Abstract
This review article summarizes the epidemiological findings published between 2011 and 2016 concerning bisphenol A (BPA), phthalates, dioxins, pesticides, air pollution, fracking chemicals, triclosan, and parabens and fertility parameters in men (i.e., semen volume, sperm concentration, sperm motility, and sperm morphology) as well as fertility parameters in women (i.e., cyclicity, fertility treatment outcomes), pregnancy outcomes (i.e., preterm birth,miscarriage), and reproductive disorders (i.e., polycystic ovary syndrome, endometriosis, and uterine fibroids). Overall, this review indicates that several environmental toxicants are significantly associated with reduced fertility parameters in men and women as well as several reproductive disorders in women. Although many studies reported that the selected exposures are associated with adverse fertility outcomes, several studies reported null associations. Thus, future studies are still needed to better elucidate the associations and potential mechanisms between these environmental chemicals and fertility outcomes in men and women.
Keywords: endocrine disruptor, environmental chemical, fertility
Humans are often exposed to a wide variety of chemicals in their everyday environments. This review focuses on epidemiological and clinical research conducted in the past 5 years that investigates environmental chemicals and their associations with reproductive outcomes in both men and women (►Figs. 1 and 2). We chose to investigate bisphenol A (BPA), select phthalates, dioxins, pesticides, and air pollution be-cause of the high rate of exposure of humans to these chemicals and their strong presence in the environment. We also chose to review fracking chemicals, triclosan, and parabens because of emerging literature and interest in the impact of these chemicals on reproductive outcomes. Several studies showed contrasting results for the association be-tween many environmental exposures and reproductive outcomes. Thus, this review summarizes positive, negative, and null associations.
Fig. 1.
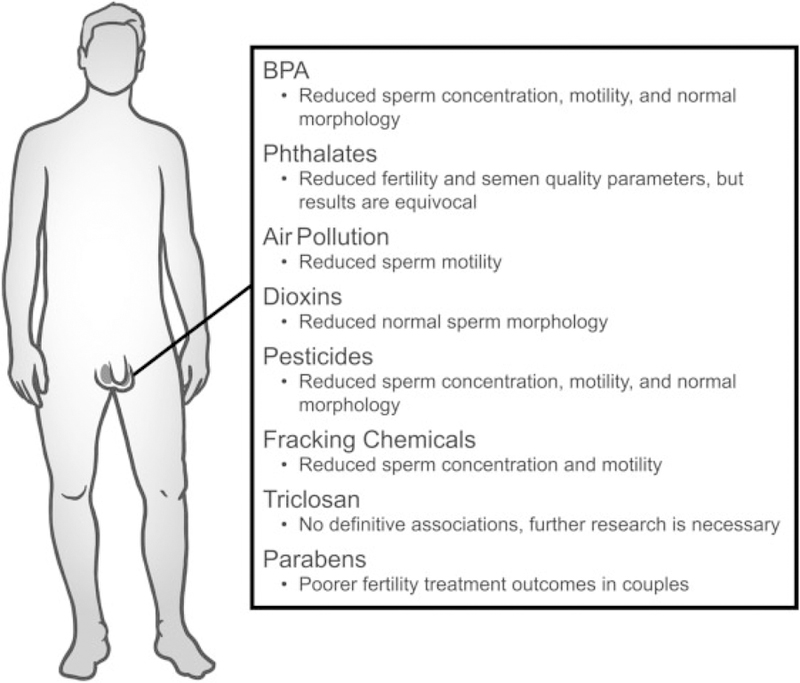
A brief summation of epidemiological research investigating endocrine disruptors and their associations with aspects of infertility in men.
Fig. 2.
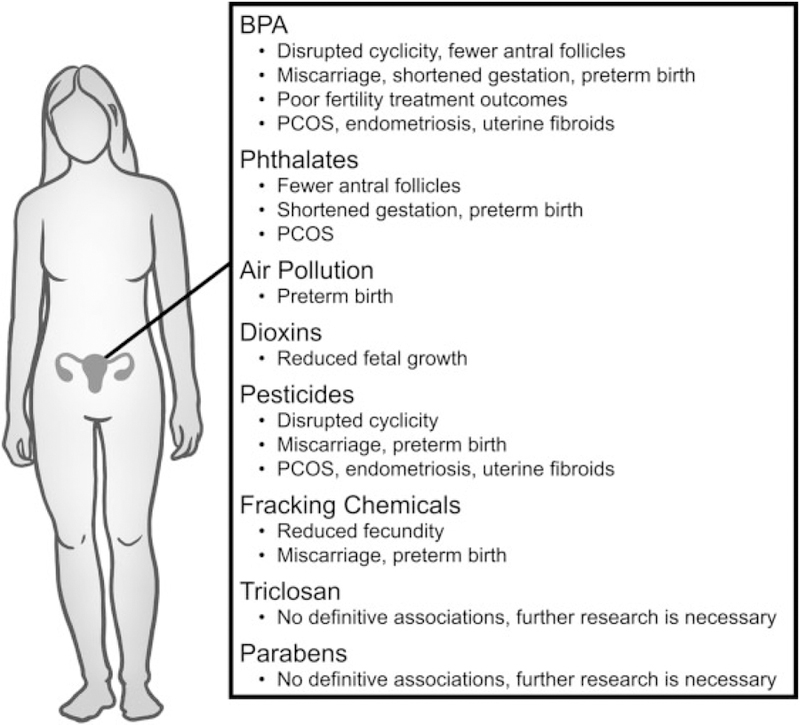
A brief summation of epidemiological research investigating endocrine disruptors and their associations with aspects of infertility and reproductive disease in women.
Bisphenol A
BPA is a chemical used in a wide variety of products such as medical equipment, epoxy resins lining aluminum cans, polycarbonate plastics, and dental sealants.1–5 Although BPA can be absorbed via inhalation, ingestion, and dermal contact, the most common route of exposure is via ingestion of food or beverages contaminated with BPA.1 After exposure, BPA can be distributed throughout the body to various bodily fluids such as colostrum, ovarian follicular fluid, breast milk, urine, semen, blood, and plasma.1,5–7 Due to BPA’s ability to interact with hormone receptors2 and the extensive exposure of the general population to BPA, the associations between BPA exposure and reproductive outcomes have been widely investigated and the results are summarized below.
