Abstract
Orthodontic tooth movement can lead to temporary hypoxia of periodontal tissues. Periodontal ligament cells (PDLCs) react to hypoxia, releasing various biological factors to promote periodontal tissue reconstruction. Hypoxia-inducible factor-1α (HIF-1α) is one of the most sensitive factors involved in the response to hypoxia. HIF-1α has been identified to be involved in osteogenic and osteoclast differentiation in vitro; however, few studies have investigated the expression of HIF-1α in the periodontal ligament (PDL) during orthodontic movement in vivo. In a previous study, microRNA-21 (miR-21) was demonstrated to be highly expressed in a rat model of orthodontic tooth movement. Additionally, miR-21 can increase the expression of HIF-1α in certain tumor cell types and is involved in tumor bioactivities. In the present study, HIF-1α exhibited expression patterns in a similar way to miR-21 in PDL samples from a rat model of orthodontic tooth movement, with expression initially increased and followed by a decrease over time. Furthermore, human PDLCs were exposed to a hypoxic environment in vitro, which induced significant upregulation of HIF-1α and miR-21 expression. Furthermore, miR-21 mimics increased HIF-1α expression and promoted osteogenic differentiation, indicated by upregulated expression of the osteogenic markers osteopontin, runt-related gene-2 and alkaline phosphatase. miR-21 inhibitors suppressed HIF-1α expression and downregulated the osteogenic markers. In conclusion, the results revealed that miR-21 has a positive effect on HIF-1α expression in PDLCs under hypoxia and has important roles in osteogenic differentiation during orthodontic tooth movement. These findings provide a theoretical basis by which to promote tissue reconstruction during orthodontic tooth movement.
Keywords: orthodontic tooth movement, hypoxia, hypoxia-inducible factor-1α, microRNA-21, periodontal ligament cells
Introduction
Orthodontic tooth movement involves a series of periodontal tissue remodeling processes, including alveolar bone resorption and formation (1). Periodontal ligament (PDL), the connective tissue between teeth and alveolar bone, serves key roles in periodontal tissue remodeling (2). The dense vascular networks in the PDL provide abundant oxygen and nutrients for its normal biological activities. When orthodontic force is applied on the tooth, the PDL on the pressure side is compressed, while the tension side is stretched. As a result, blood supply on both sides is temporarily decreased, leading temporary hypoxia in the PDL, which may trigger remodeling of periodontal tissue (3,4).
Hypoxia-inducible factor-1α (HIF-1α) is one of the most sensitive factors that responds to hypoxia, and highly activated under low oxygen concentrations (0–2%) (5). HIF-1α is considered to be the critical factor that co-ordinates angiogenic-osteogenic coupling, and overexpression of HIF-1α in mesenchymal stem cells (MSCs) alters angiogenic and osteogenic differentiation (6,7). In human periodontal ligament cells (PDLCs), HIF-1α is associated with osteogenic differentiation under hypoxia (8). Furthermore, HIF-1α improves the bone-resorbing activity of mature osteoclasts (9). An orthodontic tooth movement model is appropriate to be used to investigate the function of HIF-1α in osteogenic and osteoclastic differentiation in vivo; however, few studies have investigated the role of HIF-1α in these processes.
MicroRNA-21 (miR-21) is one of the miRs that is associated with hypoxia-regulated transcripts and can affect the expression of multiple genes (10,11). miR-21 promotes the osteogenic differentiation potential in MSCs, which may be associated with extracellular signal-regulated kinase (ERK)-mitogen-activated protein kinase signaling (12,13). Some studies indicate that miR-21 may regulate HIF-1α during cell adaptation to a hypoxic environment. In prostate cancer cells and in radioresistant non-small cell lung cancer cells, miR-21 increases the expression of HIF-1α and is involved in tumor bio-activities (14,15). Our previous study demonstrated that miR-21 is specifically expressed in PDL on the tension side and pressure side during orthodontic tooth movement in rats (16); however, it is still unclear whether miR-21 affects HIF-1α and osteogenic differentiation in PDLCs under hypoxia. The present study aimed to investigate the effect of miR-21 on HIF-1α during osteogenic differentiation in PDLCs under hypoxia. The results of the study provide a theoretical basis for promoting tissue reconstruction during orthodontic tooth movement.
