Abstract
Ovarian cancer is a serious threat to women's health. Multidrug resistance is a major cause of post-treatment relapse, metastasis, and even mortality. This characteristic severely restricts the survival of patients with ovarian cancer. Integrin α-6 (ITGA6) is a member of the adhesion molecule family that conducts signals through interactions between the extracellular domain and the matrix, serving important roles in cell adhesion-mediated drug resistance, which is considered to have a critical function in ovarian cancer drug resistance. The association between ITGA6 and ovarian cancer multidrug resistance has been investigated only rarely, to the best of our knowledge. Using RT-qPCR and immunohistochemistry, it was identified that ITGA6 is a central drug resistance gene, and that its expression was upregulated in cisplatin-resistant SKOV3 (SKOV3/DDP2), cisplatin-resistant A2780 (A2780/DDP) cells, and in 54 cases of drug-resistant tissues, as compared with in the controls. Furthermore, bioinformatics and text mining performed by Coremine Medical (http://www.coremine.com/medical/#search) confirmed that ITGA6 was significantly associated with ovarian cancer and drug resistance. Additionally, the high expression of ITGA6 is associated with a poor outcome. The present study provides the basis for further understanding the role of ITGA6 in the regulation of drug resistance in ovarian cancer, and demonstrates that it could be a potential marker for the prognosis of ovarian cancer.
Keywords: ovarian cancer, drug resistance, prognosis, integrin α-6
Introduction
Ovarian cancer is a malignant tumor that represents a serious threat to women's health, and has the highest mortality among all gynecological tumors (1). Although platinum-based chemotherapy is often effective in reducing tumor size in ovarian cancer, the majority of cases recur or metastasize due to the development of drug resistance (2). Drug resistance is a major cause of post-treatment relapse, metastasis, and even mortality, and thus it is a primary obstacle to the survival of patients with ovarian cancer. The development of ovarian cancer multi-resistance is complex, including abnormal cell proliferation, apoptosis, cell growth and invasion, epithelial-mesenchymal transition (3), tumor angiogenesis, the prevention of intracellular drug accumulation (4), intervention in DNA damage repair (5), and associated signaling pathways (6).
In recent years, the influence of cytokines present in the tumor microenvironment on resistance has been widely reported. Interactions between tumor cell adhesion molecules (CAM) and the extracellular matrix (ECM) have important effects on tumor drug resistance (7). Integrins are members of the adhesion molecules family, and through signaling cascades initiated by interactions between the extracellular domains and the matrix, the intracellular domains and various signaling molecules, these molecules serve important roles in regulating cell survival, proliferation, adhesion, differentiation and apoptosis (8). Previous studies have demonstrated that, when cells undergo malignant transformation, the integrin configurations on the cell surface and/or their expression levels also change, ultimately affecting tumor cell growth, differentiation, apoptosis and adhesion (8–10). ITGA6 is a member of the integrin family, that inhibits DNA damage and enhances DNA repair, which ultimately enhances the drug-resistance of tumor cells (11) such as human acute myeloid leukemia (12) and breast cancer (13). In the present study, it was investigated whether ITGA6 is a molecule that potentially controls multi-drug resistance. Exploring the full range of functions performed by ITGA6 is an important strategy for addressing the challenge of drug resistance in ovarian cancer.
Materials and methods
Cell culture
The Human ovarian cancer cell lines SKOV3 and A2780 were cultured generated in our lab (14). The stable cisplatin-resistant cell lines SKOV3/DDP and A2780/DDP were established from the parental SKOV3 and A2780 cell lines, respectively, by continuous exposure of the cells to increasing concentrations of cisplatin and maintenance in RPMI-1640 media (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Corning, Inc., Corning, NY, USA) and 2 µmol/l L-glutamine, at 37°C in a humidified (95%) atmosphere containing 5% CO2.
