Abstract
BACKGROUND
Despite the multitude of available treatments, glioblastoma (GBM) remains an aggressive and uniformly fatal tumor. Laser interstitial thermal therapy (LITT) is a novel, minimally invasive treatment that holds promise for treating patients with GBM who are not candidates for traditional open craniotomy. However, due to the recent introduction of LITT into clinical practice, large series that evaluate safety and long-term outcomes after LITT are lacking.
OBJECTIVE
To present our institution's series of over 50 GBM patients treated with LITT, with regard to safety, efficacy, and outcomes.
METHODS
We performed a retrospective descriptive study of patients with histologically proven GBM who underwent LITT. Data collected included demographics, tumor location and volume, tumor genetic markers, treatment volume, perioperative complications, and long-term follow-up data.
RESULTS
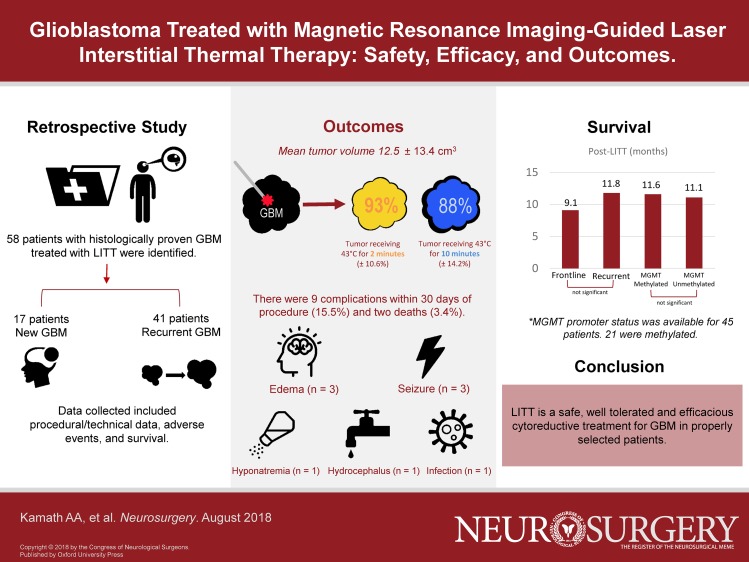
We performed 58 LITT treatments for GBM in 54 patients over 5.5 yr. Forty-one were recurrent tumors while 17 were frontline treatments. Forty GBMs were lobar in location, while 18 were in deep structures (thalamus, insula, corpus callosum). Average tumor volume was 12.5 ± 13.4 cm3. Average percentage of tumor treated with the yellow thermal damage threshold (TDT) line (dose equivalent of 43°C for 2 min) was 93.3% ± 10.6%, and with the blue TDT line (dose equivalent of 43°C for 10 min) was 88.0% ± 14.2%. There were 7 perioperative complications (12%) and 2 mortalities (3.4%). Median overall survival after LITT for the total cohort was 11.5 mo, and median progression-free survival 6.6 mo.
CONCLUSION
LITT appears to be a safe and effective treatment for GBM in properly selected patients.
Keywords: Laser ablation, Laser interstitial thermal therapy, LITT, Glioblastoma, GBM, MRI, Brain tumors, Thermoablation
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- ASA
American Society of Anesthesiologists
- CI
confidence interval
- EGFR
epidermal growth factor receptor
- GBM
glioblastoma
- ICU
intensive care unit
- IDH-1
isocitrate dehydrogenase-1 R132
- LITT
laser interstitial thermal therapy
- MGMT
O[6]-methylguanine-DNA methyltransferase
- MRI
magnetic resonance imaging
- OS
overall survival
- PFS
progression-free survival
- SRS
stereotactic radiosurgery
- TDT
thermal damage threshold
- 3D
three-dimensional
Glioblastoma (GBM) is the most common primary brain tumor and remains a uniformly fatal disease. Despite the multitude of therapies available to GBM patients, median survival is only 14 mo.1,2 Surgery is usually the initial treatment of choice, and there is mounting evidence that a greater extent of resection is associated with better overall survival (OS).3,4 However, many patients reach a point where additional craniotomies are no longer feasible. Additionally, many patients are not good candidates for traditional surgery at the time of initial diagnosis, and typically undergo biopsy followed by chemoradiation without any “direct” cytoreductive treatment.
Laser interstitial thermal therapy (LITT) has emerged as a novel treatment for a variety of intracranial lesions, including
GBM.5-8 This technology uses a laser-tip probe, inserted into the centroid of a brain lesion, to produce a controlled thermal injury by heating surrounding tissue. Real-time magnetic resonance imaging (MRI) thermometry allows for continuous monitoring of the ablation zone, and ablation can be stopped at any time. LITT is especially suitable for many patients with GBM for several reasons. The recurrent and often multifocal nature of the disease means clinicians are often faced with the problem of deep or difficult-to-access lesions. Despite the successes of multiple craniotomies for resection, factors like advancing age, comorbidity, and inevitable decline in functional status lead to a point beyond which open surgery is no longer appropriate. LITT therefore offers promise as a direct cytoreductive technique that is minimally invasive, effective, and less morbid than open craniotomy.
