Abstract
Alzheimer’s disease is more than a pure proteopathy. Chronic neuroinflammation stands out during the pathogenesis of the disease and in turn modulates disease progression. The central nervous system (CNS) is separated from the blood circulation by the blood-brain barrier. In Alzheimer’s disease, neuroinflammation heavily relies on innate immune responses that are primarily mediated by CNS-resident microglia. Apolipoprotein E (ApoE) is the strongest genetic risk factor for Alzheimer’s disease and was recently shown to affect the disease in part through its immunomodulatory function. This function of ApoE is likely linked to triggering receptor expressed on myeloid cells 2 (TREM2), which is expressed by microglia in the CNS. Here, we review the rapidly growing literature on the role of disease-associated microglia, TREM2 and ApoE in the pathogenesis of Alzheimer’s disease, and present an integrated view of innate immune function in Alzheimer’s disease.
Introduction
Alzheimer’s disease is the most common cause of dementia. It was first reported by Alois Alzheimer in 1906 in a description of a 51-year-old woman who presented with cognitive deficits and behavioral abnormalities1. Her autopsy showed brain atrophy with ‘miliary foci’ and characteristic neurofibrillary changes, which represent two distinct pathological hallmarks: extracellular deposition of amyloid plaques consisting predominantly of amyloid-β [G] peptides, and intraneuronal accumulation of neurofibrillary tangles comprising aggregated, hyperphosphorylated tau [G] protein. Alzheimer’s disease pathology begins decades prior to the onset of clinical symptoms. Extensive biomarker and imaging studies indicate that amyloid-β aggregation is the earliest pathological event in Alzheimer’s disease, followed at a later stage by pathological changes of tau aggregation extending beyond the limbic system to the neocortex, which correlates with brain atrophy and cognitive decline2,3. The amyloid hypothesis poses that amyloid-β accumulation initiates a cascade of downstream pathological events and drives Alzheimer’s disease progression4. Although Alzheimer’s disease was initially considered a cell autonomous neurodegenerative disorder, marked neuroinflammation is observed in the brains of patients with Alzheimer’s disease, alongside amyloid-β and tau pathology. Accumulating genetic and functional evidence strongly indicate an active role of brain innate immunity in Alzheimer’s disease pathogenesis and disease progression. In this Review, we summarize the exciting research progress in understanding the roles of innate immunity in Alzheimer’s disease. We first highlight the significance of the innate immune system in Alzheimer’s disease by describing their genetic linkage. We then discuss the current understanding of how brain innate immunity affects Alzheimer’s disease pathogenesis, with a particular focus on the microglia-apolipoprotein E (ApoE)-triggering receptor expressed on myeloid cells 2 (TREM2) axis.
Linking the innate immune system to Alzheimer’s disease
Genetic evidence.
Alzheimer’s disease is divided into two main categories. A small percentage (<1%) of Alzheimer’s disease is inherited within families in an autosomal-dominant fashion that manifests with early dementia onset, typically between age 30 and 60. This form of familial Alzheimer’s disease (FAD) is caused by mutations in genes affecting amyloid-β production, Aβ42/Aβ40 ratio [G], or amyloid-β structure, such as amyloid precursor protein (APP), presenilin-1(PSEN/) and presenilin-2 (PSEN2)5. Over 99% of Alzheimer’s disease cases occur later in life, typically in people over 65 years old, and are referred to as ‘late-onset’ Alzheimer’s disease (LOAD). While there is no identified ‘cause’ for LOAD, the two most important risk factors are age and genetics. To date, more than 30 Alzheimer’s disease-risk loci have been identified via a combination of genetic linkage, candidate gene, genome-wide association studies (GWAS), and whole genome/exome sequencing (WGS/WES) studies6. Of these risk loci, over 50% of gene variants validated by functional genomics are implicated in microglial and innate immune cell function, including the top two risk genes, APOE and TREM2. In addition, epigenomic analysis has shown that Alzheimer’s disease GWAS loci are preferentially enriched in enhancer sequences implicated in innate immune processes7. These findings indicate an essential role of the innate immune response in Alzheimer’s disease pathogenesis. Major Alzheimer’s disease genetic risk factors and their linkage to innate immune functions are shown in Box 1–Box 3.
Box 1: APOE as a risk factor for Alzheimer’s disease.
APOE is the first identified Alzheimer’s disease risk gene reported in 199395. It remains the strongest genetic risk factor for late-onset Alzheimer’s disease. Three common APOE alleles (ε2, ε3, and ε4) are present in the human population, with ε4 being a risk factor, and ε2 being protective relative to the ε3 allele. One copy of ε4 increases Alzheimer’s disease risk by ~3-fold and two copies by ~12-fold5. The initial discovery of ApoE as an Alzheimer’s disease risk factor was unexpected. As a lipid-carrier regulating lipid homeostasis, ApoE was considered to be primarily involved in influencing atherosclerosis. In the early 1990s, ApoE was found to be a constituent of amyloid plaques123,124, yet its significance was not clear until APOE was identified as an Alzheimer’s disease risk factor shortly afterwards. The discovery was inspired by two converging lines of evidence, the first showing an Alzheimer’s disease linkage at chromosome 19q13131 where APOE is located, and the second indicating a physical interaction of ApoE with amyloid-β95. The close link between ApoE and amyloid-β in the initial discovery studies has largely driven ApoE research in the Alzheimer’s disease field along an amyloid-β track. Important roles of ApoE in regulating amyloid-β pathogenesis have been identified101, which likely drive a large part of its linkage to Alzheimer’s disease risk. However, how ApoE’s function as a lipid-carrier is in itself related mechanistically to Alzheimer’s disease, either in relation to amyloid-β or other factors, is not entirely clear. Lipids serve more than pure nutritional purposes; they also play essential roles in immune regulation. As a major component of cell membranes, lipids regulate immune cell signalling and function by affecting membrane fluidity111. Lipids and certain lipoproteins also serve as ligands for various immune receptors to mediate immune cell activation and phagocytosis132,133. Other lipid-mediated mechanisms such as regulation of gene transcription and eicosanoid production have also been proposed to affect immune function134. Being a major protein regulating lipid homeostasis, it is not surprising that ApoE plays an immunomodulatory role. Therefore, a previously overlooked immunomodulatory function of ApoE may serve as an important mechanism in Alzheimer’s disease pathogenesis.
Box3 |. Other major genetic risk factors for Alzheimer’s disease.
Adenosine triphosphate-binding cassette transporter (ABCA7) and complement receptor 1 (CR1) are two additional highly replicated Alzheimer’s disease risk factors6. ABCA7 is abundantly expressed in myeloid cells and microglia, and is found to promote macrophage phagocytosis146. ABCA7 ablation exacerbates amyloid-β pathology in amyloid-β depositing mice147,148. CR1 is a receptor for the complement fragments C3b and C4b, and is implicated in regulating the complement cascade via interacting with C3b149. Other common Alzheimer’s disease risk genes such as clusterin (CLU, lipid metabolism, phagocytosis150), transcription factor PU.1 (SPI1, phagocytosis151, CELF1 in the same locus), and cluster of differentiation 33 (CD33, phagocytosis that influences amyloid-β deposition152,153), share a common immune function, indicating an immune-linkage with Alzheimer’s disease.
Why does innate immunity matter in Alzheimer’s disease?
Chronic glial activation is a prominent feature accompanying pathological protein accumulation in the Alzheimer’s disease brain. Prolonged neuroinflammation may induce neuronal injury and death via multiple ways. First, activated glia produce toxic substances such as reactive oxygen species (ROS) and nitric oxide (NO) that can directly damage or kill neurons; second, inflammatory stress and sub-lethal pathological insults can induce stressed-but-viable neurons to expose reversible ‘eat-me’ signals such as phosphatidylserine or calreticulin, causing the neurons to be phagocytosed and killed by activated microglia via a process called phagoptosis8; third, activation of the complement system promotes membrane attack complexes (MAC) formation, creating pores on cell membranes to induce cell lysis; fourth, activated microglia can crosstalk with astrocytes, inducing reactive astrocytes to rapidly kill neurons via secreted neurotoxic factors and loss of neurotrophic function9, and fifth, neuronal NLRP1 (NACHT, LRR and PYD domains-containing protein 1) inflammasome and caspase-1 activation in Alzheimer’s disease can induce neuronal injury and death10,11. In addition to a direct effect on neuronal viability, innate immunity also affects amyloid-β and tau pathology buildup, which in turn can influence neurodegeneration. Therefore, innate immunity is an indispensable component in Alzheimer’s disease pathogenesis and may constitute a driving force for disease progression.
As the principle immune cell type in the brain, microglia play a key role in mediating brain innate immunity. Microglial cell function in the pathogenesis in Alzheimer’s disease will be discussed in the following sections.
Microglia biology
Origin and functions in the brain.
