Abstract
The interest and demand for nutraceuticals are rapidly increasing in many industrialized countries due to the emergence of health risks associated with the increased consumption of processed foods. Several wild Mediterranean plants used as traditional foods are an extraordinary source of nutraceutical substances with antioxidant properties. This study has two main aims: (1) to quantify the antioxidant properties of traditional wild food plants and (2) to determine if their use in soups (i.e., the cooking process) can alter their beneficial properties. We have evaluated the antioxidant capacity (ABTS, DPPH) and the Total Phenolic Content (Folin-Ciocalteu) of five herbaceous plants traditionally consumed in several areas of Central Italy: (A) Reichardia picroides (L.) Roth, (B) Hypochaeris radicata L., (C) Cichorium intybus L., (D) Tordylium apulum L., and (E) Helminthotheca echioides (L.) Holub. Our analyses show good levels of antioxidant capacity for all plants, with Reichardia picroides and Helminthotheca echioides having the highest levels. There is a high correlation between the antioxidant activity and the Total Phenolic Content especially in Reichardia picroides (R2=0.92) and Hypochaeris radicata (R2=0.93). Boiling the species caused a general decrease in the antioxidant activity and polyphenols. Our study confirms the health benefits of consuming wild plants, especially raw ones in salads. It also supports the use of ethnobotanical information to study and then promote the consumption of wild food plants.
1. Introduction
Wild food plants used in the traditional Mediterranean diet have received much attention in recent years for their nutraceutical properties and in particular for their content of antioxidant compounds [1–8]. Indeed, many studies have highlighted that a dietary antioxidant intake has a protective effect against free radical-related pathologies, such as cardiovascular diseases [9], diabetes [10], cancer [11, 12], and neurodegenerative diseases [13]. The morbidity of these diseases has increased in the last few decades in many industrialized countries [14] and has been often related, among other things, to shifts from traditional to western diets [15].
In the Mediterranean basin, ethnobotanical research has identified about 2,300 different wild plants and fungi taxa, which are still gathered and consumed as food [16]. Although in decline, the consumption of wild edible plants is still common in various areas in Italy, where they are consumed because they are considered healthy and tasty [17–19], and also because they are linked to tradition and culture [18, 20]. The traditional uses of wild food plants may contribute to the health benefits associated with the Mediterranean diet and, as a consequence, studies on their phytochemistry can validate their nutraceutical properties [5–7]. This is also supported by the fact that recent studies have highlighted that the protective effect of nutraceuticals against various diseases is linked to the association of several phytochemical molecules at low concentrations, as it occurs naturally in the diet, rather than to the ingestion of individual molecules at high concentrations, as occurs in pills of dietary supplements [21–23]. Research also supports the importance of investigating the antioxidant properties of the plant part that is actually consumed, rather than focusing the attention on the effects of individual compounds [1]. Finally, although several compounds may contribute to the antioxidant properties in complex systems [24, 25], polyphenols are often considered the primary source of the antioxidant activity [26–30] but few data support a precise correlation [29, 31, 32].
Consequently, this study is aimed at (i) evaluating and comparing the total antioxidant capacity and the Total Phenolic Content in different species of wild plants, traditionally consumed either raw or cooked in Central Italy; (ii) evaluating the relationship between the antioxidant capacity and the phenolic compounds contained in plant extracts to verify whether or not the phenolic constituents are responsible for the antioxidant activity of the species.
2. Materials and Methods
2.1. Sampling and Plants Collection
We sampled five wild plants: (A) Reichardia picroides (L.) Roth (Asteraceae), (B) Hypochaeris radicata L. (Asteraceae), (C) Cichorium intybus L. (Asteraceae), (D) Tordylium apulum L. (Apiaceae), and (E) Helminthotheca echioides (L.) Holub. (Asteraceae). We selected these species because they are, to different extents, purposively consumed among local communities in Italy because they are considered to have positive effects on the health [19, 33–35].
We gathered four specimens of each species (20 samples) in the Tolfa Mountain area (70 Km north-west of Rome). We selected only specimens with vigorous growth, collected in areas with similar soil characteristics, within an altitudinal range of 350-400 m a.s.l, growing in flat areas to eliminate the influence of different exposures to the sun. The samples were carefully extracted, and they were carried intactly, along with their soil, to the laboratory of Roma Tre University, to keep the leaves alive until they were cut.
2.2. Chemical Analysis
Reagents. All chemicals used were of analytical grade. The used solvents and reagents were purchased from Sigma Aldrich (Germany).
2.3. Preparation of Extracts from Crude Plants
For each sample collected, 1 g of leaves was cut from the plant, gently cleaned with some paper, and weighed. We then put the leaves in a Falcon tube and poured 10 mL of liquid nitrogen inside. The leaves immediately became hard and fragile and then they were crushed to dust. We put the Falcon tube in a lyophilizer (Christ Alpha 1-2 b. Braun biotech international. Savant refrigerated condensation trap RT 100) until the weight of the plant material was constant. We then added 5 ml of methanol to the Falcon tube. We mixed the solution using an ultrasound apparatus (Sonica Soltec 2002MH) for 60 minutes at 30°C. Afterwards, the solution was filtered in an Eppendorf test tube (final volume 10 ml), put in a nitrogen atmosphere, and left in the freezer (-20°C) until analysis. We performed three analyses for each plant species (namely, DPPH analysis, ABTS analysis, and Total Phenolic Content).
2.4. Preparation of Extracts from Cooked Plants
We collected 1 g of leaves from each plant, put the leaves in a small beaker containing 10 ml of boiling water, and left them to cook for 5 minutes. After that, the leaves were recovered and gently dried over a clean piece of paper. We then put the leaves in a Falcon tube and poured 10 mL of liquid nitrogen inside and continued the procedure as previously described for the crude material (i.e., leaves were frozen with liquid nitrogen, crushed to dust, lyophilized to remove the water, and extracted with 5 ml of methanol in an ultrasound apparatus for 60 minutes at 30°C; the solution was filtered and put in nitrogen atmosphere and then placed in the freezer). We performed three analyses for each plant species (namely, DPPH analysis, ABTS analysis, and Total Phenolic Content).
2.5. DPPH Analysis
We performed a DPPH analysis of the samples, with some adjustments, following the method described by Brand-Williams et al. [36]. We prepared a 75 μM solution of DPPH in methanol. Plant extracts were diluted and analyzed at three different final concentrations ranging from 1.5 to 5 mg/ml. We added 50 μl of each sample solution to 0.950 ml of the DPPH solution and left them in the dark. After 30 minutes, we measured the absorbance of the samples at 517 nm using the Shimadzu UV-2401 PC spectrophotometer. We used 50 μL of pure ethanol as a control. We repeated four measurements for each plant sample.