Patients and samples
Formalin-fixed, paraffin-embedded tissue specimens from 54 patients with stage Ic-IV ovarian [International Federation of Gynecology and Obstetrics (FIGO) Stage] (15) serous adeno carcinoma were collected from the Department of Gynecologic Oncology, Affiliated Tumor Hospital of Guangxi Medical University from April 2005 to December 2012. The Ethics Committees of Guangxi Medical University approved the study protocol, and all patients received an explanation of the aims of the study and provided signed informed consent. All patients had undergone cytoreductive surgeries prior to the study and the diagnosis of ovarian serous adenocarcinoma was confirmed by two pathologists. Patients received platinum-paclitaxel chemotherapy for ≥6 cycles following surgery. Classification of the response to primary chemotherapy was designated as either sensitive (S, complete remission and relapse >6 months after stopping chemotherapy) or resistant (R, complete remission and relapse <6 months after stopping chemotherapy). The clinical data for the S (n=29) and R groups (n=25) are listed in Table I.
Table I.
Association of ITGA6 with clinic-pathological parameters in 54 cases of ovarian cancer tissues.
| Characteristics | ITGA6 positive/negative | P-value |
|---|---|---|
| Total no. | 24/30 | |
| Age (years) | 0.808 | |
| <50 | 12/16 | |
| ≥50 | 12/14 | |
| Stage | 0.695 | |
| I/II | 5/5 | |
| III/IV | 19/25 | |
| Histotype | 0.325 | |
| Serous | 12/11 | |
| Other | 12/19 | |
| Grade | 0.594 | |
| G1-2 | 8/8 | |
| G3 | 16/22 | |
| Serum ca125 (U/ml) | 0.901 | |
| <400 | 10/12 | |
| ≥400 | 14/18 | |
| Primary surgery | 0.300 | |
| Optimal | 11/18 | |
| Suboptimal | 13/12 | |
| Lymph node metastasis | 0.614 | |
| Yes | 16/8 | |
| No | 8/12 | |
| Chemosensitivity | 0.033a | |
| Sensitive | 9/20 | |
| Resistant | 15/10 |
P<0.05; ITGA6, integrin α-6.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA was extracted using an RNeasy® Mini Kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer's protocol. First-strand cDNA was synthesized using 1 µg total RNA using a Transcriptor First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Primer sequences were generated according to ITGA6 gene cDNA sequences in Genbank. The ITGA6 gene-specific primers were as following: forward: 5′-CAGTGGAGCCGTGGTTTTG-3′, and reverse: 5′-CCACCGCCACATCATAGCC-3′ (product length 113 bp). GAPDH was used as control, the primer sequences are forward 5′-GTCAAGGCTGAGAACGGGA-3′, and reverse 5′-AAATGAGCCCCAGCCTTCTC-3′ (product length, 225 bp). RT-qPCR was completed with a One Step SYBR PrimeScript Plus RT-PCR kit (Takara Bio, Inc., Otsu, Japan) and a total volume of 20 µl, using an ABI 7500 Real-Time PCR System (Thermo Fisher Scientific, Inc.). The conditions were as follows: 95°C for 10 min and 40 cycles of two-step PCR (95°C for 30 sec and 60°C for 30 sec). Average fold changes were calculated according to differences in the quantification cycles (Cq) between pairs of samples to be compared. The 2−ΔΔCq method (16) was used for data quantification.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections (4 µm) were deparaffinized in xylene and rehydrated through a graded ethanol series, followed by antigen retrieval endogenous peroxidase. The primary antibody included a mouse monoclonal antibody against human ITGA6 (Abcam, Cambridge, UK; cat. no. ab181551; 1:150; 4°C, incubated overnight), secondary antibody (cat. no. KIT-5001; MaxVision™ HRP-Polymer anti-Mouse IHC kit; 1:1; 37°C, incubated for 60 min) was purchased from Fuzhou Maixin Biotech Co, Ltd, Fuzhou, China. Negative controls were generated by substituting the primary antibody with PBS. All slides were evaluated independently by two pathologists. A total of 5 microscopic fields (Olympus Corporation, Tokyo, Japan IX71/IX81, confocal, natural light ×400) per slide were selected for the evaluation of ITGA6 staining. Any section that demonstrated a detectable membranous and/or cytoplasmic positivity was defined as positive. The intensity of immunostaining was graded as follows: 0, weak; 1+, moderate; 2+, strong; and 3+, very strong. The area of positive cancer cells (%) in each microscopic field was categorized as follows: 1+, 0–10%; 2+, 11–50%; 3+, 51–75%; and 4+, 75–100%. The score for each section was calculated by multiplying the scores for both the staining intensity and the area of positive cells. Scores of 0–3 were designated as ‘low expression’; scores of 4–12 were designated as ‘high expression’.
