Abstract
Ovarian cancer (OC) is the most common and lethal gynecologic malignancy. The pathophysiology of OC tumor development is complex and involves numerous biological pathways. Previous studies suggest that circular (circ)RNAs serve important roles in OC tumor pathology. In the present study, a re-annotation strategy was performed to evaluate the expression level of circRNAs based on a microarray dataset obtained from the Gene Expression Omnibus database. Univariate and multivariate Cox regression analyses were performed to evaluate the association between survival and expression of circRNAs in each OC cohort. An expression-based risk score model was constructed to extrapolate the prognostic efficacy of this signature. In the GSE9891 dataset, the 278 OC patients were randomly divided into training and validating groups. A six-circRNA signature was significantly associated with overall survival in the training and validating datasets. The risk score model was further validated in GSE63885 and GSE26193 datasets. The six-circRNA signature was also significantly associated with patient progression-free survival and disease-free survival. Further investigation revealed that the signature had higher area under the curve values than the existing clinical and other molecular signatures in predicting survival. In conclusion, the present study revealed that the six-circRNA signature may serve as a potential prognostic biomarker of OC.
Keywords: ovarian cancer, circular RNAs, prognostic signature, survival analysis, re-annotation strategy
Introduction
Ovarian cancer (OC) is the most common gynecologic malignancy and remains the leading cause of cancer-related mortality worldwide (1). Despite advances in chemotherapy and surgical treatment, the 5 year survival rate of OC is ~30% due to its frequent recurrence (2). Negative outcome of OC are mainly due to asymptomatic stages, rapid metastasis and chemotherapy resistance (3). Although the understanding of OC is constantly progressing, its underlying molecular mechanisms remain unclear. Most patients with OC have developed metastases by the time of their first diagnosis (4). There is therefore an urgent need to identify potential prognostic biomarkers for the prediction of OC clinical outcomes.
Circular RNAs (circRNAs) are produced from back-splicing of precursor mRNAs and represent a novel class of endogenous noncoding RNAs (5). These mRNAs have been considered for decades as abnormal splicing products of RNAs due to their low expression levels. Alongside the development of high-throughput sequencing and bioinformatics technologies, recent studies have confirmed that circRNAs are abundant, stable and conserved in mammalian cells (6–8). The involvement of circRNAs in cancer pathology is therefore intensively studied. It has been reported that circRNAs negatively modulate micro (mi)RNA expression by harboring their binding sites, and subsequently further affect the levels of downstream mRNA (9). For example, the circRNA of ciRS-7 sequence works as a competing endogenous RNA that sponges the miRNA miR-7, which leads to an increase in miR-7 targets expression in various types of cancer (10). Furthermore, RNA-sequencing analyses revealed that circRNAs are enriched in cancer cell-derived exosomes (11). A recent study reported that a large amount of long RNA species, including circRNAs, are detected in human blood-derived exosomes (12). These studies indicate that circRNAs may serve as potential biomarkers in cancer diagnosis and help monitor cancer progression. Although circRNAs have roles in oncogenesis and tumor progression, their role in OC remains unclear.
The present study aimed to identify potential circRNA signatures that could predict the survival of patients with OC. A re-annotation strategy was performed to evaluate the expression level of circRNAs based on microarray datasets obtained from the Gene Expression Omnibus (GEO) database. In the GSE9891 dataset, a signature comprising six circRNAs associated with survival was identified. An expression-based risk score model was designed to extrapolate the prognostic efficacy of this signature. The risk score model was further validated in GSE63885 and GSE26193 datasets. The results demonstrated that the signature was significantly associated with patient overall survival (OS), progression-free survival (PFS) and disease-free survival (DFS). Further analysis revealed that this signature was more sensitive and specific than the existing clinical and other molecular signatures in predicting survival. In conclusion, the present study demonstrated that the six-circRNA signature may serve as a potential prognostic biomarker of OC.
Materials and methods
The expression dataset of OC
Three independent OC datasets (Affymetrix HG-U133_Plus_2.0 array platform) GSE26193 (n=107), GSE9891 (n=278) and GSE63885 (n=75), were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/).