Materials and methods
Rat orthodontic tooth movement model
Male Sprague-Dawley rats (n=30, 6–8 weeks old and 210±15 g) were purchased from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). All animals were treated under the ethical regulations for animal experiments, defined by the Institutional Ethics Committee. Rats were randomly divided into six group (n=5/group): The 1 day group, the 3 day group, the 5 day group, the 7 day group, the 14 day group and the 21 day group. During the experiment, the rats were housed in cages at 21–24°C, 60% humidity with a 12-h light/dark cycle, and food and water were available ad libitum. The current study was approved by the Ethics Committee of the Hospital of Stomatology, Sun Yat-sen University. All rats were healthy and there was no significant weight change throughout the experiment. Rats were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (40 mg/kg). Orthodontic force (50 g) was applied between the maxillary right first molar and the incisors using an elastic Ni-Ti closed-coil spring (3M Unitek, St. Paul, MN, USA). The force caused the maxillary right first molar to move mesially. The maxillary left first molar without orthodontic force application was set as the control. The distal side of the tooth root was the tension side, and the mesial side of the tooth root was the pressure side. The orthodontic tooth movement model in rats is described in detail in a previous report (16).
Immunohistochemical staining and image analysis
Rats were sacrificed at days 1, 3, 5, 7, 14 and 21. Maxillary first molars and their surrounding periodontal tissues were dissected and fixed in 4% formaldehyde for 72 h at 4°C, then decalcified with 10% ethylene diamine tetreaacetic acid for 6–8 weeks and embedded in paraffin. The tissue blocks were cut into serial mesio-distal sections (5 µm thick) and mounted on poly-L-lysine coated glass slides (Citotest Labware Manufacturing Co., Ltd., Haimen, China). Slides were deparaffinized in xylene and hydrated using a graded alcohol series. Endogenous peroxidases were inactivated by incubation with 3% H2O2 for 15 min in the dark. Once rinsed in Tris-buffered saline (TBS), the sections were blocked with 10% bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 30 min at room temperature and then incubated overnight at 4°C with rabbit anti-HIF-1α polyclonal antibodies (cat. no. ab216842; 1:200; Abcam, Cambridge, UK). Subsequently, the sections were incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin (Ig)G secondary antibodies (cat. no. 8114; Cell Signaling Technology, Inc., Danvers, MA, USA) without dilution for 1 h at room temperature. The immunoreactivity was visualized by staining with diaminobenzidine for 5 min at room temperature, then covered with a coverslip and analyzed under a light microscope. Primary antibody was replaced with PBS as a negative control. Quantification with Image Pro plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Isolation and culture of human PDLCs
Healthy premolars (n=16) were obtained from 4 orthodontic patients aged 12–16 years old at the Hospital of Stomatology, Sun Yat-sen University. Informed consent was provided. PDLCs were isolated from the PDL and co-cultured in complete culture medium consisting of α-modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.), at 37°C with 5% CO2. Cells at passage 3–4 were used for the subsequent experiments. PDLCs were incubated at a density of 1×105 cells/well in 6-well plates. When reaching 70–80% confluence, PDLCs were further cultured under hypoxia (2% O2) or normoxia (20% O2) at 37°C for 6, 12, 24 and 48 h. Hypoxia was a suitable condition for PDLCs osteogenic differentiation. During the experiment, it was revealed that the expression of HIF-1α protein increased rapidly at first few hours under hypoxia and there was an obvious decrease at 48 h when compared with 6 h, thus the limited time was set at 48 h for observation.
Transient transfection
miR-21 mimics (5-UAGCUUAUCAGACUGAUGUUGA-3′ and 3-AUCGAAUAGUCUGACUACAACU-5) and a corresponding negative control (cat. no. miR1N0000001-1-5; Guangzhou RiboBio Co., Ltd., Guangzhou, China) were transfected into PDLCs using Lipofectamine 3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) at a final concentration of 50 nM, while miR-21 inhibitors (5-UCAACAUCAGUCUGAUAAGCUA-3) and their corresponding negative control (cat. no. miR2N0000001-1-5; Guangzhou RiboBio Co., Ltd.) were transfected at final concentrations of 200 nM. The transfection mixture was dissolved in Opti-MEM serum-free media (Invitrogen; Thermo Fisher Scientific, Inc.) and at the time of transfection cells were seeded in medium with 10% FBS and no antibiotics. At 6 h post-transfection, cells were transferred to a hypoxia incubator. Following incubation for 24 h in the hypoxia incubator, cells reached 100% confluence and possessed normal morphology. RNAs and proteins were collected at this time point for further experiment. The transfection efficiency was confirmed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
RT-qPCR
Total RNA was extracted from cells that were cultured under hypoxia and normoxia for 6, 12, 24 and 48 h. Total RNA was also extracted from transfected cells to confirm the transfection efficiency. Total RNA was extracted at each time point using RNAzol RT Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA). RNA quantification and quality were analyzed using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed into cDNA using a Reverse Transcription Kit (Takara Biotechnology Co., Ltd., Dalian, China). To detect miR-21 and U6, cDNA was reverse transcribed using the Bulge-Loop microRNA qRT-PCR Starter Kit (Guangzhou RiboBio Co., Ltd.). RT-qPCR was conducted using SYBR Green Real-Time PCR Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.), which was performed at 37°C for 60 min, followed by 95°C for 5 min for 40 cycles. Specific primers for miR-21 (cat. no. MQPS0000834-1-100) and the internal control U6 (cat. no. MQPS0000002-1-100) were from obtained from Guangzhou RiboBio Co., Ltd. Primers for HIF-1α (forward, 5′-GTGGATTACCACAGCTGA-3′; reverse, 3′-GCTCAGTTAACTTGATCCA-5′) and β-actin (forward, 5′-CTCCATCCTGGCCTCGCTGT-3′; reverse, 3′-GCTCTCACCTTCACCGTTCC-5′) were synthesized by Invitrogen (Thermo Fisher Scientific, Inc.). The relative gene expression was calculated using the 2−ΔΔCq method (17), normalized to an internal reference. All RT-qPCR experiments were performed in triplicate and results were reported as the mean ± standard deviation (SD).
