Abstract
Increased aggregation of β-amyloid (Aβ) peptides induces oxidative stress, which is considered a major contributor in the development of Alzheimer's disease (AD). Prevention of Aβ-induced neurotoxicity is proposed as a possible modality for treatment of AD. The present study aimed to elucidate possible effects of ethyl vanillin (EVA), an analog of vanillin isolated from vanilla beans, on the Aβ1-42-induced oxidative injury in PC12 cells. EVA restrained the decrease in PC12 cell viability and apoptosis induction caused by treatment with Aβ1-42. In addition, EVA markedly alleviated intracellular lipid peroxidation as demonstrated by malondialdehyde levels and reactive oxygen species production in Aβ1-42-treated PC12 cells. In addition, the reduction in the activity levels of the antioxidative enzymes superoxide dismutase, catalase and glutathione peroxidase was detected in Aβ1-42-treated PC12 cells. This effect was partially reversed by treatment with EVA. Furthermore, the results indicated that EVA attenuated Aβ1-42-induced caspase-3 activation and the increase noted in the apoptosis regulator Bcl-2/apoptosis regulator Bax ratio of PC12 cells. These results indicated that EVA could be used as an efficient and novel agent for the prevention of neurodegenerative diseases via inhibition of oxidative stress and cell apoptosis.
Keywords: ethyl vanillin, neuroprotection, oxidative stress
Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder associated with pathological deposition of β-amyloid (Aβ) peptides, intracellular neurofibrillary tangles and neuronal loss caused by neuronal apoptosis. A complex contribution of several genetic and environmental risk factors is necessary for the initiation and progression of AD (1,2). Currently, due to the lack of effective treatment options, the disease ultimately results in patient mortality (3,4). Several studies have investigated the potential association between Aβ and the development of AD (5–7). Genetic studies have revealed abnormal production of Aβ peptides in cell culture and animal models (5–7). A vast body of evidence suggests that the alterations in physicochemical properties and concentration of Aβ1-42 may initiate the transition of neurons from the physiological to the pathological state (8–10).
The etiology and pathogenesis of AD have remained elusive, and the majority of the observations have confirmed that Aβ deposition in the brain leads to the intracellular accumulation of reactive oxygen species (ROS). Although oxidative stress does not show specific clinical symptoms, the overproduction of ROS may induce cell injury via lipid peroxidation, DNA damage and regulation of apoptotic proteins (11–13). Subsequently, apoptosis and cell cycle arrest further result in neuronal cell death (14). In addition, several studies have demonstrated an association between oxidative stress and apoptosis in AD (14,15). Therefore, it has been proposed that compounds that ameliorate Aβ-induced oxidative stress may be used for the treatment and/or prevention of AD. Antioxidants that prevent or delay Aβ-induced apoptosis may be a promising therapeutic strategy against AD (16–19).
Vanillin is a primary active component extracted from vanilla beans, which has long been used as a component of perfume and for food and medical applications (20). Ethyl vanillin (EVA), an analogue of vanillin, is widely used as a food additive (Fig. 1), and the safety and long-established properties of EVA have been previously investigated (21). Furthermore, it is reported that EVA at doses <0.5 mM is unable to diminish viability of the macrophage cells (22). Due to its safety as a food additive, a number of studies have investigated the multifunctional effects of EVA, including antioxidative, antimutagenic, antiangiogenetic, anti-sickling and analgesic activities (23–26). It has been reported that EVA serves a protective role against protein oxidation and induction of apoptosis caused by rotenone in a rotenone-induced rat model of Parkinson's disease (27). A previous study suggested that the antioxidative activity of EVA is more potent compared with vanillin, as demonstrated by oxidative hemolysis inhibition assays (28). In addition, EVA can reduce the increase in ROS levels and metalloproteinase-9 expression levels in lipopolysaccharide (LPS)-stimulated macrophages, indicating that it could protect from neurodegeneration and oxidative damage (21,29). Based on the above evidence, the current study investigated the pro-survival activity of EVA against the oxidative damage caused by Aβ1-42-induced toxicity in PC12 cells. The ability of EVA to protect against Aβ-induced neurotoxicity was assessed in PC12 cells and initial experiments were performed to investigate the potential mechanism of its action.
Figure 1.
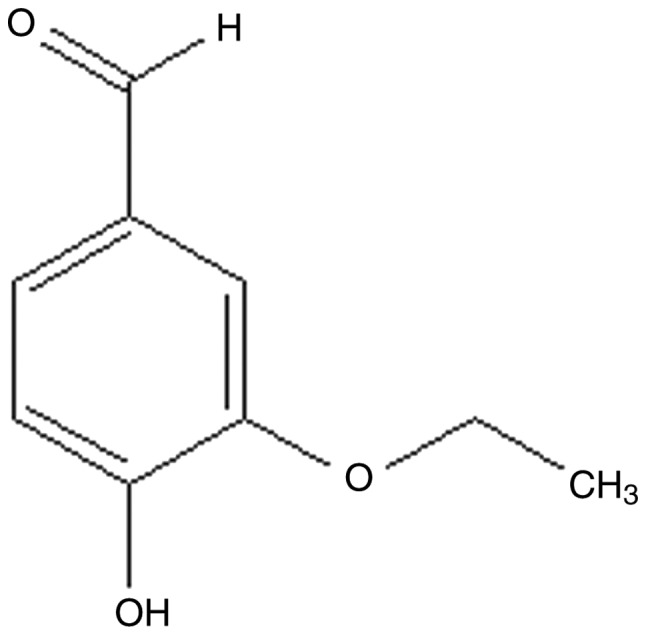
Chemical structure of ethyl vanillin.
Materials and methods
Reagents
Ethyl vanillin (EVA), nerve growth factor (NGF), Aβ1-42, Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS) and horse serum (HS) were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). TUNEL Detection Kit (#11684817910) was purchased from Roche (Basel, Switzerland). The kits for malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and lactate dehydrogenase (LDH) detection were provided from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). ROS assay kit and mitochondrial membrane potential with JC-1 kit were purchased from Beyotime Institute of Biotechnology (Haimen, China). Polyclonal antibodies against cleaved caspase-3 (#9661), apoptosis regulator Bcl-2 (#2876s) and apoptosis regulator Bax (#2772) were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Horseradish peroxidase conjugated goat anti-rabbit antibodies (#GB23303) and bovine serum albumin (#G5001) were obtained from Servicebio, Inc. (Woburn, MA, USA).
