Abstract
Silent information regulator 1 (SIRT1), a member of the sirtuin family, is involved in the development of various types of tumor. Previous studies have revealed that SIRT1 has dual functions, as a promoter and an inhibitor, in certain tumors. However, the role of SIRT1 in invasion and metastasis of glioma cells and its associated signaling pathway remain unclear. The aim of the present study was to determine the effects of SIRT1 on these processes and on the epithelial-mesenchymal transition (EMT) in human glioma and adjacent tissues, and in the human glioma cell lines U87 and U251. SIRT1 expression in tissues was investigated using the reverse transcription-quantitative polymerase chain reaction, western blotting and immunohistochemistry. The U87 and U251 cell lines were divided into control and SIRT1-small interfering RNA (siRNA) groups. The Cell Counting Kit-8, cell invasion assays were used to evaluate the effects of SIRT1 silencing on cell viability, invasion and EMT. Results indicated that SIRT1 was highly expressed in glioma tissues compared with in adjacent brain tissues. In addition, SIRT1-siRNA significantly inhibited the viability and invasion of U87 and U251 cells. Furthermore, EMT analysis revealed that the expression levels of the mesenchymal markers fibronectin and vimentin were significantly lower in the SIRT1-siRNA group compared with in the control group. Conversely, expression levels of the epithelial markers epithelial cadherin and β-catenin were significantly higher in the SIRT1-siRNA group compared with in the control group. In conclusion, the results of the present study indicated that SIRT1 was positively associated with viability and invasion of U87 cells, potentially through EMT. These results suggested that SIRT1 may serve a crucial role in the proliferation and development of glioma.
Keywords: silent information regulator 1, gliomas, viability, epithelial-mesenchymal transition
Introduction
Glioma is one of the most common malignant tumors observed in China with a high degree of malignancy (1). Gliomas account for between 35 and 50% of all intracranial brain tumors in adult patients (2). Silent information regulator 1 (SIRT1) is a conserved class III deacetylase that deacetylates the lysine residues of nucleoproteins to influence their stability and transcriptional activities (3,4). In addition, SIRT1 regulates cellular stress responses and lifespan (5). Despite great progresses in surgical treatment options for patients with gliomas, including chemotherapy and radiotherapy, the prognosis remains poor (6). Further investigation is therefore crucial to develop novel gene therapies for the treatment of glioma, and to elucidate the underlying molecular mechanisms of glioma development and progression.
SIRT1 is associated with cell viability and inhibition of apoptosis (7,8), and is involved in the growth of certain tumors (9,10). SIRT1 overexpression promotes tumor progression by regulating tumor growth-associated signaling pathways, including Wnt signaling (11,12). A previous study reported that SIRT1 serves numerous roles in cancer biology (13). On the one hand, SIRT1 is upregulated in tumors, while cancer cells can downregulates the expression of tumor suppressor genes (14). On the other hand, SIRT1 can be proapoptotic (15) and anti-proliferative (16), and consequently has been proposed to behave as a tumor suppressor in vivo.
The epithelial-mesenchymal transition (EMT) is a biological process in which epithelial cells lose their polarity and adhesion properties, and differentiate into mesenchymal cells that possess migratory and invasive properties (17). EMT is a crucial biological process involved in the migration and invasion of certain tumor cells (18), and serves an important role in cancer progression and fibrosis (19). EMT is associated with tumorigenesis and metastasis. Previous studies identified that SIRT1 promotes prostate cancer growth and migration through EMT (20), and stimulates EMT and metastasis in colorectal cancer (21). Conversely, other studies revealed that SIRT1 suppresses EMT in cancer metastasis, organ fibrosis and nasal polypogenesis (22,23). SIRT1 promotes the viability and inhibits apoptosis of human glioma cells (24). SIRT1 is also vital for neural stem cells maintenance and oncogenic transformation (25).
Numerous studies have identified various associations of SIRT1 with certain processes involved in cancer; however, its effects on invasion and EMT in human glioma remain unclear. Since controversies exist regarding the role of SIRT1 in tumors, as SIRT1 can downregulate the expression of tumor suppressor genes (14), SIRT1 also has been proposed to behave as a tumor suppressor in vivo (16), therefore, further investigation is required. To the best of our knowledge, the present study was the first to examine the effect of SIRT1 silencing on EMT in glioma. To do so, the expression levels of SIRT1 were analyzed in human glioma tissue samples together with the effects of SIRT1 on human glioma cell invasion. Previous studies reported that matrix metalloproteinase-9 (MMP-9) (26), Twist family basic helix-loop-helix transcription factor 1 (Twist1) and Snail family transcriptional repressor 1 (Snail1) serve important roles in tumor invasion (27). Therefore, these protein expression levels were also detected. The results indicated that SIRT1 was highly expressed in human glioma tissue samples compared with in adjacent tissues, and that SIRT1 silencing inhibited human glioma U87 and U251 cell line viability and invasion. In addition, SIRT1 silencing suppressed EMT in U87 and U251 cell lines, which suggested that SIRT1 may serve a role in EMT. In conclusion, the results of the present study provide an important foundation for further investigation of the underlying molecular mechanism of SIRT1 in glioma growth.