Subsequently, we plotted the percentages of DPPH inhibition vs the antioxidant concentrations and elaborated linear regressions using the Graphpad Prism 4.1 program (http://www.graphad.com). From each graph, we extrapolated IC50 values as the concentration of the sample that halves the DPPH radical absorbance. Plants with a lower IC50 value contained higher levels of antioxidants. We performed statistical analyses applying Student's t-test and ANOVA as analyses of variance for the IC50 values. We also calculated the Antiradical Activity (ARA), as the inverse of IC50. We calculated all values, including relative errors, through the propagation of uncertainty.
2.6. ABTS Analysis
To measure the antioxidant capacity of all samples, we followed the method of Pellegrini et al. [37], with some adjustments. We prepared the ABTS radical cation solution mixing 10 ml of 7.0 mM aqueous solution of ABTS with 10 ml of 2.28 mM aqueous solution of K2S2O8 and diluting it to a 25 ml final volume (ABTS+• solution). Then, we left the solution at room temperature overnight. Before the analysis, we diluted the ABTS+• solution with ethanol to reach an absorbance of 0.70±0.20. In each analysis, we added 10 μl of plant extracts to 1 ml of the diluted ABTS+• solution. All plant extracts were analyzed in ethanol (0.2% of water) at room temperature using three different final concentrations ranging from 0.1 to 1.0 mg/ml. We measured the extent of colour fading after 3 minutes at λ=734 nm using a Shimadzu UV-2401 PC spectrophotometer. We performed four measurements for each concentration. We also run solvent blanks, and we used Trolox (6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) as a reference antioxidant (Trolox is the hydrophilic derivative of alpha-tocopherol).
We calculated the dose-response curves as the percentage of absorbance decrease (% ABTS inhibition) against the amount of antioxidant concentration for each plant collected. We performed linear regressions and extrapolated slopes of the dose-response relationship using the Graphpad Prism 4.1 program (http://www.graphad.com). The level of significance for linear regressions was p <0.005 for all datasets for either the crude or the cooked samples. We reported the antioxidant capacities as Trolox Equivalent Antioxidant Capacity (TEAC), defined as the concentration (mmol/l) of Trolox having the equivalent antioxidant capacity of 1 kgfw/l solution of the plant extract under investigation. We calculated the average TEAC values for each group of plants, and we performed statistical analyses applying Student's t-test and ANOVA as analyses of variance of the TEAC values.
2.7. Total Phenolic Content (TPC)
The Folin-Ciocalteu reagent assay [38] was used to determine the Total Phenolic Content (TPC). As a first step, we diluted 2.5 ml of Folin-Ciocalteu commercial reagent to 25 ml with deionized water obtaining a new solution (solution A). We mixed 0.10 ml of each plant sample with 0.75 ml of solution A and let to rest for three minutes at 25°C before adding 0.75 ml of a saturated sodium carbonate solution. We let the new mixed solution rest for another 120 minutes before measuring the absorbance at 725 nm. Analyses were performed in quadruplicate for each plant collected. We used Gallic acid as a standard for the calibration curve. The Total Phenolic Content (TPC) was expressed as Gallic Acid Equivalents (GAE) i.e., the mg of Gallic acid corresponding to the polyphenols present in l g of dry plant material. As for the other analyses, we calculated the average TPC value for each plant and performed Student's t-test and analysis of variance (ANOVA).
2.8. Correlation between Antioxidant Capacity and Total Phenolic Content
We correlated the antioxidant capacity and Total Phenolic Content. Specifically, we used TPC and Antiradical Activity (ARA) to calculate the antioxidant capacity as a function of the presence of phenolic compounds in the plants. We analyzed the data as a whole and then as disaggregated sets for the four plant samples of each species. We elaborated linear regressions and determined slopes using the Graphpad Prism 4.1 program (http://www.graphad.com).
2.9. Statistical Analyses and Literature Search
All data were expressed as mean ± standard error (SE). To test differences among DPPH, ABTS, and TBC, we performed a series of one-way analyses of variance (ANOVA) and Bonferroni's Multiple Comparison Test. Statistical analyses were performed using the Graphpad Prism 4.1 program (http://www.graphad.com).
We carried out a literature search on the antioxidant capacity and phenolic content of the selected plants. We used common scientific literature search engines and databases (i.e., Google Scholar, Pubmed, and Science Direct) using as keywords the scientific names of the plants and the names of the various analytical tests. Subsequently, when possible, we compared the antioxidant activity and polyphenol contents with literature data and possible reasons behind significant differences in values were discussed.
3. Results and Discussion
3.1. Ethnobotanical Sampling and Plants Collection
In Italy, the gathering and use of wild edible species, even if it is decreasing and practiced mainly by older people, is still widespread throughout the entire country, mostly in rural areas. The plants we selected are commonly consumed raw or cooked in Central Italy to prepare various traditional dishes [17, 18, 39]. The Tolfa area, where we gathered the samples, is considered very important from an ethnobotanical point of view [40, 41]. In the area, the leaves of these plants are commonly consumed fresh in salads or cooked in a tasty soup called “Acquacotta”. For this reason, we sampled and analyzed four plants from each of the five wild species Reichardia picroides (L.) Roth (Asteraceae) (A), Hypochaeris radicata L. (Asteraceae) (B), Cichorium intybus L. (Asteraceae) (C), Tordylium apulum L. (Apiaceae) (D), and Helminthotheca echioides (L.) Holub. (Asteraceae) (E). Furthermore, three plants Hypochaeris radicata (BC), Cichorium intybus (CC), and Helminthotheca echioides (EC) were also analyzed after cooking. In Italy, all five plants are traditionally used, besides as food, also for their medicinal properties; i.e., they are used to treat heart problems, infections, and diabetes and as a depurative [7, 17, 42]. These plants have a wide distribution in Italy and can grow in many different habitats, mostly arid and ruderal [43]. As such, their cultivation could be promoted in marginal and arid lands or abandoned fields [44, 45].