Cell culture
Undifferentiated PC12 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured with 5 ng/ml NGF in DMEM containing 10% HS, 5% FBS and 1% penicillin-streptomycin in a CO2 incubator (37°C and 5% CO2). The medium was changed every other day. The cells were seeded at a density of 1×104 cells/ml and allowed to grow for 24 h prior to experimentation.
Cell viability and LDH assay
To assess the protective effect of EVA against Aβ1-42-induced cytotoxicity, cell viability was detected by the MTT reduction assay, as previously described. Aβ1-42 aggregates were prepared as previously described (30). PC12 cells were cultured at a density of 1×104 cells/well in poly-L-lysine-coated 96-well microplates for 24 h at 37°C. The cells were pretreated with various doses of EVA (0, 10, 30 and 100 µM) for 12 h and then exposed to 20 µM of Aβ1-42 for 12 h. The EVA group was treated with 100 µM of EVA for 24 h. At the end of the drug treatment, 20 µl of MTT solution (5 mg/ml) was added to each well and the cells were incubated at 37°C for 4 h. The supernatants were subsequently replaced with 150 ml of dimethylsulfoxide. Cell viability was determined at a wavelength of 570 nm using an ELISA reader (Varioskan™ Flash Multimode Reader; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The LDH activity is an in vitro cellular toxicity marker that was determined in the present study using a commercial assay kit. In brief, PC12 cells were cultured at a density of 1×104 cells/well in poly-L-lysine-coated 96-well microplates for 24 h at 37°C. The cells were pretreated with various doses of EVA (0, 10, 30 and 100 µM) for 12 h and then exposed to 20 µM of Aβ1-42 for 12 h. At the end of the drug treatment, 100 µl of the incubation medium was collected for the extracellular LDH activity assay. The adherent cells were washed with phosphate buffered saline (PBS) three times and subsequently homogenized. The homogenate was centrifuged at 4,000 × g for 30 min at 37°C and the supernatant was collected for the intracellular LDH activity assay. The absorbance of each sample was measured at a wavelength of 490 nm using a microplate reader according to the manufacturer's protocol. LDH release was calculated as follows:
Terminal deoxynucleotidyl-transferase-mediated dUTP nick end labelling (TUNEL) assay
Apoptotic cells were observed by TUNEL assay. Briefly, PC12 cells were cultured and treated on circular glass coverslips. The cells were pretreated with 100 µM of EVA for 12 h and then exposed to 20 µM Aβ1-42 for 12 h. At the end of drug treatment, the cells were fixed in 4% paraformaldehyde for 15 min at 4°C and washed with PBS. The samples were further incubated in a blocking solution (obtained from the assay kit) for 20 min at 37°C and permeabilizing solution (0.1% Triton X-100 in 0.1% sodium acetate) for 30 min at 4°C. Subsequently, the cells were stained by TUNEL reaction solution for 1 h at 37°C, followed by cultured with 3% H2O2 solution for 15 min and rinsed with PBS for 10 min. The cells were finally treated with 3,3′-diaminobenzidine (DAB) substrate to produce a dark brown precipitate. Coverslips were stained with hematoxylin for 1 min at 37°C and mounted with neutral balsam. The number of TUNEL-positive cells was counted at 6 high-power (magnification, ×400) fields per slide.
Lipid peroxidation assay
The MDA content, an index of lipid peroxidation, was detected using a commercial assay kit according to the manufacturer's protocol. Briefly, PC12 cells were cultured at a density of 4×105 cells/well in 6-well microplates for 24 h at 37°C. The cells were pretreated with various doses of EVA (0, 10, 30 and 100 µM) for 12 h and then exposed to 20 µM Aβ1-42 for 12 h. At the end of the drug treatment, the cells were washed with PBS and lysed in radio immunoprecipitation assay (RIPA) lysis buffer. The mixture was centrifuged at 12,000 × g for 30 min at 4°C. The collected supernatant samples were used for analysis of MDA concentration.
Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay for ROS
The accumulation of intracellular ROS was monitored using the fluorescent dye DCFH-DA as a probe. Briefly, PC12 cells were cultured at a density of 4×105 cells/well in 6-well microplates for 24 h at 37°C. The cells were pretreated with various doses of EVA (0, 10, 30 and 100 µM) for 12 h and then exposed to 20 µM of Aβ1-42 for 12 h. At the end of the drug treatment, 1 ml of 10 µM DCFH-DA solution (part of the ROS kit) was added to the cells and cultured at 37°C for 30 min. In the presence of ROS, DCFH was converted to DCF, as the kit protocol states. Subsequently, the cells were rinsed three times with PBS and the fluorescence intensity was visualized by fluorescence microscopy (Carl Zeiss AG, Oberkochen, Germany). The levels of intracellular ROS of each group were normalized to those of the control group (untreated PC12 cells).
Antioxidant system assay
PC12 cells were cultured at a density of 4×105 cells/well in 6-well microplates for 24 h at 37°C. The cells were pretreated with various doses of EVA (0, 10, 30 and 100 µM) for 12 h and then exposed to 20 µM Aβ1-42 for 12 h consecutive. At the end of the drug treatment, the cells were washed with PBS and lysed in RIPA lysis buffer. The mixture was centrifuged at 12,000 × g for 30 min at 4°C. The collected supernatants were used for the following analyses.
SOD, CAT and GSH-Px activity levels in PC12 cells were measured by commercial assay kits according to the instructions provided by the manufacturer. SOD activity was detected using the xanthine oxidase method at a wavelength of 550 nm using a spectrophotometer. CAT activity was determined using by measuring the absorbance at a wavelength of 405 nm with a spectrophotometer. GSH-Px activity was measured by quantifying the rate of oxidation of reduced glutathione to oxidized glutathione by H2O2 at a wavelength of 412 nm. The activity levels were normalized to the protein concentration of each sample.
