Abstract
Survival rates for the vast majority of pediatric cancers have improved at a remarkable pace over the past four decades. Cure is now the likely outcome for most children and adolescents diagnosed with cancer. In developed countries, the current five-year survival rate is nearly 80%, ranging from 39% to 97% within age- and diagnosis-specific groups, with the overwhelming majority of these patients being cured of their original malignancy. However, the vast majority of these cancer survivors will have at least one chronic health condition by 40 years of age. With this success has come the responsibility to further understand the long-term morbidity and mortality associated with treatments responsible for this increase in survival and to act upon this knowledge. The burden of this responsibility must be borne by many, including the research and health care communities, survivor advocacy groups, and governmental and policy making entities.
Childhood/adolescent cancer population
While cancer in the first two decades of life represents only one percent of the annual cancer incidence in the United States,1 it is a distinct subgroup with respect to cancer type, biological features, response to treatment, and long-term outcomes (Figure 1).2–5 It is estimated that there are approximately 13,500 new cases per year in the US and the incidence has been increasing at an average rate of 0.5% per year since 1975.1 Approximately half of childhood/adolescent cancers are acute leukemia or cancers of the central nervous system. Included in the remaining cases are diagnoses such as retinoblastoma, neuroblastoma, Wilms tumor, and hepatoblastoma, which are primarily confined to the pediatric age range and occur almost exclusively in the first 5 years of life. For pediatric cancers, the highest age-specific incidence is within the first year of life.
Figure 1.
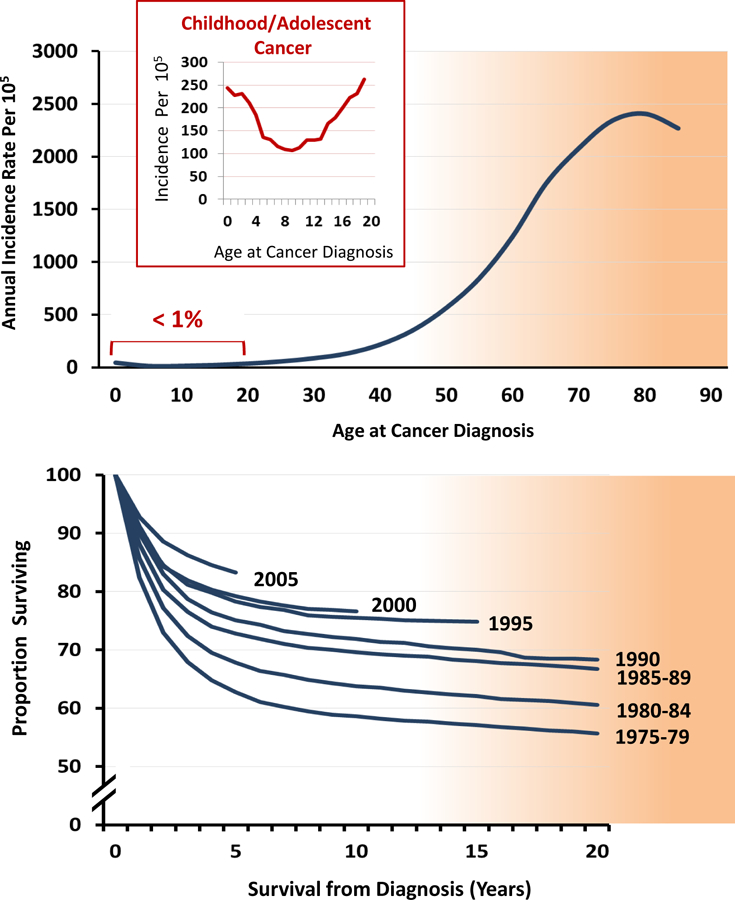
Age-specific cancer incidence rates, highlighting the small proportion represented by childhood/adolescent cancer patients (top graph). Improvements in overall survival among cancer patients diagnosed before the age of 20 years by year of cancer diagnosis (bottom graph). Source: Surveillance, Epidemiology, and End Results program of the National Cancer Institute.1
Over the past 30 years, the survival rate in children and adolescents with cancer has steadily improved (Figure 1) and the cancer-specific death rate has decreased by more than 50%. Nonetheless, cancer remains the most common cause of disease-related mortality in this young age group. Presently, eight out of every 10 children and adolescents diagnosed with cancer will survive five or more years beyond their diagnosis.1 The overwhelming majority of those achieving the five-year milestone will become long-term survivors.5 The NCI SEER program estimates that, as of January 1, 2010, there were approximately 379,100 individuals living in the US who had been diagnosed with cancer during childhood and adolescence;5 compared to the estimate of 328,650 in 2005.6 Assuming constant rates of incidence and survival post-2009, the prevalence of pediatric cancer survivors can be predicted to surpass 420,000 by the end of 2013 and will approach 500,000 by 2020. We can currently estimate that approximately 1 out of every 750 individuals in the US is a survivor of childhood/adolescent cancer. This growing population, diagnosed and treated for cancer during the early stage of life, reflects a highly vulnerable group to experience adverse health-related and quality of life outcomes during their subsequent lifetime.7–11 With extended surveillance, childhood/adolescent cancer survivors are at high-risk for early mortality from subsequent cancers (i.e., cancer other than their original diagnosis), cardiac events and pulmonary conditions.12, 13
Evaluating Outcomes of Evolving Pediatric Cancer Therapy
Pediatric cancer treatment approaches have evolved over time in response to medical advances in cancer biology, developmental therapeutics, radiation technology, diagnostic imaging, and supportive care. Surprisingly, despite the many changes undertaken over the last 50 years in efforts to improve outcomes among children with cancer, the specific agents and modalities used in early clinical trials are still included in contemporary treatment protocols.14, 15 Early pediatric-focused trials aimed to prevent developmental toxicities affecting physical and intellectual growth and development. Subsequent progress in cancer biology and therapeutics resulted in greater numbers of survivors living into adulthood and facilitated the appreciation of excess risk of organ dysfunction and secondary carcinogenesis in aging survivors. Collectively, these events stimulated reassessment of the short and long-term gains associated with the use intensive multimodality therapy in young people that produced paradigm shifts in the management of many pediatric cancers. Examples include the use of cranial irradiation, once lauded for its effectiveness in treating and preventing central nervous system disease in children with acute lymphoblastic leukemia, which now has limited indications for use in frontline treatment protocols.16 In the case of pediatric Hodgkin lymphoma treatment, doses of anthracyclines and fields/volumes of chest radiation are proactively restricted to decrease the risk of cardiovascular injury and the development of subsequent neoplasms, especially breast cancer among female survivors.17 These and similar modifications undertaken for other pediatric malignancies have reduced the occurrence of life-threatening complications presenting during childhood and adolescence, but their impact in aging adults has not been established. In contrast, contemporary therapy is still associated with many life-altering toxicities affecting neurocognitive, neurosensory, endocrine and reproductive function.8–10 Preemptive screening and surveillance of at risk treatment groups can facilitate early detection of and timely intervention for these common late effects.