Re-annotation of circRNAs from microarray dataset
A re-annotation strategy was performed to identify the circRNAs from microarray dataset (13). The circRNA transcripts were downloaded from circBase (hg19; http://www.circbase.org/) (14). The SeqMap tool was used to map probes to circRNA transcripts (15). In the mapping procedure, mismatches were not allowed in mapping probes to circRNA transcripts. Probes that were uniquely mapped to circRNAs were retained. To further purify probes that were specific to circRNAs, probes that also mapped to other transcripts were excluded based on GENCODE (v19) (https://www.gencodegenes.org/) annotation and RefSeq (GRCH37) database (https://www.ncbi.nlm.nih.gov/refseq/). With regards to probes that mapped the same circRNA, the arithmetic mean expression value was used. A total of 630 circRNAs were eventually identified from the microarray data.
Clinical information of patients with OC
Clinical characteristics of patients with OC were derived from series matrix file or supporting information of the corresponding dataset available in the GEO database. Patients with OC from GSE9891 were randomly assigned into two groups as training (n=139) and validating (n=139) datasets. There were no significant differences in the clinical characteristics between the two groups (P>0.05). Detailed clinical characteristics obtained from these datasets are presented in Table I.
Table I.
Clinical characteristics of different OC datasets.
| Number of patients with OC | |||||
|---|---|---|---|---|---|
| Characteristic | Training set (n=139) | Validating set (n=139) | GSE26193 (n=107) | GSE63885 (n=75) | P-values |
| Stage | 0.94a | ||||
| I | 11 | 13 | 21 | 0 | |
| II | 9 | 8 | 10 | 2 | |
| III | 107 | 107 | 59 | 63 | |
| IV | 12 | 10 | 17 | 10 | |
| Age, years | 0.34b | ||||
| Mean ± SD | 60.24±10.47 | 59.04±10.68 | NA | NA | |
| Range | 23–80 | 22–80 | NA | NA | |
| Histological grade | 0.73a | ||||
| G1 | 10 | 9 | 1 | 0 | |
| G2 | 51 | 44 | 33 | 9 | |
| G3 | 77 | 84 | 67 | 48 | |
| G4 | 0 | 0 | 0 | 18 | |
| Malignancy | 1a | ||||
| Malignant | 9 | 9 | NA | NA | |
| Low malignant potential | 130 | 130 | NA | NA | |
| Recurrence | 0.90a | ||||
| Yes | 94 | 96 | 80 | 70 | |
| No | 45 | 43 | 27 | 5 | |
| Survival, months | 0.47b | ||||
| Mean ± SD | 30.83±26.61 | 32.84±18.69 | 49.92±39.60 | 42.80±28.89 | |
| Range | 0–214 | 0–113 | 0–243 | 3–136 | |
| State | 0.63a | ||||
| Survival | 85 | 80 | 31 | 9 | |
| Mortality | 54 | 59 | 76 | 66 | |
The training and validating groups were randomly derived from GSE9891 dataset. The P-values were calculated by comparing the characteristics between training and validating groups. Patients with missing stage and grade values were not listed.
P-values were determined using χ2 test or Fisher's exact test when appropriate
P-values were determined using Student's t-test. OC, ovarian cancer; SD, standard deviation.
Statistical analysis
Student's t-test was used to determine the difference of age and survival time between training and validating groups. χ2 and Fisher's exact tests were used to determine the difference of stage, grade, malignancy, recurrence and survival status between training and validating groups. Univariate and multivariate Cox regression analyses were performed to evaluate the association between survival and circRNAs expression in each OC cohort. The random survival forests variable hunting (RSFVH) algorithm was carried out to select important predictors (16). This strategy has been used in a previous study to identify prognostic lncRNAs in OC (17). The risk score for each patient with OC was calculated according to the linear combination of the expression values weighted by the coefficient from univariate Cox regression analysis as follows:
where βi is the Cox regression coefficient of a circRNA and n is the number of circRNAs regulated by the same TF. Exp(ci) is the expression value of circRNA i in the corresponding patient. The median risk score was used as the cutoff point to divide the patients into high and low risk groups. Kaplan-Meier (KM) survival curves were plotted for patients in different risk groups, and statistical significance was assessed by the log-rank test (P<0.05). A time-dependent receiver operating characteristic (ROC) curve analysis was performed using an R package named survivalROC (https://cran.r-project.org/web/packages/survivalROC/). The function roc.KM.calc returns the true positive (TP) and false positive (FP) values at the time point of interest. The sensitivity is calculated as TP/(TP + false negative), and the specificity is calculated as true negative (TN)/(TN+FP). All analyses were performed based on R framework (v3.4; http://www.r-project.org/).