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential was detected using the fluorescent JC-1 dye probe. PC12 cells were cultured at a density of 4×105 cells/well in 6-well microplates for 24 h at 37°C. The cells were pretreated with various doses of EVA (0, 10, 30 and 100 µM) for 12 h and then exposed to 20 µM of Aβ1-42 for 12 h. At the end of the drug treatment, the cells were treated with 1 ml of JC-1 for 30 min at room temperature. Fluorescence intensity alterations were determined using flow cytometry (CXP 2.1, Beckman Coulter, Inc., Brea, CA, USA). The data are presented as the ratio of red to green signal. The changes in mitochondrial membrane potential were calculated as the integral of the decrease in the ratio of JC-1 fluorescence.
Immunohistochemistry (IHC) assay
IHC analysis was used to evaluate the levels of cleaved caspase-3, Bax and Bcl-2 proteins. Briefly, PC12 cells were cultured and pretreated with 100 µM of EVA for 12 h at 37°C and subsequently exposed to 20 µM of Aβ1-42 for 12 h at 37°C. At the end of the drug treatment, the cells were fixed in 4% paraformaldehyde for 15 min at 4°C and washed with PBS. Cells were then heated for 10 min in citrate buffer (pH 6.0) for antigen retrieval. The slides were subsequently exposed to 3% H2O2 solution for 25 min and blocked in 5% bovine serum albumin at room temperature for 30 min. Fixed cells were incubated with primary antibodies diluted in PBS (anti-cleaved caspase-3, 1:1,000; anti-Bax, 1:1,000; anti-Bcl-2, 1:500) overnight at 4°C. The samples were washed and treated with secondary antibody (goat anti-rabbit, 1:3,000) diluted in PBS at 37°C for 45 min. DAB was used to produce a dark brown precipitate at 37°C for 30 sec and hematoxylin was used to stain the nuclei of the cells at 37°C for 1 min. The cells were observed and images were captured with a fluorescence microscope (magnification, ×400; Carl Zeiss AG). The ratio of brown to total area was analyzed with Image-Pro Plus in 6 fields of view (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation. The differences between two groups were assessed with one-way analysis of variance followed by Dunnett's post hoc test. Analyses were performed using SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of EVA on Aβ1-42-induced cytotoxicity in PC12 cells
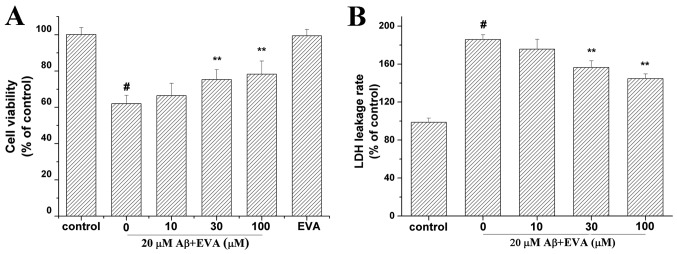
Aβ1-42 can induce both oxidative stress and apoptosis in PC12 cells (13). The viability of Aβ1-42-treated PC12 cells was assessed in the presence of EVA using the MTT assay (Fig. 2A). Cell viability was reduced to 62.07% in PC12 cells treated with 20 µM Aβ1-42 compared with the control group in the absence of Aβ1-42. In contrast to the concentration of 20 µM Aβ1-42 alone, pretreatment with different doses (10, 30 and 100 µM) of EVA increased cell viability to 66.47, 75.38 and 78.29% of the control value, respectively, in a dose-dependent manner. Furthermore, PC12 cells cultured with 100 µM of EVA exhibited a cell viability of 99.49% compared with that noted in the control group in the absence of Aβ1-42 at 24 h after the treatment. This result suggested that 100 µM of EVA alone did not reduce the viability of PC12 cells.
Figure 2.
Protective effect of EVA on the Aβ1-42-inducedcytotoxicity of PC12 cells. Cell viability was measured with (A) MTT and (B) LDH release assays. Data are presented as the percentage of the control group and expressed as the mean ± standard deviation (n=5). #P<0.01 compared with the control group untreated with Aβ1-42 and **P<0.01 compared with the Aβ1-42-treated group. LDH, lactate dehydrogenase; Aβ, β-amyloid; EVA, ethyl vanillin.
The LDH release assay was used to evaluate the effect of treatment with EVA on Aβ1-42-induced cytotoxicity (Fig. 2B). The LDH release rate was increased to 185.84% in PC12 cells incubated with 20 µM of Aβ1-42 compared with the control group untreated with Aβ1-42. Compared with the group treated with 20 µM Aβ1-42 only, pretreatment with different doses (10, 30 and 100 µM) of EVA decreased the LDH release rate to 175.66, 156.43 and 144.67% of the control value, respectively, in a dose-dependent manner.
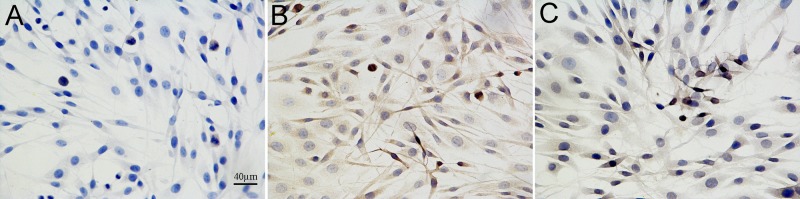
To further investigate the protective effect of EVA on the Aβ1-42-induced PC12 cell damage, the rate of apoptosis of PC12 cells was observed by the TUNEL assay (Fig. 3). The apoptotic cells were characterized by the appearance of intense brown staining. Control PC12 cells were not treated with Aβ1-42 or EVA. Treatment of PC12 cells with 20 µM of Aβ1-42 markedly increased the levels of apoptosis compared with those noted in the control cells. This effect was ameliorated by pretreatment of the cells with 100 µM of EVA. The results indicated that Aβ1-42 inducedPC12 cell injury, and that EVA possessed the ability to inhibit this change induced by Aβ1-42.
Figure 3.
Protective effect of EVA on the degree of apoptosis in Aβ1-42-treated PC12 cells as demonstrated by TUNEL staining (magnification, ×400). Brown nuclei refer to TUNEL-positive apoptotic cells. (A) Control group without Aβ1-42. (B) Group treated with 20 µM Aβ1-42. (C) Group treated with 20 µM Aβ1-42 and 100 µM EVA. TUNEL, terminal deoxynucleotidyl-transferase-mediated dUTP nick end labelling; EVA, ethyl vanillin; Aβ, β-amyloid.