Health-related and Quality of Life Outcomes
The consequences of childhood cancer on long-term health may be substantial as indicated by abundant research describing adverse outcomes involving the biomedical domains of growth and development, organ function, reproductive capacity and health of offspring, and risk of subsequent carcinogenesis (Figure 2).18, 19 In addition, the pediatric cancer experience has been associated with an increased risk of detrimental psychosocial effects impacting mental health, socialization, educational and vocational achievement, and health care access.20 A significant minority of childhood cancer survivors also experience chronic symptoms such as anxiety, fatigue, disrupted sleep, pain, and cognitive deficits after completion of therapy.21, 22 Chronic health conditions, psychosocial sequelae, and chronic symptoms may ultimately reduce the quality of survival through their impact on health and functional status.7–11 These outcomes can be significantly influenced by the developmental age at treatment, presence of co-morbid conditions antedating cancer diagnosis, and the survivor’s access to remedial and preventive services. For example, while younger pediatric patients encounter higher risks of neurocognitive injury following central nervous system-directed therapy,23 adolescent and young adult cancer survivors have been identified as a group particularly vulnerable to adverse psychosocial outcomes.11, 22 To optimize health outcomes across the age spectrum of childhood cancer survivors, providers should consider the impact of both medical and psychosocial sequelae on general health, mental health, and function pertinent to the developmental age of the survivor and facilitate their access to remedial services.
Figure 2.
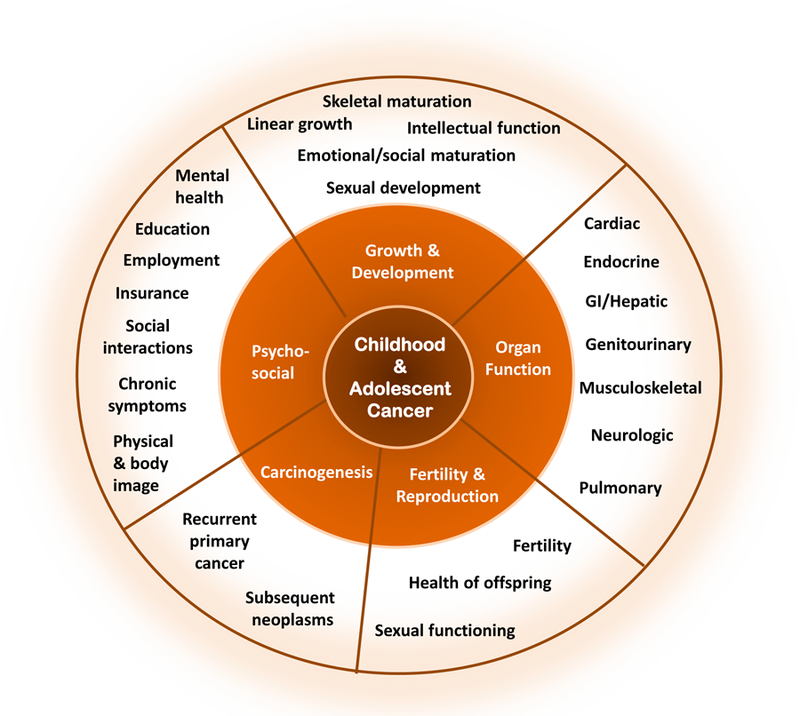
Spectrum of health-related and quality of life outcomes among long-term survivors of childhood and adolescent cancers.
Clinicians supervising the care of childhood cancer survivors should be aware of the variable latency to clinical manifestation of cancer treatment toxicities as well as patient and treatment factors that modify risk. Some treatment effects present soon after exposure and persist long-term, whereas others develop many years after completion of therapy. For example, sensorineural hearing loss associated with cisplatin typically develops as an acute toxicity that persists during long-term follow-up.24 Younger age at treatment, higher cumulative dose exposure, and combined modality therapy including ototoxic radiation, contribute to greater risk and severity of hearing deficit. Appreciation of the natural history of cisplatin-induced ototoxicity has resulted in proactive monitoring of hearing during treatment and facilitated timely preventive and remedial interventions to optimize language development and academic achievement among young survivors. In contrast, young women treated with chest radiation for childhood cancer have an increased risk of breast cancer with a median time to diagnosis 15 to 20 years after radiation; breast cancer risk becomes elevated as early as 8 years following radiation exposure.25 The dose and volume of breast in the radiation treatment field are important modifiers of risk and as well as other cancer treatments affecting ovarian function. These data directly inform the recommendations for initiation of breast cancer surveillance among at risk young women treated with chest radiation for childhood cancer.25 In general, the clinical course of normal tissue injury during the pediatric, adolescent and young adult age spectrum has been very well defined for many treatment exposures. However, further research is needed to improve understanding about the impact of aging on the health of adults treated for cancer during childhood (Figure 3).
Figure 3.
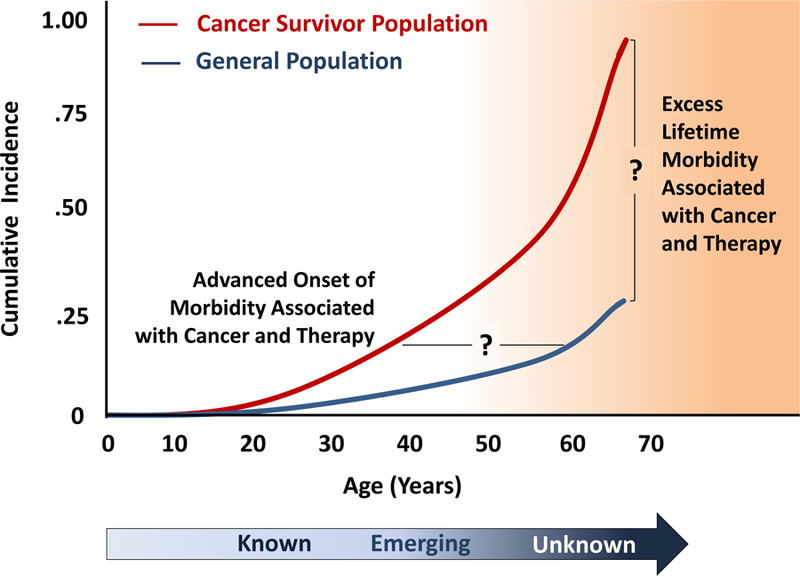
Theoretical framework regarding gaps in knowledge regarding the long-term outcomes among aging childhood and adolescent cancer survivors.