Microglia are the macrophages of the brain and play central roles in neuroinflammation. They are derived from embryonic yolk sac (YS) precursors and maintain their CNS population via local self-renewal, with little contribution from bone marrow-derived monocytes12,13. They retain many features of macrophages, but also adapt themselves to meet specific requirements of the neural tissue. During development, microglia prune complement-tagged immature synapses14,15, eliminate redundant neural precursor cells16, and promote developmental neuronal apoptosis along with removal of cell corpses17,18. In the adult brain, highly dynamic microglia processes constantly survey the local environment19,20 in a speed estimated to cover the entire parenchyma within a few hours20. Comprehensive signaling components, including major classes of neurotransmitter receptors, pattern recognition receptors, cytokine and chemokine receptors, as well as other receptor systems, converge on microglia21, making them a superior sensor and reactor to environmental cues. This grants microglia the capacity to monitor neuronal activity and modulate synaptic plasticity22. During stresses, such as brain injury, infection, or neuropathological diseases, microglia are activated in an effort to combat the insult and control damage. However, in this instance, both protective and detrimental effects can occur.
Disease-associated microglia: an updated understanding.
The signal transduction pathways for microglial cell activation have been well documented (Box 4). Initial characterization of microglial cell polarization into pro-inflammatory ‘M1’ and reparative ‘M2’ phenotypes was based on the M1/M2 macrophage classification.23 Macrophages acquire an M1 phenotype in response to interferon γ (IFNγ) and synthesize toxic substances, such ROS, NO, and lysosomal enzymes to kill microbes. By contrast, M2 macrophages are induced by interleukin-4 (IL-4) and express enzymes for collagen synthesis and fibrosis to promote tissue repair. Likewise, activated microglia are divided into an Ml phenotype, characterized by upregulation of inflammatory genes such as TNF and IL1β, and an M2 phenotype, characterized by the expression of pro-repair genes such as arginase 1 (Arg1) and chitinase-like 3 (Chi3l3, also known as Ym1)24. However, this nomenclature is now deemed inaccurate as accumulating evidence has revealed a broad diversity of macrophage activation statuses and shown that macrophages show remarkable plasticity to change phenotypes in response to environmental cues25,26.
Box 4 |. Signal transduction pathways for microglial activation.
Microglia express pattern recognition receptors (PRRs) that recognize two categories of molecular structures for activation: pathogen-associated molecular patterns (PAMPs) that are products of microbial pathogens, and damage-associated molecular patterns (DAMPs) that are derived from damaged cells, such as misfolded/aggregated proteins, or mislocalized nucleic acids. PRRs consist of toll-like receptors (TLRs) located on the cell surface and intracellular membranes, and cytosolic NOD-like receptors (NLRs). Conventional LPS-induced microglial activation involves TLR4 activation, which recruits adaptor proteins MYD88 (myeloid differentiation primary response 88) or TRIF (TIR-domain-containing adapter-inducing interferon-β) that activates NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and IRFs (interferon regulatory factors), respectively. NF-κB stimulates the expression of pro-inflammatory genes, such as tumor necrosis factor (Tnf), interleukin-1(Il1) and chemokine C-C motif ligand 2 (Ccl2), whereas IRFs promote production of type 1 interferons that are important for anti-viral responses.
In Alzheimer’s disease, the NLRP3 inflammasome is activated in microglia154. Upon ligand binding, multiple NLRP3 proteins interact to form an oligomer, where each NLRP3 molecule binds an adaptor protein called apoptosis-associated speck-like protein containing a carboxy-terminal CARD (ASC). The adaptors then bind to pro-caspase 1, an inactive precursor of caspase 1. The recruitment of pro-caspase 1 to the inflammasome induces auto-proteolytic conversion of the pro-enzyme into active caspase 1, which cleaves precursors of IL-1β and IL-18 into active forms for subsequent release. Canonical NLRP3 inflammasome activation requires two signals: the first signal is induced by microbial or endogenous molecules, typically TLR ligands such as LPS, to prime microglia for NLRP3 and pro-IL-1β expression; whereas the second signal is triggered by a variety of stimuli such as ATP, pore-forming toxins, and crystals that induce a common cellular signal (K+ efflux or others) to initiate inflammasome assembly155. In Alzheimer’s disease, protein pathologies and injured neurons may provide the two signals respectively. Cleaved IL-1β and IL-18 signal through their respective receptors, IL-1R1 and IL-18R, triggering NF-κB activation. IL-1β is a key initiator of inflammation that potently induces microglia cellular activation and cytokine production, whereas IL-18 regulates interferon-γ responses156.
In neurodegenerative diseases, the precise gene signatures of microglia that mediate pathology-and neurodegeneration-associated sterile inflammation have not been clearly elucidated. Recent single-cell RNA-seq data identified a two-stage microglial cell activation process that occurs during disease progression in an amyloid-β-depositing mouse model, where the transition from the first to the second stage is determined by TREM227. The end-stage activated microglia appear to display a conserved core-expression profile harbouring certain degrees of diversity across individual disease phenotypes27,28. The core expression profile is likely induced by neuronal cell death or damage, whereas distinct pathologies may contribute to the diversity. The common disease-associated microglial cell signature was defined by different groups as the ‘microglial neurodegenerative’ (MGnD) phenotype28 or the ‘disease-associated microglia’ (DAM) phenotype27. This signature is characterized by a suppression of microglial homeostatic genes, and an induction of pro-inflammatory genes as well as genes involved in microglial phagocytotic function targeting degenerated/injured neurons and amyloid plaques27,28. The MGnD phenotype can largely be recapitulated by injecting apoptotic neurons into mouse brain parenchyma, where the subset of microglia that are activated to phagocytose apoptotic neurons show a similar molecular phenotype to the MGnD phenotype28. The MGnD microglia can also be activated via intracerebral administration of kainic acid [G] that induces neuronal cell death28, supporting the idea that neuronal death and damage determines the central microglial cell gene signature in neurodegenerative diseases. Importantly, although the microglia that phagocytose apoptotic neurons share many molecular signatures with lipopolysaccharide (LPS) and IFNγ-stimulated M1 microglia, they also exhibit significant differences, such as contrasting gene expression patterns of secreted phosphoprotein 1(Ssp1), CC-chemokine ligand 3 (Ccl3), and early growth response 1(Egr1). But the most distinguishing difference is that the expression of Apoe — the hallmark gene of the MGnD phenotype that has been found to be upregulated in microglia in all currently-assessed neurodegenerative diseases — is suppressed in M1 microglia. In addition, certain classical M2 microglial cell markers, such as Arg1 and Ym1, are also induced in microglia that phagocytose apoptotic neurons, indicating a unique microglial activation profile in neurodegenerative diseases that is distinct from the M1/M2 classification28 (Table 1).
Table 1:
Comparison of different microglial cell phenotypes [ED:?]
| Cell phenotype | ‘M1’ microglial cell | ‘DAM’ or ‘MGnD’ microglial cell | ‘M2’ microglial cell (alternative activation) | ‘M2’ microglial cell (acquired deactivation) | |
|---|---|---|---|---|---|
| Stimulus | IFNy, TNF, LPS | Disease-associated pathologies, neuronal cell death, tissue damage | IL-4 and IL-13 | TGFβ and IL-10 | |
| Marker genes |
|
Apoe, Trem2, Clec7a, Lpl, Itgax, Axl, Csf1, Lilrb4, Spp1, Ccl2, Cst7 | Arg1, Ym1, Mrc1, Fizz1, Clec7a | Il10, Tgfb | |
| Functions |
|
|
|
||
| Genes regulated in the same direction | IL1b ↑, Cybb ↑, Lilrb4 ↑, Ccl2 ↑, Axl ↑, Itgax ↑, Tspo ↑, P2ry12 ↓, Tmem119 ↓ Olfml3 ↓, Tgfbr1 ↓, Csf1r ↓, Sall1 ↓, Hexb ↓, Rhob ↓ ⋯ | Arg1 ↑, Ym1 ↑, Clec7a ↑ ⋯ | |||
| Genes regulated in opposite directions | Apoe, Trem2, Erg1, Spp1… | Many genes… | |||
Inconclusive
Microglia and amyloid-β pathology
Global immune modulation in the brain: effects on amyloid-β pathology.
Numerous studies have examined the effect of neuroinflammation on amyloid-β pathogenesis by targeting cytokines and other inflammatory molecules to create either a pro-inflammatory or anti-inflammatory environment in the brain. The results of these studies have been inconsistent. Several studies have found that activating pro-inflammatory pathways — for example, by overexpressing TNF29, IFNγ30 or IL-631, or by inhibiting anti-inflammatory cytokines such as IL-1032 — reduces plaque deposition, and vice versa33,34. These effects have been proposed to mainly result from enhanced phagocytotic activity of microglia induced by potent inflammation, leading to greater amyloid-β clearance, whereas APP synthesis or processing is not affected. However, other studies have suggested that inhibition of inflammation attenuates amyloid-β pathology35–37. Although these studies in which brain inflammation is manipulated featuring conventional M1 or M2 microglial cell phenotypes provide important insights, it is likely that they do not truly reflect the disease-associated inflammatory signatures found in neurodegenerative diseases.
Microglia function prior to pathology onset.