Effect of EVA on oxidative stress induced in PC12 cells
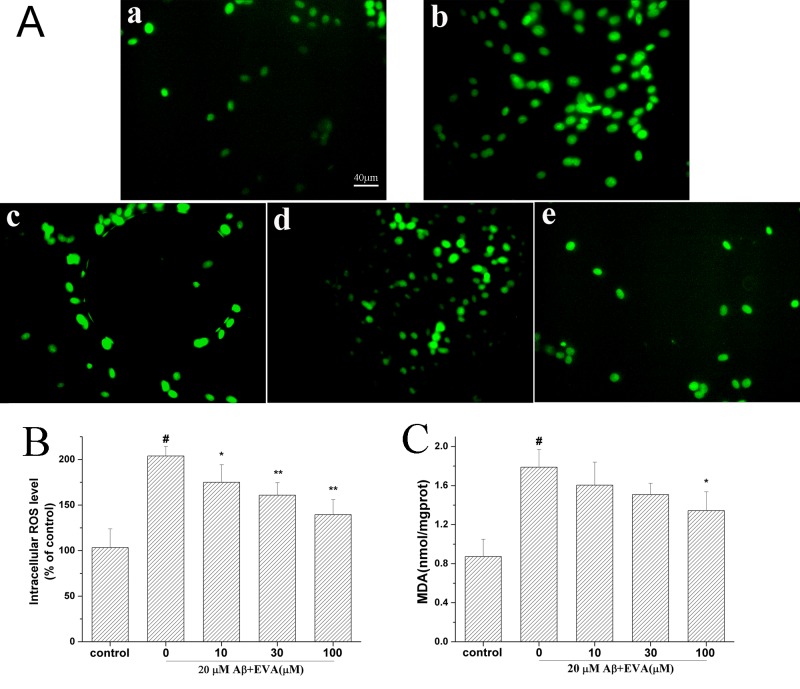
Oxidative stress serves an important role in the induction of PC12 cell apoptosis by Aβ1-42 (13). The current study investigated the effects of EVA on the MDA levels and the levels of intracellular ROS (Fig. 4) in PC12 cells following treatment with Aβ1-42. The production of ROS and the MDA levels were increased to 203.91% and 1.79 nmol/mg (control MDA value, 0.87 nmol/mg), respectively in PC12 cells induced with 20 µM of Aβ1-42, compared with the control group. Pretreatment of Aβ1-42-treated cells with different doses (10, 30 and 100 µM) of EVA decreased the intracellular ROS levels to 174.93, 160.86 and 139.51%, respectively, and the MDA levels to 1.61, 1.51 and 1.34 nmol/mg/protein, respectively, in a dose-dependent manner.
Figure 4.
Protective effect of EVA on oxidative stress in Aβ1-42-treated PC12 cells. Cells were incubated in the absence of Aβ1-42 (control) or in the presence of 20 µM of Aβ1-42 following pretreatment with different concentrations of EVA. (A) ROS production was measured with a fluorescence microscope (magnification, ×200). a, Control group. b, 20 µM Aβ1-42 single treatment. c, 20 µM Aβ1-42+10 µM EVA. d, 20 µM Aβ1-42+30 µM EVA. e, 20 µM Aβ1-42+100 µM EVA. (B) ROS levels were measured using a standard assay. Data are presented as the percentage of the control group and expressed as the mean ± standard deviation (n=3). (C) MDA content was measured with an MDA assay. The results are expressed as the mean ± standard deviation (n=5). #P<0.01 compared with the control group untreated with Aβ1-42. *P<0.05 and **P<0.01 compared with the Aβ1-42-treated group. ROS, reactive oxygen species; EVA, ethyl vanillin; Aβ, β-amyloid; MDA, malondialdehyde.
Effects of EVA on the antioxidative enzyme activities in PC12 cells treated with Aβ1-42
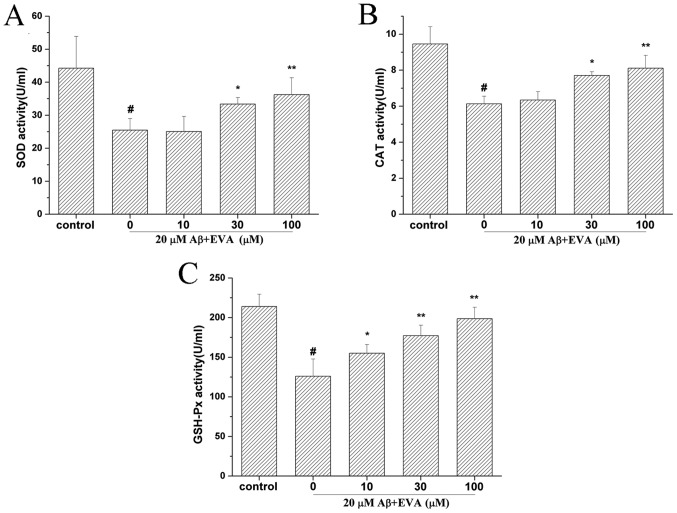
To determine the effect of EVA on the activities of antioxidative enzymes in Aβ1-42-treated PC12 cells, the activity levels of SOD (Fig. 5A), CAT (Fig. 5B) and GSH-Px (Fig. 5C) were detected. The results indicated that treatment with Aβ1-42 reduced the activities of SOD, CAT and GSH-Px to 25.47 U/ml (control SOD value, 44.25 U/ml), 6.13 U/ml (control CAT value, 9.46 U/ml) and 126.01 U/ml (control GSH-Px value, 213.91 U/ml), respectively. However, administration of different doses of EVA (10, 30 and 100 µM) ameliorated the activity levels of SOD (25.07, 33.37 and 36.24 U/ml, respectively), CAT (6.34, 7.71 and 8.11 U/ml, respectively) and GSH-Px (155.13, 177.26 and 198.63 U/ml, respectively). These activity levels of SOD, CAT and GSH-Px following pretreatment with EVA were close to those of the control cells and the protective effect was dose-dependent.
Figure 5.
