Abstract
In this study, we used three-dimensional human engineered cardiac tissue technology to directly show that phospholamban (PLN) R14del mutation impairs cardiac contractility and to demonstrate restoration of contractile properties with targeted genetic correction of this inheritable form of dilated cardiomyopathy.
Keywords: Dilated cardiomyopathy, Engineered heart tissue, Gene editing, Induced pluripotent stem cells, Phospholamban
Dilated cardiomyopathy (DCM) is a common cause of heart failure associated with ventricular dilation and deterioration of heart performance. Some forms of DCM are hereditary, and one of the genes found to be associated with inherited DCM is the one encoding phospholamban (PLN),1,2 a protein that regulates the sarcoplasmic reticulum Ca2+ ATPase (SERCA2a).3 Several PLN mutations, including a deletion of arginine 14 codon (R14del), have been linked to hereditary DCM; heterozygous patients exhibit ventricular dilation, contractile dysfunction, episodic ventricular arrhythmias, and heart failure.1 To understand the molecular mechanism underlying the pathogenesis of R14del-induced DCM, we recently examined the functional characteristics of human cardiomyocytes (hCMs) obtained by directed differentiation of induced pluripotent stem cells (iPSCs) derived from a patient with cardiomyopathy associated with PLN R14del mutation.4 Compared with iPSC-CMs derived from a healthy patient expressing wild-type PLN, the R14del-CMs exhibited Ca2+ handling abnormalities, irregular electrical activity, abnormal intracellular distributions of PLN, and increased mRNA levels for molecular markers associated with cardiac hypertrophy. Moreover, targeted correction of the PLN mutation in R14del-iPSCs, performed with transcription activator-like effector nucleases (TALENs), mitigated the disease phenotypes in iPSC-CMs derived from these isogenic iPSC clones.4 However, due to limitations of traditional cell culture methods, the effects of the PLN R14del mutation on cardiac muscle contractility were not previously tested. In this study, we used three-dimensional human engineered cardiac tissue (hECT) technology to show that PLN R14del mutation impaired cardiac contractility and to demonstrate that genetic correction restored contractile function.
Patient-specific iPSCs were corrected by gene editing using a TALEN method. As previously described,4 we developed a TALEN vector pair designed to introduce a double-strand break adjacent to the R14del mutation of the PLN gene and validated its efficiency by the surveyor assay (estimated modification efficiency 25.6%). Using low-density replating, puromycin selection, and PCR screening, we obtained homogeneous isogenic iPSC clones that differed only in the corrected R14del mutation compared with the parent clone. The corrected clones were expanded and the correction of the R14del mutation was confirmed by Sanger sequencing. Directed differentiation was then used to create human iPSC-CMs that recapitulate characteristics of healthy cardiomyocytes, DCM cardiomyocytes (PLN mutant), and TALEN-edited isogenic corrected cardiomyocytes4 (Figure 1A). After 15 days of differentiation, iPSC-CMs were harvested, suspended in culture medium, and mixed with collagen type-I and Matrigel solution. The cell-matrix suspension was transferred into polydimethylsiloxane (PDMS) moulds, with integrated cantilever end-posts for non-invasive measurement of contractile function.5
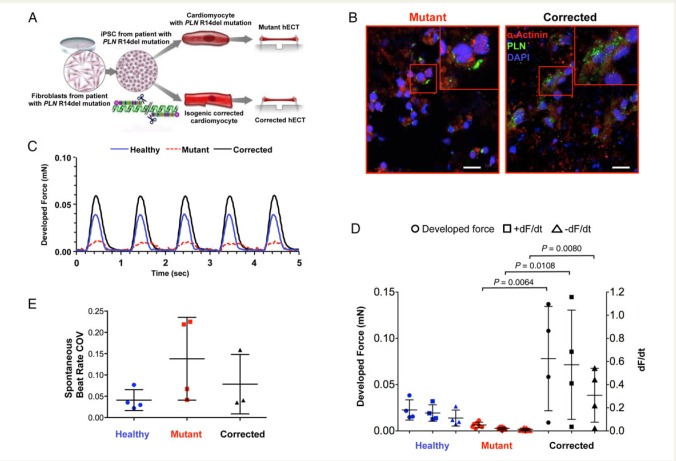
Figure 1.
