Abstract
Background
The FAbry STabilization indEX (FASTEX) is an innovative index allowing the assessment of clinical stability over time in Fabry disease patients. This index was developed in a population of 28 male patients with the classical form of Fabry disease.
Objectives
The aim of the study was to test the accuracy of the FASTEX in evaluating Fabry disease stability in 132 male and female patients with classical and non-classical Fabry disease from nine Italian centres and it also aimed to define the sensitivity and specificity of this new tool. In particular, we aimed to investigate the correlation between the FASTEX and clinical judgement in a large-scale cohort of the study population.
Methods
Statistical methods applied to this investigation included the calculation of accuracy, specificity and sensitivity, receiver operating characteristic (ROC) curves and Cohen’s κ index related to the FASTEX and clinical judgement.
Results
The patient population included 58 males (43.9%). The mean age of the overall population was 46.3 ± 15. 1 years (range 31.2–61.4). The median interval between the two multidisciplinary evaluations used for FASTEX calculation was 398 days. Since no gold standard method is available to define the overall clinical condition of Fabry patients over time, the results of the FASTEX were compared with clinical judgements given by the physicians involved in this study. In this way, the FASTEX classified 121 of 132 (92%) patients correctly. In particular, the FASTEX correctly identified 93% (41/44) of clinically ‘unstable’ and 91% (80/88) of clinically ‘stable’ patients. The area under the curve of the ROC related to the FASTEX index cut-off (20) was equal to 0.967, very close to its theoretical maximum (1), which means that it is an excellent test for classifying patients as ‘stable’ or ‘unstable’ compared with clinical judgement. In addition, the FASTEX cut-off >20 provides the most acceptable balance between sensitivity and specificity. The Cohen’s κ index value obtained in our study was 0.82, showing a highly statistically significant P-value < 0.01 related to the agreement between the FASTEX and clinical judgement.
Conclusions
The FASTEX is demonstrated here to be a specific and sensitive tool. When applied to a large cohort of Fabry patients, it was shown to be a valid instrument in helping physicians to discriminate objectively the clinical stability of individual Fabry patients.
Keywords: α-galactosidase A/α-galactosidase A deficiency, disease progression, disease stability, Fabry disease, organ dysfunction scores
INTRODUCTION
Fabry disease is a lysosomal storage disorder caused by the absence or reduced activity of the enzyme α-galactosidase A (αGal A) encoded by the GLA gene located on the X-chromosome locus Xq21.3-q22. This deficiency leads to the accumulation of glycosphingolipids, predominately globotriaosylceramide, in various cell types and organs [1, 2], leading to multisystemic clinical manifestations, including skin lesions, peripheral neuropathy, stroke, cardiomyopathy and renal failure [2]. Clinical signs and symptoms may appear in early childhood, with the earliest manifestations, such as painful peripheral acroparesthesia and angiokeratomas, occurring in the classical form of Fabry disease [3]. With age, progressive damage to affected tissues leads to organ failure [2]. End-stage renal disease and life-threatening cardiovascular or cerebrovascular complications significantly reduce life expectancy [4, 5]. Some male patients may have less-severe outcomes with a predominant organ involvement appearing as they reach adulthood (late-onset variant). Female patients with Fabry disease may develop clinical signs that range in intensity from a complete asymptomatic condition to severe clinical involvement resembling the disease manifestations of male patients with classical Fabry disease [6–9]. This clinical heterogeneity is partly explained by the random X-chromosome inactivation (lyonization) [10]. Recently a new clinical subdivision (‘classical’ and ‘non-classical’) has been proposed to describe the pleomorphism of the disease [11]. Enzyme replacement therapy (ERT) is available since 2001 and, in the absence of a clear marker, clinicians need an index to assess the therapeutic results. For this reason, in 2016, Mignani et al. [12] developed a new index called the FAbry STabilization indEX (FASTEX) that is specifically designed to monitor disease progression/stability in Fabry patients. The main feature of this index, which differs from other currently used disease severity scores, is the quantification of the clinical stability or disease progression between two consecutive multidisciplinary evaluations through analysis of a few specific parameters. In the previous FASTEX publication, which described the statistical methodology adopted for the development of the index, 28 patients were tested, leading to the identification of a cut-off value of 20% to discriminate between stable and unstable patients [12]. In the present study, we evaluate the accuracy, sensitivity and specificity of the FASTEX in a large multicentre study of patients affected by Fabry disease.