Functional analysis
Pearson correlation coefficients were used to evaluate the co-expression association between circRNAs and mRNAs [correlation coefficients >0 and false discovery rate (FDR) <0.05]. For each circRNA, the co-expressed mRNAs were used to perform gene set functional enrichment analysis. The R package named clusterProfiler was used to predict biological functions of circRNAs based on the gene sets of co-expressed mRNAs (18). Gene Ontology (GO) terms of ‘Biological Process’ and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways with FDR <0.05 were considered to be significantly enriched.
Results
Identification of potential prognostic circRNAs
To identify potential prognostic circRNAs, 278 patients with OC from GSE9891 were randomly assigned into two groups as training (n=139) and validating (n=139) datasets (Table I). There were no significant differences between the two groups in the clinical characteristics (P>0.05). A re-annotation strategy was performed to evaluate the expression of circRNAs from microarray dataset (Fig. 1A). A total of 630 circRNAs were identified from the microarray data. The univariate Cox regression analysis was performed for circRNAs expression data in the training dataset. A set of 15 circRNAs were significantly associated with patient OS at a threshold of P<0.01, of which 11 were considered as protective factors with hazard ratio (HR) values of 0–1 (Fig. 1B); however, four circRNAs were considered as risk factors for OC with HR values >1 (Fig. 1C). To provide a smaller set of circRNAs with more predictive efficacy, the RSFVH method (16) was used to select the circRNAs highly associated with OS. Subsequently, a panel of six circRNAs (circ_0031356, 0093477, 0110166, 0126526, 0130590 and 0135175) was identified (Fig. 1D and E). These six circRNAs had negative coefficients, which suggested that lower expressions were associated with poor OS (Fig. 1B). These circRNAs represented protective factors with HR values of 0–1.
Figure 1.
Pre-analysis for identification of potential prognostic circRNAs in OC. (A) Evaluation of circRNAs from microarray dataset expression by re-annotation strategy. (B) Distribution of HR values for circRNAs as protective factors. Error bars represent 95% confidence interval. (C) Distribution of HR values for circRNAs as risk factors. (D) Error rate of the random survival forests variable hunting algorithm for the selection of important circRNAs. (E) Variable importance values for the top six circRNAs. circRNA, circular RNA; HR, hazard ratio; OC, ovarian cancer.
A six-circRNA signature predicts OS in the training and validating datasets
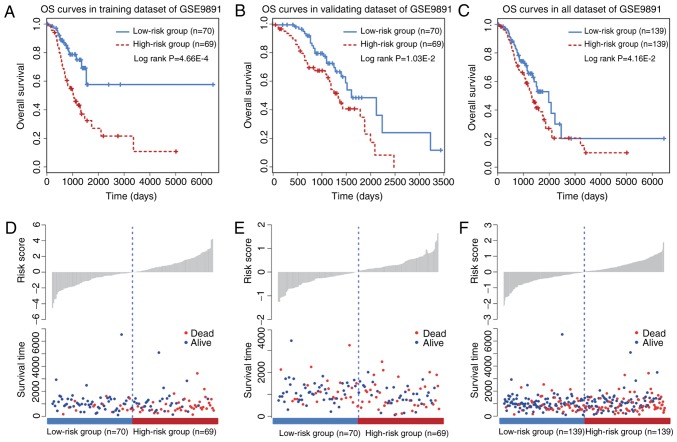
A KM survival analysis was performed for the six circRNAs based on their expression values in the training dataset. The six circRNAs presented significant differences in patient OS between high and low risk groups (log-rank test P<0.05; data not shown). To improve the predictive efficacy of the six-circRNA signature, a risk score formula was developed by integrating the expression values and corresponding coefficients derived from Cox regression analysis. A total of 139 training patients were assigned into a high (n=70) and a low risk group (n=69) by using the median risk score as a cutoff point (P=4.66×10−4; Fig. 2A). Furthermore, the prognostic efficiency of the six-circRNA signature was investigated in the validating dataset using the same risk score threshold from the training set. Based on this strategy, the validating patients were also significantly divided into high (n=70) and low risk groups (n=69; P=1.03×10−2; Fig. 2B). KM survival curves for the six-circRNA signature in the 278 patients with OC of GSE9891 are presented in Fig. 2C. By using the median risk score, the six-circRNA signature significantly divided all the OC patients of GSE9891 into high (n=139) and low risk groups (n=139) (P=4.16×10−2). Distribution of patient risk scores and survival status are presented in Fig. 2D-F. Patients in the low and high risk groups tended to have different survival rates.