A Kaplan-Meier plotter data portal (kmplot.com/analysis/index.php?p=service&cancer=ovar) was used to access 1,583 ovarian carcinoma cases. The ITGA6 mRNA expression data in the Kaplan-Meier plotter database were divided by a median into high and low expression groups. The associations between ITGA6 mRNA expression and the overall survival (OS) and the progression-free survival (PFS) were analyzed. Text mining performed by Coremine Medical software (www.coremine.com) was used to elucidate the associations of ITGA6 with drug resistance in ovarian cancer.
Statistical analysis
All data were analyzed using SPSS v.19.0 for Windows statistical software package (IBM Corp., Armonk, NY, USA). The RT-qPCR data are presented as the mean ± standard deviation, or as the median, and were analyzed with the Student's t-test and a chi-squared test. Associated factors of drug resistance were analyzed using the multivariable logistic regression method. Survival curves were constructed and compared using the Kaplan-Meier method and the log-rank test, and a Cox proportional hazards model analysis was performed to study the influence of various factors on the survival time of patients with ovarian epithelial carcinoma. P<0.05 was considered to indicate a statistically significant difference.
Results
ITGA6 is associated with drug resistance in ovarian cancer
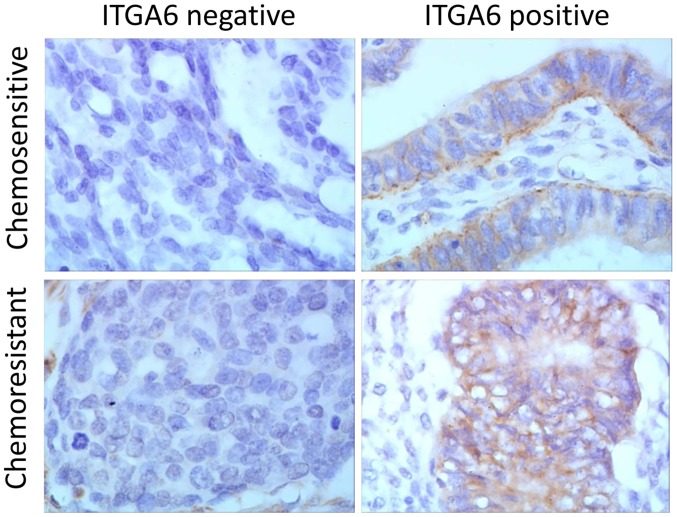
RT-qPCR analysis indicated that the expression of ITGA6 is upregulated in SKOV3/DDP2 and A2780/DDP cells, in comparison with the parental cell expression profiles (P<0.05; Fig. 1). Immunohistochemistry analysis, performed on a total of 54 ovarian cancer tissues (25 drug resistant and 29 drug sensitive tissues), indicated that ITGA6 was predominantly located in the cell membrane (Fig. 2). The percentage of chemo-resistant cases expressing ITGA6 was 60.0% (15/25), but the expression of ITGA6 in chemo-sensitive tissues was 31.0% (9/29) (P<0.05), indicating that the expression of ITGA6 was increased in chemo-resistant tissues (Table I).
Figure 1.