Materials and methods
Tissue specimen collection
A total of 20 glioma tissues and adjacent brain tissues were collected at The Second Affiliated Hospital of Kunming Medical University (Kunming, China) between April 2016 and April 2017. Tissues were collected following surgical resection. Tissue histomorphology was confirmed by pathologists. The present study was approved by the Ethics Committee of The Second Affiliated Hospital of Kunming Medical University and patients provided written informed consent.
Immunohistochemistry
Tissues are fixed in 4% paraformaldehyde for 24 h at room temperature. Fixed tissues were dehydrated with various concentrations of xylene and ethanol (50% ethanol for 4 h; 75% ethanol for 4 h; 85% ethanol for 3 h; 95% ethanol for 2 h; 100% ethanol for 1 h; 100% ethanol for 1 h; 1:1 ethanol-xylene for 1 h; xylene for 1 h; xylene for 30 min at room temperature), embedded in paraffin. Sections (4 µm thickness) were cut from a paraffin block. Sections were dewaxed with various concentrations of xylene and ethanol (xylene for 10 min; xylene for 5 min; 100% ethanol for 5 min; 95% ethanol for 2 min; 80% ethanol for 2 min; 70% ethanol for 2 min). Antigen repair was performed on the sections with 0.01 M citric acid buffer (pH 6.0) at 100°C high temperature and 80 kpa pressure. Sections were blocked by incubation with 5% goat serum (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) in PBS for 15 min at room temperature. Sections were incubated with anti-SIRT1 rabbit antibody (1:100; cat. no. 13161-1-AP; ProteinTech Group, Inc., Chicago, IL, USA) overnight at 4°C and with a HRP Goat Anti-Rabbit IgG antibody (1:200; cat. no. AS014, ABclonal Biotech Co., Ltd., Wuhan, China) for 2 h at room temperature. The reactions were visualized using a 3,3′-diaminobenzidine visualization kit (Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China). Sections were counterstained with hematoxylin to visualize nuclei, for 5–10 min at room temperature. Sections were examined under a light microscope, (×400, magnification). Brown staining indicated immunoreactive positive cells, and blue staining indicated the nuclei.
Cell culture
The human glioblastoma cell line U251 and the glioblastoma cell line U87 of unknown origin were purchased from The Kunming Cell Bank of the Chinese Academy of Sciences (Kunming, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and placed at 37°C in a humidified incubator containing 5% CO2.
SIRT1 silencing
SIRT1-small interfering RNA (siRNA) was used to target the SIRT1 gene. The nucleotide sequences were synthesized from Sangon Biotech Co., Ltd., (Shanghai, China) as follows: Forward, 5′-ACUUUGCUGUAACCCUGUA-3′ and reverse, 3′-UACAGGGUUACAGCAAAGU-3′ (28). For comparison, a random nucleotide sequence (5′-CUAGCUUAUGUGGACCUCG-3′) was used as a negative control. The transfections were carried out with Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Cells were harvested at 48 h post-transfection, and mRNA and protein levels were analyzed using the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting, respectively.
Cell viability assay
Cell viability was evaluated using the Cell Counting Kit-8 (CCK-8) (Beyotime Institute of Biotechnology, Haimen, China). Cells (1×104) were seeded in a 96-well plate. At 48 h after transfection, cell viability was assessed using the CCK-8 assay, according to the manufacturer's protocol. Briefly, cells were incubated at 37°C for 1 h, prior to measuring the optical density (OD) at 450 nm using a multiplate reader.
Cell invasion assays
A cell invasion assay was performed using 8 µm Transwell chambers (BD Biosciences, San Jose, CA, USA) precoated with Matrigel. Briefly, 300 µl cell suspension (5×105 cells/ml) was added to the upper chamber, and 500 µl DMEM containing 10% FBS was added to the lower chamber. Following 48 h transfection, cells that did not invade the Matrigel were discarded. Filters were fixed in 90% ethanol for 10 min at room temperature, stained with 0.1% crystal violet for 5 min at room temperature, and visualized under a light microscope (magnification, ×100).