Methodological and Practical Issues in Cancer Survivorship
A cancer survivor may be defined in a variety of ways, ranging from when the diagnosis is made to some post-diagnosis time point. From a vital statistics perspective, a cancer patient is considered a “survivor” starting at diagnosis, while researchers often utilize a “time from diagnosis” definition, which may be selected based upon the specific research question being addressed. For example, large cohorts like the Childhood Cancer Survivor Study26 and the British Childhood Cancer Survivor Study27 have used five-years from diagnosis, others have used three-years post-diagnosis,28 while some, such as the Bone Marrow Transplant Survivor Cohort, have applied alternative criteria using survival of two or more years from the time of hematopoietic stem cell transplant.10 The major implication of differing definitions relates directly to the generalizability of the results and conclusions from a specific population. Thus, as the body of literature increases, it is important to consider how the source population was defined when describing the status and risk profiles for childhood cancer survivors. Beyond the definition of a cancer survivor, there are a number of research-related issues that need to be considered when interpreting the cancer survivorship literature. The study design, source and eligibility criteria for the study population, the study sample size, participation rates, completeness of follow-up, approach to assign treatment-related exposures, ascertainment and characterization of outcomes, and ability to consider potential modifiers of risk can all influence how results are interpreted and translated to clinical practice.29, 30
Implementation of research in aging survivor populations poses significant challenges to researchers who must elucidate outcomes years after their discharge from pediatric cancer treatment centers. National registries and similar administrative sources can provide meaningful information about causes of mortality after childhood cancer, health care utilization, and medical events like subsequent neoplasms and pregnancy outcomes, but linkage of outcomes to patient specific data, especially types and doses of cancer treatment modalities, is required to identify groups at high risk for morbidity. Several large cohort studies have successfully used survivor (or caregiver) report of biomedical and psychosocial outcomes to describe long-term survivor health,26, 27 but these studies are limited by survivors’ potential misperceptions of health events and potential bias introduced by their variable access to health care and screening for treatment-related toxicities. Ongoing cohort studies aim to more accurately characterize the health of long-term childhood cancer survivors through systematic medical assessments based on established cancer treatment-related toxicity profiles.31, 32 The St. Jude Lifetime Cohort study identified a high prevalence of undiagnosed disease among 1713 adult (median age, 32 [range, 18–60] years) survivors of childhood cancer (median time from diagnosis, 25 [range, 10–47] years) who completed comprehensive outpatient risk-based medical testing over a 2 to 3 day period.8 In this cohort, the estimated cumulative prevalence for survivors to develop at least one chronic health condition and a serious/disabling or life-threatening chronic condition by age 45 years of age was 95.5% and 80.5%, respectively.8 Of concern, this and other studies reporting results from systematic health screening of adult survivors of childhood cancer have disclosed a high prevalence of health conditions typically observed in older individuals such as neurocognitive and neurosensory deficits, cardiovascular disease, and pulmonary dysfunction.8, 33, 34 These data suggest accelerated or premature aging as a consequence of specific cytotoxic therapies used to cure childhood cancer that deserves further study. While the contribution of cancer treatment to organ dysfunction presenting in childhood is compelling, other factors including co-morbid health problems, health habits, and natural organ senescence certainly modify risk in aging adults. Research elucidating treatment, genetic, demographic and psychosocial/behavioral predictors of adverse outcomes is critical to guide screening and surveillance of aging survivors and the development of interventions to preserve their health.
Risk of Treatment-related Adverse Health and Psychosocial Outcomes
To varying degrees, it has been shown that long-term survivors of childhood cancer experience a spectrum of adverse outcomes.12, 35–40 While it is important to identify, quantify and characterize exposure-specific risks, a priority is to characterize those survivors at highest risk, to target for intervention-based strategies.41 The causes of many adverse outcomes experienced by childhood cancer survivors may be multifactorial involving combinations of factors beyond treatment-exposures (Figure 4).42 Factors relating to the primary malignancy, demographics, premorbid conditions, underlying genetic predisposition, and health-related behaviors can modify the risk-associated impact of treatment-related exposures. Nonetheless, it is typically treatment-specific factors that primarily determine risk of adverse late-effects. Provided below are selected examples of treatment-specific adverse outcomes.
Figure 4.
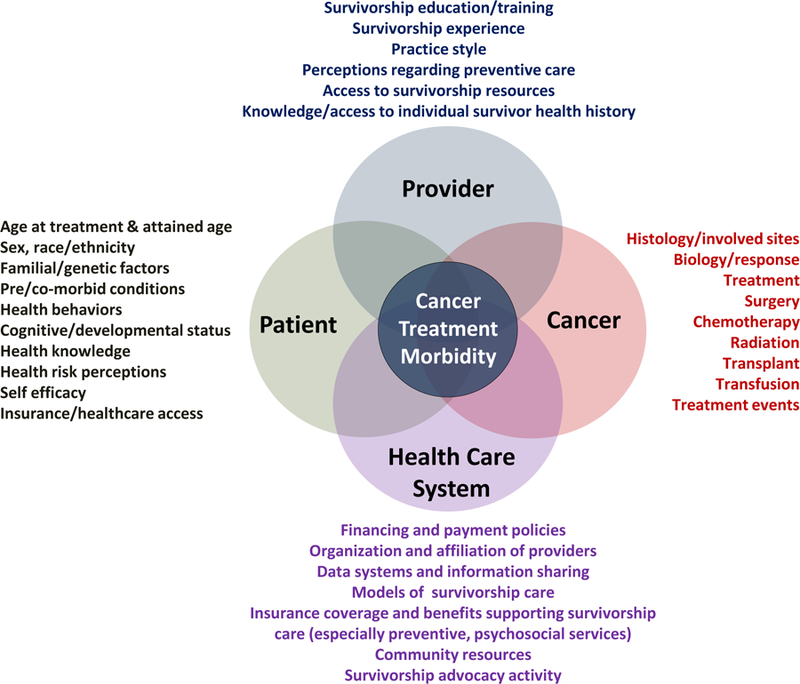
Inter-relationship of patient-, cancer-, health care system-, and provider-related issues impacting cancer treatment associated morbidity among long-term survivors of childhood and adolescent cancer.
Radiation therapy has been an essential element of treatment for many childhood malignancies.14, 15 With the expanding number of survivors and longer duration of follow-up, knowledge regarding the long-term adverse late effects associated with radiation therapy has greatly increased. There are a number of factors that can influence radiation-associated risks including radiation source, cumulative dose, volume, and fractionation, as well as demographic factors such as sex and age at radiation exposure.43 Organ-specific radiation exposure impacts the risk of organ-specific adverse outcomes, typically in a dose-dependent fashion.44 Radiation-associated outcomes include cardiovascular45–48 and cerebrovascular,49–51 endocrine,52–57 gastrointestinal,58 gonadal/reproductive,36, 59–63 hepatic,64 pulmonary,65–68 and urinary tract dysfunction,69–71 musculoskeletal growth impairment,72–74 and neurocognitive, neurosensory, and neurologic deficits 23, 24, 75–78(Table 1). Cancer survivors whose treatment included radiation therapy are at risk of invasive and non-invasive secondary neoplasms.79–93 Among childhood cancer survivors, risks have been well-described for cancers of the skin (predominantly basal cell carcinoma),79, 80, 89, 90 breast,86, 94, 95 thyroid,81, 92, 96 bone,85 and brain.87, 97, 98 Increasingly, with extended follow-up from larger pediatric cancer cohorts, data are emerging describing radiation-associated risks for subsequent neoplasms involving the colon and rectum,84, 88, 93 kidney,99 and lung.100
Table 1.