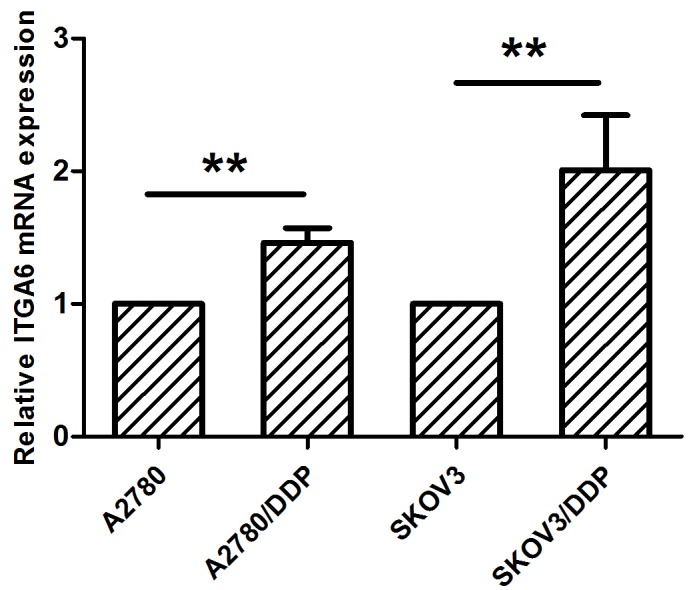
mRNA expression of ITGA6 in ovarian cancer cells. Relative expression of genes in SKOV3, cisplatin-resistant SKOV3 cells (SKOV3/DDP), A2780 cells and cisplatin resistant A2780 cells (A2780/DDP). **P<0.01. ITGA6, integrin α-6.
Figure 2.
Immunohistochemistry analysis of protein expression in 54 ovarian cancer tissues. Representative staining images from chemo-resistant and chemo-sensitive tissues are presented, at a magnification of ×400. ITGA6, integrin α-6.
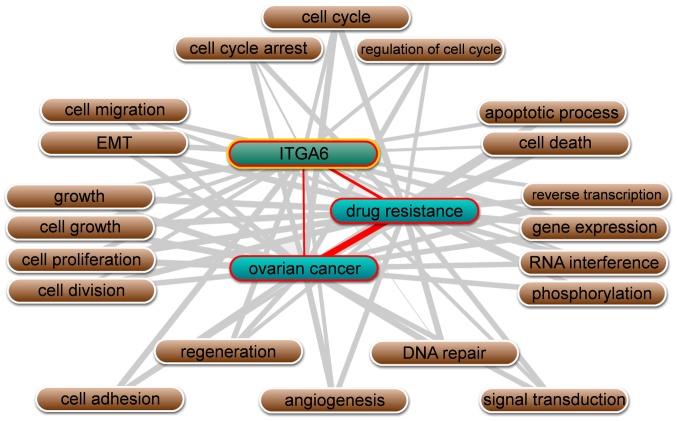
Besides, text mining performed by Coremine Medical software using the gene names and ‘ovarian neoplasms’, and ‘drug resistance’ as keywords in co-occurrence analysis, it was identified that ITGA6 is significantly associated with ovarian cancer and drug resistance, in addition to being involved in numerous biological processes, including cell adhesion, cell-matrix adhesion, epithelial-mesenchymal transition (EMT), apoptotic processes and cell proliferation (P<0.01; Fig. 3).
Figure 3.
Schematic diagram of the linear associations between ITGA6, drug resistance and ovarian cancer, as determined using Coremine Medical software. ITGA6, integrin α-6.
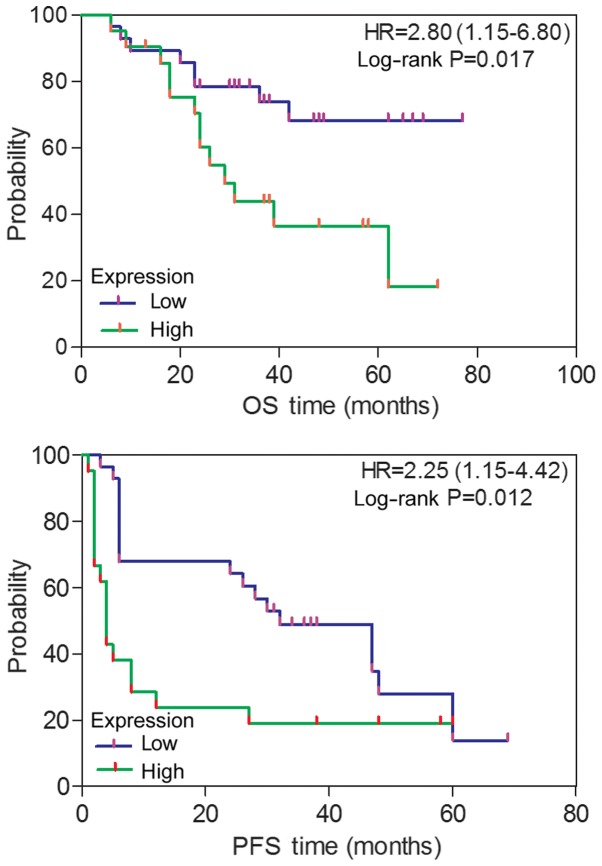
High expression levels of ITGA6 correlate with the survival of patients with ovarian cancer
Associations between ITGA6 and various clinical factors were analyzed, based on protein expression evaluated through immunohistochemistry. As depicted in Fig. 4, patients with ovarian cancer and high ITGA6 expression exhibited a poorer PFS (P=0.012) and an OS (P=0.017), compared with patients exhibiting low ITGA6 expression, as determined by Kaplan-Meier survival curves. This was further confirmed using the univariate Cox regression analysis method (HR=2.25, 95% CI, 1.15–4.42 for PFS; HR=2.80, 95% CI, 1.15–6.80 for OS). However, the associations of ITGA6 with age, FIGO stage, histological type, grade, primary surgery type, serum CA125 levels and lymph node metastasis were not statistically significant (P≥0.05; Table I).