RT-qPCR
Total RNA was isolated from cells and tissues using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was generated using a Reverse Transcription (RT) kit (Vazyme Biotech Co., Ltd, Nanjing, China), according to the manufacturer's protocol. qPCR was performed using a SYBR qPCR Master Mix kit (Vazyme Biotech Co., Ltd.) in an ABI 7300 real-time PCR machine (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols. qPCRs were performed as follows: 95°C for 5 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 30 sec. The relative expression of each mRNA of interest were normalized to endogenous control and analyzed using the 2−ΔΔCq method (29,30). Epithelial (E-)cadherin, β-catenin, fibronectin, vimentin and β-actin primers were as follows: E-cadherin, 5′-ATGCTGAGGATGATTGAGGTGGGT-3′ (forward) and 5′-CAAATGTGTTCAGCTCAGCCAGCA-3′ (reverse); β-catenin, 5′-TGCAGTTCGCCTTCACTATGGACT-3′ (forward) and 5′-GATTTGCGGGACAAAGGGCAAGAT-3′ (reverse); fibronectin, 5′-AAACTTGCATCTGGAGGCAAACCC-3′ (forward) and 5′-AGCTCTGATCAGCATGGACCACTT-3′ (reverse); vimentin, 5′-AGAACCTGCAGGAGGCAGAAGAAT-3′ (forward) and 5′-TTCCATTTCACGCATCTGGCGTTC-3′ (reverse); and β-actin, 5′-TGACGTGGACATCCGCAAAG-3′ (forward) and 5′-CTGGAAGGTGGACAGCGAGG-3′ (reverse).
Western blotting
At 48 h after transfection, total proteins from U87 and U251 cells were extracted using RIPA lysis buffer (Beyotime Institute of Biotechnology). Protein were quantified by using a bicinchoninic protein assay kit (Beyotime Institute of Biotechnology). Proteins (30 µg) were separated by SDS-PAGE (10% gel) and transferred onto a polyvinylidene fluoride membrane, which was blocked with 10% skimmed milk in Tris-buffered saline, pH 7.0, containing 0.1% Tween-20 for 2 h. Following blocking, membranes were incubated with primary antibodies at 4°C overnight (ProteinTech Group, Inc.) and followed by HRP Goat Anti-Rabbit IgG secondary antibodies (dilution 1:1,000; cat. no. AS014; ABclonal, Wuhan, China) for 2 h at room temperature. The primary antibodies as follows: SIRT1 antibody (dilution, 1:1,000; cat. no. 13161-1-AP; ProteinTech), MMP-9 antibody (dilution, 1:1,000; cat. no. 10375-2-AP; ProteinTech), Twist1 antibody (dilution, 1:1,000; cat. no. 25465-1-AP; ProteinTech), MMP-9 antibody (dilution, 1:1,000; cat. no. 10375-2-AP; ProteinTech), Snail1 antibody (dilution, 1:1,000; cat. no. 13099-1-AP; ProteinTech), E-cadherin antibody (dilution, 1:1,000; cat. no. 20874-1-AP; ProteinTech), β-catenin antibody (dilution, 1:1,000; cat. no. 51067-2-AP; ProteinTech), Fibronectin antibody (dilution, 1:1,000; cat. no. 15613-1-AP; ProteinTech), Vimentin antibody (dilution, 1:1,000; cat. no. 10366-1-AP; ProteinTech). Membrane signals were visualized with enhanced chemiluminescence reagent (Beyotime Institute of Biotechnology). The OD of the bands was determined using ImageJ 2× software (National Institutes of Health, Bethesda, MD, USA) and normalized to the expression of the internal control (β-actin).
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde for 30 min at room temperature. Following three washes with 1X PBS, cells were blocked with normal goat serum for 2 h. The cells were then incubated overnight at 4°C with anti-E-cadherin (1:100; 20874-1-AP; ProteinTech Group, Inc.) or anti-fibronectin antibodies (dilution, 1:100; cat. no. 15613-1-AP; ProteinTech Group, Inc.) and with fluorescein isothiocyanate-conjugated secondary antibody (dilution, 1:100; cat. no. SA00003-2; ProteinTech Group, Inc.) or CoraLite594-conjugated secondary antibody (dilution, 1:100; cat. no. SA00013-4; ProteinTech Group, Inc.). Nuclei were stained with 10 µg/ml DAPI in the dark for 5 min at room temperature. Cells were observed using a fluorescence microscope (magnification, ×100). Three representative fields of stained cells were analyzed using Image-Pro Plus software (version 6.0; Media Cybernetics, Inc., Rockville, MD, USA) to obtain the mean OD, which represents the staining strength per positive pixel.
Statistical analysis
Differences between two groups were analyzed using Student's t-tests with GraphPad Prism software (version 5.0a; GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as the mean ± standard deviation (n=3). P<0.05 was considered to indicate a statistically significant difference.