Microglia have long been hypothesized to regulate amyloid-β pathogenesis via phagocytosis. However, depleting microglia after amyloid-β pathology onset via pharmacological inhibition of colony-stimulating factor 1 receptor (CSF1R) signalling38 or by using the CD11b-HSVTK (TK) system39 for 4 weeks, does not affect total plaque burden. In contrast, a recent study shows that a three-month depletion of microglia via CSF1R inhibition starting prior to amyloid-β pathology onset drastically reduces plaque deposition in 5XFAD mice [G]40. This indicates that microglia may be involved in the initial seeding stage of plaque formation. Interestingly, activation of the NLRP3 inflammasome in cultured microglia leads to the release of ASC specks that rapidly bind to and cross-seed amyloid-β to promote amyloid-β aggregation41. In the brains of patients with Alzheimer’s disease and amyloid-β depositing mice, the NLRP3 inflammasome is activated and ASC specs are found to present in the core of amyloid plaques41, indicating an active role of ASC specs in plaque seeding and initiation. ASC-deficient APPSwePSEN1dE9 mice [G] have significantly reduced cerebral plaque loads, and show little seeding and spreading of amyloid-β pathology when receiving cerebral injection of brain homogenates containing amyloid-β seeds41. Consistent with this, two-photon live imaging of GFP-labelled microglia in 5XFAD mice show that microglia activated by laser-induced cerebral injury migrate to the damaged sites and induce de novo plaque formation in the injured site within two weeks42. This emerging evidence suggests that activated microglia may facilitate amyloid-β plaque formation by promoting amyloid-β seeding in the initiation stage. However, more research is needed to verify this effect and to exclude potential artefacts caused by specific animal models or off-target drug properties.
TREM2 and amyloid-β pathology.
Despite the fact that no notable changes in global plaque loads have been observed when microglia are deleted after pathology onset, genetic evidence from TREM2 studies indicate a role of microglia in regulating amyloid-β pathology post plaque formation. Microglia have long been observed to cluster around amyloid-β plaques in postmortem Alzheimer’s disease brains and in mouse models of amyloid-β deposition. Unlike non-plaque-associated microglia, which retain a more homeostatic phenotype27,43, plaque-associated microglia show major alterations in their molecular signatures and constitute the population of DAM in amyloid-β-depositing mouse models27. TREM2 was recently demonstrated to be essential for promoting microglial cell association with plaques44–49. TREM2 haplodeficiency, ablation, or the TREM2R47H mutation that causes a missense variant of TREM2, eads to a significant reduction of plaque-associated microglia compared to their TREM2-sufficient counterparts44–49. Corresponding to loss of microglial enclosure around plaques, TREM2-deficient amyloid-β depositing mice develop significantly enlarged and less compact plaques containing longer and more diffuse star-shaped amyloid fibrils that project from loosely packed cores48,49. Such morphological changes in plaques are frequently associated with a greater degree of neuritic dystrophy with enlarged, swollen axons and dendrites in close vicinity48,49, indicating that plaque-associated microglia perform plaque trimming and compacting functions that help to restrict plaque growth, and that these cells serve as a barrier to limit plaque-induced neuronal toxicity. Patients with Alzheimer’s disease who carry the TREM2R47H mutation show patterns of plaque morphology and neurite dystrophy that resemble the plaques observed in TREM2-deficient amyloid-β depositing mice49, indicating that this mutation causes a loss of function.
In terms of the mechanistic basis of these observations, TREM2 was recently identified as an amyloid-β receptor with a higher affinity for amyloid-β oligomers than monomers50. Notably, TREM2 expression is specifically upregulated in plaque-associated microglia in both mice and humans27,45. Keren-Shaul et al. showed that TREM2 expression switches on the late-stage DAM signature featuring an enhanced phagocytotic activity51, suggesting that plaque-associated microglia may be able to phagocytose plaques. Supporting this idea, plaque-associated microglia have been shown to contain plaque-derived materials27,49, indicating their capability to phagocytose plaque components. This plaque-phagocytotic capacity is impaired with TREM2 deficiency48,49, indicating that plaque-associated microglia trim and compress plaques via TREM2-dependent plaque phagocytosis.
Initial studies on TREM2 reported contradicting results regarding the effect of TREM2-deficiency on plaque loads45,46, which was subsequently identified to be a disease progression-dependent effect47. Overall, TREM2 deficiency leads to a mild reduction of plaque deposition in early-stage disease, but causes enhanced amyloid-β pathology in advanced stages of the disease45–47. This disease-stage-dependent effect was initially confusing. However, new insights into microglial cell functions suggest a possible explanation. Given the role of activated microglia in promoting amyloid-β seeding, reduced microglial association with the initial plaque seeds due to TREM2 deficiency may impede the seeding efficiency, leading to a prolonged lag phase and therefore reducing pathology in the early stage. In addition, TREM2−/− amyloid-β depositing mice consistently show downregulation of inflammatory genes, such as Tnf and Il1b45–47, and also of key genes associated with the MGnD phenotype, such as c-type lectin domain family 7 member A (Clec7a) and integrin subunit alpha X (Itgax)27,28, indicating immature polarization of microglia into the microglial neurodegenerative phenotype and insufficient microglial activation. Such incompletely activated microglia may fail to produce sufficient inflammatory factors43, such as ASCs, to effectively initiate plaque seeding.
By contrast, during the advanced disease stage plaques are already formed, hence seeding efficiency is no longer a determining factor. Instead, microglial plaque-trimming function becomes a dominating factor influencing plaque growth. Lack of TREM2 results in impaired plaque trimming, which leads to enlarged plaques with reduced compaction48,49. The increased plaque size may be the primary cause of the enhanced amyloid-β pathology observed in late-stage disease in TREM2−/− amyloid-β depositing mice revealed by amyloid-β antibody staining. Supporting this notion, fibrillar plaque staining indicated no difference in the total amount of pathology between TREM2+/+ and TREM2−/− amyloid-β depositing mice at the late stage of disease47.
Interestingly, a recent study showed that expressing human TREM2 under the endogenous human regulatory elements on top of murine TREM2 in 5XFAD mice leads to reduced fibrillar amyloid-β deposition with denser cores, accompanied by ameliorated neuritic dystrophy52. Surprisingly, plaque-associated microglia drop in number with TREM2 overexpression and exhibit elongated and ramified processes with a sharp reduction of ionized calcium binding adaptor molecule 1 (IBA1) [G] signal, indicating an altered activation status, likely reflecting less activation52. These effects are interpreted to derive from enhanced microglial phagocytotic capacity induced by TREM2 overexpression which requires less microglial activation to trim plaques. Transcription analysis from crude cortical lysates reveals mixed microglial activation patterns with suppression of certain DAM genes, but further activation of other DAM genes that appear to favour phagocytosis52. These gene signatures need to be verified in isolated microglia in future studies and the human TREM2 overexpression effect would need to be confirmed in a murine TREM2−/− background to avoid potential confounding effects.
Microglia and tau pathology
In the brain, tau is primarily an intracellular neuronal protein, but is normally secreted into the interstitial fluid (ISF)53 and is believed to transmit tau pathology in a prion-like manner54. High-molecular-weight phosphorylated tau is found in the ISF, and is proposed to be the species mediating tau propagation55. Insoluble tau isolated from postmortem Alzheimer’s disease brain is shown to be taken up by microglia in vitro and in vivo56. In a tau-spreading mouse model where human P301L tau was focally expressed in medial entorhinal cortex (MEC) layer II/III neurons, microglial cell depletion markedly suppressed tau propagation from the entorhinal cortex to the dentate gyrus by inhibiting microglial secretion of exosomes that contain tau oligomers for seeding57, indicating microglia may actively participate in tau spreading by taking in and subsequently releasing some form of tau.
Others assessed the effect of microglial activation on tau pathogenesis. Microglial activation is shown to precede tau pathology in a tauopathy mouse model58, and administering an immunosuppressant drug FK506 from an early age drastically reduces tau pathology, neurodegeneration and mortality58. In addition, chronic intraperitoneal administration of LPS to 3xTg-AD mice[G] for 6-weeks is shown to induce marked microglial activation and promote site-specific tau hyperphosphorylation59. IL-1 receptor signalling is found to play a key role in neuroinflammation-induced tau phosphorylation by enhancing the activity of tau kinases including cyclin-dependent-like kinase 5/p25 (CDK5/p25), glycogen synthase kinase 3β (GSK3β), and p38 mitogen-activated protein kinase (p38-MAPK)60,61. Importantly, deleting the microglial cell-expressed homeostatic gene CX3C chemokine receptor 1(Cx3cr1) in htau mice [G] leads to amplified microglial activation and enhanced tau hyperphosphorylation/aggregation62,63; whereas overexpressing a soluble form of CX3CL1, the ligand for CX3CR1, reduces microglial cell activation and tau pathology in rTg4510 mice [G]64. Interestingly, there also appears to be a disease-stage-dependent microglial effect on tau pathology. CX3CR1-induced differences in tau phosphorylation are only observed in early to middle disease stages62–64, but is largely absent in late-disease stage, when an overall reduction of phosphorylated-tau (p-tau) signal occurs58,63, likely due to loss of neurons from neurodegeneration. Similarly, TREM2 deficiency is reported to enhance tau hyperphosphorylation at an early disease stage in htau mice65 and a mid-disease stage in P301S mice [G]66, but does not affect tau pathology at a late-disease stage in P301S mice67, whereas overexpressing murine TREM2 in microglia is found to reduce tau hyperphosphorylation in the mid-disease stage in P301S mice68.
Microglia and neurodegeneration
Microglia directly regulate neurodegeneration.