Figure 4.
The association of ITGA6 protein expression with prognosis in 54 patients with ovarian cancer, as determined by the Kaplan-Meier survival curves. HR, hazard ratio; ITGA6, integrin α-6; OS, overall survival; PFS, progression-free survival.
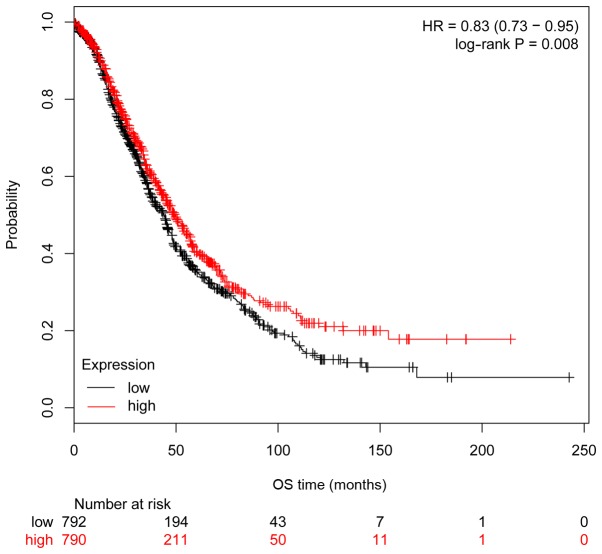
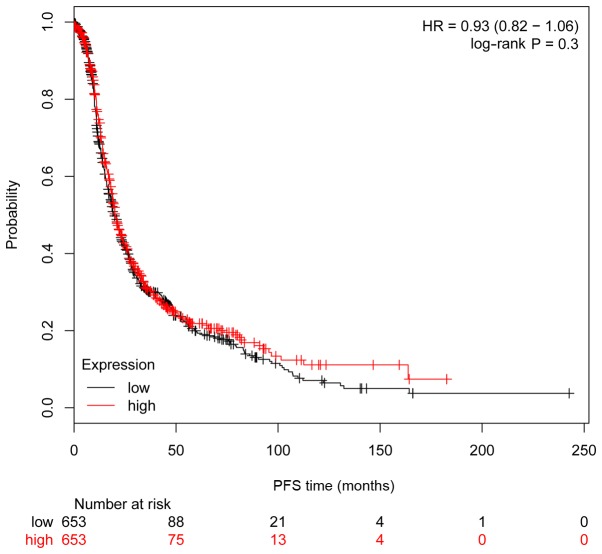
The clinical importance of ITGA6 in ovarian cancer was further investigated using Kaplan-Meier analysis, with a cohort comprising 1,583 ovarian carcinoma cases. Consistent with the results obtained from the 54 patients with ovarian cancer, high expression of ITGA6 in 1,583 patients with ovarian cancer from the Kaplan-Meier cohort was significantly associated with poorer OS (P=0.008, HR=0.83 95% CI, 0.73–0.95; Fig. 5), but not significantly associated with PFS (P=0.30, HR=0.93 95% CI, 0.82–1.06; Fig. 6).
Figure 5.