Associations with Male Reproductive Outcomes
BPA exposure has been associated with general male inferti-lity,1,6 but the results are equivocal8–10 (►Fig. 1). High levels of BPA also have been associated with reduced sexual desire, increased erectile and ejaculatory difficulties, and increased severity of infertility.1,6 Furthermore, seminal and urinary BPA levels have been negatively correlated with sperm density, total count and concentration,1,2,6,10,11 moti-lity,2,6,10,12 and morphology.1,6,10,11 However, some studies reported no association between BPA exposure and these semen quality parameters.8,10,12 In addition, although studies examining paternal exposure to BPA and assisted reproductive technologies (ART) are sparse, one study found that paternal urinary BPA levels were not associated with fertilization, embryo quality, implantation, or live birth.10 Furthermore, another study reported no association be-tween paternal urinary BPA levels and couples fecundity.13
Associations with Female Reproductive Outcomes
BPA exposure has been associated with fertility outcomes in women (►Fig. 2). Collectively, these studies have shown that elevated serum or urinary BPA levels were associated with anovulation,14 lower antral follicle counts,5,15 and inferti-lity.5 Further, BPA exposure has been associated with mis-carriage,16–18 shortened gestation, 19 and preterm birth.2 In contrast, some studies reported no association between urinary BPA levels and fecundity, time to pregnancy,5,13 or spontaneous abortions.20
As in males, BPA exposure in women has been associated with poor ART outcomes. Specifically, serum BPA levels were negatively associated with number of oocytes retrieved, oocyte maturation, fertilization rates, as well as embryo quality in women undergoing in vitro fertilization (IVF) treatment.2,5 Additionally, urinary BPA levels were associated with a decrease in successful implantation rate in women undergoing IVF.2,5 However, some studies reported null associations between urinary BPA levels and number of oocytes retrieved, embryo quality, and fertilization rates.5
Several studies investigated BPA exposure and incidence of reproductive disorders such as polycystic ovarian syndrome (PCOS), endometriosis, and fibroids. The majority of these studies showed that women with PCOS had significantly higher levels of BPA in the blood,14,21 serum,21,22 and follicular fluid22,23 compared with women without PCOS. Similarly, women with endometriosis had higher levels of BPA than women without endometriosis,2,24 and women with fibroids had higher levels of BPA than women without fibroids.25,26 However, some studies have found no associations between serum BPA concentrations and incidence of PCOS,27 endometriosis,28 or fibroids.29
Phthalates
Phthalates are synthetic chemicals used in cosmetics, medications, plastic toys, and construction material. Phthalate exposure can occur via ingestion, inhalation, and dermal contact,3,4,30 and phthalate metabolites can be detected in more than 99% of urine samples.30 The phthalates described here include di-(2-ethylhexyl) phthalate (DEHP), diethyl phthalate (DEP), dibutyl phthalate (DBP), and benzyl butyl phthalate (BBP). DEHP is commonly found in materials such as surgical tubing and gloving as well as plastic food storage containers, roofing, and carpeting.31 DEP and DBP are mostly found in personal care products such as lotions, nail polishes, and fragrances, whereas BBP is used in products such as vinyl flooring.32 The associations between exposure to these phthalates and reproductive outcomes in men and women (►Figs. 1 and 2) are summarized below.
Di-(2-Ethylhexyl) Phthalate
Associations with Male Reproductive Outcomes
Several studies, but not all, have found that levels of DEHP or DEHP metabolites were associated with reduced fertility in men. Specifically, seminal DEHP levels and urinary levels of the DEHP metabolites mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), but not the DEHP metabolites mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl-5-oxo-hexyl) phthalate (MEOHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), or mono-(2-carboxymethylhexyl) phthalate (MCMHP), have been associated with male infer-tility.13,33,34 Further, serum concentrations of the sum of DEHP metabolites or the individual metabolites (MEOHP, MEHHP, and MECPP) have been significantly associated with decreased semen volume and sperm count,11,35–38 sperm concentration,37,39,40 sperm motility,11,36,39–42 percent of morphologically normal sperm,36,43 and acrosin activity.42 In contrast, several studies reported no associations between DEHP exposure and risk of subfertility,9 fecundity,13 sperm count, concentration, motility, or morphology.9,35,37–39,43–45
Associations with Female Reproductive Outcomes
Several studies have examined DEHP exposure and fertility in women. Overall, these studies have shown that urinary DEHP metabolites were significantly associated with decreased19,46 as well as increased gestational length,46 in-creased risk of preterm birth,30,47,48 and intrauterine growth restriction.49 Furthermore, urinary DEHP metabolites were associated with reduced antral follicle counts, a potential indicator of subfertility.50 However, some studies reported null associations when examining urinary levels of DEHP metabolites and fertility parameters such as time to preg-nancy,51 infertility,34,45 and fecundity.13,34
Similar to studies on BPA, DEHP exposure has been associated with ART outcomes. In women undergoing IVF, urinary levels of the sum of DEHP metabolites were significantly associated with reduced total oocyte yield and a reduced probability of achieving pregnancy and live birth.52 In contrast, a Chinese study found no association between urinary MEHP levels and clinical pregnancy loss.53
Additionally, some studies on DEHP have shown that urinary metabolites of DEHP were associated with reproductive disorders such as PCOS, endometriosis, and fibroids.2,27,46,54 In contrast, one study found that urinary levels of MEHP were inversely associated with endometriosis risk,55 and one study reported urinary levels of MEHP, MEHHP, or MEOHP were not associated with endometriosis.56 Further, one study found that urinary levels of MEHP, MEOHP, MEHHP, and MECPP were not associated with uterine fibroids.25
Diethyl Phthalate
Associations with Male Reproductive Outcomes
A few studies have examined the associations between DEP exposure and fertility in men. Specifically, seminal levels of DEP and urinary levels of its metabolite monoethyl phthalate (MEP) have been significantly associated with male infertility,33,45 reduced sperm count and concentration,37,45 reduced sperm motility,37 and reduced numbers of morphologically normal sperm.36,37,45 However, a few studies have found no associations between urinary levels of MEP and subfertility in men,9,13 as well as sperm count and concentration, motility, viability, and abnormal morphology.9,37,43–45
Associations with Female Reproductive Outcomes
Studies investigating DEP exposure and indicators of general fertility in women are scarce, but some studies have indicated that urinary MEP levels were significantly higher in females from infertile couples than in females from fertile couples45 and that urinary MEP levels were significantly associated with clinical pregnancy loss53 and preterm delivery.57 However, some studies have found no associations between urinary MEP concentrations and time to pregnancy,13,51 clinical pregnancy loss,46 and preterm birth.46,48 Further, urinary levels of MEP were not associated with the probability of pregnancy or odds of live birth in women undergoing IVF or intracytoplasmic sperm injection (ICSI).52