Apart from regulating Alzheimer’s disease pathologies, microglia likely play a more crucial role in modulating neurodegeneration. This capacity is partially attributed to their pathology-modulating functions, as greater pathology may cause more neuronal injury and death. However, end-stage MGnD microglia that harbour high phagocytotic and inflammatory capacity may directly kill stressed neurons, such as tau-loaded neurons, via phagoptosis; or damage and kill healthy neurons by secreting neurotoxic substances, independent of their effects on Alzheimer’s disease pathology. Interestingly, microglia are shown to intimately associate with tau-loaded neurons in human brain tissue56. End-stage TREM2−/− P301S mice that exhibit significantly reduced microgliosis and downregulated MGnD genes such as ApoE and cystatin 7 (Cst7), show significantly attenuated brain atrophy compared to TREM2+/+ P301S mice despite no change of tau pathology67. Similarly, overexpression of soluble CX3CL1 reduces microglial activation and neuronal loss in rTg4510 mice64. The same principle also applies to other neurodegenerative diseases. In mouse models of Parkinson’s disease and amyotrophic lateral sclerosis (ALS), CX3CR1 ablation leads to marked microglial activation and loss of dopaminergic or motor neurons compared to CX3CR1+/+ mice. Hence, activated microglia actively modulate neurodegeneration.
Microglia-astrocyte crosstalk.
In addition to showing a direct impact, microglia also indirectly regulate neurodegeneration by communicating with astrocytes, another major CNS cell type constituting approximately 30% of CNS cells. Unlike microglia that are derived from yolk sac precursors, astrocytes resemble neurons in that they originate from ectodermal precursors and are not conventional immune cells. However, astrocytes are immune-competent. They respond to a wide range of brain insults and are activated in a wide range of neurodegenerative diseases including Alzheimer’s disease9,69–71. In physiological conditions, astrocytes provide essential nutritional and growth support to neurons to maintain their survival and function. They regulate extracellular ion and neurotransmitter concentrations and likely modulate synaptic transmission and synaptic plasticity72. In addition, astrocytes actively regulate synaptic pruning during development73,74 and in adulthood74,75 to maintain synaptic homeostasis and neuronal circuit function. They also form the glia limitans that serves as a critical barrier separating neural and non-neural tissue along perivascular spaces, meninges, and tissue lesions in the CNS to maintain brain homeostasis76.
Astrocytes are highly heterogeneous populations that differ in a variety of dimensions including morphology, function, developmental origin, and activation profiles72. A recent study identified two distinct populations of reactive astrocytes induced by inflammation or ischemia, termed as A1 astrocytes and A2 astrocytes, respectively77. A1 astrocytes strongly upregulate genes involved in classical complement activation which are shown to be destructive to synapses, therefore are considered harmful; whereas A2 astrocytes upregulate neurotrophic genes that promote neuronal survival and growth, and are deemed protective77. Interestingly, LPS-induced inflammatory microglia are found to activate A1 astrocytes by producing a collection of molecules: TNF, IL-1α and complement 1q (C1Q)9. A1 astrocytes demonstrate strong neurotoxicity by secreting toxic factors that can rapidly induce neuronal death, and by losing normal functions to support neuronal survival, growth or functionality and to maintain environmental homeostasis9. Complement 3 (C3), a secreted marker for A1 astrocytes, is found to signal through the C3a receptor (C3aR) expressed on neurons to disrupt neuronal morphology and function78. In APP-transgenic mice, blocking A1 astrocyte activation rescues cognitive impairment78. A1 astrocytes are found in post-mortem brain tissues from a broad panel of neurodegenerative diseases including Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, ALS, and multiple sclerosis9, indicating a common mechanism through which these reactive astrocytes affect neurodegeneration.
Interestingly, activated A1 astrocytes are shown to signal back to microglia by binding to microglial C3aR to regulate microglial function79. In vitro, acute C3/C3a activation promotes, whereas chronic C3/C3a treatment reduces microglial phagocytosis. In vivo, C3 elevation via NF-κB hyperactivation significantly exacerbates amyloid-β pathology and neuroinflammation in APP-transgenic mice, whereas treatment with a C3aR antagonist ameliorates plaque load and microgliosis79. Microglia-astrocyte crosstalk is essential for the immune regulation of Alzheimer’s disease pathogenesis and neurodegeneration, and should be further explored in future studies.
Other innate immune cells in Alzheimer’s disease
Three additional CNS macrophage cell types other than classical microglia, namely perivascular, meningeal, and choroid plexus macrophages, are present at the interface between the parenchyma and the circulation. Perivascular and meningeal macrophages are generated from yolk sac precursors and are maintained by self-renewal, whereas choroid plexus macrophages have dual embryonic and adult hematopoietic origins80. These macrophages express high levels of CD45 and MHCII as opposed to low expression of these markers in parenchymal microglia80, and are speculated to play a role in CNS surveillance and parenchyma-peripheral immune connection, but their functions are still not clear. The role of potential-infiltrating monocytes in affecting amyloid-β pathology is assessed in a microglia-repopulation model, where depleting microglia using the CD11b-HSVTK (TK) system is followed by a rapid repopulation by peripherally derived myeloid cells in the absence of additional stimuli such as irradiation81. Monocyte repopulation for up to 6 months does not modify amyloid load82,83, indicating peripheral monocytes may have no additional effects on parenchymal amyloid-β pathology. However, patrolling monocytes are reported to be actively involved in vascular amyloid-β clearance84. Others report that neutrophils infiltrate brain parenchyma of amyloid-β depositing mouse models and human Alzheimer’s disease brain, and migrate towards amyloid-β plaques85,86. Neutrophil infiltration is shown to exacerbate tau phosphorylation, microgliosis, and behavioral deficits in 3xTg-AD mice that develop both amyloid-β and tau pathology86.
Overview of neuroinflammation in Alzheimer’s disease
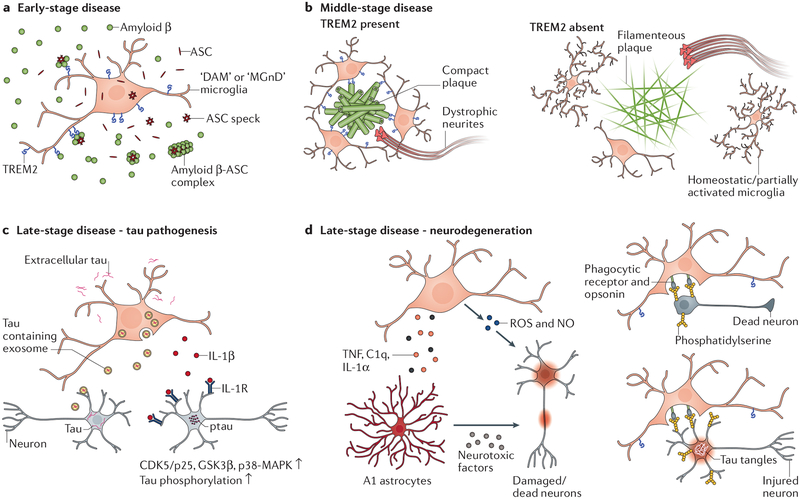
Neuroinflammation in Alzheimer’s disease can be beneficial or detrimental depending on disease stages and targets of activated microglia. There appear to be three major disease stages during the course of Alzheimer’s disease (FIG. 1). The early stage (FIG. 1a) features plaque initiation, when microglial activation appears to facilitate plaque formation via secreting inflammatory factors, therefore inflammation at this stage may be harmful. It’s unclear why microglia may have evolved to promote amyloid-β seeding during the plaque initiation stage. While amyloid-β deposition is likely an incidental event as a price paid for aging beyond active evolutionary selection, one perspective holds that amyloid-β peptides act as antimicrobial molecules that bind to and trap invading pathogens via oligomerization to inhibit their binding to host cells87. Hence, enhanced seeding may expedite pathogen insulation. Although intriguing, this theory needs more evidence to support. The mid-stage (FIG. 1b) is characterized by plaque accumulation, during which time activated microglia target growing plaques to restrict their expansion and toxicity to neighboring neurons, hence playing a protective role. The late stage (FIG. 1c) is represented by accumulating tau pathology with plateaued amyloid-β pathology. During this time, cell death increases and MGnD microglia are primarily involved in removal of dead neurons and targeting stressed-but-viable neurons injured by tau pathology for phagoptosis. Activated microglia may also exacerbate tau pathology by activating tau kinases and enhancing tau spreading. Meanwhile, they may generate ROS and NO and induce A1 astrocytes to aggravate neuronal loss. The massive neuronal death may further exacerbate neuroinflammation to kill more neurons, forming a vicious circle. This stage may be similar in all neurodegenerative diseases, and detrimental neuroinflammation during this stage may constitute a major mechanism for neurodegeneration across different diseases.