Results
SIRT1 expression in glioma tissues
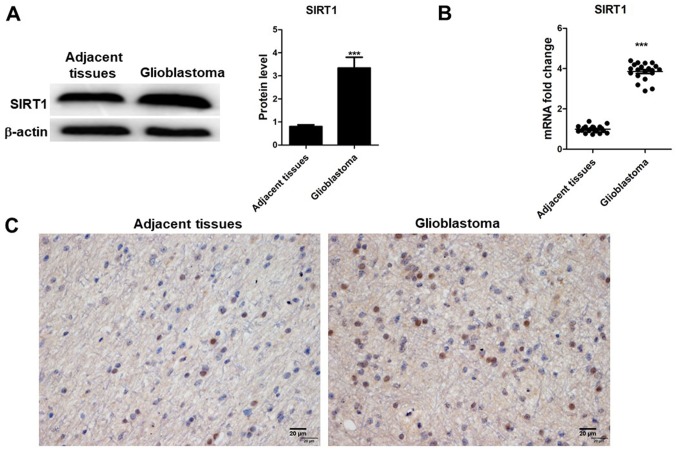
SIRT1 expression was detected in 20 glioblastoma tissues and their corresponding adjacent non-tumor tissues using RT-qPCR, western blotting and immunohistochemistry. The results indicated that SIRT1 mRNA and protein expression levels were upregulated in tumor tissues compared with in adjacent non-tumor tissues (Fig. 1A and B), which was determined to be significant. Results from immunohistochemistry indicated that SIRT1 was present in the nucleus of glioma tissues (Fig. 1C). SIRT1 levels were different in glioma tissues compared with adjacent tissues. These results indicated that SIRT1 expression was associated with glioma development.
Figure 1.
SIRT1 expression in glioma tissues and adjacent brain tissues. SIRT1 (A) protein expression, and (B) mRNA levels in glioma and adjacent brain tissues determined by western blotting and reverse transcription-quantitative polymerase chain reaction. (C) SIRT1 immunohistochemistry of glioma tissues and adjacent tissues (magnification ×400). Results are presented as the mean ± standard deviation of three independent experiments. ***P<0.001. SIRT1, silent information regulator 1.
SIRT1 silencing inhibits glioma cell viability
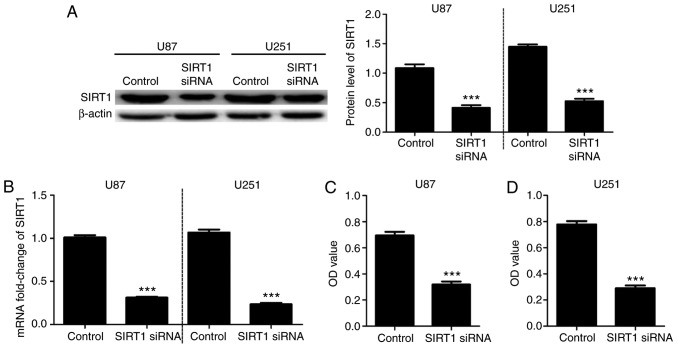
To investigate the effects of SIRT1 on U87 and U251 cell viability, cells were transfected with SIRT1-siRNA. RT-qPCR and western blotting confirmed the successful SIRT1 silencing in U87 and U251 cell lines (Fig. 2A and B). A CCK-8 assay was used to detect the cell viability of U87 and U251 cells at 48 h after transfection with SIRT1-siRNA. Results indicated that U87 and U251 cell viabilities were significantly decreased following SIRT1-siRNA transfection compared with control transfection (Fig. 2C and D). These data suggested that SIRT1 expression may be involved in U87 and U251 cell viability.
Figure 2.
SIRT1 silencing inhibits glioma cell viability. SIRT1 (A) protein and (B) mRNA expression levels in U87 and U251 cells following transfection with SIRT1-siRNA. Cell viability of (C) U87 and (D) U251 cells following SIRT1-siRNA transfection. Results are presented as the mean ± standard deviation of three independent experiments. ***P<0.001. vs. control. OD, optical density; siRNA, small interfering RNA; SIRT1, silent information regulator 1.
Silencing SIRT1 inhibits invasion of glioma cells
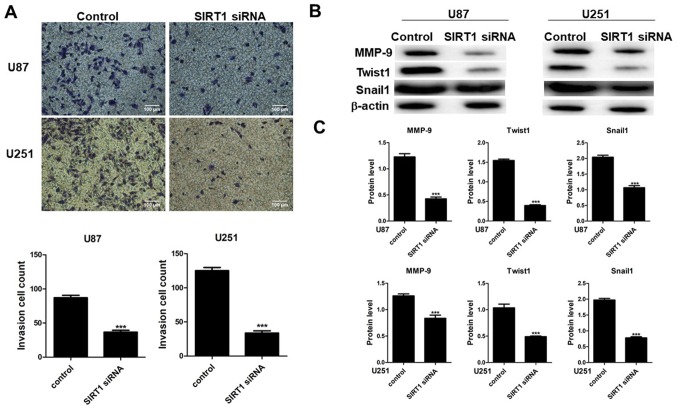
To determine the effect of SIRT1 on cell invasion, Transwell invasion assays were performed on U87 and U251 cells transfected with SIRT1-siRNA. Results indicated that SIRT1-siRNA suppressed U87 and U251 cell compared with cells transfected with control siRNA (Fig. 3A). Images of cells present on the lower membranes of the invasion assay chambers and quantification of crystal violet-positive cells are presented in Fig. 3A, which indicated a significant difference for the two cell lines. Results indicated that SIRT1-siRNA inhibited U87 and U251 cell invasion. The expression levels of MMP-9, Twist1 and Snail1 were therefore determined in U87 and U251 cell lines by western blotting. Results indicated that SIRT1-siRNA decreased the protein levels of MMP-9, Twist1 and Snail1 in the two cell lines (Fig. 3B and C), which suggested that SIRT1 may have a beneficial effect on glioma cell invasion.