3.2. DPPH Assay
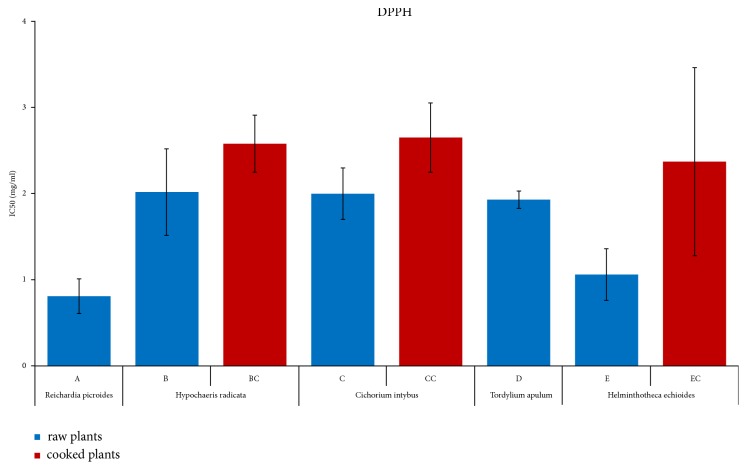
The DPPH radical scavenging assay is one of the most extensively used methods for estimating the antioxidant efficacy of molecules and plant samples [46]. We reported graphically the extent of the antioxidant capacity that was determined as the amount of antioxidant that halves the DPPH radical concentration (IC50) of the four samples of each species in Figures S1 and S2 of Supporting Information (S.I.). In Figure 1 and Tables 1 and 2, we showed the obtained IC50 average values for the five fresh plants (A-E) and the three cooked plants (BC, CC, and EC). All the analyzed plants showed antioxidant properties. In Table 1, it is possible to observe that, in the fresh samples, IC50 ranges from 2.69±0.05 mg/ml for Hypochoeris radicata (B1) to the higher 0.57±0.02 mg/ml for the antioxidant capacity of Reichardia picroides (A3). The cooking caused a general lowering of the antioxidant activity in all samples. In this case, IC50 ranged from 3.1±0.3 mg/ml of Cichorium intybus (CC3) to 1.73±0.07 mg/ml of Helminthotheca echioides (EC4) (Table 2). The comparison of fresh Cichorium intybus IC50 values with those reported in literature was, in some cases, difficult as published data dealt with antioxidant analyses of different parts of the plant (0.2-1 mg/ml for roots [47]) or cultivated varieties (5.7 nmol Trolox/mg of fresh weight for red chicory [48]). Some works, instead, reported lower values of IC50 for wild plants of Cichorium intybus (1.11 mg/ml [6]). The literature on the antioxidant capacity of cooked plants was instead quite scarce (i.e., [49]).
Figure 1.
DPPH assay of the raw and cooked plant extracts. The average antioxidant capacity for four plants of each species either raw (A-E) or cooked (BC, CC, and EC) is presented as IC50, i.e., the inhibition concentration that halves the DPPH radical activity. Lower IC50 values indicate higher antioxidant capacity. Statistical analyses of all average data were performed using ANOVA.
Table 1.
Summary table with all the results obtained from the DPPH, ABTS, and Folin-Ciocalteu assays on the extracts of the crude plants.
| Plant | Sample | DPPH IC50 | ARA | ABTS TEAC | FOLIN GAE |
|---|---|---|---|---|---|
| (mg/ml) | (ml/mg) | (mmol/kgfw) | (mg /gdw) | ||
| Reichardia picroides | A1 | 0.86 ± 0.03 | 1.17 ± 0.04 | 11.2 ± 0.3 | 20.0 ± 0.7 |
| A2 | 1.06 ± 0.03 | 0.94 ± 0.03 | 6.2 ± 0.2 | 13.8 ± 0.5 | |
| A3 | 0.57 ± 0.02 | 1.76 ± 0.05 | 6.4 ± 0.2 | 30 ± 1 | |
| A4 | 0.75 ± 0.07 | 1.33 ± 0.12 | 6.2 ± 0.2 | 25.8 ± 0.9 | |
| Hypochaeris radicata | B1 | 2.69 ± 0.05 | 0.37 ± 0.01 | 4.5 ± 0.1 | 12.2 ± 0.4 |
| B2 | 1.90 ± 0.04 | 0.53 ± 0.01 | 5.6 ± 0.2 | 18.4 ± 0.7 | |
| B3 | 1.39 ± 0.07 | 0.72 ± 0.04 | 8.8 ± 0.3 | 22.4 ± 0.8 | |
| B4 | 2.10 ± 0.07 | 0.48 ± 0.02 | 5.9 ± 0.2 | 14.0 ± 0.5 | |
| Cichorium intybus | C1 | 2.23 ± 0.02 | 0.45 ± 0.00 | 3.9 ± 0.1 | 15.2 ± 0.6 |
| C2 | 1.69 ± 0.08 | 0.59 ± 0.03 | 6.1 ± 0.2 | 22.4 ± 0.8 | |
| C3 | 1.76 ± 0.03 | 0.57 ± 0.01 | 5.8 ± 0.2 | 14.2 ± 0.5 | |
| C4 | 2.30 ± 0.03 | 0.44 ± 0.01 | 3.7 ± 0.1 | 10.5 ± 0.4 | |
| Tordylium apulum | D1 | 2.00 ± 0.05 | 0.50 ± 0.01 | 6.1 ± 0.2 | 13.8 ± 0.5 |
| D2 | 1.79 ± 0.01 | 0.56 ± 0.00 | 8.0 ± 0.3 | 17.8 ± 0.7 | |
| D3 | 1.91 ± 0.12 | 0.52 ± 0.03 | 6.1 ± 0.2 | 21.9 ± 0.8 | |
| D4 | 2.03 ± 0.06 | 0.49 ± 0.01 | 6.0 ± 0.2 | 12.4 ± 0.5 | |
| Helminthotheca echioides | E1 | 0.91 ± 0.01 | 1.09 ± 0.01 | 15.4 ± 0.5 | 33 ± 1 |
| E2 | 1.16 ± 0.04 | 0.86 ± 0.03 | 10.7 ± 0.3 | 19.8 ± 0.7 | |
| E3 | 0.71 ± 0.02 | 1.42 ± 0.04 | 12.1 ± 0.4 | 27.6 ± 1.0 | |
| E4 | 1.47 ± 0.07 | 0.68 ± 0.03 | 11.6 ± 0.4 | 20.2 ± 0.7 |
Table 2.
Summary table with all the results obtained from the DPPH, ABTS, and Folin-Ciocalteu assays on the extracts of the cooked plants.