Figure1 |. Roles of neuroinflammation in different stages of Alzheimer’s disease.
a | At an early stage disease prior to or just following the initiation of plaque deposition, NLRP3 inflammasome activation by pathological changes results in release of ASC specks from microglia that promote amyloid-β seeding. Activated microglia may capture amyloid-β seeds via TREM2-amyloid-β interaction and boost plaque formation by generating local high concentrations of ASC specks around amyloid-β seeds to facilitate amyloid-β aggregation for plaque core initiation. b | In the mid-stage disease when plaques accumulate, microglia cluster around plaques in a TREM2-dependent fashion and trim amyloid-β fibrils on the edge to compact the plaque. Plaque-associated microglia also serve as a barrier between the plaque and surrounding tissues to reduce amyloid-β-induced toxicity to neurites. TREM2 deficiency hinders full activation of DAM/MGnD microglia and significantly reduces the number of plaque-associated microglia. This results in enlarged amyloid-β plaques with wispy fibre-like structures projecting from loosely packed cores and greater neuritic dystrophy in close vicinity. c | In the late-stage disease, tau pathology accumulates. Microglia may accelerate tau pathology spreading by taking in extracellular tau and subsequently releasing certain tau species. Activated microglia also secret IL-1β that signals on neurons and likely indirectly through astrocytes as well to enhance neuronal tau phosphorylation by activating tau kinases. d | Along with tau pathology buildup, neurodegeneration occurs. DAM/MGnD microglia appear to exacerbate neuronal loss. Activated microglia induce A1 astrocytes, which kill/damage neurons via secreting neurotoxic factors and loss of neurotrophic functions. The large amounts of ROS/NO generated during microglia activation can also directly injure/kill neurons. With increased neuronal death, DAM/MGnD microglia are engaged in removing neuron corpses. However, they can also target stressed-but-viable neurons that present ‘eat-me’ signals for phagoptosis, causing more neuronal loss.
Where does ApoE fit in the picture?
ApoE biology and current view of ApoE as an Alzheimer’s disease risk factor.
ApoE as an apolipoprotein forms lipoprotein particles with lipids and cholesterol to mediate their transport. It delivers lipids and cholesterols to cells via receptor-mediated endocytosis of lipoproteins and also mediates cellular cholesterol efflux in a process called reverse cholesterol transport, where cellular cholesterol is transferred to the lipoprotein via ATP-binding cassette transporter (ABCA1)88,89. ApoE is most highly expressed in the liver and the CNS. In the brain, ApoE is the most abundantly produced apolipoprotein, and is synthesized primarily by astrocytes and to a lesser extent by microglia90,91.
APOE is by far the strongest genetic risk factor for late-onset Alzheimer’s disease. The ε4 allele is significantly enriched in patients with Alzheimer’s disease, and ε4-carrying patients show earlier disease onset92, heavier amyloid-β plaque load in the brain93, greater brain atrophy94, and accelerated disease progression69 compared to patients who do not carry this allele. The prominent effect of ApoE on Alzheimer’s disease triggered broad enthusiasm in understanding how ApoE contributes to Alzheimer’s disease. As ApoE was initially identified as an amyloid-β-binding protein95, extensive follow-up studies have focused on its relationship with amyloid-β pathology. To date, substantial evidence has demonstrated ApoE affects amyloid-β deposition in a dosage and isoform-specific fashion (ApoE4>ApoE3>ApoE2)96–100, and we recently reported that ApoE4 exacerbated tau pathogenesis and tau-mediated neurodegeneration relative to other ApoE isoforms independent of amyloid-β69. Mechanistically, ApoE isoform-dependent effects on ApoE-amyloid-β interaction, amyloid-β aggregation, and amyloid-β clearance have been comprehensively investigated101, and ApoE isoform-dependent interaction with tau is also reported102,103. Interestingly, ApoE effects do not appear to be restricted to Alzheimer’s disease. APOE4 has also been reported to be a risk factor for other neurodegenerative diseases, such as frontotemporal dementia104, pure synucleinopathies including pure dementia with Lewy bodies and Parkinson disease dementia105, although the data in these instances is less clear. Notably, genetic ablation of ApoE in various neurodegenerative mouse models — including tauopathy69, synucleinopathy106, and experimental autoimmune encephalomyelitis (EAE)107 — shows a similar protective effect against neurodegeneration and disease severity, indicating a prevalent function of ApoE in neurodegenerative conditions based on a potential common mechanism in addition to its specific effects in individual pathologies. We think this shared mechanism is likely linked to ApoE’s immunomodulatory function, as we discuss below.
Immunomodulatory function of ApoE: insights from LPS studies.
In fact, the capacity of ApoE to modulate inflammation has long been observed. In healthy humans challenged with intravenous LPS infusion, ε4-carriers demonstrate significantly higher elevation of body temperature and plasma TNF levels compared to ε4 non-carriers108. Similarly, when whole blood isolates from human subjects were stimulated ex vivo with TLR ligands, enhanced production of a wide panel of cytokines and chemokines was observed in blood from ε4+ donors compared with ε4− donors108. A greater immune response associated with the ε4 allele is also observed in human ApoE-targeted replacement (APOE-TR) mice [G] and in cultured microglia/macrophages upon LPS stimulation108–110.
How ApoE achieves this is not well understood. Evidence shows that lipid rafts play an essential role in immune activation by serving as platforms for signalling complex assembly; hence minor intervention on membrane lipids such as cholesterol loading or efflux is sufficient to induce membrane remodelling and subsequent signal transduction111. Higher retention of cholesterol in lipid rafts due to lack of the cholesterol efflux transporters ABCA1 or ABCG1, enhances TLR signalling in macrophages111 and induces leukocytosis under hypercholesterolemia conditions112, whereas myeloid cell surface-bound ApoE promotes cholesterol efflux in a cell-autonomous fashion to inhibit myelopoiesis113. ApoE4 is reported to be less effective than ApoE3 in inducing cholesterol efflux from macrophages114, which causes cholesterol accumulation on cell membranes108,114. This mechanism has been proposed to explain the higher immune reactivity associated with ApoE4. Supporting this theory, ApoE-deficient mice behave similarly to ApoE4-targeted replacement (ApoE4-TR) mice (mice with the human APOE4 coding sequence replacing the murine apoe coding sequence) in response to intracerebroventricular LPS stimulation with enhanced gliosis and cytokine production compared with ApoE2-TR and ApoE3-TR mice110. However, this study is based on LPS stimulation, which has key differences from neurodegeneration-associated sterile neuroinflammation. Above all differences, the lack of ApoE induction may determine the distinct nature of the response.
Immune functions of ApoE in neurodegeneration
Contrary to LPS treatment where ApoE deletion amplifies the immune response, under neurodegenerative or neuroinflammatory conditions, ApoE deficiency attenuates inflammation28,69,97,107 and disease severity69,106,107. In a tauopathy mouse model, genetic ablation of ApoE markedly reduces activation of microglia and astrocytes activation and ameliorates brain atrophy69. In a mouse model of Parkinson’s disease featuring synucleinopathy, ApoE deletion significantly attenuates the loss of spinal cord motor neurons, delays disease onset, and improves survival106. ApoE ablation in EAE mice also reduces inflammation and disease severity107.
How can one explain this apparent contradiction? Interestingly, in EAE, the disease-exacerbating effect of ApoE only occurs in the effector phase107 when recruited effector immune cells, primarily macrophages, are activated by helper T cells and start to attack and ingest myelin. In the initiation stage, however, T cell priming by myelin antigens is enhanced in ApoE-deficient mice compared to wild-type mice107. These results indicate multi-faceted immunomodulatory functions of ApoE. ApoE may reduce the excitability of immune cells by modulating lipid raft content on cell membranes, but may also be required for intact functionality of MGnD microglia. ApoE deletion prevents full acquisition of the microglial neurodegenerative phenotype with suppression of key MGnD genes28, and therefore may prohibit disease-associated microglia from being fully developed to carry out their pro-inflammatory and phagocytotic functions. In addition, as neurons express a number of ApoE receptors, such as low-density lipoprotein receptor-related protein 1 (LRP1), heparan sulfate proteoglycans (HSPGs), and low-density lipoprotein receptor (LDLR) on cell membranes, we propose that ApoE may accumulate on the surfaces of damaged and dead neurons as a result of impaired neuronal uptake function, and serve as an opsonin to promote neuronal phagocytosis by microglia. The opsonization effect may be achieved via ApoE interacting with TREM2 on microglia, as TREM2 has been demonstrated to be an ApoE receptor116–119. Supporting this hypothesis, ApoE binding to cell surface was shown to be significantly enhanced in apoptotic N2a cells, a murine neuroblastoma cell line, compared to healthy cells, and cultured microglia phagocytose significantly more apoptotic N2a cells with increased levels of ApoE in the medium116. TREM2−/− microglia, in contrast, show impaired phagocytosis of apoptotic N2a cells, supporting an ApoE-TREM2 axis in mediating microglial phagocytotic function.
Hence, despite higher excitability of microglia in the absence of ApoE, their impaired neurodegenerative functionality may hinder their capacity to kill and damage neurons via phagoptosis or inflammatory mechanisms, which may underlie the protective effect of ApoE deficiency in various neurodegenerative settings. Supporting this view, global or conditional deletion of ApoE in microglia reduces neuronal loss in the facial motor nucleus after facial nerve axotomy28. Interestingly, TREM2 shares the same pathways with ApoE in regulating the microglial neurodegenerative phenotype. TREM2 deletion in APPPS1–21 [G] or superoxide dismutase 1 (SOD1) mice [G] results in ApoE downregulation and a similar suppression of core inflammatory MGnD genes and restoration of homeostatic genes28. Targeting TREM2 signaling in the facial nerve axotomy model ameliorates neuronal loss28, and ablation of TREM2 in tauopathy mice attenuates neuroinflammation and neurodegeneration67, similar to the effect of ApoE deletion. Whether ApoE isoform-specifically regulates MGnD microglia activation or potential opsonization for neuronal phagocytosis are intriguing questions to be further investigated in future studies.