Western blotting
Total protein was extracted using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) supplemented with protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The protein concentration was determined using a BCA protein quantitation kit (Beyotime Institute of Biotechnology). Equal amounts of protein extracts (40 µg/lane) were separated by SDS-PAGE on an 8–10% gel and electrophoretically transferred to polyvinyldifluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked in 1X TBS with Tween 20 (TBST) containing 5% skimmed milk at room temperature for 1 h. Subsequently, the blocked membranes were probed with 1:1,000 diluted anti-HIF-1α (rabbit monoclonal; cat. no. 36169; Cell Signaling Technology, Inc.), anti-osteopontin (OPN; cat. no. 22952-1-AP; rabbit polyclonal; ProteinTech Group, Inc., Chicago, IL, USA), anti-runt-related gene-2 (RUNX-2; cat. no. 12556; rabbit monoclonal; Cell Signaling Technology, Inc.), anti-alkaline phosphatase (ALP; rabbit polyclonal; cat. no. ab83259; Abcam) and anti-β-actin (rabbit monoclonal; cat. no. 4970; Cell Signaling Technology, Inc.) antibodies overnight at 4°C, and then washed with 1X TBST. Following washing, the membranes were incubated with anti-rabbit horseradish peroxidase-conjugated IgG (1:2,000; cat. no. 7074; Cell Signaling Technology, Inc.) at room temperature for 1 h. The secondary antibodies were detected using the Western chemiluminescent ECL reagent (Tiangen Biotech Co., Ltd., Beijing, China). The protein expression level was quantified using Image Pro Plus 6.0 software (Media Cybernetics, Inc.). Relative protein levels were calculated as the protein of interest to β-actin ratio in each sample.
Statistical analysis
All statistical calculations were performed using SPSS 10.0 (SPSS, Inc., Chicago, IL, USA). Data were presented as the mean ± SD (n=3). Paired samples of groups were compared using Student's t-test. Multiple comparisons within and between groups were determined by one-way analysis of variance followed by Bonferroni's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of HIF-1α in the rat orthodontic tooth movement model
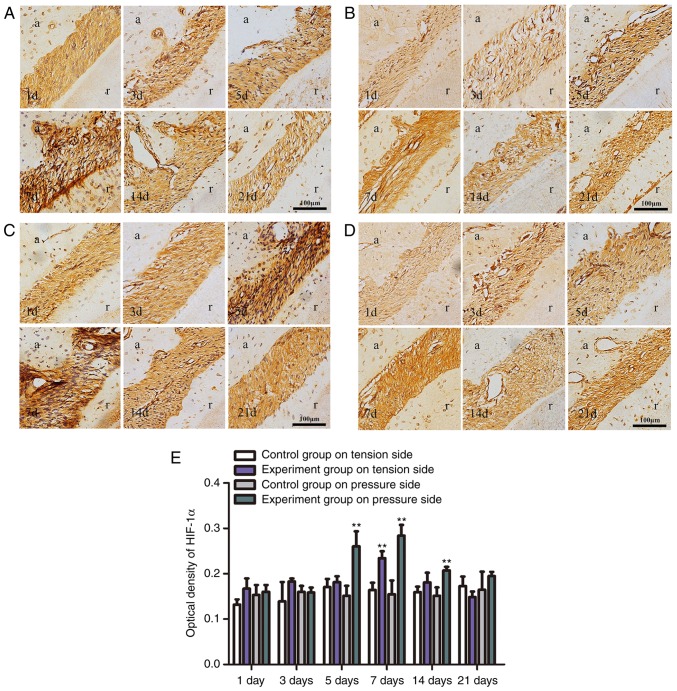
An experimental rat tooth movement model was used to determine whether HIF-1α is involved in orthodontic tooth movement. The results of immunohistochemical staining demonstrated that orthodontic tooth movement induced the expression of HIF-1α protein in the PDL on the tension side and pressure side. HIF-1α expression exhibited certain trends over time. On the tension side, the positive expression of HIF-1α in experimental groups was stronger compared with the control group, with a significant increase at day 7 (P<0.01; Fig. 1), then a decrease over time. On the pressure side, the expression of HIF-1α was significantly increased at day 5 and reached its peak at day 7, and subsequently decreased (P<0.01; Fig. 1).