| Plant | Sample | DPPH IC50 | ARA | ABTS TEAC | FOLIN GAE |
|---|---|---|---|---|---|
| (mg/ml) | (ml/mg) | (mmol/kgfw) | (mg /gdw) | ||
| Hypochaeris radicata | BC1 | 2.48 ± 0.05 | 0.40 ± 0.01 | 1.79 ± 0.06 | 8.1 ± 0.3 |
| BC2 | 2.2 ± 0.2 | 0.45 ± 0.04 | 2.20 ± 0.07 | 11.8 ± 0.4 | |
| BC3 | 2.0 ± 0.3 | 0.50 ± 0.08 | 3.2 ± 0.1 | 8.5 ± 0.5 | |
| BC4 | 2.64 ± 0.01 | 0.38 ± 0.00 | 2.07 ± 0.06 | 9.2 ± 0.3 | |
| Cichorium intybus | CC1 | 2.70 ± 0.04 | 0.37 ± 0.01 | 2.68 ± 0.08 | 12.9 ± 0.5 |
| CC2 | 2.37 ± 0.06 | 0.42 ± 0.01 | 2.53 ± 0.08 | 9.7 ± 0.4 | |
| CC3 | 3.1 ± 0.3 | 0.32 ± 0.03 | 2.05 ± 0.06 | 8.4 ± 0.3 | |
| CC4 | 2.3 ± 0.1 | 0.43 ± 0.02 | 3.8 ± 0.1 | 12.2 ± 0.4 | |
| Helminthotheca echioides | EC1 | 3.0 ± 0.4 | 0.33 ± 0.04 | 3.5 ± 0.1 | 11.4 ± 0.4 |
| EC2 | 1.8 ± 0.1 | 0.56 ± 0.04 | 2.91 ± 0.09 | 13.2 ± 0.7 | |
| EC3 | 2.5 ± 0.1 | 0.40 ± 0.02 | 2.95 ± 0.09 | 8.0 ± 0.3 | |
| EC4 | 1.73 ± 0.07 | 0.58 ± 0.02 | 4.2 ± 0.1 | 12.8 ± 0.5 |
Statistical analyses, performed applying Student's t-test to the samples, gave a level of significance of p < 0.005 for all the fresh samples and p < 0.05 for the cooked samples (blue bars in Figures S1 and S2). The average IC50 value for each group of crude samples showed a level of significance of p < 0.001 and of p < 0.05 for the cooked samples (Figure 1). The Statistical Analysis of Variation (ANOVA) of IC50 values showed a significant difference between the fresh plant species: the IC50 value of A and E proved to be similar to each other but significantly different from B, C, and D. Instead, the B, C, and D species showed IC50 values that were not statistically different from each other (Figure S1). For the cooked plants, the differences among the IC50 values of all the samples were not statistically significant (Figure S2).
3.3. ABTS Assay
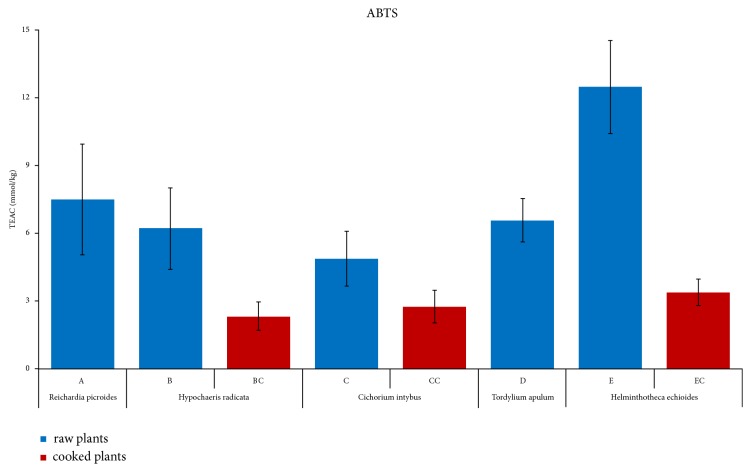
The ABTS assay also supported the antioxidant properties of the selected plants. This method of analysis is generally more sensitive than the DPPH assay to phenolic and flavonoid contents [50]. In Figure 2, we showed the relative average values expressed as Trolox Equivalent Antioxidant Capacity (TEAC) for each species either crude or cooked and their relative errors calculated with the propagation of uncertainty (see also Tables 1 and 2). The TEAC values of crude samples ranged from the lower 3.7±0.1 mmol/kgfw of Cichorium intybus (C4) to 15.4±0.5 mmol/kgfw of Helminthotheca echioides (E1) (Table 1), while for the cooked leaves, TEAC values spanned from 1.79±0.06 mmol/kgfw of Hypochaeris radicata (BC1) to 4.16±0.1 mmol/kgfw of Helminthotheca echioides (EC4) (Table 2). As in the DPPH analysis, the lower TEAC values of the cooked samples could be explained by the instability of phenolic antioxidants at high temperatures. The average TEAC value of 4.9±1 mmol/kgfw obtained from the analysis of the four samples of the crude Cichorium intybus is coherent with literature data (see also [51] for the wild specimens). Since Cichorium intybus is generally considered as having a significant level of antioxidant capacity [51], the higher TEAC values obtained for all the other examined species (crude samples) supported their good antioxidant capacity. Nevertheless, the average TEAC value for each species showed a high standard deviation (Figures S3 and S4). Other statistical analyses, instead, confirmed the robustness of the data and attested the antioxidant capacity of the plants. The level of significance for the Student's t-test was p < 0.01 for the crude samples and p < 0.05 for the cooked samples. The Analysis of Variation (ANOVA) revealed that significant differences in TEAC values only exist between E and other crude plant samples. In fact, Helminthotheca echioides (E) showed an average TEAC value of 12±2 mmol/kgfw, more than 2.5 times higher than that of Cichorium intybus (Figure 2), supporting its good antioxidant capacity. All the other TEAC values of crude (A, B, C, and D) or cooked (BC, CC, and EC) samples were not statistically different from each other.
Figure 2.
ABTS assay of the raw and cooked plant extracts. The average antioxidant capacity for four plants of each species either raw (A-E) or cooked (BC, CC, and EC) is presented as TEAC, i.e., mmol of Trolox equivalent per kg of fresh weight (kgfw). Statistical analyses of all average data were performed using ANOVA.
3.4. Total Phenolic Content
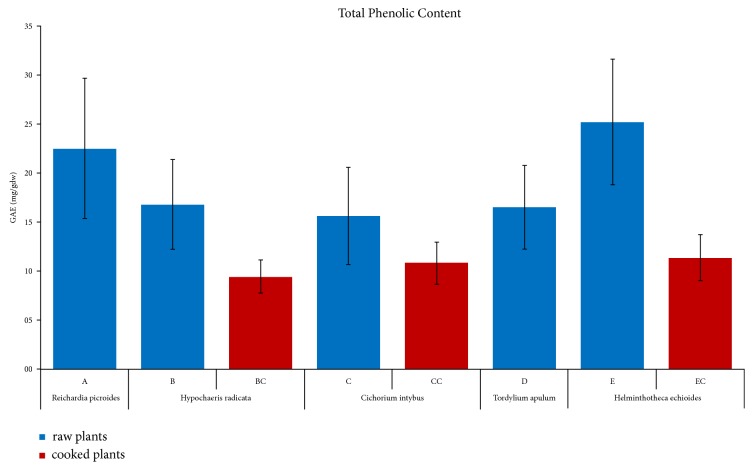
The average Total Phenolic Content (TPC) of the plant extracts is presented in Figure 3, while the TPC values for all the samples are provided in the Figures S5 and S6. The crude plant extracts showed a polyphenol level ranging from 10.5±0.4 mg GAE/gdw in Cichorium intybus (C4) to 33±1 mg GAE/gdw in Helminthotheca echioides (E1) (Table 1). On the other hand, cooked leaves displayed a lower TPC ranging from 8.0±0.3 mg GAE/gdw (EC3) to 13.2±0.7 (EC2) mg GAE/gdw, both measured in samples of Helminthotheca echioides. Literature data about crude extracts of Cichorium intybus indicated a lower phenolic content (0.66 mg GAE/gdw [6] or higher (22.6±1.0 mg GAE/gdw [52]) than the mean value of the four crude plant extracts analyzed (16±5 mg GAE/gdw). This could be due either to the intrinsic differences in the growing environment of the plant (i.e., soil, exposure to solar radiation, and hydric supply [52]) or to different sample preparations that could have safeguarded polyphenols from degradation.