Figure 3.
SIRT1 silencing inhibits invasion of glioma cells. (A) Invasive ability of U87 and U251 cells following transfection with SIRT1-siRNA determined using Transwell assays (magnification ×100). (B) Western blotting of MMP-9, Twist1 and Snail1 in U87 and U251 cells following transfection with SIRT1-siRNA. (C) Quantification of protein levels. Results are presented as the mean ± standard deviation of three independent experiments. ***P<0.001. MMP-9, matrix metalloproteinase-9; siRNA, small interfering RNA; SIRT1, silent information regulator 1, Snail1, snail family transcriptional repressor 1; Twist1, family basic helix-loop-helix transcription factor 1.
SIRT1 silencing inactivates EMT pathway in glioma cells
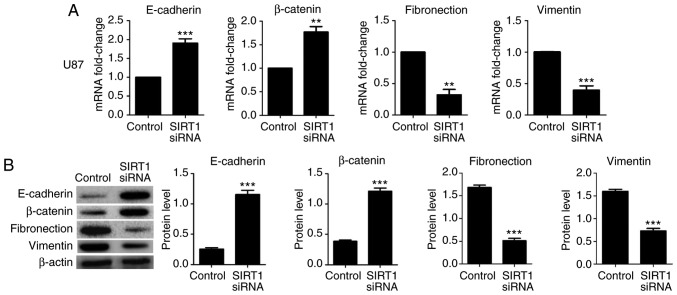
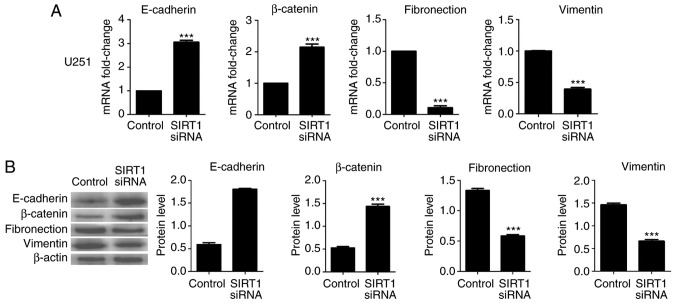
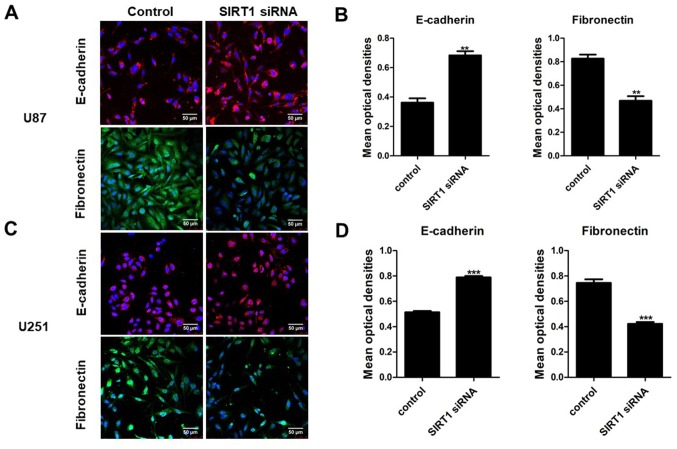
The aforementioned results suggested that SIRT1 may promote glioma cell viability invasion. EMT is a crucial biological process for the invasion of certain types of malignant tumor cells. To identify the effect of SIRT1 on EMT in U87 and U251 cells, RT-qPCR and western blotting were used to detect the expression levels of the epithelial markers E-cadherin and β-catenin, and the mesenchymal markers fibronectin and vimentin. Results indicated that SIRT1 silencing significantly increased the mRNA and protein levels of E-cadherin and β-catenin in U87 and U251 cells; conversely, SIRT1-siRNA significantly decreased the mRNA and protein levels of fibronectin and vimentin in U87 and U251 cells (Figs. 4 and 5, respectively). In addition, immunofluorescence analysis for E-cadherin and fibronectin revealed a similar significant fluorescence decrease following SIRT1 silencing (Fig. 6). These results indicate that SIRT1 may promote EMT in glioma cells.