Selected examples of established radiation-associated late effects
| Radiation Exposure | Established Late Effects | References |
|---|---|---|
| Cardiovascular | Cardiomyopathy Carotid/subclavian artery disease Coronary artery disease Dysrhythmias/conduction disorders Heart valve abnormalities Pericardial fibrosis/pericarditis |
45–48 |
| Central nervous system | Neurocognitive deficits including diminished IQ, learning deficits, executive function, sustained attention, memory processing speed, and visual motor integration. Cerebrovascular disease including stroke, moyamoya, occlusive cerebral vasculopathy Clinical leukoencephalopathy including spasticity, ataxia, dysarthria, dysphagia, hemiparesis, seizures Neurologic and neurosensory deficits |
23, 24, 49–51, 75–78 |
| Endocrine | Pituitary dysfunction including altered pubertal timing, growth hormone, TSH, ACTH, LH and FSH deficiency, altered body composition (reduced lean muscle mass, overweight/obesity), metabolic syndrome Thyroid abnormalities including hypothyroid, hyperthyroid, thyroid nodules Diabetes mellitus |
52–57 |
| Gastrointestinal | Esophageal stricture Chronic enterocolitis Bowel obstruction Gastrointestinal fistula/stricture |
58 |
| Gonadal/reproductive (females) | Uterine vascular insufficiency predisposing to spontaneous abortion, neonatal death, low-birth weight infant, fetal malposition, and premature labor Ovarian dysfunction resulting in delayed/arrested puberty, premature menopause, infertility |
36, 61–63 |
| Gonadal/reproductive (males) | Leydig cell dysfunction resulting in delayed/arrested puberty androgen insufficiency Germ cell failure oligospermia, azoospermia, Infertility |
59, 60 |
| Hepatobiliary | Hepatic fibrosis Cholelithiasis |
64 |
| Musculoskeletal | Hypoplasia/Fibrosis Reduced or uneven growth (resulting in shortened trunk height, limb length discrepancy, kyphoscoliosis) |
72–74 |
| Pulmonary | Pulmonary fibrosis Interstitial pneumonitis Restrictive lung disease Obstructive lung disease |
65–68 |
| Urinary tract | Bladder fibrosis Dysfunctional voiding Vesicoureteral reflux Hydronephrosis Renal insufficiency Hypertension |
69–71 |
| Any organ system | Subsequent neoplasms including skin (predominantly basal cell carcinoma), breast, thyroid, bone, brain. Increasing data on risk of radiation-associated colorectal cancers. | 79–90, 92, 93 |
Specific classes of chemotherapeutic agents used in the successful treatment of children and adolescents with cancer14, 15 are also associated with a broad spectrum of potential long-term late effects including alkylating agents,60, 61, 65, 70, 71, 101–104 anthracycline antibiotics, 48, 105, 106 antimetabolites,23, 64, 70, 107 corticosteroids,78, 107, 108 epipodophyllotoxins103 and vinca alkaloids109, 110 (Table 2).Generally the risk for adverse long-term outcomes associated with chemotherapy is dependent on cumulative dose, but may also differ according to route of administration and scheduling, patient’s sex and age.
Table 2.
Selected examples of established chemotherapy-associated late effects
| Class of Chemotherapy | Chemotherapeutic Agents | Established Late Effects | References |
|---|---|---|---|
| Alkylating agents | Busulfan, Carboplatin, Carmustine, Chlorambucil, Cisplatin, Cyclophosphamide, Ifosfamide, Lomustine, Mechlorethamine, Melphalan, Procarbazine, Thiotepa; plus the non-classical alkylators Dacarbazine and Temozolomide | Secondary myelodysplasia or acute myeloid leukemia Gonadal dysfunction and Infertility Pulmonary fibrosis (with exposure to Busulfan, Carmustine or Lomustine) Urinary tract abnormalities (with exposure to Cyclophosphamide or Ifosfamide) Renal dysfunction (with exposure to Cisplatin/Carboplatin and Ifosfamide) Ototoxicity (with exposure to Cisplatin or very high dose Carboplatin) Dyslipidemia (with exposure to Cisplatin) |
60, 61, 65, 70, 71, 101–104 |
| Anthracyclines | Daunorubicin, Doxorubicin, Epirubicin, and Idarubicin | Left ventricular dysfunction Cardiomyopathy Dysrhythmias |
48, 105, 106 |
| Corticosteroids | Dexamethasone, Prednisone | Reduced bone mineral density Osteonecrosis Cataracts |
78, 107, 108 |
| Vinca Alkaloids | Vincristine, Vinblastine | Peripheral sensory and motor neuropathy | 109, 110 |
| Antimetabolites | Methotrexate | Neurocognitive impairment Leukoencephalopathy Liver dysfunction Renal toxicity Decreased bone mineral density |
23, 64, 70, 107 |
| Epipodophyllotoxins | Etoposide Teniposide | Acute myeloid leukemia |
Surgical procedures carried out as part of the diagnosis or treatment of cancer in children and adolescents can have long-term effects on health status and quality of life.19 Examples of late effects of surgery include amputation and limb-sparing procedures that can directly impact physical function and mobility;39, 111 enucleation and craniofacial development;112 oophorectomy or orchiectomy and reproduction;60, 61 cystectomy and bladder function;71 nephrectomy and subsequent renal function;70 splenectomy and risk of infection;19 and, neurosurgical procedures that may result in neurocognitive, neuroendocrine, or motor sensory deficits, and seizures, as well as spinal cord injury resulting in incontinence or sexual dysfunction.76, 113
Beyond the adverse physical and chronic health outcomes associated with cancer therapy during childhood and adolescences, long-term survivors are at risk of experiencing a variety of psychological and social outcomes,20, 21, 40, 114 which may result in decreased overall quality of life. Studies of long-term survivors have investigated the prevalence of and risk factors associated with educational and occupational attainment, 115–118 insurance,119, 120 marriage,121–123 depression, anxiety and somatic distress,124–126 post-traumatic distress,127, 128 post-traumatic growth,129 fatigue,130–132 and pain.133 To varying degrees, cancer- and treatment-related factors have identified high-risk populations. However, only limited data are available describing longitudinal changes, and predictors of change, for psychosocial functioning of aging survivors of childhood/adolescent cancer.124, 127
Translation of Outcomes-based Research
The ideal approach to childhood cancer survivor care involves a risk-based paradigm that integrates a personalized plan of surveillance/screening and management and prevention of late effects predisposed by cancer and its treatment into the context of routine health care.19 Health outcomes research among childhood cancer survivors has yielded compelling data linking adverse outcomes with specific treatment modalities that permit clinicians to identify potentially at risk survivors. Several groups have used this evidence to inform clinical practice guidelines with the goal of facilitating early detection of cancer-treatment morbidity and survivor access to preventive/remedial interventions that can preserve health.134–137 A hybrid approach featuring an evidence- and consensus-based design has been utilized for guideline development related to childhood cancer survivor long-term follow-up care. This approach has been considered reasonable because of the strength of the evidence supporting many cancer treatment-related adverse outcomes and the critical need for a resource for clinicians managing the care of medically vulnerable survivors. Because pediatric cancer survivors represent a relatively rare entity in primary care practices, most community providers lack knowledge about complications that may arise as a result of treatment for pediatric malignancies, which may lead to their discomfort in supervising the care of survivors.138 This is compounded by the fact that many survivors and their families may also lack this knowledge. Currently available clinical practice guidelines provide information about potential late effects risks associated with specific cancer treatment modalities, targeted health screening, suggested methods of risk reduction, and educational resources to assist providers in coordinating risk-based survivor care.134–137
It should be noted that optimal health screening after treatment for childhood, adolescent and young adult cancers has yet to be defined. While published late effects research provides insight into who may potentially benefit from screening and early detection after specific treatment exposures, further research is required to determine the time to initiate screening, frequency of screening, most efficacious and cost-effective modality of screening, and overall risks, benefits and harms to the health care system and survivor. The relatively small size of the pediatric cancer survivor population represented by heterogeneous histological subtypes and treatment approaches, the diverse health risks associated with these treatments, and the frequency and delayed time to onset of many treatment complications often preclude implementation of high quality clinical studies assessing the impact of screening on the morbidity and mortality associated with the late effect. Notwithstanding these limitations, in addition to standardizing follow-up care to respond to the unique health care needs of childhood cancer survivors, the currently available clinical practice guidelines provide an important platform for research to begin to address knowledge gaps in survivorship care. In this regard, recently published research has focused on evaluating the yield of specific diagnostic studies in identifying late effects.8, 34, 139, 140 Pertinent considerations in interpreting the results of these studies include variability in the cohort’s age at treatment, age at screening, time from cancer treatment, and representativeness to source population. Collectively, these studies demonstrate that screening identifies a substantial proportion with previously unrecognized, treatment-related health complications of varying degrees of severity. Specifically, risk-based screening among participants in the St. Jude Lifetime Cohort identified a high prevalence of newly discovered neurocognitive and neurosensory deficits, heart valve disorders, and pulmonary dysfunction that may benefit from remedial and preventive interventions to reduce future decline in function.8
Until recently, national groups have worked independently in the development of clinical practice guidelines with resulting variation in screening recommendations, patient risk groups, diagnostic tests, and screening intervals.134–137 Recognizing the inefficient use of resources resulting from this non-integrated approach of guideline development, the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) was established in 2010 with the goal of establishing a common vision and integrated strategy for the surveillance of late effects in childhood, adolescent and young adult cancer survivors throughout the world.141 This collaboration will provide a unique forum to address knowledge gaps related to survivorship care and methods to optimize implementation of clinical practice guidelines and their impact on quality of survivorship care.