Previous series of LITT are limited by small sample sizes and short follow-up, primarily due to the fairly recent introduction of this technology in neurosurgery. The object of this study is to report on safety, efficacy, and outcomes of LITT for GBM on a cohort of 54 patients for whom follow-up data are largely complete.
METHODS
We retrospectively reviewed all patients with a diagnosis of GBM who underwent LITT between 2010 and 2016 at our institution. Institutional Review Board (IRB) approval was obtained for human subject research. Individual patient consents were not sought, as this was a descriptive retrospective study with all data anonymized. All patients with histologically proven GBM who underwent LITT were eligible. Information was collected from electronic records, deidentified, and included age, sex, tumor location, prior treatments, indication for LITT, complications, and follow-up data. Genetic data were collected from pathology reports as available. Two patients in whom the procedure was aborted due to issues unrelated to the procedure itself were excluded.
The database was maintained in Microsoft Excel (Microsoft Corporation, Redmond, Washington). Statistical analysis was performed in Excel and MATLAB (Mathworks Inc., Natick, Massachusetts). The Student's t-test was used to compare continuous variables between groups, and the log-rank test for groups was used to compare survival curves. A threshold (alpha) of 0.05 was used to indicate statistical significance.
Our institution primarily utilizes the Monteris® laser ablation system (Monteris Medical Corporation, Plymouth, Minnesota). Using the stereotactic MRI on the workstation, a three-dimensional (3D) tumor volume is constructed for volumetric analysis. During ablation, real-time MRI thermometry tracks the progress of the expanding ablation zone. The ablation zone is characterized by the yellow thermal damage threshold (TDT) line, signifying a dose equivalent of 43°C for 2 min, and by the blue TDT line, signifying a dose equivalent of 43°C for 10 min (Figure 1). The yellow and blue zones, taken over many slices, are used to construct a 3D model of the ablation zone. The overlap of the tumor volume and ablation zones (yellow and blue) yields an ablation percentage.
FIGURE 1.
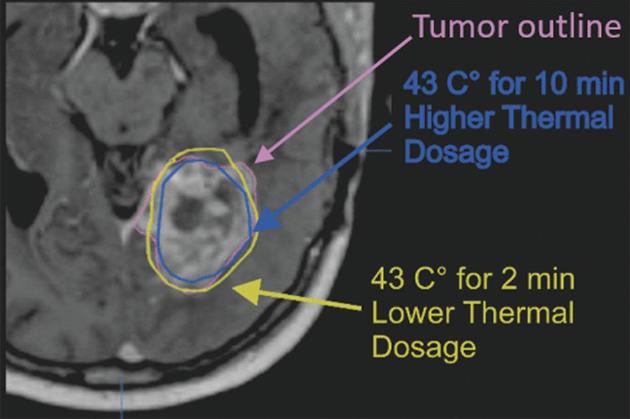
TDT lines. Image during laser ablation, demonstrating tumor volume (constructed manually on Monteris® [Plymouth, Minnesota] proprietary software at the time of surgery) and yellow and blue thermal damage threshold (TDT) lines (derived from real-time MRI thermometry).
Our paradigm for LITT candidate selection has been previously described9,10 and will be briefly reviewed here. Broadly speaking, lesions that are favorable for LITT are (1) deep seated, (2) spherical or oblong such that the laser probe can be passed through the long axis of the lesion, (3) well circumscribed, and (4) positioned such that a safe trajectory avoiding critical structures/tracts can be designed. Transgression of vascular planes (Sylvian fissure, sulci) and ventricles is avoided. Lesions unfavorable for LITT include hypervascular lesions, diffuse lesions involving bilateral or multiple lobes, and very large lesions in which treatment would be subtotal. Lesions in eloquent or constricted locations (basal ganglia, posterior fossa) are more difficult to target, although this was not a contraindication to treatment. Finally, technical considerations include patient positioning, the profile of the laser apparatus, and patient body habitus, which together must fit into the MRI bore.
GBMs in deep or difficult-to-access areas that fit the above criteria were ideal candidates for frontline LITT. If a tissue diagnosis had not yet been obtained, stereotactic needle biopsy was performed through the same trajectory prior to placement of the laser probe. In the case of recurrent GBMs, most had already undergone at least 1 open surgery and chemoradiation. Although there is evidence for the utility of multiple craniotomies for recurrent GBM,11-13 most patients reach a point where surgical morbidity is too great. While these lesions could technically be resected again via open surgery, patient factors such as declining functional status, chronic steroid use, deconditioning from chronic illness, and thinned scalp made open surgery unfavorable. These patients typically go on to receive bevacizumab or other second-line agents.14-16 Selected patients from this group were offered LITT for their recurrent GBM to be followed by second-line agents, after multidisciplinary review at our institutional tumor board.