Protective effects of EVA on the activities of the antioxidative enzymes in Aβ1-42-treated PC12 cells. Cells were incubated in the absence of Aβ1-42, as the control, or in the presence of 20 µM of Aβ1-42 following pretreatment with different concentrations of EVA. Activities of (A) SOD, (B) CAT and (C) GSH-Px were measured using a standard assay. The results are presented as the mean ± standard deviation (n=5). #P<0.01 compared with the control group untreated with Aβ1-42. *P<0.05 and **P<0.01 compared with the Aβ1-42-treated group. EVA, ethyl vanillin; Aβ, β-amyloid; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase.
Effect of EVA on the mitochondrial membrane potential in PC12 cells following treatment with Aβ1-42
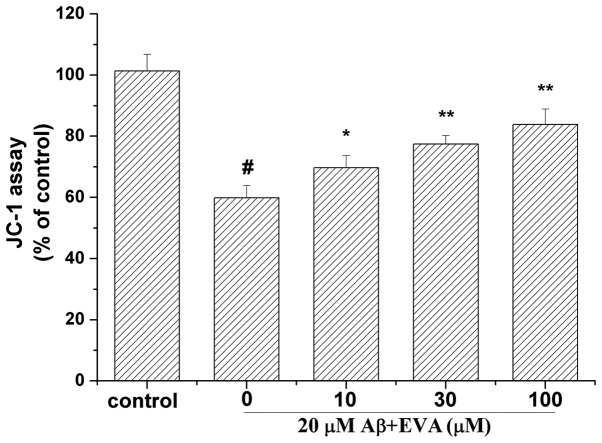
To determine whether EVA could stabilize the mitochondrial function, the mitochondrial membrane potential was measured using the JC-1 assay (Fig. 6). Treatment of PC12 cells with 20 µM Aβ1-42 for 12 h caused a reduction in the mitochondrial membrane potential to 59.91% compared with the control cells. Pretreatment of PC12 cells with different doses of EVA (10, 30 and 100 µM), caused a significant increase in the mitochondrial membrane potential (69.72, 77.45 and 83.85% compared with the control value, respectively) in a dose-dependent manner.
Figure 6.
Protective effect of EVA on depolarization of the mitochondrial membrane potential in Aβ1-42-treated PC12 cells. Cells were incubated in the absence of Aβ1-42, as the control, or in the presence of 20 µM Aβ1-42 following pretreatment with different concentrations of EVA. Mitochondrial membrane potential was measured by JC-1 staining. The results are presented as the mean ± standard deviation (n=5). #P<0.01 compared with the control group untreated with Aβ1-42. *P<0.05 and **P<0.01 compared with the Aβ1-42-treated group. EVA, ethyl vanillin; Aβ, β-amyloid.
Effects of EVA on Aβ1-42-induced apoptotic protein expression in PC12 cells
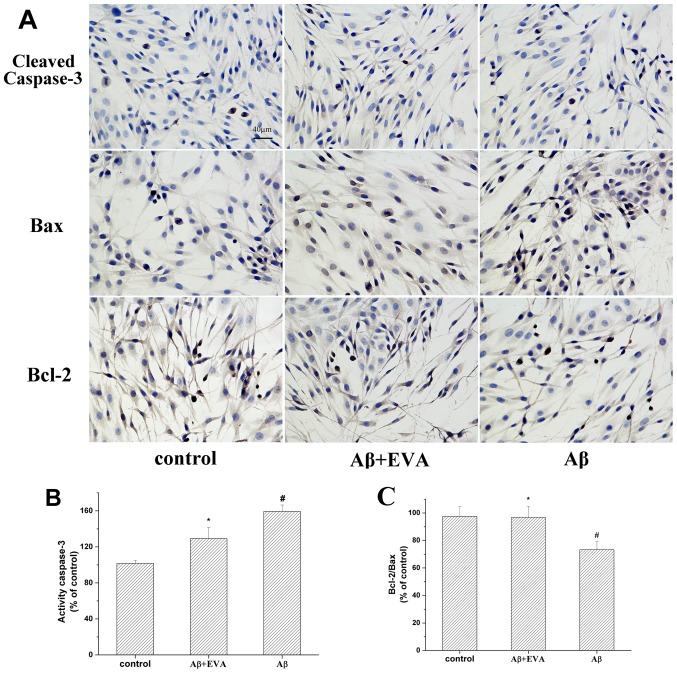
The relative expression levels of Bcl-2/Bax and caspase-3 serve an important role in the induction of cell apoptosis (30). The effects of EVA on the apoptotic proteins in Aβ1-42-treated PC12 cells were determined by the investigation of the expression levels of Bax, Bcl-2 and cleaved-caspase-3 proteins (Fig. 7). The results revealed that treatment with Aβ1-42 reduced the ratio of Bcl-2/Bax to 73.26%. Pretreatment with 100 µM of EVA partially reversed this change in Aβ1-42-treated PC12 cells. Furthermore, treatment with Aβ1-42 increased the levels of cleaved-caspase-3 to 159.44% in PC12 cells compared with those of the normal control cells. Pretreatment with 100 µM of EVA inhibited the increase of the expression levels of cleaved-caspase-3 induced by Aβ1-42. These results suggested that pretreatment with EVA may be effective for the prevention of Aβ1-42-induced cell apoptosis in PC12 cells.
Figure 7.
Protective effect of EVA on the Bcl-2/Bax ratio and caspase-3 activity in PC12 cells. Cells were incubated in the absence of Aβ1-42 or in the presence of 20 µM Aβ1-42 following pretreatment with EVA. (A) Levels of cleaved caspase-3, Bax and Bcl-2 were observed under a microscope (6 fields of view; magnification, ×400). Semiquantitative image analysis of (B) cleaved caspase-3 levels, and (C) Bcl-2/Bax ratio. Data are presented as the percentage of the control group and expressed as the mean ± standard deviation (n=6). #P<0.01 compared with the control group untreated with Aβ1-42. *P<0.05 compared with the Aβ1-42-treated group. EVA, ethyl vanillin; Aβ, β-amyloid; Bax, apoptosis regulator Bax; Bcl-2, apoptosis regulator Bcl-2.