Figure 2.
OS analysis for the six-circular RNA signature in GSE9891 dataset. (A) OS curves for training dataset in GSE9891. (B) OS curves for validating dataset in GSE9891. (C) OS curves for all dataset in GSE9891. (D-F) Distribution of risk scores and survival time in low and high risk groups of patients with ovarian cancer. OS, overall survival.
Validation of the six-circRNA signature in other independent cohorts
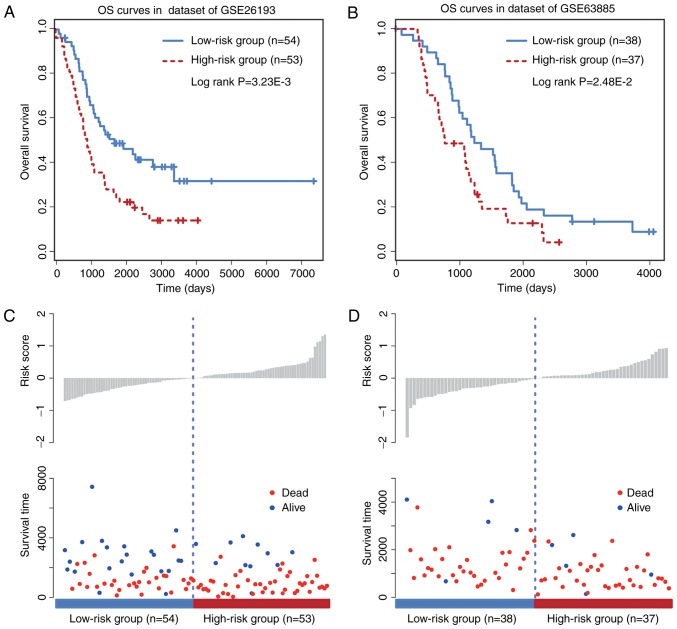
To further evaluate the prognostic efficacy of the six-circRNA signature, a survival analysis was performed in the two other independent OC datasets GSE26193 (n=107) and GSE63885 (n=75) by using the same model construction as for the GSE9891 dataset. Patients in these independent datasets were given risk scores and classified into high or low risk groups. The six-circRNA signature was significantly associated with prognosis in GSE26193 (HR=2.48, 95% CI=1.43–4.28, P=1.17×10−3) and GSE63885 (HR=2.63, 95% CI=1.40–4.96, P=2.28×10−3) datasets. In addition, significant association between the risk score and OS was observed in the two independent datasets (Fig. 3A and B). The distribution of patient risk scores and survival status is presented in Fig. 3C and D. Patients with higher risk scores were prone to have shorter survival time, whereas patients with lower risk scores tended to have longer survival time. These observations were consistent with findings obtained from the training and validating datasets.
Figure 3.
OS analysis for the six-circular RNAs signature in other independent datasets. (A) OS curves for GSE26193. (B) OS curves for GSE63885. (C and D) Distribution of risk scores and survival time in low and high risk groups of patients with ovarian cancer. OS, overall survival.
Prognostic performance of six-circRNA signature on PFS/DFS
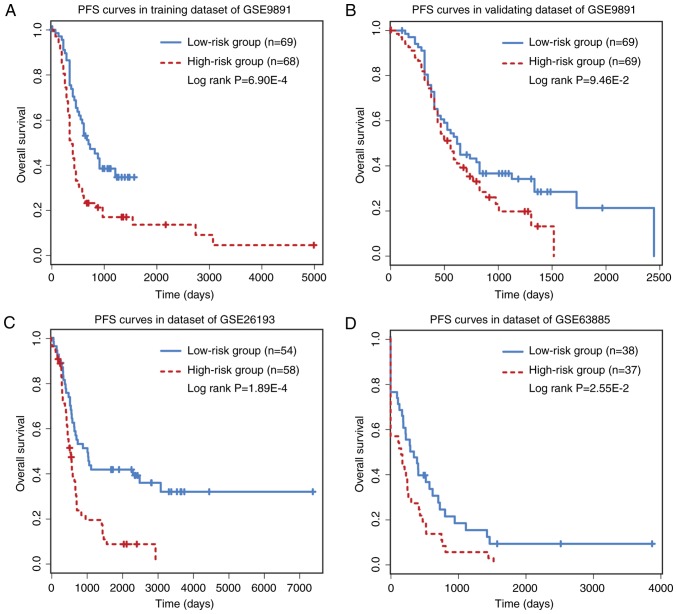
The prognostic performance of the six-circRNA signature on PFS and DFS was analyzed. PFS analysis was made on GSE9891 (n=275) and GSE26193 (n=107) datasets, while DFS analysis was performed on GSE63885 (n=75) dataset. The six-circRNA signature was significantly associated with patient PFS in the GSE9891 (HR=1.76, 95% CI=1.16–2.68, P=7.58×10−3) and GSE26193 (HR=2.17, 95% CI=1.30–3.61, P=2.83×10−3) datasets. In GSE63885, the six-circRNA signature was significantly associated with patient DFS (HR=2.76, 95% CI=1.38–5.54, P=4.13×10−3). In addition, a significant association between risk scores and PFS/DFS was observed in the three datasets (Fig. 4). In PFS analysis, the six-circRNA signature successfully divide patients from the GSE9891 and GSE26193 datasets into high and low risk groups, respectively (Fig. 4A-C). In DFS analysis, the six-circRNA signature could also significantly separate GSE63885 patients into high and low risk groups (Fig. 4D).