All patients were observed in the neuro intensive care unit (ICU) postoperatively and received perioperative antibiotics, antiepileptic medication, and dexamethasone. Dexamethasone was generally tapered over 1 to 3 wk based on surgeon's clinical judgment, accounting for factors such as tumor/ablation volume, surrounding edema, and steroid side effects.
RESULTS
A total of 58 LITT treatments were performed in 54 patients with a histological diagnosis of GBM (Table 1). All cases were performed by one of two surgeons (ECL—77% and AHK—23%). There were 37 males and 17 females. Average age was 58.8 (± 10.8) yr, with a range of 35 to 78 yr. Average American Society of Anesthesiologists (ASA) grade (1-6) preoperatively was 2.6 (± 0.6); ASA grade was not significantly different between the primary and recurrent groups (mean 2.7 vs 2.5, P = 0.15). Mean follow-up time was 11.5 ± 7 mo; only 8 patients in the cohort are still alive at present (14%) and only one was lost to follow-up. Of 58 treatments, 17 were frontline for primary GBM as determined by biopsy, either previously or concurrently with LITT. Forty-one treatments were for recurrent GBM; the majority (33, 80.4%) had surgical resection(s) and standard chemoradiation prior to LITT. Four patients had only chemoradiation prior to LITT. Four patients had prior LITT treatments; of these, 3 underwent repeat ablation for recurrence and 1 was the second stage of a planned two-stage procedure.
TABLE 1.
Demographic and Clinical Information for 54 GBM Patients Treated With LITT
| n | % | ||
|---|---|---|---|
| Patients | 54 | 100.0% | |
| Male | 37 | 68.5% | |
| Female | 17 | 31.5% | |
| Age (mean ± SD) | 58.8 ± 10.8 | ||
| Lesions treated | 58 | 100.0% | |
| Primary | 17 | 29.3% | |
| Recurrent | 41 | 70.7% | |
| First recurrence | 35 | 85.4% | |
| Second recurrence | 6 | 14.6% | |
| Location | |||
| Frontal | 14 | 24.1% | |
| Temporal | 8 | 13.8% | |
| Parietal | 9 | 15.5% | |
| Occipital | 1 | 1.7% | |
| Parieto-occipital | 4 | 6.9% | |
| Temporo-parietal | 4 | 6.9% | |
| Corpus callosum | 8 | 13.8% | |
| Insular | 2 | 3.4% | |
| Thalamic | 8 | 13.8% | |
| Prior treatments | |||
| None/biopsy only | 17 | 29.3% | |
| Chemoradiation only | 4 | 6.9% | |
| Resection(s) + | 33 | 56.9% | |
| chemoradiation | |||
| Prior LITT only | 1 | 1.7% | |
| Chemoradiation + LITT | 1 | 1.7% | |
| Resection(s) + | 2 | 3.4% | |
| chemoradiation + LITT | |||
| Post-LITT treatments | |||
| Chemotherapy | 53 | 91.4% | |
| Radiotherapy | 15 | 25.9% | |
| Surgery | 3 | 5.2% | |
| Other (eg, | 10 | 17.2% | |
| tumor-treating | |||
| fields, vaccines) |
LITT, laser interstitial thermal therapy
Anatomic locations of lesions were widely variable (Figure 2A and 2B). The majority of lesions were lobar, located in the frontal (n = 14), temporal (n = 8), parietal (n = 9), occipital (n = 1), or in multiple contiguous lobes (n = 8). Other locations included corpus callosum (n = 8), insular (n = 2), and thalamic (n = 8) GBMs
FIGURE 2.
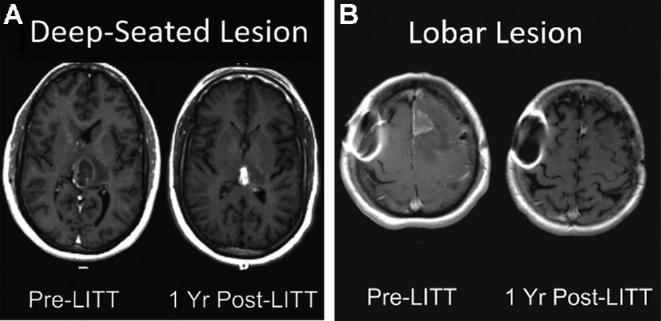
Lesion locations. Pre- and postoperative imaging demonstrating successful radiographic response for tumors in A, left posterior thalamic and B, left frontal locations. Magnetic resonance-safe metal artifact is noted on the patient's right side in B.
Certain genetic studies were available for subgroups of GBM patients (Table 2). These included isocitrate dehydrogenase-1 R132 (IDH-1) mutation, O[6]-methylguanine-DNA methyltransferase (MGMT) promoter methylation, chromosome 1p and 19q codeletion, and epidermal growth factor receptor (EGFR) amplification. Only 2 patients harbored IDH-1 mutations out of 45 whose mutation status was known (4.4%). Similarly, 21 patients out of 45 showed MGMT methylation (46.6%), 4 out of 23 showed 1p19q deletion (17.4%), and 12 out of 27 showed EGFR amplification (44.4%).