Associations between gene expression and prognosis in 1,583 patients with ovarian cancer from the Kaplan-Meier analysis cohort, as determined by Kaplan-Meier survival curves. High expression of ITGA6 was significantly associated with poorer OS. HR, hazard ratio; ITGA6, integrin α-6; OS, overall survival.
Figure 6.
Associations between gene expression and prognosis in 1,307 patients with ovarian cancer from the Kaplan-Meier analysis cohort, as determined by Kaplan-Meier survival curves. High expression of ITGA6 was not significantly associated with PFS. HR, hazard ratio; ITGA6, integrin α-6; PFS, progression-free survival.
Discussion
Ovarian cancer is a type of malignancy with the highest mortality rate among all gynecological tumors (1). Paclitaxel plus platinum-based chemotherapy is the current standard treatment strategy for ovarian cancer, with cisplatin as the preferred agent (2). Although platinum-based chemotherapy is often effective in reducing tumor size in ovarian cancer, the majority of cases present with recurrence or metastasis due to the development of drug resistance (17). Therefore, cisplatin resistance remains a serious obstacle that greatly affects patient survival. One of the causes of tumor recurrence is the frequent acquisition of drug resistant phenotypes during long-term chemotherapy. Thus, the identification of potential multi-drug resistance genes and their functions may be one effective strategy to meet the challenges of drug-resistant ovarian cancer.
A previous study demonstrated that the combination of ovarian cancer cells and ECM can increase the susceptibility of cancer cells to chemotherapy drugs; this mechanism was designated cell adhesion-mediated drug resistance (CAM-DR), and is principally mediated by adhesion molecules (18).
Integrins are an important family among cell surface adhesion molecules. They are transmembrane proteins comprised of two glycoprotein α and β subunits, that may also serve as receptors for various ECM components (8,19). Previous studies have reported that integrins can regulate cell growth, differentiation and metastasis through transmembrane signal transduction pathways (17). The growth and metastasis of tumor cells are closely associated with drug resistance, and metastatic tumor cells are more likely to become drug resistant (9,20,21).
The association between integrins and drug resistance has attracted global attention. Prior studies have demonstrated that integrins are highly expressed in drug-resistant tumor cells, including esophageal cancer (17), myeloid leukemia (12) and colon cancer (18). It was demonstrated that ITGA6 is significantly dysregulated in drug resistant ovarian cancer cells and tissues (22), and that it is overexpressed in ovarian cancer SKOV3/DDP2 cells, compared with in SKOV3 cells; these expression trends were the same as those observed in A2780/DDP and A2780 cells. Immunohistochemical analysis of a series of ovarian cancer cases demonstrated that ITGA6 expression is closely associated with ovarian cancer drug resistance. Another study reported that, when the ECM is combined with integrins, it can activate the downstream Akt2 signaling pathway, resulting in the activation of various downstream factors involved in regulating cell function and promoting survival (9). The signaling pathway linking integrins and the ECM is critical to cell homeostasis and survival. The lack of cell adhesion leads to disordered integrin signaling pathways (including the PI3K/AKT, MEK/ERK, FAK and NF-κB pathways), ultimately inducing cell apoptosis and drug-resistance (9,23). ITGA6 not only mediates interactions with the ECM, but it also drives intracellular signaling events of the tumor microenvironment and inside the tumor cell, promoting the metastasis and infiltration (13). Bioinformatics and text mining data indicated that ITGA6 is associated with drug resistance in ovarian cancer, which is consistent with the results obtained by the present study. This involves numerous biological processes, including cell adhesion, EMT, cell-matrix adhesion, apoptotic processes and cell proliferation. Thus, ITGA6 was selected for further studies into whether there are potential important genes associated with the regulation of drug resistance in ovarian cancer, as the available data indicated that the ECM and the integrin family are critical to the development of ovarian cancer cell resistance.
Associations between ITGA6 and cancer prognosis have been poorly investigated previously, with only a limited number of studies conducted and all reporting that its high expression was associated with a significantly poorer OS in patients with colorectal (24) and prostate cancer (25). Yamakawa et al (12) demonstrated that ITGA6 is associated with drug resistance through increasing cell adhesion, resulting in a poor prognosis for patients with acute myeloid leukemia. According to the Kaplan-Meier analysis data used in the present study, patients with ovarian cancer with high ITGA6 expression exhibited a significantly poorer OS. Additionally, 59 patients with ovarian cancer and high ITGA6 expression, who were enrolled in the present study, exhibited significantly a poorer PFS and OS.