Recent epidemiological studies investigating the associations between DEP exposure and reproductive disorders are limited in number, but one study reported that urinary concentrations of MEP were significantly associated with an increased incidence of PCOS.27 Further, a few studies reported no associations between urinary MEP concentrations and endometriosis46,55 or uterine fibroids.25,46
Dibutyl Phthalate
Associations with Male Reproductive Outcomes
A handful of studies have investigated the potential associations between DBP exposure and indicators of general fertility in men. These studies indicated that seminal levels of DBP33 and urinary levels of one of its metabolites, mono-n-butyl phthalate (MnBP),45 were significantly higher in infertile men when compared with fertile men. Further, urinary levels of MnBP, DBP, or another metabolite, monobutyl phthalate (MBP), were associated with lowered sperm concentration,37,43–45,58 reduced total sperm count,43 reduced sperm motility,37,41,44,45,58 reduced proportions of morphologically normal sperm,36,42 and reduced acrosin activity.42 Additionally, paternal urinary concentrations of MBP have been significantly associated with an increased time to pregnancy in couples.13 Interestingly, some studies reported that urinary MnBP levels were significantly associated with increased semen volume and sperm concentration.45,59 In contrast, some studies showed that urinary MnBP levels were not associated with risk of subfertility9 and that DBP exposure was not associated with sperm count, concentration, motility, or morphology.9,37,40–43,45
Associations with Female Reproductive Outcomes
Studies investigating the associations between DBP exposure and general indicators of female fertility are scarce. However, urinary MnBP levels were significantly higher in women from infertile couples than women from fertile couples,45 and urinary levels of MnBP from pregnant mothers were significantly associated with clinical pregnancy loss.53 Further, urinary MBP levels as well as cord blood concentrations of DBP were significantly associated with odds of preterm birth.30,47,48,57 Contrarily, some studies have found that urinary MBP levels were not significantly associated with time to pregnancy,13,51 gestational age,46 and pregnancy loss.46 Similarly, one study reported no significant associations between urinary levels of MBP and probability of pregnancy or live birth in women undergoing IVF or live births resulting from ICSI.52
Few studies have examined associations between expo-sure to DBP and PCOS, endometriosis, and fibroids. Interestingly, women with PCOS were more likely to have lower levels of urinary MBP than those without PCOS.27 Further, urinary MBP levels were significantly associated with nearly a twofold increase in odds of an endometriosis diagnosis,2 and urinary MnBP levels as well as plasma DBP levels were significantly associated with endometriosis.46 However, several studies reported null associations between urinary MBP46 and MnBP46,54,55 and endometriosis. Similarly, studies consistently reported no association between urinary MBP46 or MnBP25,46 and uterine fibroids.
Benzyl Butyl Phthalate
Associations with Male Reproductive Outcomes
Few studies have examined the associations between expo-sure to BBP and fertility in men. These studies showed that seminal levels of BBP were significantly higher in a group of infertile men when compared with a group of fertile men33 and that paternal urinary levels of metabolite monobenzyl phthalate (MBzP) were significantly associated with an increased time to pregnancy,13 decreased sperm count and concentration,36,42,44 and reduced numbers of morphologically normal sperm.36,42 Interestingly, some studies reported that urinary MBzP levels were associated with higher semen volume43,59 as well as increased sperm concentration.45 How-ever, some studies reported no association between urinary MBzP and risk of subfertility,9,45 semen volume, or sperm count, concentration, morphology, or motility.9,37,42–45
Associations with Female Reproductive Outcomes
Very few recent studies have investigated the associations between BBP exposure and general indicators of female fertility. Although one study indicated that BBP exposure is associated with preterm birth,48,57 some studies reported no association between urinary MBzP concentrations and risk of infertility,45 time to pregnancy,13,51 and gestational age.46 Further, urinary levels of MBzP were not associated with odds of pregnancy or live birth in women undergoing IVF or live births in women undergoing ICSI.52 Finally, urinary MBzP levels were inversely associated with an increased odds of PCOS,27 but not with endometriosis46,54,55 or uterine fibroids.25,46
Air Pollutants
Air pollution has significant harmful effects on environmental and human health worldwide. Inhalation exposure to air pollutants such as particulate matter (PM), ozone (O3), sulfur dioxide (SO2), nitrogen oxides (NOx), carbon monoxide (CO), polycyclic aromatic hydrocarbons (PAHs), and volatile organic compounds (VOCs) has been associated with various health problems, including reproductive disease.60–62
Associations with Male Reproductive Outcomes
Several cross-sectional, longitudinal, and ecological studies have found that air pollution was significantly negatively associated with sperm quality, which may contribute to male-mediated infertility (►Fig. 1). For example, exposure to PM 10 µM or less in size (PM10) was associated with increased sperm aneuploidy, DNA fragmentation, abnormal chromatin and sperm morphology, and decreased sperm motility.61 Exposure to fine PM less than 2.5 µM (PM2.5) was associated with sperm aneuploidy and decreased sperm motility, with 2- to 3-month lags. Ambient O3 levels were significantly associated with low average sperm concentrations at all biologically relevant time points during spermatogenesis. Other gaseous pollutants such as SO2, NOx, and CO were associated with reduced sperm motility and kinetics and increased morphological abnormalities.61 Further, traffic police and tollgate workers exposed to motor vehicle exhaust had an increased proportion of sperm with damaged chromatin and fragmented DNA compared with unexposed, healthy men.62 In contrast, one study found no associations between exposure to O3 or PM2.5 at levels below the current National Ambient Air Quality Standards and sperm quality parameters.61 Similarly, one study found that exposure to CO, NO2, and PM10 was not associated with sperm quality.62
Associations with Female Reproductive Outcomes
Although limited information is available on air pollutants and female reproductive outcomes (►Fig. 2), one study indicated an association between residential proximity to major roads (as an index of air pollution exposure) and preeclampsia, premature rupture of membrane, and preterm birth, but not gestational diabetes mellitus, placenta abruption, placenta previa, or preterm labor.63 Similarly, exposure to ambient PM2.5 increased the risk of preterm birth, particularly if exposure occurred during early or mid-pregnancy.64 Further, maternal exposure to emissions from municipal solid-waste incinerators throughout pregnancy was associated with preterm birth.65 Ambient air pollution was also correlated with spontaneous abortion66 as well as decreased fecundability.67
Dioxins