Figure 4.
PFS or DFS analysis for the six-circRNA signature. (A) PFS for the training dataset of GSE9891. (B) PFS for validating dataset of GSE9891. (C) PFS for GSE26193. (D) DFS for GSE63885. DFS, disease-free survival; PFS, progression-free survival.
Independence of six-circRNA signature from clinical characteristics
To evaluate whether the six-circRNA signature was associated with other clinical variables, multivariate Cox regression analyses were performed in each OC cohort. The six-circRNA signature and other clinical and pathological variables, including age at diagnosis, International Federation of Gynecologists and Obstetricians (FIGO) stage and tumor grade, were analyzed as covariables (Table II). Following multivariate Cox regression analysis, the six-circRNA signature was significantly associated with patient survival in GSE9891 (P=1.08×10−4), GSE26193 (P=5.28×10−3) and GSE63885 (P=4.04×10−3). Furthermore, a time-dependent ROC analysis was performed to assess the sensitivity and specificity of OS prediction between the six-circRNA signature and other clinical and molecular variables, including a panel of eight lncRNAs obtained from a previous study (19). The median survival time was used as a cutoff point to identify positive/negative cases. Following comparison of the area under the curve (AUC) values of the ROC curves, the predictive value of the six-circRNA signature was higher than other clinical and molecular variables in different OC datasets (Fig. 5A-C). In GSE9891, the six-circRNA signature reached the highest AUC value of 0.68. The AUC value of FIGO stage variable reached 0.67 in GSE9891; however, it was only 0.59 and 0.43 in GSE26193 and GSE63885 datasets, respectively. These results demonstrated that the six-circRNA signature was more sensitive and specific than the existing clinical and molecular signatures in predicting the survival of patients with OC.
Table II.
Univariate and multivariate Cox regression analyses of the six-circRNA signature and other clinical variables.
| A, GSE9891 (Training set) | ||||
|---|---|---|---|---|
| Univariate Cox analysis | Multivariate Cox analysis | |||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Grade (G1/G2/G3) | 1.154 (0.741–1.797) | 5.26×10−01 | 0.679 (0.408–1.130) | 1.36×10−01 |
| Stage (I/II/III/IV) | 3.047 (1.787–5.193) | 4.24×10−05 | 3.529 (1.939–6.422) | 3.67×10−05 |
| Age | 1.020 (0.990–1.051) | 1.83×10−01 | 1.023 (0.993–1.053) | 1.41×10−01 |
| Six-circRNA signature | 1.509 (1.260–1.806) | 7.46×10−06 | 1.488 (1.242–1.784) | 1.69×10−05 |
| B, GSE9891 (Validating set) | ||||
| Univariate Cox analysis | Multivariate Cox analysis | |||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Grade (G1/G2/G3) | 1.618 (1.015–2.578) | 4.31×10−02 | 1.264 (0.772–2.068) | 3.52×10−01 |