TABLE 2.
Genetic Markers
| Genetic marker | n | Total number with known status | % |
|---|---|---|---|
| IDH-1 (R132) mutation | 2 | 45 | 4.4 |
| MGMT methylation | 21 | 45 | 46.6 |
| 1p19q codeletion | 4 | 23 | 17.4 |
| EGFR amplification | 12 | 27 | 44.4 |
LITT was chosen primarily due to lesion location in 19 cases (32.8%), while advanced age, recurrence after prior treatments, and/or poor functional status were the primary reason for LITT in 39 cases (67.2%).
Multiple trajectories were used during some LITT treatments (Table 3). The majority of treatments involved one trajectory only (n = 49, 84.4%). Seven cases involved two trajectories (12.0%), and 2 cases involved three trajectories (3.4%). Operating room time—defined as the time from skin puncture to the application of the final dressing—varied from 93 to 648 min (mean 240 min, median 223 min). Volumetric data were available for 50 of 58 cases; the following data refer to this subgroup. Average tumor volume was 12.5 ± 13.4 cm3. Average percentage of tumor treated with the yellow TDT line was 93.3 ± 10.6%, and with the blue TDT line, 88.0 ± 14.2%. Thirty-eight patients (76%) had at least 90% of tumor volume treated by the yellow TDT line, and 33 patients (66%) had at least 90% of tumor volume treated by the blue TDT line. Twenty-one patients (42%) had total or supratotal ablation by the yellow TDT boundary, and 10 patients (17%) had total or supratotal ablation by the blue TDT boundary. Ablation was terminated in case of encroachment on eloquent regions or the inability to reach parts of the lesion due to fiber placement. MRI was routinely obtained on postablation day one for evaluation of ablation success, and to establish a post-LITT baseline. Average ICU length of stay for all patients was 1.7 ± 2.8 d, and average total hospital stay was 3.2 ± 4.6 d; this figure includes the earliest LITT patients at our institution. We have previously reported that the length of stay tends to decline as providers become more familiar with post-LITT patients’ clinical course.8
TABLE 3.
Surgical Information
| n | % | ||
|---|---|---|---|
| Number of trajectories used | |||
| (in a single treatment) | |||
| 1 | 49 | 84.5% | |
| 2 | 7 | 12.1% | |
| 3 | 2 | 3.4% | |
| Number of cases with | 4 | 6.9% | |
| multiple LITT treatments | |||
| Surgery time (min) | 240 ± 125 | ||
| Tumor volume (mL) | 12.5 ± 13.4 | ||
| Min | 0.27 | ||
| Max | 62.77 | ||
| Yellow boundary | 93.2 ± 10.6 | ||
| treated (%) | |||
| Min | 54.1 | ||
| Max | 100 | ||
| Blue boundary | 88.0 ± 14.2 | ||
| treated (%) | |||
| Min | 45.5 | ||
| Max | 100 |
LITT, laser interstitial thermal therapy; IDH-1 (R132), isocitrate dehydrogenase-1, arginine 132; MGMT, O[6]-methylguanine-DNA methyltransferase promoter; 1p19q, chromosomes 1p and 19q; EGFR, epidermal growth factor receptor
There were 9 complications within 30 d of procedure leading to further procedures, unexpected readmission, or transfer back to the ICU, or a rate of 15.5%. These consisted of complications from cerebral edema (n = 3), seizures (n = 3), hydrocephalus (n = 1), hyponatremia (n = 1), and infection (n = 1). There were 2 mortalities (3.4%). In cases without complications, mean tumor volume was 11.7 cm3; in cases with complications, the mean volume of tumor was 15.5 cm3; this difference was not significant (P = 0.20).
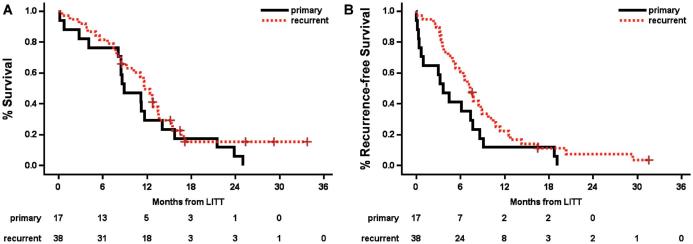
Median OS after LITT for the total cohort was 11.5 mo (95% confidence interval (CI) 8.5-12.8 mo, range 0-34.2 mo), and median progression-free survival (PFS) was 6.6 mo (95% CI 4.3-7.7 mo, range 0-32.0 mo). These groups were further broken down as follows. For primary GBMs treated with LITT as a frontline therapy (n = 17), OS was 9.1 mo (95% CI 4.2-14.2 mo, range 0-25.4 mo) and PFS was 3.6 mo (95% CI 0.37-7.67 mo, range 0-19.3 mo). Two of 17 patients in this cohort had no further therapy due to perioperative mortality and 15 had standard chemoradiation post-LITT. For recurrent GBMs (n = 41), OS was 11.8 mo (95% CI 8.6-13.8 mo, range 0-34.2 mo) and PFS was 7.3 mo (95% CI 5.1-8.9 mo, range 0-32 mo; Figure 3A and 3B). Of these, 36 had prior open craniotomy and all 41 had prior standard of care chemoradiation. When calculated from time of initial histologic diagnosis of GBM, OS for the recurrent group was 22.3 mo (95% CI 16.2-26.8 mo), compared to 9.1 mo for the primary group (P < 0.0001; Figure 4). The difference in survival from time of LITT between primary and recurrent tumors was not significant (P = 0.31).