MATERIALS AND METHODS
Patient population
The patient population included 132 Fabry patients from nine Italian referral centres with complete records of yearly multidisciplinary evaluation and with the most recent follow-up visit having been performed between January 2017 and June 2017. Both ERT-treated and untreated patients were included in the analysis. Treatment with supportive therapies, including blood pressure–lowering drugs, antiarrhythmic drugs, Angiotensin-converting-enzyme inhibitor, angiotensin-receptor blockers, anticoagulants and analgesic drugs, did not represent an exclusion criterion. The clinical criteria used to define patients as stable or unstable refer to a worsening of parameters that are well accepted by the international community for each organ (for instance, proteinuria, left ventricular mass, new white matter lesions etc.) [13–15]. In order to homogenize the clinical assessment of all Fabry patients, a recent minireview published in 2018 by Ortiz et al. [16] was used as the main guidelines to be taken into account by all treating physicians. For all patients, signed informed consent for the analysis of their clinical records was obtained.
Study protocol and FASTEX assessment
Multiple parameters required to calculate the FASTEX were provided for each patient by participating centres. For each patient, data obtained at the latest follow-up multidisciplinary evaluation and at the previous evaluation performed at least 6–12 months before were provided. In addition, the investigators from each centre provided their clinical judgement on disease stability, classifying each enrolled patient as clinically ‘stable’ or ‘unstable’, blinded to the FASTEX value. Indeed, the investigators were unable to independently calculate the FASTEX to guide their clinical judgement since Ibis Informatica calculated the FASTEX independently.
Statistical analysis
Analysis of data and FASTEX index calculation for each patient were performed by Ibis Informatica, an independent statistical advisor based in Milan, Italy, blinded to clinical judgement. Subsequently Ibis Informatica performed the statistical analysis, matching calculated FASTEX values with the clinical judgement previously provided by the clinical investigators.
Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and charts were drawn using Excel 2016 (Microsoft, Redmond, WA, USA).
From a clinical point of view, patients were defined as stable if the levels of organ involvement and symptoms were not significantly changed at the second visit compared with the previous visit. In contrast, patients were considered unstable at the second visit if there was single or multiorgan involvement that deteriorated the global condition of the patient.
Correlation between clinical judgement and FASTEX values was assessed. Accuracy, sensitivity, specificity and unstable and stable predictive values were calculated based on the correlation between clinical judgement and the FASTEX. ‘Accuracy’ indicates the percentage of patients correctly classified as stable or unstable using the FASTEX index. ‘Sensitivity’ was defined as the proportion of patients defined as ‘unstable’ according to clinical judgement and who were correctly classified as ‘unstable’ by the FASTEX. ‘Specificity’ was defined as the proportion of patients defined as ‘stable’ according to clinical judgement who were correctly classified as ‘stable’ by the FASTEX. ‘Unstable’ predictive value was defined as the probability that a patient classified as ‘unstable’ according to clinical judgement was correctly classified as ‘unstable’ by the FASTEX. ‘Stable’ predictive value was defined as the probability that a patient classified as ‘stable’ according to clinical judgement was correctly classified as ‘stable’ by the FASTEX. Moreover, two statistical tests were also evaluated: the receiver operating characteristics (ROC) curve and the Cohen’s κ index.
The ROC is often used to describe the discriminative abilities of different tests or raters or may be used to explore the trade-offs between sensitivity and specificity for a test. An advantage of ROC analysis over traditional sensitivity and specificity analyses is that the ROC’s area under the curve (AUC) is independent both from the cut-off point criterion chosen (a threshold of 20% for the FASTEX) and the prevalence of the outcome of interest. In particular, the ROC is constructed from a set of (x, y) points, where x is the proportion of false positives (1 − specificity) and y is the proportion of true positive results (sensitivity). The x and y axes of the ROC plot both range from 0 to 1. The typical curve will be convex and located above the diagonal from (0, 0) and (1, 1), the ‘chance line’. An ideal curve, representing an effective operator or test, will pass close to the point with coordinates (0, 1), whereas a curve near the diagonal represents an operator or test that is a poor predictor of the true outcome. The AUC is the most commonly used quantitative index used to describe the ROC, ranging from 0 (worst predictor) to 1 (best predictor). The ROC in this clinical research was used to assess the specificity and sensitivity of the FASTEX to predict clinical judgement classification as ‘stable’ or unstable’.