Beyond translation into clinical care guidelines, it is now important to also focus greater attention to translating research into development and testing of intervention approaches designed to avoid or ameliorate adverse outcomes. The future portfolio of intervention-based research can, and should, encompass a wide spectrum of approaches and outcomes. Specific interventions may include social, behavioral, and/or pharmacologic approaches. With the often multifactorial nature of known or anticipated risk factors for most adverse outcomes (e.g., cardiotoxic therapy, obesity, tobacco use and risk for cardiac disease), interventions may be most effective using a combination of approaches. Outcomes for intervention research may relate to (1) changes in health behaviors such as diet, exercise, and tobacco use; (2) health care practices, such as ongoing medical surveillance and compliance with recommended risk-based screening; (3) prevention or amelioration of adverse health outcomes such as cancer, congestive heart failure, obesity, fatigue, or hypertension; and, (4) promotion of positive social/quality of life outcomes such as education, employment, insurance, or mental health.
Future Challenges
With the rapid expansion of evidence regarding health risks associated with pediatric cancer survivorship, medical and research communities have the responsibility to translate research findings into clinical practice guidelines to optimize follow-up care and outcomes of this growing population. The implementation and dissemination of outcomes and intervention research must consider potential barriers existing at the level of the survivor, provider, or health care environment impacting access to quality survivorship care (Figure 4).
Survivor-related Barriers –
Lack of knowledge regarding cancer treatment history and its associated long-term health risks represents an important barrier to survivor participation in follow-up care that pediatric late effects programs have aimed to remediate through longitudinal health counseling and provision of treatment summaries and survivorship care plans.142 Complicating the educational process is that transition of care typically occurs when survivors reach a developmental age at which they may be more cognizant of cancer-related health risks and personally responsible for health behaviors. Published research related to these initiatives is largely limited to descriptive studies of clinic interventions.42, 143, 144 Few studies feature assessment of the impact of clinic interventions on survivor health knowledge, health perceptions, and health behaviors, including their ongoing participation in care.145–147 Despite the absence of evidence to support specific benefits from the counseling and resources routinely provided in late effects programs, consensus remains that such interventions represent good clinical care.148 However, there is a critical need for future research to define effective and efficient methods of health risk counseling for this population that is developmentally and culturally appropriate.
Provider-related Barriers –
Knowledge deficits among providers regarding pediatric survivorship health issues can also pose barriers to the delivery of quality survivorship care. Surprisingly, lack of familiarity with recommended screening for pediatric cancer treatment toxicities is not limited to primary care providers. In a study evaluating preferences and knowledge gaps among pediatric oncologists regarding the care of childhood cancer survivors, only 33% of respondents correctly answered vignette-based questions regarding surveillance recommendations for breast cancer, cardiomyopathy and thyroid function.149 These providers related increasing discomfort in managing the care of pediatric survivors 21 years of age or older, but a significant proportion (38%) preferred to observe their survivors for as long as possible. In a related study evaluating the same outcomes among family physicians, only 2% of respondents correctly answered the same vignette-based questions regarding surveillance.138 The vast majority (85%) of these family physicians preferred to care for survivors in consultation with clinicians from a cancer treatment center or late effects program. Access to clinical care guidelines and receipt of a patient-specific letter detailing surveillance recommendations were perceived as the modalities most likely to assist them in survivorship care. Both studies highlight the need to expand health professional education and training programs related to survivorship care, improve dissemination of survivorship clinical practice guidelines, and evaluate methods to enhance communication and collaboration among oncology and primary care providers sharing survivorship care.
Barriers Related to the Health Care Environment –
Potential barriers to quality survivorship care imposed by the health care environment relate to availability of providers and survivorship resources, specialized late effects clinic, and operational models of survivorship care, which are to a great degree influenced by provider/payer relationships and health care policy. Survivorship care is generally a non-revenue-generating service because of the limited or lack of reimbursement for significant components of the care.150 This reality represents a significant threat to survivor access to specialized late effects clinics that have multidisciplinary staff with expertise in late effects and health screening/surveillance focused on educating survivors, promoting their access to resources to remediate or prevent treatment-related toxicities, and facilitating communication and care transitions with community providers. Because specialized pediatric late effects programs are not universally available, and when available, usually have institutional age limitations that preclude follow-up beyond adolescence,151 most pediatric cancer survivors ultimately have their care transitioned to community providers. This transition of care can be complicated by suboptimal communication among members of the treating oncology team and primary care providers who lack awareness about the unique health risks associated with treatment for cancer during childhood and screening/surveillance recommendations. Various models of survivorship care have been implemented to facilitate care transitions and assure that the health care needs of childhood cancer survivors are optimally addressed.152 Among these, a shared-care model that utilizes a risk-stratified approach based on treatment intensity or risk for late effects has been favored by late effects specialists as this model promotes ongoing communication throughout the spectrum of cancer care and takes advantage of the expertise of the oncology team and the primary care provider in delivery of care.152 Research is required to delineate the essential elements of survivorship care and flexible models of care delivery that can enhance survivor access to interventions that proactively address cancer-related morbidity.
Insurance and Policy Barriers –
In countries like the U.S., where government-based health care is not provided, lack of health insurance or health policy exclusions and restrictions represent a significant barrier to survivorship care that may disproportionately impact individuals with racial/ethnic minority or low socioeconomic status. National health legislation like the Patient Protection and Affordability Act provides many policy changes to ensure that pediatric cancer survivors have access to appropriate health care services.153 This legislation provides mechanisms to enhance access and coverage to components of survivorship care, but additional measures will be required to achieve the goal of high quality comprehensive, coordinated survivorship care. To achieve this goal, health care policy change is needed to define the essential metrics of quality care that should be accessible to all survivors and to improve provider reimbursement for comprehensive care coordination that includes assessment for medical and psychosocial sequelae, delivery of interventions to remediate or prevent treatment complications, counseling regarding methods of risk reduction, and referral to resources to address medical, psychosocial and practical needs.