Figure 3.
Total Phenolic Content of the raw and cooked plant extracts determined using the Folin-Ciocalteu assay. The averages data of all raw (A-E) and cooked (BC, CC, and EC) species are presented as Gallic Acid Equivalents (GAE); i.e., mg of Gallic acid corresponding to the polyphenols contained per gram of dry weight (gdw). Statistical analyses of all average data were performed using ANOVA.
TPC values and their relative errors, calculated through the propagation of uncertainty, are reported in Tables 1 and 2. The level of significance is p < 0.05 for all data. The ANOVA test for the average TPC values confirmed a low variation in phenolic concentrations among the various plants either crude or cooked: these concentrations were not statistically different from each other except to some marginal differences between E and B or C and D (crude samples).
3.5. Correlation between Antioxidant Capacity and Total Phenolic Content
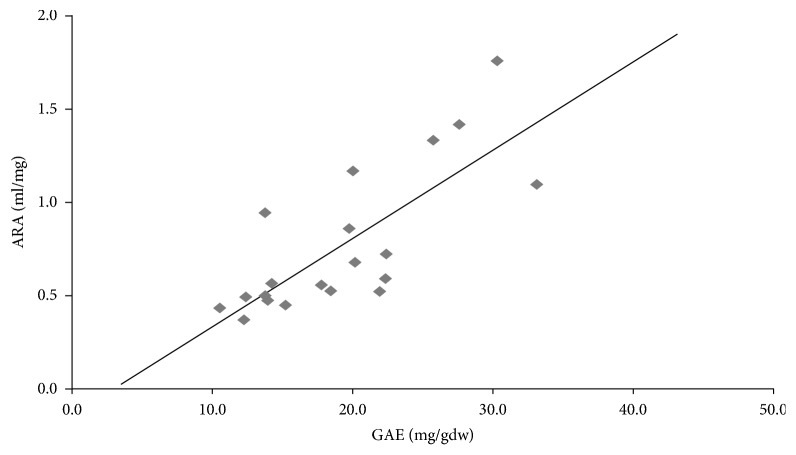
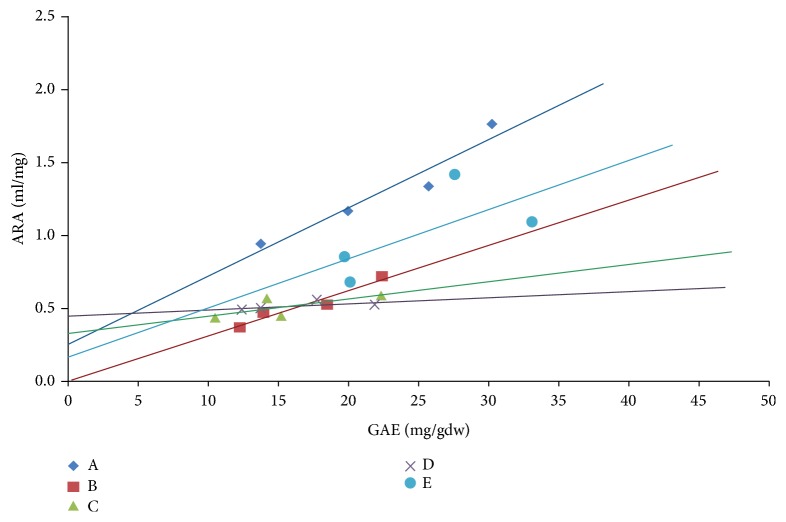
The Antiradical activity (ARA), as the inverse of IC50, is reported in Table 1 and was used to correlate TPC with the antioxidant capacity of crude samples (Figures 4 and 5). In Figure 4, we showed the graph plotting the values of ARA and TPC of all the crude plant samples under study. The graph showed a linear correlation (p<0.0001) with a slope of 0.047±0.009 and -0.1±0.2 as y intercept. As regards Figure 4, the value of R2 (0.59) suggests a good linear correlation between the two variables (see [6]). In Figure 5, we showed the same linear correlations between ARA and TPC but with data disaggregated for the four plant samples of each species. For Reichardia picroides A (R2=0.92) and Hypochaeris radicata B (R2=0.93) the antioxidant capacity proved to be linearly dependent on TPC while a weaker correlation was observed with Cichorium intybus (C), Tordylium apulum (D), and Helminthotheca echioides (E). The slope of each curve correlates the antioxidant capacity with the polyphenols contained in the plant: the higher the value of the slope the higher the antioxidant capacity of the polyphenols in the samples. Furthermore, Hypochaeris radicata (B) exhibited an antioxidant activity completely dependent on polyphenolic compounds, as shown by y intercepts tending towards zero in the absence of polyphenolic compounds (x=0), whereas the antioxidant capacity of Reichardia picroides (A), Cichorium intybus (C), Tordylium apulum (D), and Helminthotheca echioides (E) is probably due to other kinds of antioxidants (i.e., carotenoids), as shown by y intercepts different from zero at x=0.
Figure 4.
Correlation between the Antiradical Activity (ARA) and Total Phenolic Content (TPC) for all data of the crude plant extracts: y = 0.047x - 0.14; R2 = 0.59; p <0.0001. Linear regressions and best fit values were calculated using the Graphpad Prism 4.1 program (http://www.graphad.com).
Figure 5.
Correlation between the Antiradical Activity (ARA) and Total Phenolic Content for each raw species (A-E). A Reichardia picroides (◆) y = 0.047x +0.25; R2 = 0.92; B Hypochaeris radicata (■) y = 0.031x +0.009; R2 = 0.93; C Cichorium intybus (Δ) y = 0.011x +0.34; R2 = 0.52; D Tordylium apulum (x) y = 0.004x +0.45; R2 = 0.33; and E Helminthotheca echioides (•) y = 0.03x+0.25; R2 = 0.45. A (p< 0.05), B (p< 0.05), C (p< 0.1), D (p< 0.3), and E (p< 0.1). Linear regressions and best fit values were calculated using the Grahpad Prism 4 program (http://www.graphad.com).