The authors declare that all procedures followed were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients included in the study.
Cohen’s κ measures the agreement between two tests (in this case the FASTEX versus clinical judgement). Cohen’s κ ranges between 0 (no agreement at all) and 1 (perfect agreement).
RESULTS
Characteristics of the patient population are summarized in Figures 1 and 2. The patient population included 58 males (43.9%). The mean age of the overall population was 46.3 ± 15.1 years (range 31.2–61.4), with a mean age of 45.8 in males (range 14–77) and 46.8 in females (range 17–82) (Figure 1). Figure 2 reports the results of the FASTEX according to age and gender across the whole patient population.
FIGURE 1.
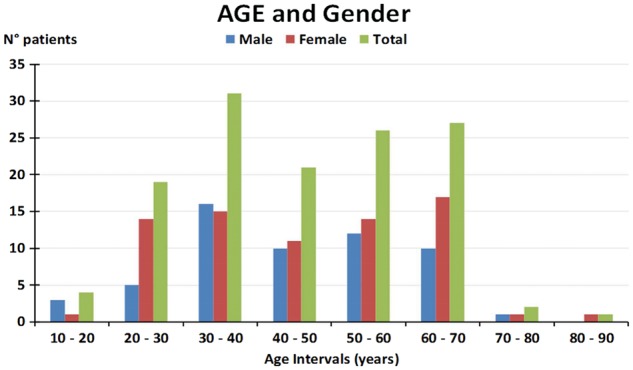
Age and gender population stratification.
FIGURE 2.
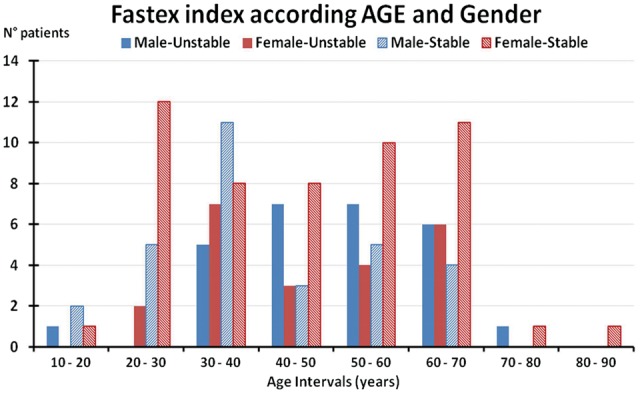
FASTEX related to patients’ age and gender stratification. Stable: FASTEX index <20% Unstable: FASTEX index ≥20%.
All patients involved in this clinical research have been classified as classical phenotype, late-onset phenotype and ‘others’, where ‘others’ means patients carrying a genetic variant of unknown significance (Table 1). The classification of patients as classical, late-onset phenotype or others was based on the genotyping reported in the Fabry database (www.fabry-database.org).
Table 1.
Population phenotype
| Phenotype | n (%) | FASTEX <20% | FASTEX ≥20% |
|---|---|---|---|
| Classical | 101 (76.5) | 53 | 48 |
| Late onset | 19 (14.4) | 14 | 5 |
| Other | 12 (9.1) | 8 | 4 |
| Total | 132 (100) | 75 | 57 |
Classical phenotypes were observed in 76.5% of patients, with 14.4% presenting the late-onset variant of the disease. Ninety-nine patients were under treatment with ERT (58 patients treated with agalsidase α 0.2 mg/kg of body weight every other week and 38 patients treated with agalsidase β 1.0 mg/kg of body weight every other week), 3 switched patients were in treatment with oral chaperone migalastat (123 mg migalastat once every other day) and 33 patients were untreated (Table 2). The FASTEX was calculated at the second visit for all patients regardless of what treatment was being received. As reported in Table 2, within the untreated patient population (n = 28), 10 patients showed a FASTEX ≥20%. Within the patient population treated with agalsidase α at the second visit (n = 54), 23 patients (43%) reported a FASTEX ≥20%. Regarding the patient population treated with agalsidase β (n = 47) at the second visit, 16 patients (34%) reported a FASTEX index ≥20%. In this clinical research, three patients were receiving migalastat, none of which reported a FASTEX ≥20% at the second visit. The median interval between the two multidisciplinary evaluations used for FASTEX calculation for all patients involved was 398 days.