Dioxins and dioxin-like compounds are a group of chemicals with similar structural and biological properties and include polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and certain polychlorinated biphenyls. They are released into the environment as by-products of combustion (e.g., municipal and commercial waste incineration, burning of fuels, and forest fires), chemical manufacture (e.g., pesticide and herbicide production, and chlorine bleaching of paper and pulp), and metal industry operations. These compounds are highly stable, persistent pollutants in the environment that bioaccumulate in the food chain. Consumption of contaminated food and water is the major source of human exposure. Dioxins are a public health threat worldwide and have been associated with serious health issues, including reproductive problems.3,68
Associations with Male Reproductive Outcomes
Dioxin exposure has been associated with adverse male reproductive outcomes (►Fig. 1). Specifically, dioxin content in semen was associated with male infertility.69 Further, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) content in semen was associated with abnormal spermiograms (characterized by teratozoospermia, oligospermia, and asthenospermia).69 However, not all studies found associations between dioxin exposure and semen quality parameters or risk of subfertility in men.9
Associations with Female Reproductive Outcomes
As in males, dioxin exposure in females has been associated with adverse reproductive outcomes (►Fig. 2). Maternal exposure to dioxins was associated with reduced fetal growth at intakes below the tolerable weekly intake of 14 picograms toxic equivalents/kilogram body weight/week.70 However, the Seveso Women’s Health Study, a retrospective cohort of TCDD exposure and reproductive health that assessed pregnancy outcomes over 30 years following a chemical explosion resulting in the highest known residential exposure to TCDD, found no significant associations between maternal exposure and spontaneous abortion, fetal growth, gestational length, or endometriosis.71 Similarly, a Japanese study found no significant association between serum dioxin levels and endometriosis in infertile women diagnosed with the condition.72
Pesticides
Pesticides, which include herbicides, insecticides, and fungicides, play a major role in agriculture, horticulture, livestock production, and disease control. Their use has been associated with adverse effects on human and environmental health worldwide. Certain pesticides such as organochlorines are persistent pollutants in the environment and show bioaccumulation in the food web.73 Exposure to pesticides and pesticide residues occurs through ingestion of contaminated food, water, and soil, inhalation, as well as dermal and ocular contact. Pesticides are thought to act as endocrine disruptors and may be associated with reproductive problems.74
Associations with Male Reproductive Outcomes
Collectively, various pesticide exposures have been associated with reduced sperm numbers, motility, or volume (►Fig. 1). Specifically, agricultural pesticide exposure was associated with a decline in semen quality, decreased sperm count, decreased sperm motility, and increased teratospermia.75 Similarly, organophosphate pesticides were associated with reduced sperm motility, seminal volume, and increased sperm morphological abnormalities.75 Further, occupational exposure to the herbicide 2,4-dichlorophenoxyacetic acid was associated with asthenospermia, necrospermia, and teratospermia.75 Occupational exposure to the carbamate insecticide carbaryl was associated with low sperm counts and abnormal sperm morphology, and occupational exposure to dibromo-chloropropane was associated with male subfertility, oligospermia, and genetic alterations in sperm.74 Exposure to organophosphate pesticides (dimethyl phosphate, dimethyl thiophosphate, dimethyl dithiophosphate) was negatively associated with sperm concentration, total sperm count, and percentage of motile sperm.76 Exposure to pyrethroid pesticides was positively associated with the percentage of medium sperm DNA fragmentation index and high sperm DNA fragmentation index77 as well as abnormal sperm morphology and low sperm concentration.78 Increased serum levels of oxychlordane (a metabolite of the organochlorine pesticide, chlordane) were associated with an increased risk of subfertility and decreased sperm concentration and motility.9 Environmental exposures to dichlorodiphenyldichloroethylene (DDE) and lindane were negatively associated with sperm motility,79 and DDE exposure was associated with increased sperm sex-chromosome disomy.80 Finally, high plasma abamectin levels were associated with decreased sperm motility and sperm maturity.81
Associations with Female Reproductive Outcomes
Similar to men, organochlorine pesticide exposures have been associated with adverse reproductive outcomes in women such as low fecundability, miscarriage, preeclampsia, intrauterine growth restriction, poor weight gain during fetal development, and preterm birth73,82 (►Fig. 2). Further, organochlorine pesticide exposure has been associated with an increased risk of PCOS,22 endometriosis,83 and fibroids.84 Interestingly, exposure to organochlorine pesticides was associated with shorter menstrual cycles, whereas exposure to non-organochlorine pesticides was associated with longer menstrual cycles or absence of cycles.14 Similarly, exposure to the commonly used herbicide atrazine via drinking water was associated with menstrual cycle irregularity and longer follicular phases.85 Finally, exposure to pyrethroids in women living in rural South Africa was negatively associated with plasma levels of anti-Müllerian hormone, a marker of ovarian reserve.86 However, one study reported no association between glyphosate, another commonly used herbicide, and adverse reproductive effects at environmentally relevant concentrations.87
Fracking Chemicals
Fracking chemicals are used and produced during the process of drilling and hydraulic fracturing for oil and gas manufacture. Fracking chemicals from oil and gas extraction, processing, transport, and disposal of waste water contaminate the air, surface water, and ground water. Oral, inhalation, and dermal exposure to these chemicals can occur through residential proximity or occupational exposure. Several fracking chemicals have been associated with endocrine disrupting activities and adverse reproductive outcomes.88,89
Associations with Male Reproductive Outcomes
A few studies reported that occupational exposure to fracking operations was associated with decreased sperm concentration and motility as well as increased DNA damage88 (►Fig. 1). Further, exposures to fracking chemicals such as benzene, toluene, ethylbenzene, and xylene were associated with low sperm count, motility, and viability; abnormal sperm morphology; and abnormal semen viscosity.90
Associations with Female Reproductive Outcomes
Several studies have reported a significant association between maternal exposure to fracking operations and preterm birth as well as miscarriage, but not the incidence of still-births88 (►Fig. 2). Further, the fracking chemical toluene was associated with reduced fecundity, conception failure, miscarriage, and premature menopause.90
Triclosan
Triclosan is a phenolic compound with antimicrobial activity that is commonly used in products such as soaps, disinfectants, and toothpaste.8 Exposure to triclosan occurs via oral, inhalation, and dermal routes.18 Research on triclosan is sparse and epidemiological data regarding its association with reproductive outcomes are lacking (►Figs. 1 and 2).