Figure 1.
Immunohistochemical staining (magnification, ×200). (A) HIF-1α protein expression in rat PDL on the tension side in the experimental group. HIF-1α expression was initially increased and then decreased, with a maximum increase indicated at day 7. (B) HIF-1α protein expression in rat PDL on the tension side in the control group. (C) HIF-1α protein expression in rat PDL on the pressure side in the experimental group. HIF-1α expression initially increased and then decreased, with a maximum increase at day 7. (D) HIF-1α protein expression in rat PDL on the pressure side in the control group. (E) Optical density of HIF-1α protein expression levels in tooth movement by immunohistochemical staining. Significantly high expression was identified at day 7 on tension sides and at day 5, 7 and 14 on pressure sides (**P<0.01 vs. the control group). PDL, periodontal ligament; HIF-1α, hypoxia-inducible factor-1α; d, day; a, alveolar; r, root.
Our previous study (16) that detected miR-21 via in situ hybridization in a rat model of orthodontic tooth movement also indicated that miR-21 was highly expressed in the PDL on both sides, and the HIF-1α expression trend was similar, which suggested that the expression of HIF-1α may be associated with miR-21. Thus, the effect of miR-21 on HIF-1α in PDLCs required further study.
Expression of HIF-1α and miR-21 in human PDLCs under hypoxia in vitro
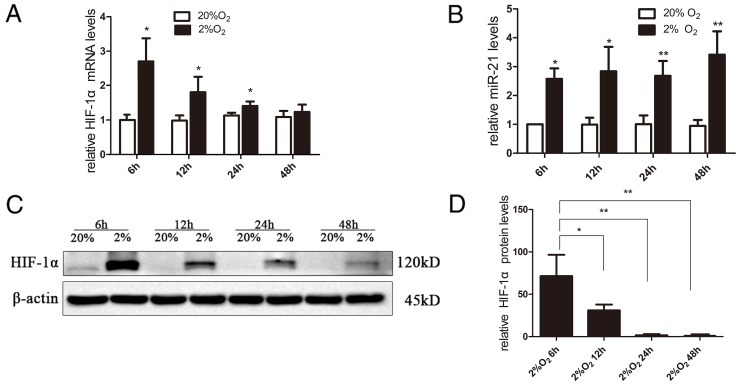
The expression levels of HIF-1α mRNA were rapidly and significantly increased at 6 h under hypoxia compared with normoxic conditions (P<0.05; Fig. 2A). Subsequently, the expression of HIF-1α mRNA decreased slowly over time (Fig. 2A). miR-21 was also significantly increased in PDLCs when exposed to hypoxia and remained significantly higher under hypoxia from 6–48 h compared with the normoxia group (P<0.05 and P<0.01; Fig. 2B). Under normoxia, HIF-1α protein was hardly detectable. Compared with 48 h, HIF-1α protein exhibited significantly higher expression at 6 and 12 h, and decreased slowly after 6 h (Fig. 2C and D).
Figure 2.
Expression of HIF-1α and miR-21 in periodontal ligament cells under hypoxia. (A) Expression of HIF-1α mRNA was rapidly and significantly increased under hypoxia at 6 h compared with the normoxia group, then the expression of HIF-1α mRNA slowly decreased. (B) miR-21 was significantly increased in periodontal ligament cells when exposed to hypoxia and remained significantly higher under hypoxia from 6 to 48 h compared with the normoxia group. (C and D) HIF-1α protein exhibited significantly higher expression at 6 and 12 h, and also decreased slowly (*P<0.05, **P<0.01 vs. the 48 h group). HIF-1α, hypoxia-inducible factor-1α; miR, microRNA.