Our results show that the examined wild food plants have a good antioxidant activity (see [53]), although with some differences among plants, and a certain variability among samples of the same species. Among the analyzed plants, Helminthotheca echioides (E) and Reichardia picroides (A) have shown the best performances. Helminthotheca echioides (E) exhibited the highest antioxidant capacity using either the ABTS or the DPPH assays, coupled with the highest polyphenolic content.
The antioxidant capacity and the phenolic content are correlated in the plants under study. A strong correlation exists in Reichardia picroides A (R2=0.92) and Hypochaeris radicata B (R2=0.93), instead, in Cichorium intybus (C), Tordylium apulum (D), and Helminthotheca echioides (E) the correlation decreases due to the likely presence of nonpolyphenolic antioxidants. Similarly, Dalar et al. [54] found a correlation between total phenolics and antioxidant capacity in their experiments, but they also detected a significant level of other redox-active compounds besides phenolics. In another study, instead, the contribution of phenolic components to the antioxidant capacity was found to be at only 58% [55].
4. Conclusions
Our study demonstrated that the examined wild food plants, consumed in traditional recipes in Central Italy, are indeed rich in antioxidant compounds. Ethnobotanical research might guide the study and then the revitalization of a healthy Mediterranean diet that incorporates wild plants with antioxidant capacity. We also verified that this antioxidant capacity is mainly correlated with the presence of phenolic compounds although further analysis (i.e., the identification of the individual constituents of the mixture by HPLC or LC-MS) would better determine the presence of other antioxidant compounds. We were also able to assess that the way food is consumed (raw or cooked) can significantly alter the health benefits of the ingested plants. In view of the growing demand for food rich in natural antioxidants by the global market, the use of ethnobotanical information to study and then promote new agronomic products from wild food plants could constitute an important way to produce income in many rural regions.
Acknowledgments
The authors would like to thank Paolo Maria Guarrera for providing useful comments and suggestions.
Abbreviations
- DPPH:
2,2-diphenyl-1-picrylhydrazyl radical
- ANOVA:
Analysis of variance
- ARA:
Antiradical activity
- ABTS:
2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
- TEAC:
Trolox equivalent antioxidant capacity
- Trolox:
6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
- TPC:
Total phenolic content
- GAE:
Gallic acid equivalents
- kgfw:
Kilograms fresh weight
- gdw:
Grams dry weight
- SE:
Standard error
Contributor Information
Valentina Savo, Email: valentina.savo@uniroma3.it.
Daniela Tofani, Email: daniela.tofani@uniroma3.it.
Data Availability
The data used to support the findings of this study are included within the article and the supplementary information files.
Conflicts of Interest
The authors declare no financial conflicts of interest.
Supplementary Materials
Figure S1. DPPH assay of the crude plant extracts. Data of each plant sample and averages of all species (A-E) are presented as IC50 i.e., the inhibition concentration that halves the DPPH radical activity. Lower IC50 values indicate higher antioxidant capacity. Statistical analyses of all average data were performed using ANOVA. Values for A and E resulted not statistically different from each other but different from all other values (p < 0.01) except E versus B (p<0.05). Figure S2. DPPH assay of the cooked plant extracts. Data of plant samples and averages of species (BC, CC, and EC) are presented as IC50 i.e., the inhibition concentration that halves the DPPH radical activity. Lower IC50 values indicate higher antioxidant capacity. Statistical analyses of all average data were performed using ANOVA. Values resulted not statistically different from each other. Figure S3. ABTS assay of the crude plant extracts. Data of each plant sample and averages of all species (A-E) are presented as TEAC i.e., mmoles of Trolox equivalent per kilogram of fresh weight (kgfw). Statistical analyses of all average data were performed using ANOVA. All average data resulted not statistically different from each other except for E that was statistically different from A (p< 0.05), B (p< 0.01), C (p< 0.001), and D (p< 0.01). Figure S4. ABTS assay of the cooked plant extracts. Data of plant samples and averages of species (BC, CC and EC) are presented as TEAC, i.e., mmoles of Trolox equivalent per kilogram of fresh weight (kgfw). Statistical analyses of all average data were performed using ANOVA. All average data resulted not statistically different from each other. Figure S5. Total Phenolic Content of the crude plant extracts determined using the Folin-Ciocalteu assay. Data of each plant sample and averages of all species (A-E) are presented as mg of Gallic acid per gram of dry weight (gdw). Statistical analyses of all average data were performed using ANOVA. All average data resulted not statistically different from each other except to some marginal differences between E and B or C and D. Figure S6. Total Phenolic Content of the cooked plant extracts determined using the Folin-Ciocalteu assay. Data of plant samples and averages of species (BC, CC and EC) are presented as mg of Gallic acid per gram of dry weight (gdw). Statistical analyses of all average data were performed using ANOVA. All average data resulted not statistically different from each other.