| Stage (I/II/III/IV) | 1.568 (0.939–2.619) | 8.55×10−02 | 1.716 (0.939–3.136) | 7.90×10−02 |
| Age | 1.031 (1.004–1.059) | 2.18×10−02 | 1.032 (1.005–1.059) | 2.03×10−02 |
| Six-circRNA signature | 2.202 (1.357–3.575) | 1.40×10−03 | 2.110 (1.267–3.513) | 4.10×10−03 |
| C, GSE26193 (n=107) | ||||
| Univariate Cox analysis | Multivariate Cox analysis | |||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Grade (G1/G2/G3) | 1.207 (0.829–1.759) | 3.26×10−01 | 0.819 (0.537–1.247) | 3.51×10−01 |
| Stage (I/II/III/IV) | 2.057 (1.546–2.738) | 7.54×10−07 | 2.212 (1.588–3.083) | 2.72×10−06 |
| Six-circRNA signature | 2.475 (1.432–4.279) | 1.17×10−03 | 2.134 (1.253–3.635) | 5.28×10−03 |
| D, GSE63885 (n=75) | ||||
| Univariate Cox analysis | Multivariate Cox analysis | |||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Grade (G2/G3/G4) | 1.707 (1.129–2.582) | 1.13×10−02 | 1.639 (1.043–2.575) | 3.20×10−02 |
| Stage (II/III/IV) | 2.315 (1.239–4.323) | 8.45×10−03 | 2.018 (1.087–3.748) | 2.62×10−02 |
| Six-circRNA signature | 2.630 (1.396–4.957) | 2.78×10−03 | 2.643 (1.363–5.128) | 4.04×10−03 |
CI, confidence interval; circRNA, circular RNA; HR, hazard ratio.
Figure 5.
Time-dependent ROC analysis of the sensitivity and specificity for survival prediction of the six-circRNA signature and other factors. (A) ROC curves in GSE9891. (B) ROC curves in GSE26193. (C) ROC curves in GSE63885. CircRNAs, circulating RNAs; FIGO, International Federation of Gynecologists and Obstetricians; lncRNAs, long non-coding RNAs; ROC, receiver operating curve.
Function prediction of six-circRNA signature
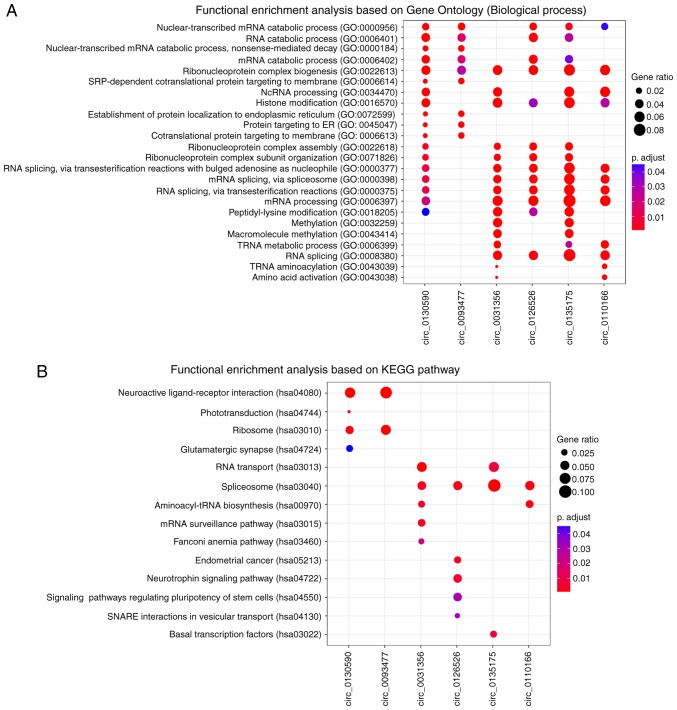
To explore the potential functional roles of these six prognostic circRNAs, a ‘guilt-by-association’ analysis (20) was performed to identify co-expressed circRNA-mRNA pairs on GSE9891 dataset, which has the largest samples size. For the six circRNAs, functional enrichment analysis was performed based on their co-expressed mRNAs by using clusterProfiler R package (18). The results from GO enrichment analysis revealed that the six circRNAs were significantly enriched in 24 GO terms based on ‘biological processes’ ontology (Fig. 6A). These related GO terms could be organized into different functional clusters, including ‘RNA catabolic processes’, ‘ribonucleoprotein complex biogenesis’, ‘histone modification’ and ‘methylation’. KEGG pathway enrichment analysis revealed that circ_0130590 and 0093477 were significantly enriched in ‘neuroactive ligand-receptor interaction’ and ‘ribosome’ pathways (Fig. 6B). Circ_0031356, 0126526, 0135175 and 0110166 were significantly enriched in ‘spliceosome pathway’. ‘Endometrial cancer’ and certain other signaling pathways were enriched by circ_0126526. These results suggested that the six circRNAs may participate in numerous biological processes involved in OC tumorigenesis.