Figure 4.
SIRT1 silencing inhibits epithelial-mesenchymal transition in U87 cells. The (A) mRNA and (B) protein levels of E-cadherin, β-catenin, fibronectin and vimentin in U87 cells following transfection with SIRT1-siRNA were determined using the reverse transcription-quantitative polymerase chain reaction and western blotting, respectively. **P<0.01 and ***P<0.001. siRNA, small interfering RNA; SIRT1, silent information regulator 1; E-cadherin, epithelial cadherin.
Figure 5.
SIRT1 silencing inhibits epithelial-mesenchymal transition in U251 cells. The (A) mRNA and (B) protein levels of E-cadherin, β-catenin, fibronectin and vimentin in U251 cells following transfection with SIRT1-siRNA were determined using the reverse transcription-quantitative polymerase chain reaction and western blotting, respectively. ***P<0.001. siRNA, silencing RNA; SIRT1, silent information regulator 1; E-cadherin, epithelial cadherin.
Figure 6.
Immunofluorescence detection of E-cadherin and fibronectin. (A) Immunofluorescence and (B) quantification of results of E-cadherin and fibronectin in U87, following transfection with SIRT1-siRNA (magnification, ×100). (C) Immunofluorescence and (D) quantification of results of E-cadherin and fibronectin in U251 cells following transfection with SIRT1-siRNA (magnification ×100). Results are presented as the mean ± standard deviation of three independent experiments. **P<0.01 and ***P<0.001. siRNA, silencing RNA; SIRT1, silent information regulator 1; E-cadherin, epithelial cadherin.
Discussion
Glioma is one of the most common malignancies in China. In addition, it is the most frequent type of intracranial tumor and the most primary malignant brain tumor (31,32). The World Health Organization classifies glioma according to its malignancy with grades ranging from I to IV (33). Although microsurgical tumor resection, adjuvant chemotherapy and radiotherapy are available, 75% of patients diagnosed with glioma succumb within 18 months of diagnosis (34). Further investigation aiming to develop novel therapeutic targets to prevent glioma metastasis is therefore crucial. To the best of our knowledge, the effect of SIRT1 on glioma remains poorly documented and the role of SIRT1 in EMT in glioma remains unclear.
SIRT1 is a class III histone deacetylase that belongs to the sirtuin family. The sirtuins have been reported to serve crucial roles in genome stability, stress responses and tumorigenesis (35). However, they serve a dual role in cancer. SIRT1 is a bifunctional sirtuin that is involved in tumor suppression and other oncogenic factors (36); however, the mechanisms underlying these contradictory functions remain unclear. SIRT1 is upregulated in lung adenocarcinoma, colorectal, hepatocellular and prostate cancer (10,36,37). SIRT1 inhibition can suppress tumor growth (38). In addition, the tumor suppressor p53 downregulates SIRT1 expression in glioma tumor cells (39). SIRT1 also induces p53 inactivation and inhibits p53-dependent apoptosis (7). Furthermore, SIRT1 promotes viability and inhibits apoptosis of glioma tumor cell lines (24). However, the effects of SIRT1 on invasion and metastasis and its underlying molecular mechanisms in glioma remain unknown. In the present study, the expression levels of SIRT1 in glioma tissues and adjacent brain tissue were determined using RT-qPCR, western blotting and immunohistochemistry. Results revealed that SIRT1 was overexpressed in glioma tissues, compared with adjacent brain tissues that only exhibited weak signal. In addition, SIRT1 silencing was used to investigate SIRT1 effects on invasion and metastasis of glioma U87 cells.
EMT is an important process involved in tumor metastasis (40,41). Previous studies have identified that increased migration and invasion are positively associated with EMT (42,43). In addition, decreased expression of E-cadherin and β-catenin, and increased expression of fibronectin and vimentin serve crucial roles in tumor progression (44). Furthermore, it was reported previously that EMT serves a crucial role in glioma (45,46) and that SIRT1 induces EMT in various types of tumor (47). However, it was identified previously that SIRT1 suppresses EMT in cancer metastasis (22). In the present study, SIRT1 silencing suppressed viability and invasion of glioma U87 and U251 cells, increased E-cadherin and β-catenin expression, and decreased fibronectin and vimentin expression.
To the best of our knowledge, the present study was the first to assess the mRNA and protein expression levels of SIRT1 in human glioma and adjacent brain tissues. To do so, the effects of SIRT1 on viability and invasion of glioma U87 and U251 cells were investigated. Results indicated that SIRT1 expression was higher in glioma tissues compared with in adjacent brain tissues, and that SIRT1 silencing inhibited cell viability, invasion and metastasis in glioma U87 and U251 cells. Furthermore, SIRT1 silencing significantly decreased EMT in U87 and U251 cells, which suggested that EMT may contribute to the inhibition of viability, invasion and metastasis of glioma cells.