References
- 1.The local Food-Nutraceuticals Consortium. Understanding local Mediterranean diets: a multidisciplinary pharmacological and ethnobotanical approach. Pharmacological Research. 2005;52:353–366. doi: 10.1016/j.phrs.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Salvatore S., Pellegrini N., Brenna O. V., et al. Antioxidant characterization of some Sicilian edible wild greens. Journal of Agricultural and Food Chemistry. 2005;53(24):9465–9471. doi: 10.1021/jf051806r. [DOI] [PubMed] [Google Scholar]
- 3.Heinrich M., Müller W. E., Galli C. Local Mediterranean Food plants. Basel, Switzerland: Karger; 2006. [Google Scholar]
- 4.Martins D., Barros L., Carvalho A. M., Ferreira I. C. F. R. Nutritional and in vitro antioxidant properties of edible wild greens in Iberian Peninsula traditional diet. Food Chemistry. 2011;125(2):488–494. doi: 10.1016/j.foodchem.2010.09.038. [DOI] [Google Scholar]
- 5.Morales P., Carvalho A. M., Sánchez-Mata M. C., Cámara M., Molina M., Ferreira I. C. F. R. Tocopherol composition and antioxidant activity of Spanish wild vegetables. Genetic Resources and Crop Evolution. 2012;59(5):851–863. doi: 10.1007/s10722-011-9726-1. [DOI] [Google Scholar]
- 6.Morales P., Ferreira I. C. F. R., Carvalho A. M., et al. Mediterranean non-cultivated vegetables as dietary sources of compounds with antioxidant and biological activity. LWT - Food Science and Technology. 2014;55(1):389–396. doi: 10.1016/j.lwt.2013.08.017. [DOI] [Google Scholar]
- 7.Ranfa A., Maurizi A., Romano B., Bodesmo M. The importance of traditional uses and nutraceutical aspects of some edible wild plants in human nutrition: the case of Umbria (central Italy) Plant Biosystems. 2014;148(2):297–306. doi: 10.1080/11263504.2013.770805. [DOI] [Google Scholar]
- 8.Barros L., Morales P., Carvalho A. M., Ferreira I. C. F. R. Antioxidant potential of wild plant foods. In: Sánchez-Mata M. C., Tardío J., editors. Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables. New York, NY, USA: Springer; 2016. pp. 209–232. [Google Scholar]
- 9.Hertog M. G. L., Feskens E. J. M., Kromhout D. Antioxidant flavonols and coronary heart disease risk. The Lancet. 1997;349(9053):p. 699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- 10.Montonen J., Knekt P., Järvinen R., Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004;27(2):362–366. doi: 10.2337/diacare.27.2.362. [DOI] [PubMed] [Google Scholar]
- 11.Trichopoulou A., Lagiou P., Kuper H., Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiology, Biomarkers & Prevention. 2000;9(9):869–873. [PubMed] [Google Scholar]
- 12.La Vecchia C. Association between Mediterranean dietary patterns and cancer risk. Nutrition Reviews. 2009;67(1):S126–S129. doi: 10.1111/j.1753-4887.2009.00174.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilgun-Sherki Y., Melamed E., Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. Journal of Neurology. 2004;251(3):261–268. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 14.Koene R. J., Prizment A. E., Blaes A., Konety S. H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuckler D., Nestle M. Big food, food systems, and global health. PLoS Medicine. 2012;9(6):p. e1001242. doi: 10.1371/journal.pmed.1001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadjichambis A. C., Paraskeva-Hadjichambi D., Della A., et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. International Journal of Food Sciences and Nutrition. 2008;59(5):383–414. doi: 10.1080/09637480701566495. [DOI] [PubMed] [Google Scholar]
- 17.Guarrera P. M. Usi e Tradizioni Della Flora Italiana. Rome, Italy: Aracne editrice; 2006. [Google Scholar]
- 18.Ghirardini M., Carli M., del Vecchio N., et al. The importance of a taste. A comparative study on wild food plant consumption in twenty-one local communities in Italy. Journal of Ethnobiology and Ethnomedicine. 2007;3(1):p. 22. doi: 10.1186/1746-4269-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guarrera P. M., Savo V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: a review. Journal of Ethnopharmacology. 2013;146(3):659–680. doi: 10.1016/j.jep.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Guarrera P. M., Savo V. Wild food plants used in traditional vegetable mixtures in Italy. Journal of Ethnopharmacology. 2016;185:202–234. doi: 10.1016/j.jep.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 21.Liu R. H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. American Journal of Clinical Nutrition. 2003;78(3):517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 22.Mehta R. G., Murillo G., Naithani R., Peng X. Cancer chemoprevention by natural products: how far have we come? Pharmaceutical Research. 2010;27(6):950–961. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 23.Liu R. H. Health benefits of phytochemicals in whole foods. In: Temple N. J., Wilson T., Jacobs D. R., editors. Nutritional Health: Strategies For Disease Prevention. New York, NY, USA: Springer Science; 2012. pp. 293–310. [Google Scholar]
- 24.Simopoulos A. P. Omega-3 fatty acids and antioxidants in edible wild plants. Biological Research. 2004;37(2):263–277. doi: 10.4067/s0716-97602004000200013. [DOI] [PubMed] [Google Scholar]
- 25.Brewer M. S. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Comprehensive Reviews in Food Science and Food Safety. 2011;10(4):221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- 26.Zheng W., Wang S. Y. Antioxidant activity and phenolic compounds in selected herbs. Journal of Agricultural and Food Chemistry. 2001;49(11):5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 27.Kapiszewska M., Soltys E., Visioli F., Cierniak A., Zajac G. The protective ability of the Mediterranean plant extracts against the oxidative DNA damage. The role of the radical oxygen species and the polyphenol content. Journal of Physiology and Pharmacology Journal. 2005;56:183–197. [PubMed] [Google Scholar]
- 28.Heimler D., Isolani L., Vignolini P., Tombelli S., Romani A. Polyphenol content and antioxidative activity in some species of freshly consumed salads. Journal of Agricultural and Food Chemistry. 2007;55(5):1724–1729. doi: 10.1021/jf0628983. [DOI] [PubMed] [Google Scholar]
- 29.Conforti F., Sosa S., Marrelli M., et al. The protective ability of Mediterranean dietary plants against the oxidative damage: the role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chemistry. 2009;112(3):587–594. doi: 10.1016/j.foodchem.2008.06.013. [DOI] [Google Scholar]
- 30.Vanzani P., Rossetto M., de Marco V., Sacchetti L. E., Paoletti M. G., Rigo A. Wild mediterranean plants as traditional food: a valuable source of antioxidants. Journal of Food Science. 2011;76(1):C46–C51. doi: 10.1111/j.1750-3841.2010.01949.x. [DOI] [PubMed] [Google Scholar]
- 31.Shan B., Cai Y. Z., Sun M., Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of Agricultural and Food Chemistry. 2005;53(20):7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- 32.Hinneburg I., Damien Dorman H. J., Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chemistry. 2006;97(1):122–129. doi: 10.1016/j.foodchem.2005.03.028. [DOI] [Google Scholar]
- 33.Guarrera P. M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium) Fitoterapia. 2003;74(6):515–544. doi: 10.1016/S0367-326X(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 34.Nebel S., Heinrich M. The use of wild edible plants in the Graecanic area in Calabria, Southern Italy. In: Pardo-de-Sanatayana M., Pieroni A., Puri R. K., editors. Ethnobotany of the New Europe. Berghahn, NY, USA: 2010. pp. 172–188. [Google Scholar]