Figure 6.
Functional prediction of the six prognostic circular RNAs. (A) Functional analysis of enriched biological processes. (B) Functional analysis of enriched Kyoto Encyclopedia of Genes and Genomes pathways. GO, gene ontology.
Discussion
In the present study, a re-annotation strategy was performed to evaluate circRNAs expression from microarray dataset of OC. Following univariate Cox regression analysis, 15 circRNAs were significantly associated with patients OS. Amongst these prognostic candidates, 11 circRNAs were determined as protective factors with HR values of 0–1, while 4 circRNAs were determined as risk factors with HR values >1. In addition, the RSFVH method revealed that a panel of six circRNAs (circ_0031356, circ_0093477, circ_0110166, circ_0126526, circ_0130590 and circ_0135175) was associated with patient OS. Subsequently, an expression-based risk score model was constructed to extrapolate the prognostic efficacy of these circRNAs. The results revealed that the six-circRNA signature was significantly associated with patient survival in the training and validating datasets of GSE9891. The risk score model was further validated in the independent cohorts of GSE26193 and GSE63885. Furthermore, PFS analysis was performed on GSE9891 and GSE26193 dataset, while DFS analysis was performed on GSE63885. The six-circRNA signature was significantly associated with patient PFS/DFS in GSE9891, GSE26193 and GSE63885 datasets. In addition, consensus cluster analysis (21) was performed on GSE9891 and GSE26193 datasets by considering the expression correlation of the six-circRNA signature. The clustering results revealed that patients in GSE9891 and GSE26193 could be clustered into different risk groups, which was consistent with the results found by using risk scores of the six-circRNA signature (data not shown).
To further evaluate the independence of the six-circRNA signature, multivariate Cox regression analyses were performed based on the six-circRNA signature and other clinical and pathological variables. Results demonstrated that the six-circRNA signature was significantly associated with patient survival in GSE9891, GSE26193 and GSE63885 datasets. In addition, certain clinical variables, including patient age, stage and tumor grade, were significantly associated with OS in different OC cohorts (Table II). Additional stratification analyses according to these factors therefore require further investigation. With regards to OC stage, patients from different OC datasets were divided into early (I/II) and late (III/IV) stage subgroups. Results revealed that the six-circRNA signature significantly subdivided patients in late stage subgroups but not in early stage groups (data not shown). With regards to OC age at diagnosis, patients from validating GSE9891 dataset were stratified into younger and older groups according to the age median value. Results revealed that the six-circRNA signature significantly subdivided patients at different age levels (data not shown). With regards to OC tumor grade, patients in the GSE63885 dataset were stratified into low (G1/G2) and high (G3/G4) grade subgroups; however, P-values were not significant with regards to OC survival analysis in the two subgroups (data not shown). This last finding may be due to unknown confounding factors or the small size of each subgroup.
In the present study, only 630 circRNAs were identified from the microarray dataset; the re-annotation strategy of circRNA expression from the microarray dataset may not have been able to cover all circRNA transcripts. By considering this limitation, it is possible that certain potential signatures may have been ignored. For example, blood or exosome-derived biomarkers are crucial for the prediction of cancer survival (22,23). However, validation of the prognostic biomarkers from peripheral blood or exosome populations requires expression dataset tested previously in the human circulation system and well-annotated follow up information. The follow up procedure may take 3–5 years (24). The acquisition of fast growing RNA-sequencing datasets may allow the discovery of novel noncoding signatures associated with survival. Future work will consider blood or exosome datasets to validate circRNA biomarkers.