Table 2.
Population treatment
| Treatment | First visit, n (%) | Second visit, n (%) | FASTEX ≥20% at second visit, n (%) | ||
|---|---|---|---|---|---|
| Untreated (no ERT; no chaperone treatment) | 33 (25) | 28 (21.2) | 10 (36) | ||
| Agalsidase α | 58 (43.9) | 54 (40.9) | 23 (43) | ||
| Agalsidase β | 38 (28.8) | 47 (35.6) | 16 (34) | ||
| Migalastat | 3 (2.3) | 3 (2.3) | 0 (0) | ||
| Total | 132 (100) | 132 (100) | 49 | ||
From a clinical point of view, the treating physicians judged 88 patients as stable and 44 patients as unstable; the FASTEX correctly classified 121 of 132 (92%) patients. In particular, the FASTEX correctly identified 93% (41/44) of patients classified as unstable by clinical judgement and 91% (80/88) of patients classified as stable by clinical judgement (Table 3). The unstable predictive value of the FASTEX was 0.84 (41/49) while the stable predictive value was 0.96 (80/83) (Table 4). The AUC of the ROC related to the FASTEX cut-off (20) was equal to 0.967, very close to its theoretical maximum (1), which means that it is an excellent test for categorizing patients as stable and unstable according to clinical judgement (Figure 3). In addition, the FASTEX cut-off >20 provides the most acceptable balance between sensitivity and specificity. The Cohen’s κ value obtained in our study was 0.82, showing high statistical significance (P < 0.01) related to agreement between the FASTEX and clinical judgement (Table 5).
Table 3.
FASTEX versus clinical judgement
| Clinical judgement |
|||
|---|---|---|---|
| FASTEX index | Unstable, n (%) | Stable, n (%) | Total, n |
| ≥20 | 41 (93) | 8 | 49 |
| <20 | 3 | 80 (91) | 83 |
| Total | 44 (100) | 88 (100) | 132 |
Table 4.
Accuracy, sensitivity, specificity and predictive values
| Statistical values | Value (95% confidence interval) |
|---|---|
| Accuracy | 91.7 (85.6–95.8) |
| Sensitivity | 93.2 (81.3–98.6) |
| Specificity | 90.9 (82.9–96.0) |
| ‘Unstable’ predictive value | 83.7 (70.3–92.7) |
| ‘Stable’ predictive value | 96.4 (89.8–99.2) |
FIGURE 3.
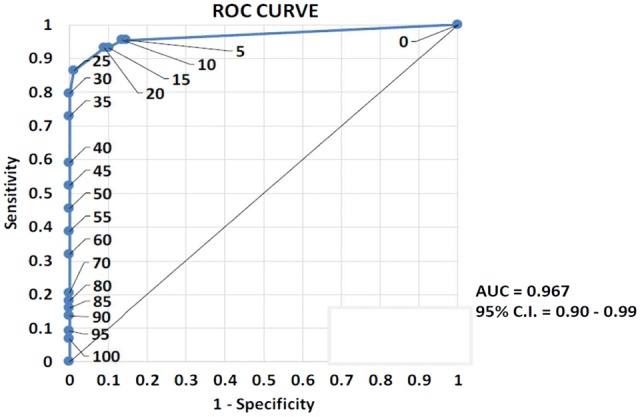
ROC. The AUC of the ROC curve related to the FASTEX index cut-off (20) is equal to 0.967, very close to its theoretical maximum (1), which means that it is an excellent test for discriminating between ‘stable’ and ‘unstable’ patients.
Table 5.