ApoE and Alzheimer’s disease pathologies
ApoE and amyloid-β pathology.
ApoE has long been observed to affect amyloid-β pathogenesis. Compared with other ApoE isoforms, ApoE4 exacerbates the deposition of amyloid plaques in the brain98–100. Furthermore, regardless of ApoE isoforms, ablating one allele or both alleles of ApoE all lead to reduction of fibrillar amyloid-β deposits96,97,120. These effects were attributed to ApoE affecting amyloid-β fibrillization and clearance101. However, recent data suggest new mechanisms of ApoE function. ApoE was found to be essential for plaque formation during the initial seeding stage121,122. Targeting ApoE4 with antisense oligonucleotides (ASOs) from birth reduces amyloid-β plaque deposition, whereas ASO treatment after pathology onset in adult mice does not affect fibrillar plaque load121. Vice versa, induction of ApoE4 expression during the initial seeding stage, but not during the plaque growing phase, increases amyloid-β pathology122. However, although no change in fibrillar plaques was observed in adult ASO-treated mice when X-34 staining (which stains for bona fide fibrillary beta-sheet structures) was used, staining by amyloid-β antibodies revealed significantly increased plaque sizes121. Interestingly, ApoE ablation in APPSwePSEN1dE9 [G] or APPPS1–21 [G] mice also results in enlarged plaques with wispy fibre-like structures projecting from loosely packed cores120, similar to the plaques observed in TREM2-deficient amyloid-β-depositing mice48,49. Corresponding to loose and enlarged plaques, ApoE deletion leads to impaired microglial cell response to plaques120, the same phenotype observed with TREM2 deficiency44,46. These findings indicate that the ApoE-TREM2 axis implicated in neurodegeneration may share similar mechanisms in regulating microglial cell responses to amyloid-β pathology.
Microglia-mediated compaction and insulation of amyloid-β plaques may require phagocytotic MGnD microglia activation that is dependent on ApoE, and the abundance of ApoE in amyloid plaques123,124 may also play an important role in opsonizing plaques for targeting by activated microglia. In addition, microglia are found to present in the core of ApoE-labelled senile plaques in areas of ApoE-like immunoreactivity91, suggesting microglial cell targeting amyloid-β seeds to promote plaque seeding during the plaque initiation stage may also involve ApoE participation as an opsonin to enhance microglia-plaque interaction. The ApoE-TREM2-microglia connection is strongly supported by the observation that the presence of ApoE, TREM2, or microglia similarly enhances amyloidogenesis during the early amyloid-depositing stage, and that ApoE and TREM2 deficiency share the same plaque phenotype mediated by impaired microglial cell function at the late amyloid-depositing stage. Of note, ApoE4 is associated with higher microglial cell reactivity around amyloid-β plaques than ApoE3 in deep cortical layers125. The effect of ApoE isoforms on plaque deposition may be related to their different capacities in mediating microglial cell activation and opsonization, in addition to direct isoform-dependent effects on amyloid-β aggregation or clearance.
ApoE and tau pathology.
Early studies showed that ApoE3, but not ApoE4, avidly binds to human tau in vitro102,103, indicating a potential link between ApoE and tau. ApoE is normally produced by glia and is secreted into the extracellular space, but rare ApoE immunoreactivity is also observed in neuronal cell bodies in brain tissues of Alzheimer’s disease and tauopathy patients123,126,127. Specific overexpression of human ApoE4 in neurons, but not astrocytes, increases tau phosphorylation in mouse brains compared to ApoE3 overexpression128,129. In addition, amyloid-β pathology is reported to induce higher levels of endogenous murine tau phosphorylation in ApoE4 amyloid-β depositing mice compared to their ApoE3 counterparts130. We recently found that human ApoE4 expressed under the endogenous murine ApoE promoter exacerbates tau pathology compared to other ApoE isoforms in a tauopathy mouse model independent of amyloid-β pathology69. However, the key point is that in addition to affecting tau pathology, ApoE also potently modulates neurodegeneration in the setting of tauopathy. ApoE4 significantly exacerbates neurodegeneration whereas ApoE ablation strongly attenuates the damage compared to the presence of ApoE regardless of ApoE isoforms69. While ApoE may directly regulate tau pathogenesis and affect neurodegeneration via tau-induced cell-autonomous neuronal toxicity, we think the effects of ApoE on both tau pathology and neurodegeneration at least partially fit in the broader picture of the immunomodulatory function of ApoE. The specific lipid composition of ApoE4 lipoprotein may affect lipid raft structures on microglial cell membranes and induce a stronger MGnD phenotype that exacerbates ptau pathogenesis and neurodegeneration. It may also make ApoE4 a more competent opsonin to potentiate neuronal loss via enhanced phagoptosis. In contrast, failure to acquire a complete MGnD phenotype and loss of ApoE opsonization in the absence of ApoE may underlie its protective effect in tauopathy.
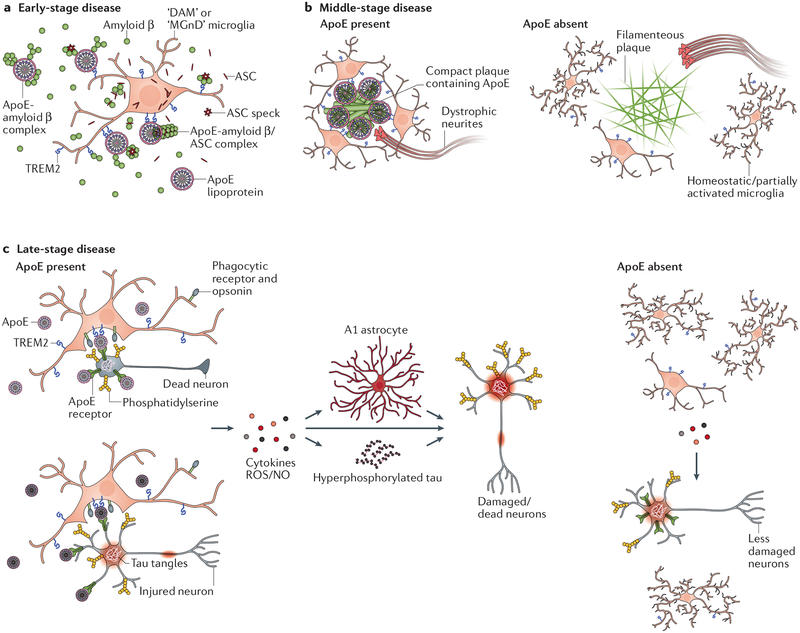
An integrated view of ApoE function in Alzheimer’s disease is summarized in FIG. 2.
Figure 2: Model of immunomodulatory functions of ApoE in Alzheimer’s disease pathogenesis and neurodegeneration along disease progression.
a | ApoE exacerbates amyloid-β pathology during the initial amyloid-β seeding stage. This is likely partially due to a direct pro-aggregating effect of ApoE on amyloid-β through ApoE-amyloid-β interactions. In addition, ApoE may serve as an opsonin bridging microglia with amyloid-β seeds by binding to TREM2 on microglia. This may result in recruitment of more activated microglia to amyloid-β seeds that promote plaque formation via secreting ASC specks or other mechanisms. b | During the plaque accumulation phase, DAM/MGnD microglia clustering around amyloid-β plaques for plaque trimming and insulation is strictly regulated by ApoE. ApoE is required for full DAM/MGnD microglial activation that allows them to associate with plaques. In addition, ApoE is abundantly present in amyloid plaques and may promote DAM/MGnD microglia to target plaques via ApoE-TREM2 interaction. c | In the late-stage disease when intracellular pathological tau accumulation and other factors result in more neuronal injury and death, ApoE may accumulate on the cell surface of jeopardized neurons likely due to their impaired cellular uptake function, and may opsonize these neurons for phagoptosis by DAM/MGnD microglia via interaction with TREM2, thus exacerbating neurodegeneration. In addition, ApoE is required for full DAM/MGnD microglial polarization. Fully activated DAM/MGnD microglia in the presence of ApoE may further aggravate neuronal death by elevating oxidative stress, enhancing A1 astrocytic activation and exacerbating tau pathology.
Concluding remarks and future directions
Substantial genetic and functional evidence point to a central role of innate immunity in neurodegenerative diseases. In Alzheimer’s disease, neuroinflammation plays different roles depending on disease stages. Activated microglia appear to accelerate amyloid plaque formation during the plaque initiation stage, but serve to restrain plaque growth and plaque-associated neuronal damage during the plaque deposition phase. In the late-disease stage when pathological tau accumulates and neuronal death increases, activated microglia exacerbate tau pathology and may serve as a major driving force for neurodegeneration. Targeting neuroinflammation may be an effective therapeutic approach to intervene with disease progression, but therapeutic designs should take into account the disease stage to target the appropriate pathophysiology.
ApoE affects Alzheimer’s disease risk in large part due to its effects on amyloid-β seeding and clearance; it is also a core immune regulator that may influences Alzheimer’s disease through its immunomodulatory functions. The shared features between ApoE and TREM2 suggest an ApoE-TREM2 axis linking ApoE to microglial function. Deeper characterization of disease-associated microglia signatures and functions will be key to understanding the role of microglia-mediated neuroinflammation in disease. Further investigation connecting ApoE-dependent lipid metabolism with microglia profiling will provide crucial insights into mechanisms underlying ApoE immunomodulatory functions.