The preliminary results of the present study highlight the potential association of SIRT1 with EMT during human glioma progression; however, the underlying molecular mechanisms remain unclear and further investigation is required. Future work will investigate how SIRT1 influences the EMT associated pathway in human glioma.
In conclusion, the results of the present study may serve to further understand SIRT1 functions and mechanisms in glioma cells. These results suggested that SIRT1 may be considered as a potential novel therapeutic target for glioma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and are freely available to any researchers.
Authors' contributions
YL, XC and YC guided the experiments; XW and SC designed the experiments; XC, YC, QW and YL performed the experiments and analyzed the data; and YL wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All human tissue samples were collected by The Second Affiliated Hospital of Kunming Medical University and written informed consent was obtained from all patients. All methods were approved by the Research Medical Ethics Committee of Kunming Medical University and were performed in accordance with the approved guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Penas-Prado M, Armstrong S, Gilbert MR. Glioblastoma. Handb Clin Neurol. 2012;105:485–506. doi: 10.1016/B978-0-444-53502-3.00004-5. [DOI] [PubMed] [Google Scholar]
- 3.Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci USA. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu T, Liu PY, Marshall GM. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009;69:1702–1705. doi: 10.1158/0008-5472.CAN-08-3365. [DOI] [PubMed] [Google Scholar]
- 5.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 6.Marumoto T, Saya H. Molecular biology of glioma. Adv Exp Med Biol. 2012;746:2–11. doi: 10.1007/978-1-4614-3146-6_1. [DOI] [PubMed] [Google Scholar]
- 7.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/S0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 8.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/S0092-8674(01)00527-X. [DOI] [PubMed] [Google Scholar]
- 9.Lim CS. Human SIRT1: A potential biomarker for tumorigenesis? Cell Biol Int. 2007;31:636–637. doi: 10.1016/j.cellbi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Choi HN, Bae JS, Jamiyandorj U, Noh SJ, Park HS, Jang KY, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression and role of SIRT1 in hepatocellular carcinoma. Oncol Rep. 2011;26:503–510. doi: 10.3892/or.2011.1301. [DOI] [PubMed] [Google Scholar]
- 11.Holloway KR, Calhoun TN, Saxena M, Metoyer CF, Kandler EF, Rivera CA, Pruitt K. SIRT1 regulates Dishevelled proteins and promotes transient and constitutive Wnt signaling. Proc Natl Acad Sci USA. 2010;107:9216–9221. doi: 10.1073/pnas.0911325107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, et al. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 13.Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruitt K, Zinn RL, Ohm JE, McGarvey KM, Kang SH, Watkins DN, Herman JG, Baylin SB. Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet. 2006;2:e40. doi: 10.1371/journal.pgen.0020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chua KF, Mostoslavsky R, Lombard DB, Pang WW, Saito S, Franco S, Kaushal D, Cheng HL, Fischer MR, Stokes N, et al. Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell Metab. 2005;2:67–76. doi: 10.1016/j.cmet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Zavadil J, Haley J, Kalluri R, Muthuswamy SK, Thompson E. Epithelial-mesenchymal transition. Cancer Res. 2008;68:9574–9577. doi: 10.1158/0008-5472.CAN-08-2316. [DOI] [PubMed] [Google Scholar]
- 18.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyazono K. Transforming growth factor-β signaling and cancer: The 28th sapporo cancer seminar, 25–27 June 2008. Cancer Sci. 2009;100:363–365. doi: 10.1111/j.1349-7006.2008.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui Y, Li J, Zheng F, Ouyang Y, Chen X, Zhang L, Chen Y, Wang L, Mu S, Zhang H. Effect of SIRT1 Gene on epithelial-mesenchymal transition of human prostate cancer PC-3 Cells. Med Sci Monit. 2016;22:380–386. doi: 10.12659/MSM.895312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng F, Su L, Yao C, Liu L, Shen J, Liu C, Chen X, Luo Y, Jiang L, Shan J, et al. SIRT1 promotes epithelial-mesenchymal transition and metastasis in colorectal cancer by regulating Fra-1 expression. Cancer Lett. 2016;375:274–283. doi: 10.1016/j.canlet.