Taken together, these studies indicated that ITGA6 expression is closely associated with multi-drug resistance in ovarian cancer. Therefore, we hypothesized that the mechanism underlying the involvement of ITGA6 in ovarian epithelial carcinoma drug-resistance may mediating CAM-DR.
In conclusion, based on bioinformatics analysis and molecular biology data, the present study demonstrated that ITGA6 may function as a regulatory gene in ovarian cancer cells, participating in the development of multi-drug resistance, and may be a prognostic risk factor for patients with OS. The investigation of IGTA6 expression in the present study may be involved in the regulation of drug resistance and prognosis in ovarian cancer cells; however, further studies are required to confirm the present findings.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science Foundation of China (grant. nos. 81572579, 81302283 and 81560424), the Ministry of Education Special Fund for the Doctoral Program in Colleges and Universities (grant. no. 20124503110003), the Guangxi Science and Technology Development Program (grant. no. 14124004-1-24), and the Key Laboratory of High-Incidence Tumor Prevention and Treatment, Guangxi Medical University (grant. no. GK2015-TKF01).
Availability of data and materials
The datasets generated and analyzed in the present study are included in this published article.
Authors' contributions
LW, FY and LL made substantial contributions to the conception or design of the work; CC had substantial contributions to the analysis of data for the present study; LL gave thier final approval of the version to be published.
Ethics and consent to participate
The ethics committees of Guangxi Medical University (Nanning, China) approved the study. All patients received an explanation of the aims of the study and provided written informed consent.
Consent for publication
The study participants provided consent for the data to be published.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3.Groeneweg JW, Foster R, Growdon WB, Verheijen RH, Rueda BR. Notch signaling in serous ovarian cancer. J Ovarian Res. 2014;7:95. doi: 10.1186/s13048-014-0095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kathawala RJ, Gupta P, Ashby CR, Jr, Chen ZS. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist Updat. 2015;18:1–17. doi: 10.1016/j.drup.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Tuorkey MJ. Curcumin a potent cancer preventive agent: Mechanisms of cancer cell killing. Interv Med Appl Sci. 2014;6:139–146. doi: 10.1556/IMAS.6.2014.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han X, DU F, Jiang L, Zhu Y, Chen Z, Liu Y, Hong T, Wang T, Mao Y, Wu X, et al. A2780 human ovarian cancer cells with acquired paclitaxel resistance display cancer stem cell properties. Oncol Lett. 2013;6:1295–1298. doi: 10.3892/ol.2013.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan L, Wang C, Lin B, Liu J, Liu D, Hou R, Wang Y, Gao L, Zhang S, Iwamori M. Lewis y enhances CAM-DR in ovarian cancer cells by activating the FAK signaling pathway and upregulating Bcl-2/Bcl-XL expression. Biochimie. 2015;113:17–25. doi: 10.1016/j.biochi.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 8.De Marco R, Tolomelli A, Juaristi E, Gentilucci L. Integrin ligands with α/β-hybrid peptide structure: Design, bioactivity, and conformational aspects. Med Res Rev. 2016;36:389–424. doi: 10.1002/med.21383. [DOI] [PubMed] [Google Scholar]
- 9.Blandin AF, Renner G, Lehmann M, Lelong-Rebel I, Martin S, Dontenwill M. β1 Integrins as therapeutic targets to disrupt hallmarks of cancer. Front Pharmacol. 2015;6:279. doi: 10.3389/fphar.2015.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkhalter RJ, Symowicz J, Hudson LG, Gottardi CJ, Stack MS. Integrin regulation of beta-catenin signaling in ovarian carcinoma. J Biol Chem. 2011;286:23467–23475. doi: 10.1074/jbc.M110.199539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott T, Sethi T. Integrins and extracellular matrix: A novel mechanism of multidrug resistance. Expert Rev Anticancer Ther. 2002;2:449–459. doi: 10.1586/14737140.2.4.449. [DOI] [PubMed] [Google Scholar]