Discussion
EVA has been used as an antioxidant agent for oxidative injury. This compound has been examined for antioxidant properties via in vivo and in vitro studies (21,28,29). The present study investigated the effect of EVA on Aβ1-42-induced PC12 cells, a common in vitro model of AD (31). Although the precise molecular mechanism underlying the Aβ-mediated neuronal apoptosis remains unclear, the majority of the studies indicated that oxidative stress is a hallmark of Aβ-induced neuronal toxicity in AD (11–13,32). The PC12 cell line is a useful model system to study Aβ1-42-induced cytotoxicity due to its particular sensitivity to Aβ peptides (33). Furthermore, PC12 cells are relatively easy to culture and survive longer than primary cultured neurons (33). Therefore, PC12 cells were selected to investigate the neuroprotective effects of EVA against Aβ1-42-induced cell damage. Furthermore, several animal models have been used to determine whether compounds such as Aβ1-42 may protect against AD, including a transgenic animal model and a rat model induced by intrahippocampal injection of Aβ1-42 in the brain (34,35). The main aim addressed by the present study was whether EVA could protect against Aβ1-42-induced injury in PC12 cells and the investigation of the associated mechanism underlying this process.
Aβ is considered an inducer of neuronal damage (5). In the present study, treatment with Aβ1-42resulted in a decrease in the percentage of viable cells. Furthermore, the increase in LDH activity of Aβ1-42-treated PC12 cells implied an increase in the number of damaged plasma membranes and an increase in the overall cell death (36). However, treatment with EVA increased the viability of cells treated withAβ1-42 in a dose-dependent manner. In addition, the morphological characteristics of Aβ1-42-treated PC12 cells were investigated by the TUNEL assay. Aβ1-42-treated PC12 cells that were pretreated with EVA exhibited a decrease in the level of apoptosis. These results indicated that pretreatment with different concentrations of EVA could efficiently prevent cytotoxicity and apoptosis induction by Aβ1-42 in PC12 cells. Additionally, no cellular toxicity was induced by treatment with 100 µM of EVA, which demonstrated good biocompatibility of this compound. Additionally, it was previously reported that EVA alone did not alter the ROS levels and metalloproteinase-9 expression in the LPS-stimulated RAW264.7 macrophage cells (22). Therefore, the group treated with EVA alone was not included in the other experiments in the current study.
Oxidative stress is one of the most important factors that induce cellular injury and apoptosis in several neurodegenerative disorders (17). Excessive levels ROS and lipid peroxidation are markers of oxidative stress (17). Aβ induced cytotoxicity through the overproduction of intracellular ROS following superoxide accumulation in the mitochondria of PC12 cells (37). The levels of MDA, a decomposition product of lipid hydroperoxides, are representative of oxidative damage to the cells and tissues (38). In the present study, EVA reduced the production of ROS and MDA in a dose-dependent manner. This result demonstrated that EVA may induce protective effects on Aβ1-42-treated PC12 cells by reducing the overproduction of ROS.
Excessive production of ROS is associated with oxidative stress, resulting in cellular injury and apoptosis in a wide variety of cell types (13). Under physiological conditions, intracellular ROS levels are accurately regulated by free radical scavengers and by a precise antioxidant defense system (39). The clearance of ROS is achieved with various antioxidants (39,40). The most widely investigated cellular antioxidant system comprises the enzymes SOD, CAT and GSH-Px, which are associated with a direct removal of ROS (41–43). It has been demonstrated that SOD can transform superoxide anions to hydrogen peroxide, which is subsequently scavenged by CAT (41,42). GSH-Px converts lipid hydroperoxides to their corresponding alcohols and free hydrogen peroxide to water (42). Following administration of Aβ1-42, overproduction of ROS led to disturbances in the endogenous antioxidant balance of PC12 cells. According to the current results, enzyme activities of SOD, CAT and GSH-Px were decreased in Aβ1-42-induced PC12 cells. However, EVA could attenuate the activity levels of SOD, CAT and GSH-Px in a dose-dependent manner, which suggested that the protective effect of EVA may be partially involved in its antioxidative effect.
It is generally accepted that ROS is required for the activation of the mitochondria-dependent apoptotic pathways in neurodegenerative diseases (44). Mitochondrial membrane potential is an indicator of mitochondrial dysfunction to cells and tissues (45). Mitochondrial dysfunction is caused by an irreversible depolarization of the mitochondria (30). This process is usually encountered in various neurodegenerative diseases in association with Aβ1-42-induced neuronal toxicity (39). Concomitantly, mitochondrial dysfunction further initiates ROS production in the mitochondria and their subsequent release into the cytoplasm, leading to oxidative stress via activation of apoptotic signaling (46). In the present study, the mitochondrial membrane potential of Aβ1-42-treated PC12 cells was decreased. However, EVA was found to be effective in improving Aβ1-42-induced mitochondrial membrane depolarization in PC12 cells. This result provides evidence that EVA may exert neuroprotective effects by stabilizing the mitochondrial membrane potential.
The decrease in the mitochondrial membrane potential influences the regulation of apoptotic proteins, including the Bcl-2 family proteins (45). Caspase-3, Bax and Bcl-2 proteins regulate the mitochondrial apoptotic pathway (47,48). Bcl-2 is an anti-apoptotic protein, while Bax is a pro-apoptotic protein which can enhance programmed cell death (13). Furthermore, under oxidative stress and mitochondrial membrane depolarization conditions, the decreased Bcl-2/Bax ratio can directly lead to the activation of caspase-3 (19,30). Several studies have suggested that caspase-3 is activated in the hippocampus of AD rats as well as in Aβ1-42-induced PC12 cells (13,14,34). It has been previously demonstrated that activation of caspase-3 is involved in the cytotoxicity and apoptosis of PC12 cells (49). The present study indicated that the ratio of Bcl-2/Bax was significantly decreased in Aβ1-42-induced cells, and that the expression levels of the cleaved form of caspase-3 were significantly increased following Aβ1-42 administration. However, pretreatment with EVA reversed the alterations induced by Aβ1-42. These results suggested that EVA may prevent Aβ1-42-induced cytotoxicity and apoptosis in PC12 cells via regulation of the protein Bcl-2, Bax and caspase-3.
In conclusion, the present study investigated the protective effect of EVA on Aβ1-42-induced neuronal toxicity. EVA protected Aβ1-42-induced PC12 cell damage and this protective effect was mediated by the inhibition of oxidative stress and prevention of cell apoptosis. The present study provides novel data with regard to the effect of EVA and may provide a novel strategy for the treatment of AD. Furthermore, a future study will involve an intrahippocampal injection of Aβ1-42 in the brain of a rat model to verify the appropriate concentration and administration route of EVA in vivo.