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Simic P, Williams EO, Bell EL, Gong JJ, Bonkowski M, Guarente L. SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis. Cell Rep. 2013;3:1175–1186. doi: 10.1016/j.celrep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee M, Kim DW, Yoon H, So D, Khalmuratova R, Rhee CS, Park JW, Shin HW. Sirtuin 1 attenuates nasal polypogenesis by suppressing epithelial-to-mesenchymal transition. J Allergy Clin Immunol. 2016;137:87–98.e7. doi: 10.1016/j.jaci.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Qu Y, Zhang J, Wu S, Li B, Liu S, Cheng J. SIRT1 promotes proliferation and inhibits apoptosis of human malignant glioma cell lines. Neurosci Lett. 2012;525:168–172. doi: 10.1016/j.neulet.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Park JR, Kwon OS, Lee TH, Nakano I, Miyoshi H, Chun KH, Park MJ, Lee HJ, Kim SU, Cha HJ. SIRT1 is required for oncogenic transformation of neural stem cells and for the survival of ‘cancer cells with neural stemness’ in a p53-dependent manner. Neuro Oncol. 2015;17:95–106. doi: 10.1093/neuonc/nou145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu WB, Wang W, Du YH, Li H, Xia SJ, Liu HT. MicroRNA-3713 regulates bladder cell invasion via MMP9. Sci Rep. 2016;6:32374. doi: 10.1038/srep32374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun T, Fu J, Shen T, Lin X, Liao L, Feng XH, Xu J. The Small C-terminal domain phosphatase 1 inhibits cancer cell migration and invasion by dephosphorylating Ser(P)68-Twist1 to accelerate Twist1 protein degradation. J Biol Chem. 2016;291:11518–11528. doi: 10.1074/jbc.M116.721795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford J, Jiang M, Milner J. Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res. 2005;65:10457–10463. doi: 10.1158/0008-5472.CAN-05-1923. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Long X, Chao C, Yan C, Wu Q, Hua S, Zhang Y, Wu A, Fang W. Knocking down CDK4 mediates the elevation of let-7c suppressing cell growth in nasopharyngeal carcinoma. BMC Cancer. 2014;14:274. doi: 10.1186/1471-2407-14-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Qu Q, Qu J, Luo WM, Wang SY, He YZ, Luo QS, Xu YX, Wang YF. Association between XRCC1 polymorphisms and glioma risk among Chinese population. Med Oncol. 2014;31:186. doi: 10.1007/s12032-014-0186-2. [DOI] [PubMed] [Google Scholar]
- 32.Sai K, Zhong MG, Wang J, Chen YS, Mou YG, Ke C, Zhang XH, Yang QY, Lin FH, Guo CC, et al. Safety evaluation of high-dose BCNU-loaded biodegradable implants in Chinese patients with recurrent malignant gliomas. J Neurol Sci. 2014;343:60–65. doi: 10.1016/j.jns.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q. WHO classification of tumors of central nervous system (2007): An introduction. Zhonghua Bing Li Xue Za Zhi. 2008;37:5–7. (In Chinese) [PubMed] [Google Scholar]
- 34.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 35.Bosch-Presegué L, Vaquero A. The dual role of sirtuins in cancer. Genes Cancer. 2011;2:648–662. doi: 10.1177/1947601911417862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Hokka D, Maniwa Y, Ohbayashi C, Itoh T, Hayashi Y. Sirt1 is a tumor promoter in lung adenocarcinoma. Oncol Lett. 2014;8:387–393. doi: 10.3892/ol.2014.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu DF, Jiang SJ, Pan ZP, Cheng WD, Zhang WJ, Yao XK, Li YC, Lun YZ. Expression and clinical significance of Sirt1 in colorectal cancer. Oncol Lett. 2016;11:1167–1172. doi: 10.3892/ol.2015.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oon CE, Strell C, Yeong KY, Östman A, Prakash J. SIRT1 inhibition in pancreatic cancer models: Contrasting effects in vitro and in vivo. Eur J Pharmacol. 2015;757:59–67. doi: 10.1016/j.ejphar.2015.03.064. [DOI] [PubMed] [Google Scholar]
- 39.Yuan F, Liu L, Lei Y, Tang P. p53 inhibits the upregulation of sirtuin 1 expression induced by c-Myc. Oncol Lett. 2017;14:4396–4402. doi: 10.3892/ol.2017.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: Mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 42.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 43.Kang Y, Massagué J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 45.Liu ZJ, Liu HL, Zhou HC, Wang GC. TIPE2 Inhibits Hypoxia-Induced Wnt/β-catenin pathway activation and EMT in glioma cells. Oncol Res. 2016;24:255–261. doi: 10.3727/096504016X14666990347356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Wu Y, Wang Y, Jin Y, Ma X, Zhang Y, Ren H. Matrine inhibits the invasive properties of human glioma cells by regulating epithelial-to-mesenchymal transition. Mol Med Rep. 2015;11:3682–3686. doi: 10.3892/mmr.2015.3167. [DOI] [PubMed] [Google Scholar]
- 47.Byles V, Zhu L, Lovaas JD, Chmilewski LK, Wang J, Faller DV, Dai Y. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene. 2012;31:4619–4629. doi: 10.1038/onc.2011.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and are freely available to any researchers.