- 35.Ranfa A., Orlandi F., Maurizi A., Bodesmo M. Ethnobotanical knowledge and nutritional properties of two edible wild plants from Central Italy: Tordylium apulum L. and Urospermum dalechampii (L.) F.W. Schmid. Journal of Applied Botany and Food Quality. 2015;88:249–254. [Google Scholar]
- 36.Brand-Williams W., Cuvelier M. E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 37.Pellegrini N., Visioli F., Buratti S., Brighenti F. Direct analysis of total antioxidant activity of olive oil and studies on the influence of heating. Journal of Agricultural and Food Chemistry. 2001;49(5):2532–2538. doi: 10.1021/jf001418j. [DOI] [PubMed] [Google Scholar]
- 38.Singleton V. L., Rossi J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16:144–158. [Google Scholar]
- 39.Pieroni A., Janiak V., Dürr C. M., Lüdeke S., Trachsel E., Heinrich M. In vitro antioxidant activity of non-cultivated vegetables of ethnic Albanians in southern Italy. Phytotherapy Research. 2002;16(5):467–473. doi: 10.1002/ptr.1243. [DOI] [PubMed] [Google Scholar]
- 40.Guarrera P. M. Il Patrimonio Etnobotanico Del Lazio. Le Piante Del Lazio Nell’Uso Terapeutico, Alimentare, Domestico, Religioso E Magico. Etnobotanica Laziale E Della Media Penisola Italiana A Confronto. Rome, Italy: Regione Lazio, Assessorato Alla Cultura E Dipartimento Di Biologia Vegetale, Università La Sapienza; 1994. [Google Scholar]
- 41.Guarrera P. M., Savo V., Caneva G. Traditonal uses of plants in the Tolfa-Cerite-Manziate area (central Italy) Ethnobiology Letters. 2015;6(1):119–161. doi: 10.14237/ebl.6.1.2015.288. [DOI] [Google Scholar]
- 42.Cornara L., La Rocca A., Marsili S., Mariotti M. G. Traditional uses of plants in the Eastern Riviera (Liguria, Italy) Journal of Ethnopharmacology. 2009;125(1):16–30. doi: 10.1016/j.jep.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Pignatti S. Flora d’Italia. Bologna, Italy: Edagricole; 1982. [Google Scholar]
- 44.Spina M., Cuccioloni M., Sparapani L., et al. Comparative evaluation of flavonoid content in assessing quality of wild and cultivated vegetables for human consumption. Journal of the Science of Food and Agriculture. 2008;88(2):294–304. doi: 10.1002/jsfa.3089. [DOI] [Google Scholar]
- 45.Heimler D., Isolani L., Vignolini P., Romani A. Polyphenol content and antiradical activity of Cichorium intybus L. from biodynamic and conventional farming. Food Chemistry. 2009;114(3):765–770. doi: 10.1016/j.foodchem.2008.10.010. [DOI] [Google Scholar]
- 46.Kedare S. B., Singh R. P. Genesis and development of DPPH method of antioxidant assay. Journal of Food Science and Technology. 2011;48(4):412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H., Wang Q., Liu Y., Chen G., Cui J. Antimicrobial and antioxidant activities of cichorium intybus root extract using orthogonal matrix design. Journal of Food Science. 2013;78(2):M258–M263. doi: 10.1111/1750-3841.12040. [DOI] [PubMed] [Google Scholar]
- 48.Lavelli V. Antioxidant activity of minimally processed red chicory (Cichorium intybus L.) evaluated in xanthine oxidase-, myeloperoxidase-, and diaphorase-catalyzed reactions. Journal of Agricultural and Food Chemistry. 2008;56(16):7194–7200. doi: 10.1021/jf801913v. [DOI] [PubMed] [Google Scholar]
- 49.Boari F., Cefola M., Di Gioia F., Pace B., Serio F., Cantore V. Effect of cooking methods on antioxidant activity and nitrate content of selected wild Mediterranean plants. International Journal of Food Sciences and Nutrition. 2013;64(7):870–876. doi: 10.3109/09637486.2013.799125. [DOI] [PubMed] [Google Scholar]
- 50.Floegel A., Kim D.-O., Chung S.-J., Koo S. I., Chun O. K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis. 2011;24(7):1043–1048. doi: 10.1016/j.jfca.2011.01.008. [DOI] [Google Scholar]
- 51.Montefusco A., Semitaio G., Marrese P. P., et al. Antioxidants in varieties of chicory (Cichorium intybus L.) and wild poppy (Papaver rhoeas L.) of Southern Italy. Journal of Chemistry. 2015;2015:8. doi: 10.1155/2015/923142.923142 [DOI] [Google Scholar]
- 52.Dalar A., Konczak I. Cichorium intybus from eastern anatolia: phenolic composition, antioxidant and enzyme inhibitory activities. Industrial Crops and Products. 2014;60:79–85. doi: 10.1016/j.indcrop.2014.05.043. [DOI] [Google Scholar]
- 53.Pellegrini N., Serafini M., Colombi B., et al. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. Journal of Nutrition. 2003;133(9):2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- 54.Dalar A., Uzun Y., Turker M., Mukemre M., Konczak I. Health attributes of ethnic vegetables consumed in the Eastern Anatolia region of Turkey: antioxidant and enzyme-inhibitory properties. Journal of Ethnic Foods. 2016;3(2):142–149. doi: 10.1016/j.jef.2016.05.005. [DOI] [Google Scholar]
- 55.Fraisse D., Felgines C., Texier O., Lamaison L. J. Caffeoyl derivatives: major antioxidant compounds of some wild herbs of the Asteraceae family. Journal of Food and Nutrition Sciences. 2011;2(3):181–192. doi: 10.4236/fns.2011.230025. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. DPPH assay of the crude plant extracts. Data of each plant sample and averages of all species (A-E) are presented as IC50 i.e., the inhibition concentration that halves the DPPH radical activity. Lower IC50 values indicate higher antioxidant capacity. Statistical analyses of all average data were performed using ANOVA. Values for A and E resulted not statistically different from each other but different from all other values (p < 0.01) except E versus B (p<0.05). Figure S2. DPPH assay of the cooked plant extracts. Data of plant samples and averages of species (BC, CC, and EC) are presented as IC50 i.e., the inhibition concentration that halves the DPPH radical activity. Lower IC50 values indicate higher antioxidant capacity. Statistical analyses of all average data were performed using ANOVA. Values resulted not statistically different from each other. Figure S3. ABTS assay of the crude plant extracts. Data of each plant sample and averages of all species (A-E) are presented as TEAC i.e., mmoles of Trolox equivalent per kilogram of fresh weight (kgfw). Statistical analyses of all average data were performed using ANOVA. All average data resulted not statistically different from each other except for E that was statistically different from A (p< 0.05), B (p< 0.01), C (p< 0.001), and D (p< 0.01). Figure S4. ABTS assay of the cooked plant extracts. Data of plant samples and averages of species (BC, CC and EC) are presented as TEAC, i.e., mmoles of Trolox equivalent per kilogram of fresh weight (kgfw). Statistical analyses of all average data were performed using ANOVA. All average data resulted not statistically different from each other. Figure S5. Total Phenolic Content of the crude plant extracts determined using the Folin-Ciocalteu assay. Data of each plant sample and averages of all species (A-E) are presented as mg of Gallic acid per gram of dry weight (gdw). Statistical analyses of all average data were performed using ANOVA. All average data resulted not statistically different from each other except to some marginal differences between E and B or C and D. Figure S6. Total Phenolic Content of the cooked plant extracts determined using the Folin-Ciocalteu assay. Data of plant samples and averages of species (BC, CC and EC) are presented as mg of Gallic acid per gram of dry weight (gdw). Statistical analyses of all average data were performed using ANOVA. All average data resulted not statistically different from each other.
Data Availability Statement
The data used to support the findings of this study are included within the article and the supplementary information files.