Effect of miR-21 on HIF-1α and osteogenic markers in human PDLCs under hypoxia
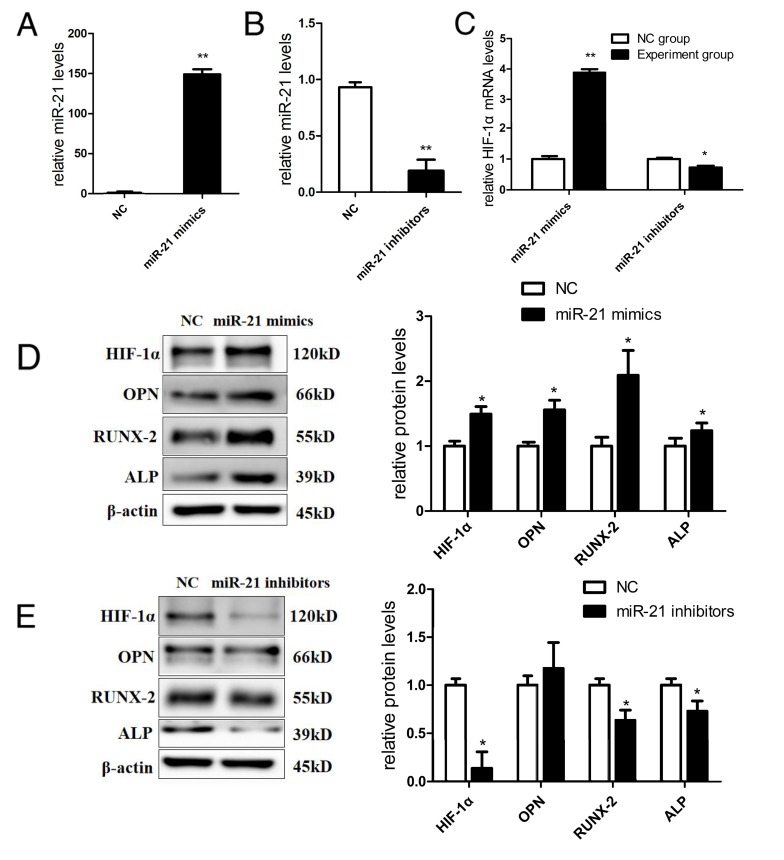
To investigate whether miR-21 could affect HIF-1α and influence osteogenic differentiation in PDLCs under hypoxia, PDLCs were transiently transfected with miR-21 mimics and miR-21 inhibitors to overexpress and inhibit miR-21, respectively, in vitro. The transfection efficiency was analyzed by RT-qPCR, confirming that miR-21 mimics and inhibitors were effective. miR-21 expression was elevated by ~149-fold when transfected with miR-21 mimics (Fig. 3A). However, miR-21 expression was decreased to 0.1-fold when transfected with miR-21 inhibitors (Fig. 3B).
Figure 3.
Effect of miR-21 on HIF-1α and osteogenic differentiation. (A) Transfection efficiency of miR-21 mimics. RT-qPCR confirmed that miR-21 mimics increased the expression of miR-21. (B) Transfection efficiency of miR-21 inhibitors. RT-qPCR confirmed that miR-21 inhibitors significantly decreased the expression of miR-21. (C) miR-21 mimics significantly increased the expression of HIF-1α by 3.9-fold, and miR-21 inhibitors decreased the expression of HIF-1α by 0.7-fold. (D) miR-21 mimics upregulated HIF-1α protein by 1.5-fold, and significantly increased the protein expression of osteogenic markers-OPN by 1.7-fold, RUNX-2 by 2.1-fold and ALP by 1.2-fold. (E) miR-21 inhibitors suppressed HIF-1α protein by 0.1-fold, and significantly decreased the protein expression of RUNX-2 by 0.6-fold and ALP by 0.7-fold. No significant difference was identified in OPN expression between the control and miR-21 inhibitors group (*P<0.05, **P<0.01 vs. the control group). miR, microRNA; HIF-1α, hypoxia-inducible factor-1α; OPN, osteopontin; RUNX-2, runt-related gene-2; ALP, alkaline phosphatase; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
miR-21 mimics significantly upregulated HIF-1α mRNA by 3.9-fold (P<0.01; Fig. 3C) and upregulated HIF-1α protein by 1.5-fold (P<0.05; Fig. 3D); furthermore, miR-21 inhibitors suppressed HIF-1α mRNA by 0.7-fold (P<0.05; Fig. 3C) and suppressed HIF-1α protein by 0.1-fold (P<0.05; Fig. 3E) under hypoxia, demonstrating that miR-21 regulates the expression of HIF-1α. Additionally, miR-21 mimics increased the protein expression of the osteogenic markers. OPN was increased by 1.7-fold, RUNX-2 by 2.1-fold and ALP by 1.2-fold (P<0.05; Fig. 3D). miR-21 inhibitors decreased the protein expression of RUNX-2 by 0.6-fold and ALP by 0.7-fold (P<0.05; Fig. 3E); however, there was no significant difference in OPN expression between the control and miR-21 inhibitors group. These results indicated that miR-21 affects the expression of HIF-1α and is involved in the osteogenic differentiation of PDLCs.