Cohen’s κ index value
| Cohen’s κ index | |||
|---|---|---|---|
| Value (95% confidence interval) | Probability | Significance | |
| 0.82 (0.72–0.92) | < 0.0001 | P < 0.01 | |
DISCUSSION
Fabry disease is a chronic storage disease characterized by progressive organ damage and major clinical events. ERT has been demonstrated to be effective in preventing major complications and in slowing disease progression, mostly when administered early in the disease course. Early diagnosis of organ damage and early treatment are essential for optimal management. On the other hand, multidisciplinary follow-up and periodic assessment of target organ damage is mandatory to optimize treatment. In fact, there is growing evidence that the therapeutic approach must be optimized in terms of ERT timing and dosage and in terms of additional therapies, according to genotype, metabolic profile and the rate of disease progression. Thus, defining disease stabilization or progression is mandatory to optimize Fabry patients’ management. However, from our point of view, no biomarker or clinical score has so far been demonstrated to be reliable enough for monitoring of the disease’s course [12, 17, 18]. This also happens because the changes of indices, such as the reduction of estimated glomerular filtration rate or left ventricular hypertrophy, which are commonly used to assess patients, occur well after tissue injury has occurred. Therefore clinical laboratory data need to be collected to monitor patient’s outcomes.
We recently introduced the new FASTEX in a small patient population in order to quantify disease progression and define clinical stability. In that study we hypothesized a possible cut-off of the stability value of <20% [12]. However, this study enrolled a limited number of patients and it was therefore mandatory for us to validate the FASTEX in a larger cohort of patients.
In the present study we have demonstrated the high accuracy of the FASTEX in defining disease stability or progression when compared with the treating physician’s clinical judgement in a larger and heterogeneous population of Fabry disease patients. The cut-off of 20% was revealed to be effective in distinguishing stable and unstable patients with very high specificity and sensitivity (91% and 93%, respectively). In addition, the ROC was shown to be very close to a value of 1, further confirming that 20% can be considered to be a realistic cut-off to discriminate between ‘stable’ and ‘unstable’ patients. These results are also confirmed by the Cohen’s κ value of 0.82, which shows high statistically significant (P < 0.01) agreement between the FASTEX and clinical judgement. In this larger study, the value of the FASTEX was confirmed, notwithstanding an increase of heterogeneity of the data due to the number of different participating centres with different medical specialties and the non-uniform approach of the disease.
Moreover, given the heterogeneity of the study population, the performance of the FASTEX in both males and females with classical and non-classical disease and in both early and advanced disease was excellent. The reliability of the FASTEX was also confirmed in treated and untreated patients. The main advantage of the application of the FASTEX in routine clinical practice refers to the possibility of all physicians treating Fabry patients, particularly those in peripheral centres, having a standardized way to monitor the condition of Fabry patients, enabling the early detection of disease progression as well the assessment of disease stability or progression through periodic follow-up, particularly in patients with advanced organ involvement at baseline.
Finally, the need for indices for assessment of the stability and progression of Fabry disease was noted in a recent publication of the European expert consensus statement on therapy in Fabry disease [19], where maintenance of the clinical stability of several parameters (pain, left ventricular hypertrophy, proteinuria, GFR etc.) was listed as an important therapeutic goal for research on Fabry disease.
Limitations of the study
Since there are, to our knowledge, no other publications in the literature that evaluate the clinical stability of Fabry patients, one of the limitations of this study could be the lack of data for comparison with our results. More comparative studies involving clinical judgement are required to better evaluate the potential of this innovative tool in routine clinical practice. Clinical judgement is currently the only method of evaluating the overall clinical condition of Fabry patients. However, the main bias of this approach is the subjectivity of clinical judgement among physicians treating Fabry patients. The FASTEX aims to standardize clinical evaluation and thus avoid any subjective judgement.
Another limitation is that the study is retrospective; therefore our findings did not allow us to determine whether the FASTEX may also represent a prognostic stratification tool. Prospective studies may provide new insights into the prognostic value of disease stability versus progression as assessed by the FASTEX.
In the present study, a possible correlation between the FASTEX and changes in globotriaosylsphingosine was not evaluated, as this parameter was not available in a sufficient number of cases.