Conclusion
While the many individuals who have played a role in achieving the remarkable increase in survival of childhood/adolescent cancers should be gratified; with success comes responsibility. Simply focusing on the cure of the cancer cannot be an acceptable objective when considering the life-long risk survivors experience for development of treatment-related complications. Because of the young age of these cancer survivors, and thus their potential longevity, the delayed consequences of therapy will likely have a greater impact on their lives, families, and on society at-large, than the acute complication of the cytotoxic and surgical therapies they have already experienced. Thus, there is a role not only for researchers and health care providers, but also for survivors and their families, governing bodies, and advocacy groups to help understand and overcome the barriers that prevent survivors from receiving optimal care to minimize adverse health-related and quality of life outcomes.
Key Points Box.
Over 80% of childhood/adolescent cancer patients will survive five or more year from diagnosis, with the majority being cured of their original malignancy.
An estimated 420,000 individuals living in the U.S. have been diagnosed with cancer prior to 20 years of age.
Long-term survivors of childhood/adolescent cancer are at increased risk of treatment-related morbidity and mortality.
By 50 years of age, the vast majority of childhood/adolescent survivors will have a serious/disabling or life-threatening chronic health condition.
Research is providing the foundation for development of risk-based clinical care guidelines for survivors of childhood/adolescent cancer.
Targeted intervention strategies are needed to prevent or ameliorate late effects of therapy.
There are significant challenges to providing long-term surveillance and care for aging survivors of childhood/adolescent cancer.
Acknowledgments
Research grant support: Drs. Robison and Hudson are supported in part by the Cancer Center Support (CORE) grant CA 21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC).
Contributor Information
Leslie L. Robison, Departments of Epidemiology and Cancer Control and Oncology, St. Jude Children’s Research Hospital, Memphis, TN
Melissa M. Hudson, Departments of Epidemiology and Cancer Control and Oncology, St. Jude Children’s Research Hospital, Memphis, TN
References
- 1.Howlader N et al. (National Cancer Institute, Bethesda, MD: ). [Google Scholar]
- 2.Gajjar A et al. Children’s Oncology Group’s 2013 blueprint for research: central nervous system tumors. Pediatr Blood Cancer 60, 1022–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norris RE & Adamson PC Challenges and opportunities in childhood cancer drug development. Nat Rev Cancer 12, 776–82 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Carroll WL, Meshinchi S & Arceci RJ Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol 29, 551–65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MA et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28, 2625–34 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariotto AB et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev 18, 1033–40 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Hudson MM et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA 290, 1583–92 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Hudson MM et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 309, 2371–81 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oeffinger KC et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355, 1572–82 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Sun CL et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood 116, 3129–39; quiz 3377 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tai E et al. Health status of adolescent and young adult cancer survivors. Cancer 118, 4884–91 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong GT, Pan Z, Ness KK, Srivastava D & Robison LL Temporal trends in cause-specific late mortality among 5-year survivors of childhood cancer. J Clin Oncol 28, 1224–31 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mertens AC et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 100, 1368–79 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DM et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer 60, 1083–94 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson MM et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer 58, 334–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pui CH Prophylactic cranial irradiation: going, going, gone. Lancet Oncol 10, 932–3 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Hodgson DC, Hudson MM & Constine LS Pediatric hodgkin lymphoma: maximizing efficacy and minimizing toxicity. Semin Radiat Oncol 17, 230–42 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Hudson MM Survivors of childhood cancer: coming of age. Hematol Oncol Clin North Am 22, 211–31, v-vi (2008). [DOI] [PubMed] [Google Scholar]
- 19.Oeffinger KC & Hudson MM Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin 54, 208–36 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Nathan PC et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children’s Oncology Group. Arch Pediatr Adolesc Med 161, 798–806 (2007).17679663 [Google Scholar]
- 21.Lund LW, Schmiegelow K, Rechnitzer C & Johansen C A systematic review of studies on psychosocial late effects of childhood cancer: structures of society and methodological pitfalls may challenge the conclusions. Pediatr Blood Cancer 56, 532–43 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Schultz KA et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 25, 3649–56 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Winick N Neurocognitive outcome in survivors of pediatric cancer. Curr Opin Pediatr 23, 27–33 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Grewal S et al. Auditory late effects of childhood cancer therapy: a report from the Children’s Oncology Group. Pediatrics 125, e938–50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson TO et al. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med 152, 444–55; W144–54 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robison LL et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 27, 2308–18 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkins MM et al. The British Childhood Cancer Survivor Study: Objectives, methods, population structure, response rates and initial descriptive information. Pediatr Blood Cancer 50, 1018–25 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Menu-Branthomme A et al. Radiation dose, chemotherapy and risk of soft tissue sarcoma after solid tumours during childhood. Int J Cancer 110, 87–93 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Hawkins MM & Robison LL Importance of clinical and epidemiological research in defining the long-term clinical care of pediatric cancer survivors. Pediatr Blood Cancer 46, 174–8 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Leisenring WM et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol 27, 2319–27 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson MM et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer 56, 825–36 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieswerda E et al. The EKZ/AMC childhood cancer survivor cohort: methodology, clinical characteristics, and data availability. J Cancer Surviv 7, 439–54 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Geenen MM et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297, 2705–15 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Landier W et al. Yield of screening for long-term complications using the children’s oncology group long-term follow-up guidelines. J Clin Oncol 30, 4401–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diller L et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol 27, 2339–55 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green DM et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 27, 2677–85 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurney JG et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol 27, 2390–5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meadows AT et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol 27, 2356–62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ness KK et al. Physical performance limitations in the Childhood Cancer Survivor Study cohort. J Clin Oncol 27, 2382–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeltzer LK et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol 27, 2396–404 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudson MM et al. High-risk populations identified in Childhood Cancer Survivor Study investigations: implications for risk-based surveillance. J Clin Oncol 27, 2405–14 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hudson MM A model for care across the cancer continuum. Cancer 104, 2638–42 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Armstrong GT, Stovall M & Robison LL Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the childhood cancer survivor study. Radiat Res 174, 840–50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Dijk IW et al. Dose-effect relationships for adverse events after cranial radiation therapy in long-term childhood cancer survivors. Int J Radiat Oncol Biol Phys 85, 768–75 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Galper SL et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood 117, 412–8 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Schellong G et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer 55, 1145–52 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Tukenova M et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 28, 1308–15 (2010). [DOI] [PubMed] [Google Scholar]