Discussion
Orthodontic force loading causes local hypoxia of the PDL, initiating an aseptic inflammatory cascade culminating in osteoblast deposition in areas of tension and osteoclast resorption in areas of pressure (18). In the early stages of tooth movement, blood vessels are narrowed and the oxygen level is decreased because the PDL is stretched on the tension side and compressed on the pressure side. By studying the PDL vascular changes during tooth movement in rhesus monkeys and dogs, Khouw et al (19) identified that blood vessels on both sides were expanded following 7 days of loading, and new bone formation was observed on the tension side, with bone resorption fossa on the pressure side. The 50 g force can lead to effective tooth movement of first molars without causing periodontal damage, which has been proven to be a suitable force and applied widely in experimental tooth movement models (20,21). Franzen et al (20) applied 50 g force to move rats' first molars, and then removed the orthodontic appliances to study the periodontal tissue reaction to during orthodontic relapse in rats. Wei et al (22) used 30 g force to study the reaction of dental pulp tissues by examining HIF-1α and vascular endothelial growth factor; they revealed that the expression of HIF-1α was markedly increased in the 1, 3, 7 day and 2 week groups. In the present study, 50 g force was also applied between the maxillary right first molar and incisors of rats, and hypoxia-sensitive factor HIF-1α was highly expressed in PDL, which could also indicate that the model used was suitable. The present results demonstrated that HIF-1α was significantly elevated in the pressure and tension PDL areas, exhibiting a trend of an initial increase followed by a subsequent decrease on both sides, which indicated that HIF-1α may be involved in PDL tissue remodeling. Our previous in vitro study also demonstrated that HIF-1α enhances osteogenic differentiation of PDLCs under hypoxia (8). Jiang et al (23) reported that HIF-1α protein could increase the bone mineralization density and bone mineralization content in the distraction osteogenesis zone. Additionally, knockdown of HIF-1α enhances adipogenesis and suppresses hypoxia-induced osteogenesis in MSCs (24).
Previous evidence has demonstrated that specific miRs are essential elements in regulating gene expression through post-transcriptional mechanisms in response to hypoxia, and this specific group of miRs was termed ‘hypoxamiRs’ (4). Hypoxia could either activate or repress hypoxamirs via several mechanisms (4). miR-21 was one of the hypoxamiRs reportedly involved in cellular adaption to low oxygen tension (25). It was indicated that miR-21 was significantly increased in human PDLCs under hypoxia in the present study. Notably, a previous study demonstrated that miR-21 was involved in tooth movement in a normal and inflammatory micro-environment in vivo (26). Additionally, miR-21 was previously indicated to be mechano-sensitive and had a role in the osteogenic differentiation of PDLCs when exposed to stretch (27). Our previous study also demonstrated that miR-21 was expressed in the PDL during experimental tooth movement (16). These studies suggested that miR-21 may be associated with osteogenic differentiation in hypoxia. PDLCs are clusters of multiple cell types, including periodontal ligament stem cells (PDLSCs). PDLSCs are a type of MSC that have the potential to differentiate into adipocytes, cementoblast-like cells and collagen-forming cells under defined culture conditions; thus, PDLSCs are critical in periodontal remodeling (28). In the present study, it was indicated that overexpression of miR-21 significantly elevated the expression of osteogenic markers (OPN, RUNX-2 and ALP), while knockdown of miR-21 decreased the expression of osteogenic markers (RUNX-2 and ALP). Meng et al (29) reported that overexpression of miR-21 increased the levels of the osteogenesis-associated genes in human umbilical cord MSCs, and that miR-21 acts by altering the phosphoinositide 3-kinase-protein kinase B (AKT)-glycogen synthase kinase 3β pathway.
As the present in vivo and in vitro studies revealed that the expression pattern of HIF-1α was similar to that of miR-21 under hypoxia, it was hypothesized that miR-21 may regulate HIF-1α and osteogenic differentiation of PDLCs under hypoxia. Furthermore, miR-21 mimics significantly increased the expression of HIF-1α and miR-21 inhibitors significantly suppressed the expression of HIF-1α, which suggested that miR-21 could affect HIF-1α in PDLCs under hypoxia. In the present study, miR-21 mimics increased the expression of HIF-1α mRNA by 3.9-fold, while miR-21 inhibitors decreased the expression of HIF-1α mRNA by 0.7-fold. The difference in fold change may be due to the difference in concentration and potency of miR-21 mimics and miR-21 inhibitors. Previous studies have also reported that HIF-1α is regulated by other miRs (14,15,30). However, the detailed mechanism by which miR-21 regulates HIF-1α is still unknown, and the regulation may involve several signaling pathways. In prostate cancer cells, miR-21 increases the activation of the AKT and ERK1/2 signaling pathways by targeting the phosphatase and tensin homolog deleted on chromosome ten (PTEN) gene, increasing the expression of HIF-1α and vascular endothelial growth factor, resulting in tumor angiogenesis (14). In radioresistant non-small cell lung cancer cells, HIF-1α was upregulated by miR-21, resulting in increased expression of key glycolytic enzymes (15). Liu et al (31) indicated there is a regulatory feedback loop between HIF-1α and miR-21 in response to hypoxia in cardiomyocytes; whereby, HIF-1α transcriptionally enhances miR-21 promoter activity by binding to its promoter, while miR-21 inhibition reduces HIF-1α expression and modulates the PTEN/AKT signaling pathway. However, the specific mechanism by which miR-21 regulates HIF-1α under hypoxia in PDLCs requires further exploration.
