FJC.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holdenrieder S, Stieber P, Förg T, Kühl M, Schulz L, Busch M, Schalhorn A, Seidel D. Apoptosis in serum of patients with solid tumours. Anticancer Res 19A. 1999:2721–2724. [PubMed] [Google Scholar]
- 16.Sõti C, Sreedhar AS, Csermely P. Apoptosis, necrosis and cellular senescence: Chaperone occupancy as a potential switch. Aging Cell. 2003;2:39–45. doi: 10.1046/j.1474-9728.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Gan X, Wang Y, Zhang X, Ding X, Chen L, Du J, Luo Q, Wang T, Shen J, et al. Toxoplasma gondii prevalent in China induce weaker apoptosis of neural stem cells C17.2 via endoplasmic reticulum stress (ERS) signaling pathways. Parasit Vectors. 2015;8:73. doi: 10.1186/s13071-015-0670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun XY, Qin HJ, Zhang Z, Xu Y, Yang XC, Zhao DM, Li XN, Sun LK. Valproate attenuates diabetic nephropathy through inhibition of endoplasmic reticulum stress-induced apoptosis. Mol Med Rep. 2016;13:661–668. doi: 10.3892/mmr.2015.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Büssing A, Vervecken W, Wagner M, Wagner B, Pfüller U, Schietzel M. Expression of mitochondrial Apo2.7 molecules and caspase-3 activation in human lymphocytes treated with the ribosome-inhibiting mistletoe lectins and the cell membrane permeabilizing viscotoxins. Cytometry. 1999;37:133–139. doi: 10.1002/(SICI)1097-0320(19991001)37:2<133::AID-CYTO6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 20.Wang JD, Takahara S, Nonomura N, Ichimaru N, Toki K, Azuma H, Matsumiya K, Okuyama A, Suzuki S. Early induction of apoptosis in androgen-independent prostate cancer cell line by FTY720 requires caspase-3 activation. Prostate. 1999;40:50–55. doi: 10.1002/(SICI)1097-0045(19990615)40:1<50::AID-PROS6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.