Associations with Male Reproductive Outcomes
A case–control study that examined whether exposure to various endocrine disrupting chemicals was associated with subfertility in men found no associations between triclosan and semen quality parameters or risk of subfertility.9 Another case–control study that examined the relationship between exposure to phenols and idiopathic male infertility found that urinary triclosan concentrations were not significantly associated with idiopathic male infertility.8
Associations with Female Reproductive Outcomes
Little information is available about the relationship between triclosan and female fertility. However, a retrospective time to pregnancy study in women recruited from the Maternal-Infant Research on Environmental Chemicals (MIREC) study suggested that elevated urinary triclosan levels may be associated with decreased fecundity.51
Parabens
Parabens are a group of p-hydroxybenzoic acid esters that are commonly found in cosmetics as preservatives. They can also be found in foods where they are used as antimicrobials to help preserve foods and increase shelf life. Humans are most commonly exposed to parabens via dermal contact or ingestion.91 Studies investigating the potential associations between parabens and fertility outcomes in men and women are sparse (►Figs. 1 and 2).
Associations with Male Reproductive Outcomes
Although limited information exists on parabens and male reproductive outcomes, one study reported that paternal urinary methylparaben concentrations were associated with a decrease in live birth after intrauterine insemination.92 In contrast, paternal urinary concentrations of methyl-, propyl-or butylparaben were not associated with fertilization rate, embryo quality, or odds of implantation in couples who underwent IVF.92 Similarly, urinary concentrations of methyl-, propyl-, and butylparaben were not associated with sperm concentration or motility.93
Associations with Female Reproductive Outcomes
Few epidemiological studies have investigated parabens and female reproductive health. One such study found no significant associations between urinary concentrations of methyl-, propyl-, or butylparaben with total and mature oocyte yields, embryo quality, fertilization rates, implantation, clinical pregnancy, or live birth rates in women undergoing IVF.94
Conclusion
The average person is exposed to environmental endocrine disruptors daily due to their ubiquitous presence in the general environment as well as their use in a wide variety of consumer products. Many of the studies published during the past 5 years demonstrate significant negative associations between exposure to the selected chemicals and reproductive outcomes in both men and women. As negative reproductive outcomes are associated with overall health in men, women, and offspring, it is important to better understand how environmental exposures contribute to these reproductive outcomes and in turn overall health status of those with adverse reproductive outcomes as a result of their exposures. In general, BPA exposure was associated with several negative reproductive factors in men and women with relative consistency. Similarly, dioxins and pesticides were significantly associated with reduced fertility parameters in men and women. However, not all studies reported significant associations between chemical exposures and reproductive outcomes in humans. These conflicting results could be due to the high variability of study population, sample size, methods of measuring exposure levels, as well as the reproductive outcomes measured between studies. Thus, further studies are needed to clarify associations between exposures to these chemicals, further define the possible mechanisms through which these chemicals act, and determine how they contribute to negative reproductive outcomes that may be markers of overall health.
Acknowledgments
This work was supported by grant NIH R56 ES 025147 and Environmental Toxicology Training grant NIH T32 ES 007326.
Footnotes
Conflict of Interest
The authors do not have any conflicts of interest to declare.