In conclusion, the present study demonstrated that HIF-1α was highly expressed in the PDL of rats in an experimental tooth movement model, sharing a similar expression pattern with miR-21 (as reported in our previous study). Hypoxia significantly upregulated HIF-1α and miR-21 in PDLCs. Furthermore, miR-21 exerted a positive effect on HIF-1α and osteogenic differentiation in PDLCs under hypoxia. These findings provide a theoretical basis for promoting periodontal tissue reconstruction during orthodontic tooth movement; however, the detailed mechanisms of miR-21 regulating HIF-1α may involve some complex signaling pathways. Future work is required to focus on the regulation mechanism of miR-21 regulating HIF-1α in orthodontic tooth movement.
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from Natural Science Foundation of Guangdong Province (grant no. 2015A030313083).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
XZ and DC designed and conducted the experiments, analyzed the data and wrote manuscript. LD, ZC and JZ conducted the experiments and analyzed the data. LW and JL designed the experiments, analyzed the data and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animals were treated under the ethical regulations for animal experiments, defined by the Institutional Ethics Committee. All enrolled patients provided their written informed consent. The present study was approved by the Ethic Committee of the Hospital of Stomatology, Sun Yat-sen University (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Huang H, Williams RC, Kyrkanides S. Accelerated orthodontic tooth movement: Molecular mechanisms. Am J Orthod Dentofacial Orthop. 2014;146:620–632. doi: 10.1016/j.ajodo.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 2.de Jong T, Bakker AD, Everts V, Smit TH. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J Periodont Res. 2017;52:965–974. doi: 10.1111/jre.12477. [DOI] [PubMed] [Google Scholar]
- 3.Niklas A, Proff P, Gosau M, Römer P. The role of hypoxia in orthodontic tooth movement. Int J Dent. 2013;2013:841840. doi: 10.1155/2013/841840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: A master regulator of microRNA biogenesis and activity. Free Radical Biol Med. 2013;64:20–30. doi: 10.1016/j.freeradbiomed.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mingyuan X, Qianqian P, Shengquan X, Chenyi Y, Rui L, Yichen S, Jinghong X. Hypoxia-inducible factor-1α activates transforming growth factor-β1/Smad signaling and increases collagen deposition in dermal fibroblasts. Oncotarget. 2017;9:3188–3197. doi: 10.18632/oncotarget.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddle RC, Khatri R, Schipani E, Clemens TL. Role of hypoxia-inducible factor-1α in angiogenic-osteogenic coupling. J Mol Med (Berl) 2009;87:583–590. doi: 10.1007/s00109-009-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lampert FM, Kütscher C, Stark GB, Finkenzeller G. Overexpression of Hif-1α in mesenchymal stem cells affects cell-autonomous angiogenic and osteogenic parameters. J Cell Biochem. 2016;117:760–768. doi: 10.1002/jcb.25361. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Wu L, Liu L, Gong Q, Zheng J, Peng C, Deng J. Comparison of HIF1A-AS1 and HIF1A-AS2 in regulating HIF-1α and the osteogenic differentiation of PDLCs under hypoxia. Int J Mol Med. 2017;40:1529–1536. doi: 10.3892/ijmm.2017.3138. [DOI] [PubMed] [Google Scholar]
- 9.Hulley PA, Bishop T, Vernet A, Schneider JE, Edwards JR, Athanasou NA, Knowles HJ. Hypoxia-inducible factor 1-alpha does not regulate osteoclastogenesis but enhances bone resorption activity via prolyl-4-hydroxylase 2. J Pathol. 2017;242:322–333. doi: 10.1002/path.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8:212–227. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorospe M, Tominaga K, Wu X, Fähling M, Ivan M. Post-transcriptional control of the hypoxic response by RNA-binding proteins and MicroRNAs. Front Mol Neurosci. 2011;4:7. doi: 10.3389/fnmol.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mei Y, Bian C, Li J, Du Z, Zhou H, Yang Z, Zhao RCH. miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J Cell Biochem. 2013;114:1374–1384. doi: 10.1002/jcb.24479. [DOI] [PubMed] [Google Scholar]