Despite all the exciting findings deciphering roles of neuroinflammation in disease pathogenesis and progression with mechanistic and therapeutic implications, the fundamental problem is not solved. Alzheimer’s disease, like many other neurodegenerative diseases, begins with disruption of proteostasis. This process, once initiated, cannot currently be self-resolved and will inevitably lead to pathological protein accumulation that eventually progresses to neurodegeneration. The failure to effectively maintain brain proteostasis is likely due to dampened innate immunity and lack of adaptive immunity in brain parenchyma, which jeopardize the brain’s clearance machinery compared to peripheral tissues. Boosting immune activity in the brain to enhance waste clearance under homeostatic or pathological status without damaging neurons may be an alternative strategy. Specific reprogramming of microglia to enhance the clearance of amyloid-β seeds and plaques without amplifying inflammatory stress or targeting neurons; or connecting adaptive immunity with brain innate immunity, such as pathology-directed antibody treatment or T cell-based strategies, are promising fields that should be worked out with caution to avoid potential side effects. For intracellular tau pathology that may be difficult to target by immune cells without damaging neurons, activating intrinsic or applying extrinsic neuronal protein degradation programmes could be considered.
Box 2: TREM2 as a risk factor for Alzheimer’s disease.
TREM2 is second only to APOE in terms of the magnitude of its effects as a late-onset Alzheimer’s disease risk factor. The most common TREM2 variant R47H increases Alzheimer’s disease risk by 2- to 3-fold135,136. TREM2 is a cell surface receptor of the immunoglobulin superfamily that is expressed by tissue macrophages, such as bone osteoclasts, CNS microglia, alveolar macrophages, peritoneal macrophages and intestinal macrophages137. Upon ligand binding, TREM2 associates with an adaptor protein DNAX-activation protein 12 (DAP12), which contains a cytosolic immunoreceptor tyrosine-based activation motif (ITAM) that upon phosphorylation, recruits the spleen tyrosine kinase (SYK)137. SYK activation initiates a cascade of signalling that promotes cell anabolic metabolism138, survival138,139, proliferation140 and phagocytosis140,141. Therefore, TREM2 activation is essential for macrophage viability and immune function. Homozygous TREM2 mutations that cause a complete loss of function of TREM2 are associated with Nasu-Hakola disease (NHD), an autosomal recessive disorder featured by a presenile form of frontal-lobe dementia and bone cysts with fractures142. Heterozygous TREM2 variants leading to partial TREM2 loss of function are found to confer higher Alzheimer’s disease risk142. Mutations that cause NHD or increase Alzheimer’s disease risk lead to premature protein truncation (Q33X), impaired receptor trafficking and surface expression (T66M, Y38C), defective ligand binding (R47H, R62H, T66M, Y38C) and signalling (R47H, R62H), and reduced phagocytosis (R47H, R62H, T66M, Y38C)143. Conversely, a missense variant S144G in TREML2, a TREM-like gene located within the same TREM gene cluster that encodes a structurally similar protein showing opposite effects on microglial cell proliferation and inflammatory responses as TREM2144, was reported to be protective against Alzheimer’s disease145. These genetic findings suggest that immune functions mediated by TREM2-expressing immune cells critically regulate Alzheimer’s disease pathogenesis.
Acknowledgements
This work was funded by National Institutes of Health NIH grants R01AG047644, R01 NS090934, R01NS034467, the JPB Foundation, the Tau Consortium, and the Cure Alzheimer’s Fund to DMH.
Glossary terms:
- Amyloid-β
Small peptides that are major components of amyloid deposits in the brain and cerebrovasculature. They are generated from cleavage of the amyloid precursor protein (APP). Depending on the C-terminus cleavage site, amyloid-β of varying lengths (36–43 amino acids, denoted as Aβ36-Aβ43) can be generated, among which Aβ40 and Aβ42 are the most prevalent species.
- Tau
A microtubule-binding protein synthesized primarily in neurons. Under normal conditions, tau is most abundantly located in axons and serves to stabilize microtubules. In Alzheimer’s disease, tau becomes hyperphosphorylated, dissociates from microtubules, translocates from axons to neuronal cell bodies and dendrites, and self-aggregates to form neurofibrillary tangles.
- Aβ42/Aβ40 ratio
Aβ42 is more fibrillogenic than Aβ40. The level of Aβ42 produced relative to Aβ40 determines the propensity of amyloid plaque formation.
- Kainic acid
A potent neuroexcitatory amino acid that serves as an agonist for kainate-class ionotropic glutamate receptors. High doses of kainic acid induce neuronal death by overexcitation of neurons.
- APPSwePSEN1dE9 mice
An amyloid-β depositing mouse model that expresses a chimeric mouse/human APP transgene carrying the Swedish mutation (K670N, M671L) and a mutant PSEN1 transgene lacking exon 9 (dE9) under the prion promoter. These mice begin to develop amyloid-β pathology around six months of age.
- 5XFAD mice
An amyloid-β depositing mouse model that overexpresses mutant human APP carrying the Swedish (K670N, M671L), Florida (I716V), and London (V717I) FAD mutations along with human PS1 carrying the M146L and L286V mutations. Both transgenes are controlled under the Thy1 promoter. These mice accumulate high levels of intraneuronal Aβ42 around six weeks of age, followed by plaque deposition around two months of age.
- APPPS1–21 mice
An amyloid-β depositing mouse model that harbours mutant human transgenes of APP (K670N, M671L) and PSEN1 (L166P), both under the control of the Thy1 promoter. These mice begin to develop amyloid plaques in the neocortex at approximately six weeks of age, followed by deposits in the hippocampus at three to four months of age.
- Ionized calcium binding adaptor molecule 1 (IBA1)
A microglia marker protein in the CNS that binds calcium and actin. It is involved in RacGTPase-dependent membrane ruffling and phagocytosis during microglial cell activation.
- 3xTg-AD mice
This mouse model harbours three mutant human genes (APP K670N M671L, pS1 M146V, and MAPTP301L), and sequentially develop amyloid-β pathology and tau pathology starting at six months of age.
- htau mice
This mouse model expresses all six human tau isoforms, including 3R and 4R tau, under the endogenous human MAPT promoter, and expresses no murine tau. These mice start to develop tau pathology around nine months of age.
- rTg4510 mice
A tauopathy mouse model overexpressing the 0N4R human tau isoform carrying the P301L mutation linked to familial frontotemporal dementia. Tau transgene expression is largely restricted to the forebrain by the Camk2a promoter, and is regulatable by doxycycline. These mice develop tau pathology around three to four months of age and show significant neuronal loss by six months of age.
- P301S mice
A tauopathy mouse model overexpressing the 1N4R human tau isoform carrying the P301S mutation found in frontotemporal dementia patients. These mice begin to accumulate tau pathology at four to five months of age and develop severe brain atrophy at 9 months of age.
- ApoE-targeted replacement (APOE-TR) mice
These mice express human APOE in place of the murine ApoE under the endogenous murine Apoe promoter. Therefore, the expression level and pattern of human APOE is maintained in a physiological form.
- superoxide dismutase 1 (SOD1) mice
A mouse model for ALS that overexpresses human Cu-Zn superoxide dismutase (SOD1) carrying the G93A mutation. These mice recapitulate phenotypes in human ALS patients showing motor neurons loss in the spinal cord and paralysis in one or more limbs.
Footnotes
Competing interests statement
D.M.H. co-founded and is on the scientific advisory board of C2N Diagnostics. D.M.H. is on the scientific advisory board of Denali, Genentech, and Proclara. D.M.H. consults for AbbVie and Eli Lilly.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer A Über eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psychiatr 64, 146–148 (1907). [Google Scholar]
- 2.Jack CR Jr. et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9, 119–128, doi: 10.1016/s1474-4422(09)70299-6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman RJ et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367, 795–804, doi: 10.1056/NEJMoa1202753 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy J & Selkoe DJ The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science (New York, N.Y.) 297, 353–356, doi: 10.1126/science.1072994 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Holtzman DM, Morris JC & Goate AM Alzheimer’s disease: the challenge of the second century. Sci Transl Med 3, 77sr71, doi: 10.1126/scitranslmed.3002369 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimenova AA, Raj T & Goate AM Untangling Genetic Risk for Alzheimer’s Disease. Biol Psychiatry, doi: 10.1016/j.biopsych.2017.05.014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gjoneska E et al. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature 518, 365–369, doi: 10.1038/nature14252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown GC & Neher JJ Microglial phagocytosis of live neurons. Nat Rev Neurosci 15, 209–216, doi: 10.1038/nrn3710 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Liddelow SA et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487, doi: 10.1038/nature21029 (2017).This study defines the roles of A1 astrocytes in neurodegenerative diseases and demonstrates an essential role of microglia-astrocytes crosstalk in A1 astrocytic activation.