FIGURE 3.
A, Overall and B, PFS in primarily treated vs recurrent GBM after LITT. OS for primarily treated vs recurrent GBM was 9.1 and 11.8 mo, respectively; PFS was 3.6 and 7.3 mo, respectively. There were no statistically significant differences.
FIGURE 4.
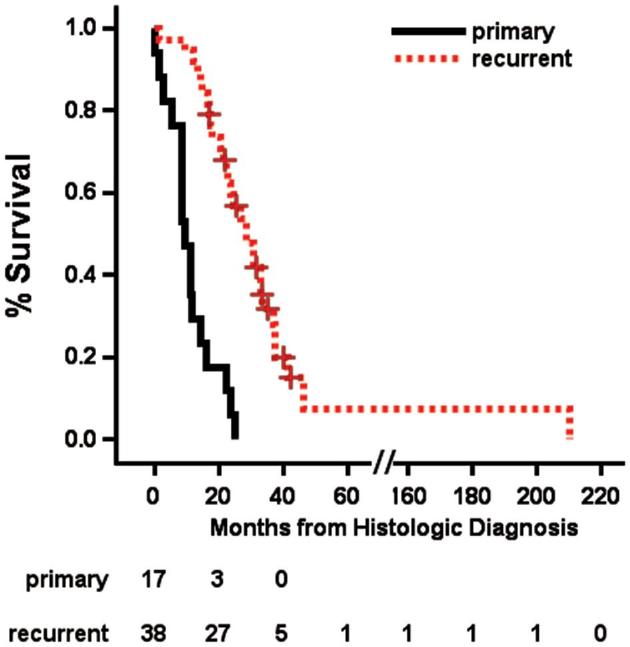
OS in primarily treated vs recurrent GBM from time of histologic diagnosis. OS for the primarily treated group was 9.1 mo, and for the recurrent group, 22.3 mo (P < 0.0001).
Lesions that were deep-seated (corpus callosum, thalamus, insula) were compared to those in lobar locations. There were 18 deep-seated and 40 lobar lesions. Of the deep lesions, 9 (50%) were primary treatments and 9 (50%) were recurrences. Of the lobar lesions, 9 (23%) were primary treatments and 31 (77%) were recurrences. Median OS after LITT was 11.3 mo (range 0-34.2 mo) for lobar and 11.5 mo (range 0-25.4 mo) for deep-seated GBMs (P = 0.68). Median PFS after LITT was 6.8 mo (range 0-32 mo) for lobar and 6.0 mo (range 0-19.3) for deep GBMs (P = 0.44).
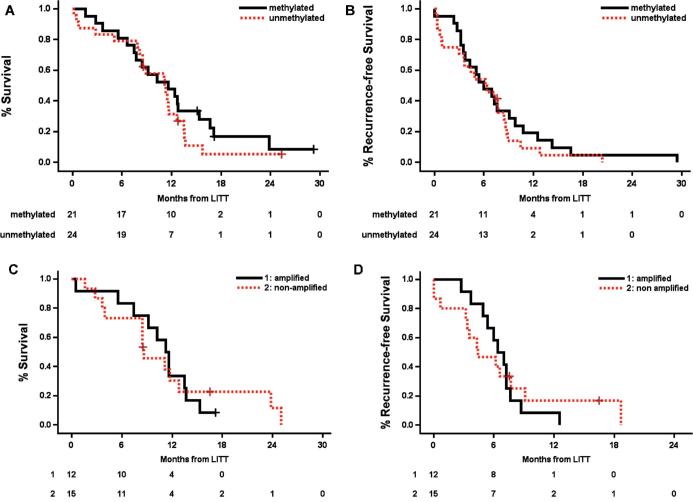
In patients with MGMT, methylation OS was 11.6 mo and PFS was 6.0 mo, while for unmethylated MGMT OS was 11.1 and PFS was 6.3 mo (P = 0.32 and 0.44, respectively; Figure 5A and 5B). In patients with EGFR amplification, OS and PFS were 11.4 and 6.7 mo, respectively, and for those without amplification, 8.6 and 4.4 mo (P = 0.99 and 0.84, respectively; Figure 5C and 5D). We did not have sufficiently high numbers of other genetic markers (such as IDH-1 mutation) to permit survival analyses.