Acknowledgements
The authors would like to thank Dr Manda Williams (Department of Bioengineering, University of Washington, Seattle, WA, USA) for language editing.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81603050), the Foundation of Sichuan Provincial People's Hospital (grant no. 2016LY01) and the National Key Specialty Construction Project of Clinical Pharmacy (grant no. 30305030698).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LZ and YZ conceived and designed the current study. YT, LZ and LB collected samples and performed the experiments. YT and JC analyzed the data. LZ and JS drafted the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer's disease: Genes, proteins and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.Epperly T, Dunay MA, Boice JL. Alzheimer disease: Pharmacologic and nonpharmacologic therapies for cognitive and functional symptoms. Am Fam Physician. 2017;95:771–778. [PubMed] [Google Scholar]
- 4.Gao LB, Yu XF, Chen Q, Zhou D. Alzheimer's disease therapeutics: Current and future therapies. Minerva Med. 2016;107:108–113. [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Zheng WH, Bastianetto S, Mennicken F, Ma W, Kar S. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience. 2002;115:201–211. doi: 10.1016/S0306-4522(02)00404-9. [DOI] [PubMed] [Google Scholar]
- 7.Choi SJ, Kim JK, Kim HK, Harris K, Kim CJ, Park GG, Park CS, Shin DH. 2,4-Di-tert-butylphenol from sweet potato protects against oxidative stress in PC12 cells and in mice. J Med Food. 2013;16:977–983. doi: 10.1089/jmf.2012.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. doi: 10.1016/S0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 9.Crack PJ, Cimdins K, Ali U, Hertzog PJ, Iannello RC. Lack of glutathione peroxidase-1 exacerbates Abeta-mediated neurotoxicity in cortical neurons. J Neural Transm (Vienna) 2006;113:645–657. doi: 10.1007/s00702-005-0352-y. [DOI] [PubMed] [Google Scholar]
- 10.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu JF, Chu SF, Ning N, Yuan YH, Xue W, Chen NH, Zhang JT. Protective effect of (−)clausenamide against Abeta-induced neurotoxicity in differentiated PC12 cells. Neurosci Lett. 2010;483:78–82. doi: 10.1016/j.neulet.2010.07.067. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Ma R, Huang C, Tang Q, Fu Q, Liu H, Hu B, Xiang J. Protective effect of erythropoietin on beta-amyloid-induced PC12 cell death through antioxidant mechanisms. Neurosci Lett. 2008;442:143–147. doi: 10.1016/j.neulet.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Xian YF, Lin ZX, Mao QQ, Ip SP, Su ZR, Lai XP. Protective effect of isorhynchophylline against beta-amyloid-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32:353–360. doi: 10.1007/s10571-012-9847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turunc Bayrakdar E, Uyanikgil Y, Kanit L, Koylu E, Yalcin A. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Aβ(1–42)-induced rat model of Alzheimer's disease. Free Radical Res. 2014;48:146–158. doi: 10.3109/10715762.2013.857018. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y, Dong Y, Tucker D, Wang R, Ahmed ME, Brann D, Zhang Q. Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer's disease. J Alzheimers Dis. 2017;56:1469–1484. doi: 10.3233/JAD-160869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deguchi K, Hayashi T, Nagotani S, Sehara Y, Zhang H, Tsuchiya A, Ohta Y, Tomiyama K, Morimoto N, Miyazaki M, et al. Reduction of cerebral infarction in rats by biliverdin associated with amelioration of oxidative stress. Brain Res. 2008;1188:1–8. doi: 10.1016/j.brainres.2007.07.104. [DOI] [PubMed] [Google Scholar]
- 17.Dumont M, Beal MF. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radical Biol Med. 2011;51:1014–1026. doi: 10.1016/j.freeradbiomed.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calabrese V, Guagliano E, Sapienza M, Panebianco M, Calafato S, Puleo E, Pennisi G, Mancuso C, Butterfield DA, Stella AG. Redox regulation of cellular stress response in aging and neurodegenerative disorders: Role of vitagenes. Neurochem Res. 2007;32:757–773. doi: 10.1007/s11064-006-9203-y. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Ji X, Zhang J, Shi G, Zhu X, Wang K. Paeoniflorin attenuates Aβ25-35-induced neurotoxicity in PC12 cells by preventing mitochondrial dysfunction. Folia Neuropathol. 2014;52:285–290. doi: 10.5114/fn.2014.45569. [DOI] [PubMed] [Google Scholar]
- 20.Wu SL, Chen JC, Li CC, Lo HY, Ho TY, Hsiang CY. Vanillin improves and prevents trinitrobenzene sulfonic acid-induced colitis in mice. J Pharmacol Exp Ther. 2009;330:370–376. doi: 10.1124/jpet.109.152835. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Lee HO, Cho YJ, Kim J, Chun J, Choi J, Lee Y, Jung WH. A vanillin derivative causes mitochondrial dysfunction and triggers oxidative stress in Cryptococcus neoformans. PLoS One. 2014;9:e89122. doi: 10.1371/journal.pone.0089122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung HJ, Song YS, Kim K, Lim CJ, Park EH. Assessment of the anti-angiogenic, anti-inflammatory and antinociceptive properties of ethyl vanillin. Arch Pharmacal Res. 2010;33:309–316. doi: 10.1007/s12272-010-0217-2. [DOI] [PubMed] [Google Scholar]
- 23.Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozuka Y, Singhirunnusorn P, Ruchirawat S, Svasti J, Saiki I. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005;25:57–65. doi: 10.1016/j.ejps.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Makni M, Chtourou Y, Fetoui H, Garoui el EM, Boudawara T, Zeghal N. Evaluation of the antioxidant, anti-inflammatory and hepatoprotective properties of vanillin in carbon tetrachloride-treated rats. Eur J Pharmacol. 2011;668:133–139. doi: 10.1016/j.ejphar.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Tai A, Sawano T, Yazama F, Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim Biophys Acta. 2011;1810:170–177. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Kwon J, Kim J, Park S, Khang G, Kang PM, Lee D. Inflammation-responsive antioxidant nanoparticles based on a polymeric prodrug of vanillin. Biomacromolecules. 2013;14:1618–1626. doi: 10.1021/bm400256h. [DOI] [PubMed] [Google Scholar]