- 13.Yang N, Wang G, Hu C, Shi Y, Liao L, Shi S, Cai Y, Cheng S, Wang X, Liu Y, et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res. 2013;28:559–573. doi: 10.1002/jbmr.1798. [DOI] [PubMed] [Google Scholar]
- 14.Liu LZ, Li CY, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai LH, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1a expression. PLoS One. 2011;6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang S, Wang R, Yan H, Jin L, Dou X, Chen D. MicroRNA-21 modulates radiation resistance through upregulation of hypoxia-inducible factor-1alpha-promoted glycolysis in non-small cell lung cancer cells. Mol Med Rep. 2016;13:4101–4107. doi: 10.3892/mmr.2016.5010. [DOI] [PubMed] [Google Scholar]
- 16.Hong H, Zheng JX, Liu L, Mai LX, Wei X, Wu LP. MicroRNA 21 and PLAP-1 regulate periodontal ligament remodeling during tooth movement of rats. Int J Clin Exp Pathol. 2016;9:4943–4952. [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Jacox LA, Little SH, Ko CC. Orthodontic tooth movement: The biology and clinical implications. Kaohsiung J Med Sci. 2018;34:207–214. doi: 10.1016/j.kjms.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Khouw FE, Goldhaber P. Changes in vasculature of the periodontium associated with tooth movement in the rhesus monkey and dog. Arch Oral Biol. 1970;15:1125–1132. doi: 10.1016/0003-9969(70)90003-8. [DOI] [PubMed] [Google Scholar]
- 20.Franzen TJ, Brudvik P, Vandevska-Radunovic V. Periodontal tissue reaction during orthodontic relapse in rat molars. Eur J Orthod. 2013;35:152–159. doi: 10.1093/ejo/cjr127. [DOI] [PubMed] [Google Scholar]
- 21.Hou J, Chen Y, Meng X, Shi C, Li C, Chen Y, Sun H. Compressive force regulates ephrinB2 and EphB4 in osteoblasts and osteoclasts contributing to alveolar bone resorption during experimental tooth movement. Korean J Orthod. 2014;44:320–329. doi: 10.4041/kjod.2014.44.6.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei F, Yang S, Xu H, Guo Q, Li Q, Hu L, Liu D, Wang C. Expression and function of hypoxia inducible factor-1α and vascular endothelial growth factor in pulp tissue of teeth under orthodontic movement. Mediators Inflamm. 2015;2015:215761. doi: 10.1155/2015/215761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang X, Zhang Y, Fan X, Deng X, Zhu Y, Li F. The effects of hypoxia-inducible factor (HIF)-1α protein on bone regeneration during distraction osteogenesis: An animal study. Int J Oral Maxillofac Surg. 2016;45:267–272. doi: 10.1016/j.ijom.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Wagegg M, Gaber T, Lohanatha FL, Hahne M, Strehl C, Fangradt M, Tran CL, Schönbeck K, Hoff P, Ode A, et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PLoS One. 2012;7:e46483. doi: 10.1371/journal.pone.0046483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N, Sui BD, Hu CH, Cao J, Zheng CX, Hou R, Yang ZK, Zhao P, Chen Q, Yang QJ, et al. microRNA-21 contributes to orthodontic tooth movement. J Dent Res. 2016;95:1425–1433. doi: 10.1177/0022034516657043. [DOI] [PubMed] [Google Scholar]
- 27.Wei F, Liu D, Feng C, Zhang F, Yang S, Hu Y, Ding G, Wang S. microRNA-21 mediates stretch-induced osteogenic differentiation in human periodontal ligament stem cells. Stem Cells Dev. 2015;24:312–319. doi: 10.1089/scd.2014.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamila PPP, Samaranayake LP, Jin LJ, Zhang C. Periodontal ligament stem cells: An update and perspectives. J Investig Clin Dent. 2014;5:81–90. doi: 10.1111/jicd.12089. [DOI] [PubMed] [Google Scholar]
- 29.Meng YB, Li X, Li ZY, Zhao J, Yuan XB, Ren Y, Cui ZD, Liu YD, Yang XJ. microRNA-21 promotes osteogenic differentiation of mesenchymal stem cells by the PI3K/β-catenin pathway. J Orthop Res. 2015;33:957–964. doi: 10.1002/jor.22884. [DOI] [PubMed] [Google Scholar]
- 30.Liu LL, Li D, He YL, Zhou YZ, Gong SH, Wu LY, Zhao YQ, Huang X, Zhao T, Xu L, et al. miR-210 protects renal cell against hypoxia-induced apoptosis by targeting HIF-1 alpha. Mol Med. 2017;23:258–271. doi: 10.2119/molmed.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Nie H, Zhang K, Ma D, Yang G, Zheng Z, Liu K, Yu B, Zhai C, Yang S. A feedback regulatory loop between HIF-1α and miR-21 in response to hypoxia in cardiomyocytes. FEBS Lett. 2014;588:3137–3146. doi: 10.1016/j.febslet.2014.05.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.