Effect of phospholamban R14del mutation on cardiac muscle contractility in three-dimensional human engineered cardiac tissues. (A) Schematic of the methods. (B) Representative immunofluorescence images of mutant and corrected human engineered cardiac tissues stained for α-actinin (red), phospholamban (green) and DAPI (blue), scale bar = 25 µm. (C) Example twitch tracings from healthy (solid blue line), mutant (dashed red line), and corrected (solid black line) human engineered cardiac tissues during pacing at 1 Hz. (D) Twitch force parameters: developed force, +dF/dt, and −dF/dt (symbols indicate individual measurements, bars represent mean ± SD) analysed by ANOVA with Scheffe's post hoc test (corrected for multiple pairwise comparisons); n = 4 healthy, n = 7 mutant, n = 4 corrected human engineered cardiac tissues. (E) Spontaneous beat rate variability expressed as coefficient of variation, recorded from: n = 4 healthy, n = 4 mutant, and n = 3 corrected human engineered cardiac tissues (P = 0.209 by ANOVA). COV, coefficient of variation; hECTs, human engineered cardiac tissues.
After 1 week in culture, the hECTs from all three conditions beat spontaneously and resembled thin trabecular muscles. In mutant hECTs, the intracellular distribution of PLN within hCMs was polarized at one side of the cell, similar to observations in cardiac tissue sections from R14del patients,4 whereas corrected hECTs showed a more homogeneous distribution throughout the hCM cytoplasm (Figure 1B). These findings suggest that the 3D environment of hECTs preserves the disease-dependent distribution of PLN observed in native tissue samples and monolayer cultures of the corresponding cell types.4 All hECTs achieved 1:1 capture during electrical field stimulation at 1 Hz (Figure 1C), but the excitation threshold potential to initiate pacing was significantly lower in mutant hECTs (85 ± 59 mV/mm) compared with corrected (239 ± 100 mV/mm, P = 0.011), possibly suggesting an increased susceptibility to arrhythmogenesis in mutant hECTs. This was corroborated by a trend toward higher intrinsic variability of the spontaneous beating rate in mutant vs. corrected (1.8-fold) and vs. healthy (3.4-fold) hECTs (Figure 1E), though the effect was not statistically significant by ANOVA (P > 0.2). In terms of contractile performance, the amplitude of the developed twitch force was dramatically lower in mutant compared with corrected hECTs (P = 0.0064) (Figure 1D). Similarly, the maximum rates of contraction and relaxation (+dF/dt and −dF/dt, respectively) were significantly slower in mutant vs. corrected hECTs (P = 0.0108 and P = 0.0080), a direct functional manifestation of the abnormal calcium handling observed in cultured R14del-CMs.4 Interestingly, the developed force, +dF/dt and −dF/dt values from corrected hECTs tended to exceed the corresponding values from healthy hECTs (P = 0.055, P = 0.085 and P = 0.113 respectively), partly reflecting the intrinsic genetic variability between individuals and/or differences in the epigenetic state among iPSC lines.6 This comparison illustrates the advantage of genome editing and tissue engineering technologies to study the effects of specific mutations within the context of a common genetic background. This study demonstrates that the PLN R14del mutation interferes with intrinsic muscle contractility in patient-specific hECTs, and lays the groundwork for targeted gene therapy for treating patients with hereditary forms of cardiomyopathy.
Conflict of interest: none declared.
References
- 1. Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, Fan GC, Tsiapras D, Parekh RR, Dorn GW II, MacLennan DH, Kremastinos DT, Kranias EG. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci USA 2006;103:1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Spaendonck-Zwarts KY, van Rijsingen IA, van den Berg MP, Lekanne Deprez RH, Post JG, van Mil AM, Asselbergs FW, Christiaans I, van Langen IM, Wilde AA, de Boer RA, Jongbloed JD, Pinto YM, van Tintelen JP. Genetic analysis in 418 index patients with idiopathic dilated cardiomyopathy: overview of 10 years’ experience. Eur J Heart Fail 2013;15:628–636. [DOI] [PubMed] [Google Scholar]
- 3. MacLennan DH, Asahi M, Tupling AR. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann N Y Acad Sci 2003;986:472–480. [DOI] [PubMed] [Google Scholar]
- 4. Karakikes I, Stillitano F, Nonnenmacher M, Tzimas C, Sanoudou D, Termglinchan V, Kong CW, Rushing S, Hansen J, Ceholski D, Kolokathis F, Kremastinos D, Katoulis A, Ren L, Cohen N, Gho JM, Tsiapras D, Vink A, Wu JC, Asselbergs FW, Li RA, Hulot JS, Kranias EG, Hajjar RJ. Correction of human phospholamban R14del mutation associated with cardiomyopathy using targeted nucleases and combination therapy. Nat Commun 2015;6:6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee JJ, Xie C, Senyei G, Gordon RE, Li RA, Akar FG, Hajjar RJ, Hulot JS, Costa KD. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J 2014;28:644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Musunuru K. Genome editing of human pluripotent stem cells to generate human cellular disease models. Dis Model Mech 2013;6:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]