- 48.van der Pal HJ et al. High risk of symptomatic cardiac events in childhood cancer survivors. Journal of Clinical Oncology 30, 1429–37 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Bowers DC et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol 24, 5277–82 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Bowers DC et al. Stroke as a late treatment effect of Hodgkin’s Disease: a report from the Childhood Cancer Survivor Study. J Clin Oncol 23, 6508–15 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Morris B et al. Cerebrovascular disease in childhood cancer survivors: A Children’s Oncology Group Report. Neurology 73, 1906–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chemaitilly W & Sklar CA Endocrine complications in long-term survivors of childhood cancers. Endocr Relat Cancer 17, R141–59 (2010). [DOI] [PubMed] [Google Scholar]
- 53.de Vathaire F et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: a retrospective cohort study. Lancet Oncol 13, 1002–10 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Laughton SJ et al. Endocrine outcomes for children with embryonal brain tumors after risk-adapted craniospinal and conformal primary-site irradiation and high-dose chemotherapy with stem-cell rescue on the SJMB-96 trial. J Clin Oncol 26, 1112–8 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Meacham LR et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med 169, 1381–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merchant TE et al. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clin Oncol 29, 4776–80 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patterson BC, Truxillo L, Wasilewski-Masker K, Mertens AC & Meacham LR Adrenal function testing in pediatric cancer survivors. Pediatr Blood Cancer 53, 1302–7 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Goldsby R et al. Survivors of childhood cancer have increased risk of gastrointestinal complications later in life. Gastroenterology 140, 1464–71 e1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green DM et al. Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 28, 332–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kenney LB et al. Male reproductive health after childhood, adolescent, and young adult cancers: a report from the Children’s Oncology Group. J Clin Oncol 30, 3408–16 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Metzger ML et al. Female reproductive health after childhood, adolescent, and young adult cancers: guidelines for the assessment and management of female reproductive complications. J Clin Oncol 31, 1239–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Signorello LB et al. Female survivors of childhood cancer: preterm birth and low birth weight among their children. J Natl Cancer Inst 98, 1453–61 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Signorello LB et al. Stillbirth and neonatal death in relation to radiation exposure before conception: a retrospective cohort study. Lancet 376, 624–30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castellino S et al. Hepato-biliary late effects in survivors of childhood and adolescent cancer: a report from the Children’s Oncology Group. Pediatr Blood Cancer 54, 663–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang TT et al. Pulmonary outcomes in survivors of childhood cancer: a systematic review. Chest 140, 881–901 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mertens AC et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer 95, 2431–41 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Motosue MS et al. Pulmonary function after whole lung irradiation in pediatric patients with solid malignancies. Cancer 118, 1450–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mulder RL et al. Pulmonary function impairment measured by pulmonary function tests in long-term survivors of childhood cancer. Thorax 66, 1065–71 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Dawson LA et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys 76, S108–15 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Jones DP, Spunt SL, Green D & Springate JE Renal late effects in patients treated for cancer in childhood: a report from the Children’s Oncology Group. Pediatr Blood Cancer 51, 724–31 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ritchey M, Ferrer F, Shearer P & Spunt SL Late effects on the urinary bladder in patients treated for cancer in childhood: a report from the Children’s Oncology Group. Pediatr Blood Cancer 52, 439–46 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Estilo CL et al. Effects of therapy on dentofacial development in long-term survivors of head and neck rhabdomyosarcoma: the memorial sloan-kettering cancer center experience. J Pediatr Hematol Oncol 25, 215–22 (2003). [DOI] [PubMed] [Google Scholar]
- 73.Merchant TE et al. Differential attenuation of clavicle growth after asymmetric mantle radiotherapy. Int J Radiat Oncol Biol Phys 59, 556–61 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Paulino AC Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys 60, 265–74 (2004). [DOI] [PubMed] [Google Scholar]
- 75.Goldsby RE et al. Late-occurring neurologic sequelae in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol 28, 324–31 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Packer RJ et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol 21, 3255–61 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Whelan K et al. Auditory complications in childhood cancer survivors: a report from the childhood cancer survivor study. Pediatr Blood Cancer 57, 126–34 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whelan KF et al. Ocular late effects in childhood and adolescent cancer survivors: a report from the childhood cancer survivor study. Pediatr Blood Cancer 54, 103–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Armstrong GT et al. Occurrence of multiple subsequent neoplasms in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 29, 3056–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bassal M et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 24, 476–83 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Bhatti P et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res 174, 741–52 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boukheris H et al. Risk of salivary gland cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys 85, 776–83 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friedman DL et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 102, 1083–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henderson TO et al. Secondary gastrointestinal cancer in childhood cancer survivors: a cohort study. Ann Intern Med 156, 757–66, W-260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henderson TO et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys 84, 224–30 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Inskip PD et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol 27, 3901–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neglia JP et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 98, 1528–37 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Nottage K et al. Secondary colorectal carcinoma after childhood cancer. J Clin Oncol 30, 2552–8 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Pappo AS et al. Melanoma as a subsequent neoplasm in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Pediatr Blood Cancer 60, 461–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perkins JL et al. Nonmelanoma skin cancer in survivors of childhood and adolescent cancer: a report from the childhood cancer survivor study. J Clin Oncol 23, 3733–41 (2005). [DOI] [PubMed] [Google Scholar]
- 91.Reulen RC et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA 305, 2311–9 (2011). [DOI] [PubMed] [Google Scholar]
- 92.Ronckers CM et al. Thyroid cancer in childhood cancer survivors: a detailed evaluation of radiation dose response and its modifiers. Radiat Res 166, 618–28 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Tukenova M et al. Second malignant neoplasms in digestive organs after childhood cancer: a cohort-nested case-control study. Int J Radiat Oncol Biol Phys 82, e383–90 (2012). [DOI] [PubMed] [Google Scholar]
- 94.De Bruin ML et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 27, 4239–46 (2009). [DOI] [PubMed] [Google Scholar]
- 95.Swerdlow AJ et al. Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: a National Cohort Study. J Clin Oncol 30, 2745–52 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Sigurdson AJ et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet 365, 2014–23 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Bowers DC et al. Subsequent neoplasms of the CNS among survivors of childhood cancer: a systematic review. Lancet Oncol 14, e321–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taylor AJ et al. Population-based risks of CNS tumors in survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol 28, 5287–93 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson CL et al. Renal carcinoma after childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst 105, 504–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ibrahim EM et al. Increased risk of second lung cancer in Hodgkin’s lymphoma survivors: a meta-analysis. Lung 191, 117–34 (2013). [DOI] [PubMed] [Google Scholar]