References
- 1.Manfo FP, Jubendradass R, Nantia EA, Moundipa PF, Mathur PP. Adverse effects of bisphenol A on male reproductive function. Rev Environ Contam Toxicol 2014;228:57–82 [DOI] [PubMed] [Google Scholar]
- 2.Peretz J, Vrooman L, Ricke WA, et al. Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect 2014;122(08):775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenardhanan P, Panneerselvam M, Mathur PP. Effect of environmental contaminants on spermatogenesis. Semin Cell Dev Biol 2016;59:126–140 [DOI] [PubMed] [Google Scholar]
- 4.Patel S, Zhou C, Rattan S, Flaws JA. Effects of endocrine-disrupting chemicals on the ovary. Biol Reprod 2015;93(01):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziv-Gal A, Flaws JA. Evidence for bisphenol A-induced female infertility: a review (2007–2016). Fertil Steril 2016;106(04):827–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitku J, Heracek J, Sosvorova L, et al. Associations of bisphenol A and polychlorinated biphenyls with spermatogenesis and steroidogenesis in two biological fluids from men attending an infertility clinic. Environ Int 2016;89–90:166–173 [DOI] [PubMed] [Google Scholar]
- 7.Cantonwine DE, Hauser R, Meeker JD. Bisphenol A and human reproductive health. Expert Rev Obstet Gynecol 2013;8(04) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M, Tang R, Fu G, et al. Association of exposure to phenols and idiopathic male infertility. J Hazard Mater 2013;250–251:115–121 [DOI] [PubMed] [Google Scholar]
- 9.Den Hond E, Tournaye H, De Sutter P, et al. Human exposure to endocrine disrupting chemicals and fertility: a case-control study in male subfertility patients. Environ Int 2015;84:154–160 [DOI] [PubMed] [Google Scholar]
- 10.Mínguez-Alarcón L, Hauser R, Gaskins AJ. Effects of bisphenol A on male and couple reproductive health: a review. Fertil Steril 2016; 106(04):864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagos-Cabré R, Moreno RD. Contribution of environmental pollutants to male infertility: a working model of germ cell apoptosis induced by plasticizers. Biol Res 2012;45(01):5–14 [DOI] [PubMed] [Google Scholar]
- 12.Lassen TH, Frederiksen H, Jensen TK, et al. Urinary bisphenol A levels in young men: association with reproductive hormones and semen quality. Environ Health Perspect 2014;122(05):478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril 2014;101(05):1359–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa EM, Spritzer PM, Hohl A, Bachega TA. Effects of endocrine disruptors in the development of the female reproductive tract. Arq Bras Endocrinol Metabol 2014;58(02):153–161 [DOI] [PubMed] [Google Scholar]
- 15.Souter I, Smith KW, Dimitriadis I, et al. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol 2013;42:224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lathi RB, Liebert CA, Brookfield KF, et al. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil Steril 2014;102(01):123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Y, Zheng Y, Jiang J, et al. Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: evidence from a case-control study in eastern China. PLoS One 2015;10(05):e0127886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan M, Bai MZ, Huang XF, et al. Preimplantation exposure to bisphenol A and triclosan may lead to implantation failure in humans. BioMed Res Int 2015;2015:184845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberger B, Vetrano AM, Archer FE, et al. Effects of maternal exposure to phthalates and bisphenol A during pregnancy on gestational age. J Matern Fetal Neonatal Med 2014;27(04):323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Chen M, Xu B, et al. Parental phenols exposure and spontaneous abortion in Chinese population residing in the middle and lower reaches of the Yangtze River. Chemosphere 2013;93(02):217–222 [DOI] [PubMed] [Google Scholar]
- 21.Palioura E, Diamanti-Kandarakis E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev Endocr Metab Disord 2015;16(04):365–371 [DOI] [PubMed] [Google Scholar]
- 22.Rutkowska AZ, Diamanti-Kandarakis E. Polycystic ovary syndrome and environmental toxins. Fertil Steril 2016;106(04):948–958 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhu Q, Dang X, He Y, Li X, Sun Y. Local effect of bisphenol A on the estradiol synthesis of ovarian granulosa cells from PCOS. Gynecol Endocrinol 2017;33(01):21–25 [DOI] [PubMed] [Google Scholar]
- 24.Simonelli A, Guadagni R, De Franciscis P, et al. Environmental and occupational exposure to bisphenol A and endometriosis: urinary and peritoneal fluid concentration levels. Int Arch Occup Environ Health 2017;90(01):49–61 [DOI] [PubMed] [Google Scholar]
- 25.Pollack AZ, Buck Louis GM, Chen Z, et al. Bisphenol A, benzophenone-type ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environ Res 2015;137:101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Y, Xu Q, Ren M, Feng X, Cai Y, Gao Y. Measurement of phenolic environmental estrogens in women with uterine leiomyoma. PLoS One 2013;8(11):e79838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vagi SJ, Azziz-Baumgartner E, Sjödin A, et al. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: a case-control study. BMC Endocr Disord 2014;14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upson K, Sathyanarayana S, De Roos AJ, Koch HM, Scholes D, Holt VL. A population-based case-control study of urinary bisphenol A concentrations and risk of endometriosis. Hum Reprod 2014; 29(11):2457–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Y, Dong YM, Lu Q, et al. Phenolic environmental estrogens in urine and blood plasma from women with uterine leiomyoma: epidemiological survey. J Obstet Gynaecol Res 2016;42(04): 440–445 [DOI] [PubMed] [Google Scholar]
- 30.Ferguson KK, O’Neill MS, Meeker JD. Environmental contaminant exposures and preterm birth: a comprehensive review. J Toxicol Environ Health B Crit Rev 2013;16(02):69–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne) 2015;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environ Int 2015; 85:27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang SY, Wang Y, Xie FQ, et al. Analysis of PAEs in semen of infertile men. Int J Occup Environ Health 2015;21(01):40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Specht IO, Bonde JP, Toft G, Lindh CH, Jönsson BA, Jørgensen KT. Serum phthalate levels and time to pregnancy in couples from Greenland, Poland and Ukraine. PLoS One 2015;10(03):e0120070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Specht IO, Toft G, Hougaard KS, et al. Associations between serum phthalates and biomarkers of reproductive function in 589 adult men. Environ Int 2014;66:146–156 [DOI] [PubMed] [Google Scholar]