FIGURE 5.
A and B, Overall and PFS by MGMT promoter status, and C and D, EGFR amplification status. OS for the MGMT-methylated vs MGMT-unmethylated group was 11.6 and 11.1 mo, respectively; PFS was 6 and 6.3 mo, respectively. There were no statistically significant differences. OS for the EGFR amplified vs EGFR nonamplified group was 11.4 and 8.6 mo, respectively; PFS was 6.7 and 4.4 mo, respectively. There were no statistically significant differences.
DISCUSSION
LITT is a minimally invasive surgical tool to treat intracranial lesions, and offers lower morbidity, shorter length of stay, and good surgical outcomes.5,17-19 We find LITT to be a safe and efficacious procedure based on our experience of 58 cases. Of the two mortalities (3.4%), one was a 78-yr old with a large parieto-occipital GBM which hemorrhaged after treatment; the patient's family elected for comfort measures only. The second patient developed fulminant Enterobacter meningitis, and analysis of the event traced this problem to a sterile-processing related contamination.
Perioperative morbidities were commonly related to cerebral edema and seizures. It is postulated that patients with larger tumor volume are predisposed to complications from cerebral edema and mass effect.20,21 Intuitively, it would seem that larger volume ablations would result in more cell death, coagulative necrosis, and release of more inflammatory mediators than smaller volume ablations. In our series, mean tumor volume in patients who had complications was 15.5 cm3 while that of uncomplicated cases was 11.7 cm3; however, this did not reach significance (P = 0.20). Similarly, tumor location may have some effect on complication rate, and it might be expected that deep-seated lesions (corpus callosum, thalamus, insula) have more complications. Only 13% (5 of 40) of patients with superficial/lobar GBMs experienced a complication, while 22% (4 of 18) patients with deep-seated lesions experienced a complication. However, this difference also was not significant (P = 0.37).
Median tumor volume was 8.8 cm3 (mean 12.5 cm3, range 0.27-62.8 cm3). Overall, we were able to achieve an average of 93% of tumor volume treatment within the yellow TDT line and 88% treatment with the blue TDT line, concordant with longer term radiographic results, suggesting that LITT is an effective cytoreductive option for GBMs that are less favorable for open surgery.
Follow-up data in this series were largely complete; at the time of this study, only 8 of 54 patients (14.8%) were alive. For the total cohort, median PFS after LITT was 6.6 mo and median OS was 11.5 mo. For frontline GBM treatment, median OS was 9.1 mo, while for recurrent GBM treatment median OS was 11.8 mo. This difference may be in part due to patient selection for these groups. Patients for whom LITT is chosen as a frontline therapy were not good candidates for craniotomy, due to deep-seated lesions, advanced age, poor functional status, or some combination thereof. Since these patients have a poorer prognosis from the start, it follows that OS in this group may be shorter.
OS in GBM patients who undergo biopsy only followed by chemoradiation has been reported by Stupp et al22 to be 9.4 mo. In our cohort, the addition of frontline LITT did not demonstrate a survival benefit (OS 9.1 mo) relative to this historical outcome data. The frontline LITT cohort had either deep-seated tumors (ie thalamic, brainstem), or were elderly/frail with poor performance status; both are poor prognostic factors in GBM. At this juncture, it is hard to ascertain whether the biopsy only group from Stupp et al22 is comparable to the upfront LITT population. It is possible that the upfront LITT patient population would have fared worse without LITT. The shorter OS in the frontline LITT group relative to the recurrence group, from time of diagnosis (9.1 mo vs 22.3 mo, P < 0.0001), is also evidence that these are very different patient populations (Figure 4). Ultimately, a randomized controlled trial would be necessary to formally answer this question.
For recurrent GBM deemed nonsurgical, treatment modalities include stereotactic radiosurgery (SRS) and additional chemotherapies. Studies have shown mixed results regarding the efficacy and safety profile of SRS, though it appears that certain subgroups of patients—such as those with small focal recurrence—may benefit.23-25 At our institution, recurrent GBM patients are typically treated with bevacizumab or repeat temozolomide, alone or in combination with other chemotherapeutic drugs. In the recurrent cohort, Friedman et al showed OS after treatment with bevacizumab to be 9.2 mo,14 and Desjardins et al26 showed OS after combination bevacizumab and temozolomide to be 9.3 mo. Numerous other studies have demonstrated shorter or similar survival figures after a variety of chemotherapeutic regimens at GBM recurrence.27-29 In our cohort, the addition of LITT at recurrence resulted in an OS of 11.8 mo. While this is only an additional 2 mo, in the context of survival after recurrent GBM, this may represent a small but meaningful survival benefit. Furthermore, this benefit comes with significantly less operative morbidity and recovery time compared to traditional craniotomy. Further controlled studies on LITT for recurrent GBM will be essential as LITT gains acceptance as a therapy for this group.