- 101.Brock PR et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol 30, 2408–17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feldman DR, Schaffer WL & Steingart RM Late cardiovascular toxicity following chemotherapy for germ cell tumors. J Natl Compr Canc Netw 10, 537–44 (2012). [DOI] [PubMed] [Google Scholar]
- 103.Hijiya N, Ness KK, Ribeiro RC & Hudson MM Acute leukemia as a secondary malignancy in children and adolescents: current findings and issues. Cancer 115, 23–35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Skinner R Nephrotoxicity--what do we know and what don’t we know? J Pediatr Hematol Oncol 33, 128–34 (2011). [DOI] [PubMed] [Google Scholar]
- 105.Lipshultz SE et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 23, 2629–36 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Mulrooney DA et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339, b4606 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wasilewski-Masker K et al. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics 121, e705–13 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Kadan-Lottick NS et al. Osteonecrosis in adult survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 26, 3038–45 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jain P et al. Vincristine-induced Neuropathy in Childhood ALL (Acute Lymphoblastic Leukemia) Survivors: Prevalence and Electrophysiological Characteristics. J Child Neurol (2013). [DOI] [PubMed] [Google Scholar]
- 110.Ness KK et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the st. Jude lifetime cohort study. Arch Phys Med Rehabil 94, 1451–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barr RD & Wunder JS Bone and soft tissue sarcomas are often curable--but at what cost?: a call to arms (and legs). Cancer 115, 4046–54 (2009). [DOI] [PubMed] [Google Scholar]
- 112.Lin HY & Liao SL Orbital development in survivors of retinoblastoma treated by enucleation with hydroxyapatite implant. Br J Ophthalmol 95, 630–3 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Turner CD et al. Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer 53, 417–23 (2009). [DOI] [PubMed] [Google Scholar]
- 114.Wakefield CE et al. The psychosocial impact of completing childhood cancer treatment: a systematic review of the literature. J Pediatr Psychol 35, 262–74 (2010). [DOI] [PubMed] [Google Scholar]
- 115.Kirchhoff AC et al. Occupational outcomes of adult childhood cancer survivors: A report from the childhood cancer survivor study. Cancer 117, 3033–44 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuehni CE et al. Educational achievement in Swiss childhood cancer survivors compared with the general population. Cancer 118, 1439–49 (2012). [DOI] [PubMed] [Google Scholar]
- 117.Lancashire ER et al. Educational attainment among adult survivors of childhood cancer in Great Britain: a population-based cohort study. J Natl Cancer Inst 102, 254–70 (2010). [DOI] [PubMed] [Google Scholar]
- 118.Mitby PA et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer 97, 1115–26 (2003). [DOI] [PubMed] [Google Scholar]
- 119.Kirchhoff AC et al. Employer-sponsored health insurance coverage limitations: results from the Childhood Cancer Survivor Study. Support Care Cancer 21, 377–83 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park ER et al. Health insurance coverage in survivors of childhood cancer: the Childhood Cancer Survivor Study. J Clin Oncol 23, 9187–97 (2005). [DOI] [PubMed] [Google Scholar]
- 121.Frobisher C, Lancashire ER, Winter DL, Jenkinson HC & Hawkins MM Long-term population-based marriage rates among adult survivors of childhood cancer in Britain. Int J Cancer 121, 846–55 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Frobisher C et al. Long-term population-based divorce rates among adult survivors of childhood cancer in Britain. Pediatr Blood Cancer 54, 116–22 (2010). [DOI] [PubMed] [Google Scholar]
- 123.Janson C et al. Predictors of marriage and divorce in adult survivors of childhood cancers: a report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev 18, 2626–35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brinkman TM et al. Longitudinal patterns of psychological distress in adult survivors of childhood cancer. Br J Cancer (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zebrack BJ et al. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the childhood cancer survivor study. J Clin Oncol 22, 999–1006 (2004). [DOI] [PubMed] [Google Scholar]
- 126.Zebrack BJ et al. Psychological outcomes in long-term survivors of childhood leukemia, Hodgkin’s disease, and non-Hodgkin’s lymphoma: a report from the Childhood Cancer Survivor Study. Pediatrics 110, 42–52 (2002). [DOI] [PubMed] [Google Scholar]
- 127.Kwak M et al. Trajectories of psychological distress in adolescent and young adult patients with cancer: a 1-year longitudinal study. J Clin Oncol 31, 2160–6 (2013). [DOI] [PubMed] [Google Scholar]
- 128.Stuber ML et al. Prevalence and predictors of posttraumatic stress disorder in adult survivors of childhood cancer. Pediatrics 125, e1124–34 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zebrack BJ et al. Perceived positive impact of cancer among long-term survivors of childhood cancer: a report from the childhood cancer survivor study. Psychooncology 21, 630–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clanton NR et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood Cancer: A report from the childhood cancer survivor study. Cancer (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meeske KA, Siegel SE, Globe DR, Mack WJ & Bernstein L Prevalence and correlates of fatigue in long-term survivors of childhood leukemia. J Clin Oncol 23, 5501–10 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Mulrooney DA et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS). Sleep 31, 271–81 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu Q et al. Pain in long-term adult survivors of childhood cancers and their siblings: a report from the Childhood Cancer Survivor Study. Pain 152, 2616–24 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dutch Childhood Oncology Group. Richtlijn follow-up na kinderkanker meer dan 5 jaar na diagnose SKION, Den Haag/Amsterdam: [online], http://www.skion.nl/ (2010). [Google Scholar]
- 135.Scottish Intercollegiate Guidelines Network. Long term follow up of survivors of childhood cancer A national clinical guideline; [online], http://www.sign.ac.uk/ (2004). [Google Scholar]
- 136.United Kingdom Children’s Cancer Study Group Late Effects Group. Therapy based long term follow up practice statement [online], http://www.cclg.org.uk/ (2011). [Google Scholar]
- 137.Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 3.0 [online], http://www.survivorshipguidelines.org/ (2008). [Google Scholar]
- 138.Nathan PC et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv 7, 275–82 (2013). [DOI] [PubMed] [Google Scholar]
- 139.Staba Hogan MJ, Ma X & Kadan-Lottick NS New health conditions identified at a regional childhood cancer survivor clinic visit. Pediatr Blood Cancer 60, 682–7 (2013). [DOI] [PubMed] [Google Scholar]
- 140.Wasilewski-Masker K, Mertens AC, Patterson B & Meacham LR Severity of health conditions identified in a pediatric cancer survivor program. Pediatr Blood Cancer 54, 976–82 (2010). [DOI] [PubMed] [Google Scholar]
- 141.Kremer LC et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer 60, 543–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salz T, Oeffinger KC, McCabe MS, Layne TM & Bach PB Survivorship care plans in research and practice. CA Cancer J Clin (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hinkle AS et al. A clinic-based, comprehensive care model for studying late effects in long-term survivors of pediatric illnesses. Pediatrics 113, 1141–5 (2004). [PubMed] [Google Scholar]
- 144.Kenney LB et al. The current status of follow-up services for childhood cancer survivors, are we meeting goals and expectations: a report from the Consortium for New England Childhood Cancer Survivors. Pediatr Blood Cancer 57, 1062–6 (2011). [DOI] [PubMed] [Google Scholar]
- 145.Ford JS, Chou JF & Sklar CA Attendance at a survivorship clinic: impact on knowledge and psychosocial adjustment. J Cancer Surviv (2013). [DOI] [PubMed] [Google Scholar]
- 146.Hudson MM et al. Multi-component behavioral intervention to promote health protective behaviors in childhood cancer survivors: the protect study. Med Pediatr Oncol 39, 2–1; discussion 2 (2002). [DOI] [PubMed] [Google Scholar]
- 147.Klosky JL et al. Factors influencing long-term follow-up clinic attendance among survivors of childhood cancer. J Cancer Surviv 2, 225–32 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hewitt M, Greenfield S & Stovall E (eds.) From Cancer Patient to Cancer Survivor: Lost in Transition National Academies Press, Washington, D.C. (2006). [Google Scholar]
- 149.Henderson TO, Hlubocky FJ, Wroblewski KE, Diller L & Daugherty CK Physician preferences and knowledge gaps regarding the care of childhood cancer survivors: a mailed survey of pediatric oncologists. J Clin Oncol 28, 878–83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Advisory Board Company: Survivorship [online], http://www.cogenths.com/Portals/2/pdf/Survivorship_elevating_patient_experience.pdf (2008). [Google Scholar]
- 151.Eshelman-Kent D et al. Cancer survivorship practices, services, and delivery: a report from the Children’s Oncology Group (COG) nursing discipline, adolescent/young adult, and late effects committees. J Cancer Surviv 5, 345–57 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oeffinger KC & McCabe MS Models for delivering survivorship care. J Clin Oncol 24, 5117–24 (2006). [DOI] [PubMed] [Google Scholar]
- 153.The Patient Protection and Affordable Care Act. Pub Law 111–148, 2010 [Google Scholar]