- 12.Yamakawa N, Kaneda K, Saito Y, Ichihara E, Morishita K. The increased expression of integrin α6 (ITGA6) enhances drug resistance in EVI1(high) leukemia. PLoS One. 2012;7:e30706. doi: 10.1371/journal.pone.0030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks DL, Schwab LP, Krutilina R, Parke DN, Sethuraman A, Hoogewijs D, Schörg A, Gotwald L, Fan M, Wenger RH, Seagroves TN. ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models. Mol Cancer. 2016;15:26. doi: 10.1186/s12943-016-0510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin F, Liu L, Liu X, Li G, Zheng L, Li D, Wang Q, Zhang W, Li L. Downregulation of tumor suppressor gene ribonuclease T2 and gametogenetin binding protein 2 is associated with drug resistance in ovarian cancer. Oncol Rep. 2014;32:362–372. doi: 10.3892/or.2014.3175. [DOI] [PubMed] [Google Scholar]
- 15.Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;104:177–178. doi: 10.1016/j.ijgo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Luvero D, Milani A, Ledermann JA. Treatment options in recurrent ovarian cancer: Latest evidence and clinical potential. Ther Adv Med Oncol. 2014;6:229–239. doi: 10.1177/1758834014544121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia Y, Zeng ZZ, Markwart SM, Rockwood KF, Ignatoski KM, Ethier SP, Livant DL. Integrin fibronectin receptors in matrix metalloproteinase-1-dependent invasion by breast cancer and mammary epithelial cells. Cancer Res. 2004;64:8674–8681. doi: 10.1158/0008-5472.CAN-04-0069. [DOI] [PubMed] [Google Scholar]
- 19.Dransfield I, Cabanas C, Barrett J, Hogg N. Interaction of leukocyte integrins with ligand is necessary but not sufficient for function. J Cell Biol. 1992;116:1527–1535. doi: 10.1083/jcb.116.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janouskova H, Ray AM, Noulet F, Lelong-Rebel I, Choulier L, Schaffner F, Lehmann M, Martin S, Teisinger J, Dontenwill M. Activation of p53 pathway by Nutlin-3a inhibits the expression of the therapeutic target alpha5 integrin in colon cancer cells. Cancer Lett. 2013;336:307–318. doi: 10.1016/j.canlet.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Toquet C, Colson A, Jarry A, Bezieau S, Volteau C, Boisseau P, Merlin D, Laboisse CL, Mosnier JF. ADAM15 to α5β1 integrin switch in colon carcinoma cells: A late event in cancer progression associated with tumor dedifferentiation and poor prognosis. Int J Cancer. 2012;130:278–287. doi: 10.1002/ijc.25891. [DOI] [PubMed] [Google Scholar]
- 22.Maubant S, Cruet-Hennequart S, Poulain L, Carreiras F, Sichel F, Luis J, Staedel C, Gauduchon P. Altered adhesion properties and alphav integrin expression in a cisplatin-resistant human ovarian carcinoma cell line. Int J Cancer. 2002;97:186–194. doi: 10.1002/ijc.1600. [DOI] [PubMed] [Google Scholar]
- 23.Kim HI, Huang H, Cheepala S, Huang S, Chung J. Curcumin inhibition of integrin (alpha6beta4)-dependent breast cancer cell motility and invasion. Cancer Prev Res (Phila) 2008;1:385–391. doi: 10.1158/1940-6207.CAPR-08-0087. [DOI] [PubMed] [Google Scholar]
- 24.Linhares MM, Affonso RJ, Jr, Viana Lde S, Silva SR, Denadai MV, de Toledo SR, Matos D. Genetic and immunohistochemical expression of integrins ITGAV, ITGA6, and ITGA3 As prognostic factor for colorectal cancer: Models for global and disease-free survival. PLoS One. 2015;10:e0144333. doi: 10.1371/journal.pone.0144333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marthick JR, Dickinson JL. Emerging putative biomarkers: The role of alpha 2 and 6 integrins in susceptibility, treatment, and prognosis. Prostate Cancer. 2012;2012:298732. doi: 10.1155/2012/298732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in the present study are included in this published article.