- 36.Bloom MS, Whitcomb BW, Chen Z, Ye A, Kannan K, Buck Louis GM. Associations between urinary phthalate concentrations and semen quality parameters in a general population. Hum Reprod 2015;30(11):2645–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jurewicz J, Hanke W. Exposure to phthalates: reproductive outcome and children health. A review of epidemiological studies. Int J Occup Med Environ Health 2011;24(02):115–141 [DOI] [PubMed] [Google Scholar]
- 38.Lenters V, Portengen L, Smit LA, et al. Phthalates, perfluoroalkyl acids, metals and organochlorines and reproductive function: a multipollutant assessment in Greenlandic, Polish and Ukrainian men. Occup Environ Med 2015;72(06):385–393 [DOI] [PubMed] [Google Scholar]
- 39.Huang LP, Lee CC, Fan JP, Kuo PH, Shih TS, Hsu PC. Urinary metabolites of di(2-ethylhexyl) phthalate relation to sperm motility, reactive oxygen species generation, and apoptosis in polyvinyl chloride workers. Int Arch Occup Environ Health 2014;87(06):635–646 [DOI] [PubMed] [Google Scholar]
- 40.Pant N, Kumar G, Upadhyay AD, Patel DK, Gupta YK, Chaturvedi PK. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ Sci Pollut Res Int 2014;21(18):11066–11074 [DOI] [PubMed] [Google Scholar]
- 41.Axelsson J, Rylander L, Rignell-Hydbom A, Jönsson BA, Lindh CH, Giwercman A. Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environ Int 2015;85:54–60 [DOI] [PubMed] [Google Scholar]
- 42.Pan Y, Jing J, Dong F, et al. Association between phthalate metabolites and biomarkers of reproductive function in 1066 Chinese men of reproductive age. J Hazard Mater 2015;300:729–736 [DOI] [PubMed] [Google Scholar]
- 43.Wang YX, You L, Zeng Q, et al. Phthalate exposure and human semen quality: results from an infertility clinic in China. Environ Res 2015;142:1–9 [DOI] [PubMed] [Google Scholar]
- 44.Cai H, Zheng W, Zheng P, et al. Human urinary/seminal phthalates or their metabolite levels and semen quality: a meta-analysis. Environ Res 2015;142:486–494 [DOI] [PubMed] [Google Scholar]
- 45.Kay VR, Bloom MS, Foster WG. Reproductive and developmental effects of phthalate diesters in males. Crit Rev Toxicol 2014; 44(06):467–498 [DOI] [PubMed] [Google Scholar]
- 46.Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol 2013; 43(03):200–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr 2014;168(01): 61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int 2014;70:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y, Chen L, Li LX, et al. Gender-specific relationship between prenatal exposure to phthalates and intrauterine growth restriction. Pediatr Res 2014;76(04):401–408 [DOI] [PubMed] [Google Scholar]
- 50.Messerlian C, Souter I, Gaskins AJ, et al. ; Earth Study Team. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum Reprod 2016;31(01):75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vélez MP, Arbuckle TE, Fraser WD. Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril 2015;103(04):1011–1020.e2 [DOI] [PubMed] [Google Scholar]
- 52.Hauser R, Gaskins AJ, Souter I, et al. ; EARTH Study Team. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the EARTH study. Environ Health Perspect 2016;124(06): 831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mu D, Gao F, Fan Z, Shen H, Peng H, Hu J, Levels of Phthalate Metabolites in Urine of Pregnant Women and Risk of Clinical Pregnancy Loss. Levels of phthalate metabolites in urine of pregnant women and risk of clinical pregnancy loss. Environ Sci Technol 2015;49(17):10651–10657 [DOI] [PubMed] [Google Scholar]
- 54.Kim SH, Cho S, Ihm HJ, et al. Possible role of phthalate in the pathogenesis of endometriosis: in vitro, animal, and human data. J Clin Endocrinol Metab 2015;100(12):E1502–E1511 [DOI] [PubMed] [Google Scholar]
- 55.Upson K, Sathyanarayana S, De Roos AJ, et al. Phthalates and risk of endometriosis. Environ Res 2013;126:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Itoh H, Iwasaki M, Hanaoka T, Sasaki H, Tanaka T, Tsugane S. Urinary phthalate monoesters and endometriosis in infertile Japanese women. Sci Total Environ 2009;408(01):37–42 [DOI] [PubMed] [Google Scholar]
- 57.Huang Y, Li J, Garcia JM, et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One 2014;9(02):e87430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knez J Endocrine-disrupting chemicals and male reproductive health. Reprod Biomed Online 2013;26(05):440–448 [DOI] [PubMed] [Google Scholar]
- 59.Toshima H, Suzuki Y, Imai K, et al. Endocrine disrupting chemicals in urine of Japanese male partners of subfertile couples: a pilot study on exposure and semen quality. Int J Hyg Environ Health 2012;215(05):502–506 [DOI] [PubMed] [Google Scholar]
- 60.Giudice LC. Environmental toxicants: hidden players on the reproductive stage. Fertil Steril 2016;106(04):791–794 [DOI] [PubMed] [Google Scholar]
- 61.Deng Z, Chen F, Zhang M, et al. Association between air pollution and sperm quality: A systematic review and meta-analysis. Environ Pollut 2016;208(Pt B):663–669 [DOI] [PubMed] [Google Scholar]
- 62.Rengaraj D, Kwon WS, Pang MG. Effects of motor vehicle exhaust on male reproductive function and associated proteins. J Proteome Res 2015;14(01):22–37 [DOI] [PubMed] [Google Scholar]
- 63.Yorifuji T, Naruse H, Kashima S, Murakoshi T, Doi H. Residential proximity to major roads and obstetrical complications. Sci Total Environ 2015;508:188–192 [DOI] [PubMed] [Google Scholar]
- 64.Chang HH, Warren JL, Darrow LA, Reich BJ, Waller LA. Assessment of critical exposure and outcome windows in time-to-event analysis with application to air pollution and preterm birth study. Biostatistics 2015;16(03):509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Candela S, Ranzi A, Bonvicini L, et al. Air pollution from incinerators and reproductive outcomes: a multisite study. Epidemiology 2013;24(06):863–870 [DOI] [PubMed] [Google Scholar]