In conclusion, a re-annotation strategy was performed to identify circRNA expressions from microarray dataset. Following the design of a risk score model, a panel of six circRNAs was significantly associated with OS, PFS and DFS in patients with OC. In addition, the present study revealed that the six-circRNA signature was independent of other clinical characteristics and more powerful than other molecular signatures. The present study highlighted some novel, potentially powerful prognostic markers for OC.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Natural Science Foundation of China (grant no. 81772780).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GZ conceived, designed and supervised the study. YH, QG, LS, CK and YC carried out data processing and experimental analysis. GZ, YH and QG drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Rustin G, van der Burg M, Griffin C, Qian W, Swart AM. Early versus delayed treatment of relapsed ovarian cancer. Lancet. 2011;377:380–381. doi: 10.1016/S0140-6736(11)60126-8. [DOI] [PubMed] [Google Scholar]
- 3.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10:803–808. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Bonazzoli E, Bellone S, Choi J, Dong W, Menderes G, Altwerger G, Han C, Manzano A, Bianchi A, et al. Mutational landscape of primary, metastatic, and recurrent ovarian cancer reveals c-MYC gains as potential target for BET inhibitors. Proc Natl Acad Sci USA. 2019;116:619–624. doi: 10.1073/pnas.1814027116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar L, Shamsuzzam A, Haque R, Baghel T, Nazir A. Circular RNAs: The emerging class of non-coding RNAs and their potential role in human neurodegenerative diseases. Mol Neurobiol. 2017;54:7224–7234. doi: 10.1007/s12035-016-0213-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17:205–211. doi: 10.1038/nrm.2015.32. [DOI] [PubMed] [Google Scholar]
- 7.Shang Q, Yang Z, Jia R, Ge S. The novel roles of circRNAs in human cancer. Mol Cancer. 2019;18:6. doi: 10.1186/s12943-018-0934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chekulaeva M, Rajewsky N. Roles of long noncoding RNAs and circular RNAs in translation. Cold Spring Harb Perspect Biol. 2018:a032680. doi: 10.1101/cshperspect.a032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilusz JE, Sharp PA. Molecular biology. A circuitous route to noncoding RNA. Science. 2013;340:440–441. doi: 10.1126/science.1238522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–5612. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Z, Fei T, Verhaak RG, Su Z, Zhang Y, Brown M, Chen Y, Liu XS. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat Struct Mol Biol. 2013;20:908–913. doi: 10.1038/nsmb.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glazar P, Papavasileiou P, Rajewsky N. circBase: A database for circular RNAs. RNA. 2014;20:1666–1670. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H, Wong WH. SeqMap: Mapping massive amount of oligonucleotides to the genome. Bioinformatics. 2008;24:2395–2396. doi: 10.1093/bioinformatics/btn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishwaran H, Kogalur UB. Consistency of random survival forests. Stat Probab Lett. 2010;80:1056–1064. doi: 10.1016/j.spl.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhan X, Dong C, Liu G, Li Y, Liu L. Panel of seven long noncoding RNA as a candidate prognostic biomarker for ovarian cancer. Onco Targets Ther. 2017;10:2805–2813. doi: 10.2147/OTT.S128797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou M, Sun Y, Sun Y, Xu W, Zhang Z, Zhao H, Zhong Z, Sun J. Comprehensive analysis of lncRNA expression profiles reveals a novel lncRNA signature to discriminate nonequivalent outcomes in patients with ovarian cancer. Oncotarget. 2016;7:32433–32448. doi: 10.18632/oncotarget.8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li ZC, Huang MH, Zhong WQ, Liu ZQ, Xie Y, Dai Z, Zou XY. Identification of drug-target interaction from interactome network with ‘guilt-by-association’ principle and topology features. Bioinformatics. 2016;32:1057–1064. doi: 10.1093/bioinformatics/btv695. [DOI] [PubMed] [Google Scholar]
- 21.Wilkerson MD, Hayes DN. ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26:1572–1573. doi: 10.1093/bioinformatics/btq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panagopoulou M, Karaglani M, Balgkouranidou I, Biziota E, Koukaki T, Karamitrousis E, Nena E, Tsamardinos I, Kolios G, Lianidou E, et al. Circulating cell-free DNA in breast cancer: Size profiling, levels, and methylation patterns lead to prognostic and predictive classifiers. Oncogene. 2019 doi: 10.1038/s41388-018-0660-y. [DOI] [PubMed] [Google Scholar]
- 23.Varkaris A, Katsiampoura A, Davis JS, Shah N, Lam M, Frias RL, Ivan C, Shimizu M, Morris J, Menter D, et al. Br J Cancer; 2019. Circulating inflammation signature predicts overall survival and relapse-free survival in metastatic colorectal cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huelsmann E, Zighelboim I, Ahmed A, Dewdney S. The role of neoadjuvant chemotherapy in the management of patients with advanced stage ovarian cancer: Survey results from members of the society of gynecologic oncologists, a 5-year follow-up. Gynecol Oncol Rep. 2017;20:47–50. doi: 10.1016/j.gore.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.