Finally, the FASTEX was not compared with currently available and used indices (Mainz Severity Score Index, Fabry Disease Severity Scoring System) with respect to their association with clinical judgement. Thus it is unknown whether one of the three indices (and if so which) is superior in this respect.
CONCLUSIONS
In a large and heterogeneous population of patients with Fabry disease, we confirmed that the FASTEX is a reliable index for the assessment of disease progression or stability. Clinical application of the FASTEX aims to optimize the management of Fabry patients who are either untreated or are treated with specific therapies like ERT or chaperone therapies.
FUNDING
The publication was developed through financial support provided by Public Healthcare Institutions.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Desnick RJ, Ioannou YA, Eng CM.. α-Galactosidase A deficiency: Fabry disease In: Scriver CR, Beaudet AL, Sly WS. et al. (eds). The Metabolic and Molecular Bases of Inherited Disease, 8th edn, Vol. 3 New York: McGraw-Hill, 2001, 3733–3774 [Google Scholar]
- 2. Germain DP. Fabry disease. Orphanet J Rare Dis 2010; 5: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laney DA, Peck DS, Atherton AM. et al. Fabry disease in infancy and early childhood: a systematic literature review. Genet Med 2015; 17: 323–330 [DOI] [PubMed] [Google Scholar]
- 4. Mehta A, Clarke JT, Giugliani R. et al. Natural course of Fabry disease: changing pattern of causes of death in FOS - Fabry Outcome Survey. J Med Genet 2009; 46: 548−552 [DOI] [PubMed] [Google Scholar]
- 5. Waldek S, Patel MR, Banikazemi M. et al. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry Registry. Genet Med 2009; 11: 790−796 [DOI] [PubMed] [Google Scholar]
- 6. Nakao S, Takenaka T, Maeda M. et al. An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med 1995; 333: 288–293 [DOI] [PubMed] [Google Scholar]
- 7. Sachdev B, Takenaka T, Teraguchi H. et al. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation 2002; 105: 1407–1411 [DOI] [PubMed] [Google Scholar]
- 8. Wilcox WR, Oliveira JP, Hopkin RJ. et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab 2008; 93: 112−128 [DOI] [PubMed] [Google Scholar]
- 9. Deegan PB, Baehner AF, Barba Romero MA. et al. Natural history of Fabry disease in females in the Fabry Outcome Survey. J Med Genet 2006; 43: 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lyon M. Gene activation in the X-chromosome of the mouse. Nature 1961; 190: 372−373 [DOI] [PubMed] [Google Scholar]
- 11. Arends M, Wanner C, Hughes D. et al. Characterization of classical and nonclassical Fabry disease: a multicenter study. J Am Soc Nephrol 2017; 28: 1631–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mignani R, Pieruzzi F, Berri F. et al. FAbry STabilization indEX (FASTEX): an innovative tool for the assessment of clinical stabilization in Fabry disease. Clin Kidney J 2016; 9: 739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD). Kidney Int Suppl 2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliot PM, Anastasakis A, Borger MA et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014; 35: 2733–2779 [DOI] [PubMed] [Google Scholar]
- 15. Wintermark M. Imaging recommendations for acute stroke and transient ischemic attack patients: a joint statement by the American Society of Neuroradiology, the American College of Radiology and the Society of NeuroInterventional Surgery. Am J Neuroradiol 2013; 34: E117–E127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ortiz A, Germain D, Desnick RJ. et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Gent Metab 2018; 123:416–427 [DOI] [PubMed] [Google Scholar]
- 17. Giannini EH, Mehta AB, Hilz MJ. et al. A validated disease severity scoring system for Fabry disease. Mol Genet Metab 2009; 99: 289−290 [DOI] [PubMed] [Google Scholar]
- 18. Whybra C, Kampmann C, Krummenauer F. et al. The Mainz Severity Score Index: a new instrument for quantifying the Anderson–Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet 2004; 65: 299−307 [DOI] [PubMed] [Google Scholar]
- 19. Wanner C, Arad M, Baron R. et al. European expert consensus statement on therapeutic goals in Fabry disease. Mol Genet Metab 2018; 124: 189–203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