- 66.Enkhmaa D, Warburton N, Javzandulam B, et al. Seasonal ambient air pollution correlates strongly with spontaneous abortion in Mongolia. BMC Pregnancy Childbirth 2014;14:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slama R, Bottagisi S, Solansky I, Lepeule J, Giorgis-Allemand L, Sram R. Short-term impact of atmospheric pollution on fecundability. Epidemiology 2013;24(06):871–879 [DOI] [PubMed] [Google Scholar]
- 68.United States Environmental Protection Agency. An Inventory of Sources and Environmental Releases of Dioxin-Like Compounds in the United States for the Years 1987, 1995, and 2000 (EPA/600/ P-03/002F) Washington, DC: National Center for Environmental Assessment; 2006 [Google Scholar]
- 69.Galimova EF, Amirova ZK, Galimov ShN. Dioxins in the semen of men with infertility. Environ Sci Pollut Res Int 2015;22(19): 14566–14569 [DOI] [PubMed] [Google Scholar]
- 70.Papadopoulou E, Caspersen IH, Kvalem HE, et al. Maternal dietary intake of dioxins and polychlorinated biphenyls and birth size in the Norwegian Mother and Child Cohort Study (MoBa). Environ Int 2013;60:209–216 [DOI] [PubMed] [Google Scholar]
- 71.Wesselink A, Warner M, Samuels S, et al. Maternal dioxin exposure and pregnancy outcomes over 30 years of follow-up in Seveso. Environ Int 2014;63:143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soave I, Caserta D, Wenger JM, Dessole S, Perino A, Marci R. Environment and Endometriosis: a toxic relationship. Eur Rev Med Pharmacol Sci 2015;19(11):1964–1972 [PubMed] [Google Scholar]
- 73.Annamalai J, Namasivayam V. Endocrine disrupting chemicals in the atmosphere: Their effects on humans and wildlife. Environ Int 2015;76:78–97 [DOI] [PubMed] [Google Scholar]
- 74.Sengupta P, Banerjee R. Environmental toxins: alarming impacts of pesticides on male fertility. Hum Exp Toxicol 2014;33(10): 1017–1039 [DOI] [PubMed] [Google Scholar]
- 75.Mehrpour O, Karrari P, Zamani N, Tsatsakis AM, Abdollahi M. Occupational exposure to pesticides and consequences on male semen and fertility: a review. Toxicol Lett 2014;230(02):146–156 [DOI] [PubMed] [Google Scholar]
- 76.Melgarejo M, Mendiola J, Koch HM, Moñino-García M, Noguera-Velasco JA, Torres-Cantero AM. Associations between urinary organophosphate pesticide metabolite levels and reproductive parameters in men from an infertility clinic. Environ Res 2015; 137:292–298 [DOI] [PubMed] [Google Scholar]
- 77.Jurewicz J, Radwan M, Wielgomas B, et al. The effect of environmental exposure to pyrethroids and DNA damage in human sperm. Syst Biol Reprod Med 2015;61(01):37–43 [DOI] [PubMed] [Google Scholar]
- 78.Radwan M, Jurewicz J, Wielgomas B, et al. Semen quality and the level of reproductive hormones after environmental exposure to pyrethroids. J Occup Environ Med 2014;56(11):1113–1119 [DOI] [PubMed] [Google Scholar]
- 79.Pant N, Shukla M, Upadhyay AD, Chaturvedi PK, Saxena DK, Gupta YK. Association between environmental exposure to p, p′-DDE and lindane and semen quality. Environ Sci Pollut Res Int 2014; 21(18):11009–11016 [DOI] [PubMed] [Google Scholar]
- 80.McAuliffe ME, Williams PL, Korrick SA, Altshul LM, Perry MJ. Environmental exposure to polychlorinated biphenyls and p,p ′-DDE and sperm sex-chromosome disomy. Environ Health Perspect 2012;120(04):535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Celik-Ozenci C, Tasatargil A, Tekcan M, et al. Effect of abamectin exposure on semen parameters indicative of reduced sperm maturity: a study on farmworkers in Antalya (Turkey). Andrologia 2012;44(06):388–395 [DOI] [PubMed] [Google Scholar]
- 82.Chevrier C, Warembourg C, Gaudreau E, et al. Organochlorine pesticides, polychlorinated biphenyls, seafood consumption, and time-to-pregnancy. Epidemiology 2013;24(02):251–260 [DOI] [PubMed] [Google Scholar]
- 83.Upson K, De Roos AJ, Thompson ML, et al. Organochlorine pesticides and risk of endometriosis: findings from a population-based case-control study. Environ Health Perspect 2013; 121(11–12):1319–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trabert B, Chen Z, Kannan K, et al. Persistent organic pollutants (POPs) and fibroids: results from the ENDO study. J Expo Sci Environ Epidemiol 2015;25(03):278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cragin LA, Kesner JS, Bachand AM, et al. Menstrual cycle characteristics and reproductive hormone levels in women exposed to atrazine in drinking water. Environ Res 2011;111(08):1293–1301 [DOI] [PubMed] [Google Scholar]
- 86.Whitworth KW, Baird DD, Steiner AZ, et al. Anti-Müllerian hormone and lifestyle, reproductive, and environmental factors among women in rural South Africa. Epidemiology 2015;26(03):429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams AL, Watson RE, DeSesso JM. Developmental and reproductive outcomes in humans and animals after glyphosate exposure: a critical analysis. J Toxicol Environ Health B Crit Rev 2012;15(01):39–96 [DOI] [PubMed] [Google Scholar]
- 88.Balise VD, Meng CX, Cornelius-Green JN, Kassotis CD, Kennedy R, Nagel SC. Systematic review of the association between oil and natural gas extraction processes and human reproduction. Fertil Steril 2016;106(04):795–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elliott EG, Ettinger AS, Leaderer BP, Bracken MB, Deziel NC. A systematic evaluation of chemicals in hydraulic-fracturing fluids and wastewater for reproductive and developmental toxicity. J Expo Sci Environ Epidemiol 2017;27(01):90–99 [DOI] [PubMed] [Google Scholar]
- 90.Webb E, Bushkin-Bedient S, Cheng A, Kassotis CD, Balise V, Nagel SC. Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev Environ Health 2014;29(04):307–318 [DOI] [PubMed] [Google Scholar]
- 91.Andersen A Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol 2008;27(Suppl 4):1–82 [DOI] [PubMed] [Google Scholar]
- 92.Dodge LE, Williams PL, Williams MA, et al. Paternal urinary concentrations of parabens and other phenols in relation to reproductive outcomes among couples from a fertility clinic. Environ Health Perspect 2015;123(07):665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ Health Perspect 2011;119(02):252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mínguez-Alarcón L, Chiu YH, Messerlian C, et al. ; EARTH Study Team. Urinary paraben concentrations and in vitro fertilization outcomes among women from a fertility clinic. Fertil Steril 2016; 105(03):714–721 [DOI] [PMC free article] [PubMed] [Google Scholar]