- 27.Dhanalakshmi C, Janakiraman U, Manivasagam T, Justin Thenmozhi A, Essa MM, Kalandar A, Khan MA, Guillemin GJ. Vanillin attenuated behavioural impairments, neurochemical deficts, oxidative stress and apoptosis against rotenone induced rat model of Parkinson's disease. Neurochem Res. 2016;41:1899–1910. doi: 10.1007/s11064-016-1901-5. [DOI] [PubMed] [Google Scholar]
- 28.Tai A, Sawano T, Yazama F. Antioxidant properties of ethyl vanillin in vitro and in vivo. Biosci Biotechnol Biochem. 2011;75:2346–2350. doi: 10.1271/bbb.110524. [DOI] [PubMed] [Google Scholar]
- 29.Chen XM, Wei M, Zhang HM, Luo CH, Chen YK, Chen Y. Effect of vanillin and ethyl vanillin on cytochrome P450 activity in vitro and in vivo. Food Chem Toxicol. 2012;50:1897–1901. doi: 10.1016/j.fct.2012.03.060. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Sun X, Gong T, He Q, Zhang Z. antioxidant and antiapoptotic effects of 1,1,1anillin on cytochrome P450 activity in vitro and in1-one on protecting PC12 cells from Aβ-induced injury. Mol Pharmaceutics. 2013;11:428–435. doi: 10.1021/mp400395g. [DOI] [PubMed] [Google Scholar]
- 31.Kinarivala N, Shah K, Abbruscato TJ, Trippier PC. Passage variation of PC12 cells results in inconsistent susceptibility to externally induced apoptosis. ACS Chem Neurosci. 2017;8:82–88. doi: 10.1021/acschemneuro.6b00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldsbury C, Whiteman IT, Jeong EV, Lim YA. Oxidative stress increases levels of endogenous amyloid-β peptides secreted from primary chick brain neurons. Aging Cell. 2008;7:771–775. doi: 10.1111/j.1474-9726.2008.00423.x. [DOI] [PubMed] [Google Scholar]
- 33.Harper JD, Lansbury PT., Jr Models of amyloid seeding in Alzheimer's disease and scrapie: Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 34.Jantaratnotai N, Ryu JK, Schwab C, McGeer PL, McLarnon JG. Comparison of vascular perturbations in an Aβ-injected animal model and in AD brain. Int J Alzheimers Dis 2011. 2011 doi: 10.4061/2011/918280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beal MF. Oxidatively modified proteins in aging and disease. Free Radical Biol Med. 2002;32:797–803. doi: 10.1016/S0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 37.Naoi M, Maruyama W, Yi H, Inaba K, Akao Y, Shamoto-Nagai M. Mitochondria in neurodegenerative disorders: Regulation of the redox state and death signaling leading to neuronal death and survival. J Neural Transm (Vienna) 2009;116:1371–1381. doi: 10.1007/s00702-009-0309-7. [DOI] [PubMed] [Google Scholar]
- 38.Benedí J, Arroyo R, Romero C, Martín-Aragón S, Villar AM. Antioxidant properties and protective effects of a standardized extract of Hypericum perforatum on hydrogen peroxide-induced oxidative damage in PC12 cells. Life Sci. 2004;75:1263–1276. doi: 10.1016/j.lfs.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signaling. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong Y, Chuan J, Bai L, Shi J, Zhong L, Duan X, Zhu Y. The protective effect of shikonin on renal tubular epithelial cell injury induced by high glucose. Biomed Pharmacother. 2018;98:701–708. doi: 10.1016/j.biopha.2017.12.112. [DOI] [PubMed] [Google Scholar]
- 41.Xiao X, Liu J, Hu J, Zhu X, Yang H, Wang C, Zhang Y. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca(2+) antagonism and antioxidant mechanisms. Eur J Pharmacol. 2008;591:21–27. doi: 10.1016/j.ejphar.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 42.Mueller SG, Trabesinger AH, Boesiger P, Wieser HG. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology. 2001;57:1422–1427. doi: 10.1212/wnl.57.8.1422. [DOI] [PubMed] [Google Scholar]
- 43.Ghanta S, Banerjee A, Poddar A, Chattopadhyay S. Oxidative DNA damage preventive activity and antioxidant potential of Stevia rebaudiana (Bertoni) Bertoni, a natural sweetener. J Agric Food Chem. 2007;55:10962–10967. doi: 10.1021/jf071892q. [DOI] [PubMed] [Google Scholar]
- 44.Lee IK, Kang KA, Zhang R, Kim BJ, Kang SS, Hyun JW. Mitochondria protection of baicalein against oxidative damage via induction of manganese superoxide dismutase. Environ Toxicol Pharmacol. 2011;31:233–241. doi: 10.1016/j.etap.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Chen JX, Yan SD. Pathogenic role of [correction of mitochondral] amyloid-beta peptide. Expert Rev Neurother. 2007;7:1517–1525. doi: 10.1586/14737175.7.11.1517. [DOI] [PubMed] [Google Scholar]
- 46.Iijima T, Mishima T, Tohyama M, Akagawa K, Iwao Y. Mitochondrial membrane potential and intracellular ATP content after transient experimental ischemia in the cultured hippocampal neuron. Neurochem Int. 2003;43:263–269. doi: 10.1016/S0197-0186(02)00228-0. [DOI] [PubMed] [Google Scholar]
- 47.Peng HY, Du JR, Zhang GY, Kuang X, Liu YX, Qian ZM, Wang CY. Neuroprotective effect of Z-ligustilide against permanent focal ischemic damage in rats. Biol Pharm Bull. 2007;30:309–312. doi: 10.1248/bpb.30.309. [DOI] [PubMed] [Google Scholar]
- 48.Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 49.Tong Y, Bai L, Gong R, Chuan J, Duan X, Zhu Y. Shikonin protects PC12 cells against β-amyloid peptide-induced cell injury through antioxidant and antiapoptotic activities. Sci Rep. 2018;8:26. doi: 10.1038/s41598-017-18058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.