We had insufficient data to perform valid survival analyses with regard to all genetic prognostic markers in GBM except MGMT methylation. Of 45 patients for whom MGMT data were available, 21 were methylated. OS in the methylated group after LITT was 11.6 compared with 11.1 for unmethylated (log rank P = 0.32). Significantly improved survival in the MGMT-methylated group is not seen in this cohort, as may be expected from previous literature.30-32 One explanation is that MGMT methylation confers greater responsiveness to chemotherapy initially, but these cells are selectively eliminated and not present in recurrent tumors. It is also possible that this equivalence in survival between these two cohorts is related to the difference in how LITT affects the biology of the tumor and local environment. Hypermethylation of the MGMT gene has been shown to be associated with improved outcome in GBM and may be a predictive marker of sensitivity to alkylating agents and radiotherapy.22,32 Thus, the lack of methylation makes GBMs less susceptible to chemoradiation. LITT has been shown to be effective at breaking down the blood brain barrier in humans,7 and hyperthermia has been shown to enhance radiosensitivity in glioma stem cells by altering the PI3-AKT inducing pathway, which is aberrantly regulated in more than 40% of GBM and associated with poor patient prognosis.33 Taken together, the equivalent outcome for LITT in MGMT unmethylated patients may represent that this susceptibility is to some degree compensated by the biological effects of LITT.
Limitations
Limitations of this study are those inherent to a single-institution series. The cases were performed by two surgeons at a single center, and may not be generalizable to all centers that perform LITT. Additionally, treatment of GBM is a complex process individualized to the patient. We included data about surgery, radiotherapy, and chemotherapy as the standards of care; however, we did not control for the myriad clinical trials, research-phase drugs, and devices (e.g. Optune®; Novocure, St. Helier, United Kingdom) that may have played a role in these patients’ care. Finally, this is a descriptive study. While LITT offers several advantages over craniotomy, a comparative study with matched groups would directly compare the efficacy of the two treatments. Further studies examining LITT in this context will be useful.
CONCLUSION
Our experience suggests that LITT is a safe, well-tolerated and efficacious cytoreductive treatment for GBM in properly selected patients.
Disclosures
Funding was provided by the Christopher Davidson Brain Tumor Research Fund (to Dr Leuthardt). Washington University IRB ID: 201609152. Dr Leuthardt has a consulting relationship with Monteris Medical. There were no gains, financial or otherwise, related to the production of this manuscript, which was produced wholly independently of Monteris Medical. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
The authors would like to thank Corey Hollander and Scott Suppelsa for filming and producing the video abstract associated with this work.
COMMENTS
The authors present a single-center retrospective analysis of 54 GBM patients treated with MRI-guided LITT over a 6-year period. They find that LITT is an effective therapy that may extend life expectancy in patients with recurrent GBM who are not candidates for re-resection due to age, medical co-morbidities, or poor functional status. The paper extends substantively the published experience with LITT for GBM, helping to define its appropriate use.
Ron L. Alterman
Boston, Massachusetts
In this study, authors present outcomes from over 50 patients with glioblastoma treated with Laser Interstitial Thermal Therapy (LITT). This is one of the largest series available, with essentially complete follow-up data. The majority of the treatments (about 70%) were for recurrent glioblastoma, while the remainder (about 30%) were for newly-diagnosed glioblastoma, for which the pathology was determined by biopsy. Median overall survival was 11.5 months, while median progression-free survival was 6.6 months. There were 9 complications within 30 days of the procedure (15.5%), mostly related to cerebral edema and seizures. There were 2 mortalities (3.4%) secondary to post-treatment hemorrhage (possibly related to tumor size) and to infection from instrument contamination. Based on the data in this study, LITT appears to have particular value for patients with recurrent glioblastoma who may be too medically frail and/or have lesions that are too deep-seated for safe surgical resection. In these cases, average overall median survival was about 2 months longer than comparable cohorts using bevacizumab. While relatively small, the difference is nonetheless meaningful given the decreased recovery time compared to a traditional craniotomy. Overall, the study is well-written and adds to the body of literature on available treatment modalities for glioblastoma
Emanuela Binello
Boston, Massachusetts
The authors present outcomes from glioblastoma patients treated with Laser Interstitial Thermal Therapy (LITT). With 58 LITT treatments (17 for newly diagnosed GBM confirmed by biopsy either previously or concurrently with LITT) in 54 GBM patients, this is one of the largest series available, with good long-term follow-up. Progression-free survival was 6.6 months and overall survival was 11.5 months. Adverse events mostly involved cerebral edema and seizures.The 30-day treatment-related morbidity rate was 16% and there were 2 treatment-related mortalities (3%), 1 from post-LITT hemorrhage in a large tumor and the other from infection due to contaminated instruments. Choice of LITT was due to tumor location precluding surgery in one-third of LITT cases and due to age or functional status in two-thirds of cases. In these cases, average overall survival was about 2 months longer than similar published cohorts treated with bevacizumab. The authors are to be commended on this well-written study, which adds to the growing body of literature on the use of LITT for treating glioblastoma.
Manish K. Aghi
San Francisco, California
Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Hart MG, Garside R, Rogers G, Stein K, Grant R. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2013;30(4):CD007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 4. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 5. Thomas JG, Rao G, Kew Y, Prabhu SS. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg Focus. 2016;41(4):1–6, E12. [DOI] [PubMed] [Google Scholar]
- 6. Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma. J Neurosurg. 2013;118(6):1202–1219. [DOI] [PubMed] [Google Scholar]
- 7. Leuthardt EC, Duan C, Kim MJ, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):1–14, e0148613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamath AA, Friedman DD, Akbari SHA, et al. MRI-guided interstitial laser ablation for intracranial lesions: A large single-institution experience of 133 cases. Stereotact Funct Neurosurg. 2017;95(6):417–428. [DOI] [PubMed] [Google Scholar]
- 9. Hawasli AH, Bagade S, Shimony JS, Miller-Thomas M, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions. Neurosurgery. 2013;73(6):1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dadey DY, Kamath AA, Smyth MD, Chicoine MR, Leuthardt EC, Kim AH. Utilizing personalized stereotactic frames for laser interstitial thermal ablation of posterior fossa and mesiotemporal brain lesions: a single-institution series. Neurosurg Focus. 2016;41(4):1–7, E4. [DOI] [PubMed] [Google Scholar]
- 11. Perrini P, Gambacciani C, Weiss A, et al. Survival outcomes following repeat surgery for recurrent glioblastoma: A single-center retrospective analysis. J Neurooncol. 2017;131(3):585–591. [DOI] [PubMed] [Google Scholar]
- 12. Bloch O, Han SJ, Cha S, et al. Impact of extent of resection for recurrent glioblastoma on overall survival. J Neurosurg. 2012;117(6):1032–1038. [DOI] [PubMed] [Google Scholar]
- 13. Oppenlander ME, Wolf AB, Snyder LA, et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120(4):846–853. [DOI] [PubMed] [Google Scholar]
- 14. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 15. Burger MC, Breuer S, Cieplik HC, et al. Bevacizumab for patients with recurrent multifocal glioblastomas. Int J Mol Sci. 2017;18(11):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sim HW, Morgan ER, Mason WP. Contemporary management of high-grade gliomas. CNS Oncol. 2018;7(1):51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Willie JT, Laxpati NG, Drane DL, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74(6):569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: preliminary results in 16 patients. Eur J Radiol. 2006;59(2):208–215. [DOI] [PubMed] [Google Scholar]
- 19. Patel P, Patel NV, Danish SF. Intracranial MR-guided laser-induced thermal therapy: Single-center experience with the Visualase thermal therapy system. J Neurosurg. 2016;125(4):853–860. [DOI] [PubMed] [Google Scholar]
- 20. Pisipati S, Smith KA, Shah K, Ebersole K, Chamoun RB, Camarata PJ. Intracerebral laser interstitial thermal therapy followed by tumor resection to minimize cerebral edema. Neurosurg Focus. 2016;41(4):1–4, E13. [DOI] [PubMed] [Google Scholar]
- 21. Wright J, Chugh J, Wright CH, et al. Laser interstitial thermal therapy followed by minimal-access transsulcal resection for the treatment of large and difficult to access brain tumors. Neurosurg Focus. 2016;41(4):1–8, E14. [DOI] [PubMed] [Google Scholar]
- 22. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 23. Combs SE, Widmer V, Thilmann C, Hof H, Debus J, Schulz-Ertner D. Stereotactic radiosurgery (SRS). Cancer. 2005;104(10):2168–2173. [DOI] [PubMed] [Google Scholar]
- 24. Mahajan A, McCutcheon IE, Suki D, et al. Case—control study of stereotactic radiosurgery for recurrent glioblastoma multiforme. J Neurosurg. 2005;103(2):210–217. [DOI] [PubMed] [Google Scholar]
- 25. Masciopinto JE, Levin AB, Mehta MP, Rhode BS. Stereotactic radiosurgery for glioblastoma: A final report of 31 patients. J Neurosurg. 1995;82(4):530–535. [DOI] [PubMed] [Google Scholar]
- 26. Desjardins A, Reardon DA, Coan A, et al. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118(5):1302–1312. [DOI] [PubMed] [Google Scholar]
- 27. Vredenburgh JJ, Desjardins A, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. [DOI] [PubMed] [Google Scholar]
- 28. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23(23):5294–5304. [DOI] [PubMed] [Google Scholar]
- 30. Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;2000(19):1350–1354. [DOI] [PubMed] [Google Scholar]
- 31. Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26(25):4189–4199. [DOI] [PubMed] [Google Scholar]
- 32. Rivera AL, Pelloski CE, Gilbert MR, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neurooncol. 2010;12(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Man J, Shoemake JD, Ma T, et al. Hyperthermia sensitizes glioma stem-like cells to radiation by inhibiting AKT signaling. Cancer Res. 2015;75(8):1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]