- 10.Kaushal V et al. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ 22, 1676–1686, doi: 10.1038/cdd.2015.16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan MS et al. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis 5, e1382, doi: 10.1038/cddis.2014.348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginhoux F et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845, doi: 10.1126/science.1194637 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ajami B, Bennett JL, Krieger C, Tetzlaff W & Rossi FM Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10, 1538–1543, doi: 10.1038/nn2014 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Paolicelli RC et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458, doi: 10.1126/science.1202529 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Stevens B et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178, doi: 10.1016/j.cell.2007.10.036 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Cunningham CL, Martinez-Cerdeno V & Noctor SC Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33, 4216–4233, doi: 10.1523/jneurosci.3441-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakselman S et al. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci 28, 8138–8143, doi: 10.1523/jneurosci.1006-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marin-Teva JL et al. Microglia promote the death of developing Purkinje cells. Neuron 41, 535–547 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Davalos D et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8, 752–758, doi: 10.1038/nn1472 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Nimmerjahn A, Kirchhoff F & Helmchen F Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318, doi: 10.1126/science.1110647 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Kettenmann H, Hanisch U-K, Noda M & Verkhratsky A Physiology of microglia. Physiological reviews 91, 461–553, doi: 10.1152/physrev.00011.2010 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Dissing-Olesen L, MacVicar BA & Stevens B Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol 36, 605–613, doi: 10.1016/j.it.2015.08.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez FO & Gordon S The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6, 13, doi: 10.12703/p6-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boche D, Perry VH & Nicoll JA Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 39, 3–18, doi: 10.1111/nan.12011 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Mosser DM & Edwards JP Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8, 958–969, doi: 10.1038/nri2448 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray PJ et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20, doi: 10.1016/j.immuni.2014.06.008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keren-Shaul H et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290.e1217, doi: 10.1016/j.cell.2017.05.018 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Krasemann S et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47, 566–581.e569, doi: 10.1016/j.immuni.2017.08.008 (2017).This work and reference 27 raise the concept of neurodegenerative microglial phenotype. They define the molecular signatures of the phenotype and demonstrate the requirement of TREM2 and ApoE for its activation.
- 29.Chakrabarty P, Herring A, Ceballos-Diaz C, Das P & Golde TE Hippocampal expression of murine TNFalpha results in attenuation of amyloid deposition in vivo. Mol Neurodegener 6, 16, doi: 10.1186/1750-1326-6-16 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakrabarty P et al. IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J Immunol 184, 5333–5343, doi: 10.4049/jimmunol.0903382 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakrabarty P et al. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. Faseb j 24, 548–559, doi: 10.1096/fj.09-141754 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guillot-Sestier MV et al. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 85, 534–548, doi: 10.1016/j.neuron.2014.12.068 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakrabarty P et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron 85, 519–533, doi: 10.1016/j.neuron.2014.11.020 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakrabarty P et al. Hippocampal expression of murine IL-4 results in exacerbation of amyloid deposition. Mol Neurodegener 7, 36, doi: 10.1186/1750-1326-7-36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vom Berg J et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat Med 18, 1812–1819, doi: 10.1038/nm.2965 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Kiyota T et al. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP+PS1 bigenic mice. Faseb j 24, 3093–3102, doi: 10.1096/fj.10-155317 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao X, Cummins DJ & Paul SM Neuroinflammation-induced acceleration of amyloid deposition in the APPV717F transgenic mouse. Eur J Neurosci 14, 474–482 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Spangenberg EE et al. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-beta pathology. Brain 139, 1265–1281, doi: 10.1093/brain/aww016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grathwohl SA et al. Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat Neurosci 12, 1361–1363, doi: 10.1038/nn.2432 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sosna J et al. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol Neurodegener 13, 11, doi: 10.1186/s13024-018-0244-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venegas C et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 552, 355–361, doi: 10.1038/nature25158 (2017).This work shows that activated microglia promote amyloid-β seeding via secreting ASC specks that cross seed amyloid-β. This study together with reference 39 suggest that microglia participate in the initiation stage of plaque formation by affecting amyloid-β seeding.
- 42.Baik SH, Kang S, Son SM & Mook-Jung I Microglia contributes to plaque growth by cell death due to uptake of amyloid beta in the brain of Alzheimer’s disease mouse model. Glia 64, 2274–2290, doi: 10.1002/glia.23074 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Yin Z et al. Immune hyperreactivity of Abeta plaque-associated microglia in Alzheimer’s disease. Neurobiol Aging 55, 115–122, doi: 10.1016/j.neurobiolaging.2017.03.021 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Ulrich JD et al. Altered microglial response to Abeta plaques in APPPS1–21 mice heterozygous for TREM2. Mol Neurodegener 9, 20, doi: 10.1186/1750-1326-9-20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jay TR et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J Exp Med 212, 287–295, doi: 10.1084/jem.20142322 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071, doi: 10.1016/j.cell.2015.01.049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jay TR et al. Disease Progression-Dependent Effects of TREM2 Deficiency in a Mouse Model of Alzheimer’s Disease. J Neurosci 37, 637–647, doi: 10.1523/jneurosci.2110-16.2016 (2017).References 44–47 show that TREM2 promotes microglial clustering around amyloid-β plaques and demonstrate a disease-stage dependent effect of TREM2 deficiency on amyloid-β pathology.
- 48.Wang Y et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med 213, 667–675, doi: 10.1084/jem.20151948 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan P et al. TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron 90, 724–739, doi: 10.1016/j.neuron.2016.05.003 (2016).References 48 and 49 demonstrate a major TREM2-dependent function of plaque-associated microglia to trim and compact plaques, and serve as a barrier to reduce neuritic dystrophy.
- 50.Zhao Y et al. TREM2 Is a Receptor for beta-Amyloid that Mediates Microglial Function. Neuron 97, 1023–1031.e1027, doi: 10.1016/j.neuron.2018.01.031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. Journal of Neuroscience 34, 11929–11947, doi: 10.1523/JNEUR0SCI.1860-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CYD et al. Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer’s Disease Models. Neuron 97, 1032–1048.e1035, doi: 10.1016/j.neuron.2018.02.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada K et al. In Vivo Microdialysis Reveals Age-Dependent Decrease of Brain Interstitial Fluid Tau Levels in P301S Human Tau Transgenic Mice. Journal of Neuroscience 31, 13110–13117, doi: 10.1523/JNEUR0SCI.2569-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clavaguera F, Hench J, Goedert M & Tolnay M Invited review: Prion-like transmission and spreading of tau pathology. Neuropathol Appl Neurobiol 41, 47–58, doi: 10.1111/nan.12197 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Takeda S et al. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat Commun 6, 8490, doi: 10.1038/ncomms9490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolos M et al. Direct Evidence of Internalization of Tau by Microglia In Vitro and In Vivo. J Alzheimers Dis 50, 77–87, doi: 10.3233/jad-150704 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Asai H et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nature Publishing Group 18, 1584–1593, doi: 10.1038/nn.4132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshiyama Y et al. Synapse Loss and Microglial Activation Precede Tangles in a P301S Tauopathy Mouse Model. Neuron 53, 337–351, doi: 10.1016/j.neuron.2007.01.010 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Kitazawa M, Oddo S, Yamasaki TR, Green KN & LaFerla FM Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci 25, 8843–8853, doi: 10.1523/jneurosci.2868-05.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Liu L, Barger SW & Griffin WS Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci 23, 1605–1611 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitazawa M et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer’s disease model. J Immunol 187, 6539–6549, doi: 10.4049/jimmunol.1100620 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhaskar K et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68, 19–31, doi: 10.1016/j.neuron.2010.08.023 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maphis N et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 138, 1738–1755, doi: 10.1093/brain/awv081 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nash KR et al. Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy. Neurobiol Aging 34, 1540–1548, doi: 10.1016/j.neurobiolaging.2012.12.011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bemiller SM et al. TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol Neurodegener 12, 74, doi: 10.1186/s13024-017-0216-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang T et al. Silencing of TREM2 exacerbates tau pathology, neurodegenerative changes, and spatial learning deficits in P301S tau transgenic mice. Neurobiology of Aging 36, 3176–3186, doi: 10.1016/j.neurobiolaging.2015.08.019 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Leyns CEG et al. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci U S A 114, 11524–11529, doi: 10.1073/pnas.1710311114 (2017).This work shows that dampening neurodegenerative microglial activation via TREM2 deletion reduces neurodegeneration at an advanced disease stage in a tauopathy mouse model, supporting a role of microglia in modulating neurodegeneration.
- 68.Jiang T et al. TREM2 modifies microglial phenotype and provides neuroprotection in P301S tau transgenic mice. Neuropharmacology 105, 196–206, doi: 10.1016/j.neuropharm.2016.01.028 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Shi Y et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527, doi: 10.1038/nature24016 (2017).This study demonstrates an amyloid-β-independent role of ApoE in regulating neurodegeneration and neuroinflammation in the setting of a tauopathy. The protective effect of ApoE deficency on neurodegeneration is in line with that of TREM2.
- 70.Kraft AW et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. Faseb j 27, 187–198, doi: 10.1096/fj.12-208660 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liddelow SA & Barres BA Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46, 957–967, doi: 10.1016/j.immuni.2017.06.006 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y & Barres BA Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 20, 588–594, doi: 10.1016/j.conb.2010.06.005 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Vainchtein ID et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science, doi: 10.1126/science.aal3589 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung WS et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400, doi: 10.1038/nature12776 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chung WS et al. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci U S A 113, 10186–10191, doi: 10.1073/pnas.1609896113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sofroniew MV Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16, 249–263, doi: 10.1038/nrn3898 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zamanian JL et al. Genomic Analysis of Reactive Astrogliosis. The Journal of Neuroscience 32, 6391–6410, doi: 10.1523/JNEUROSCI.6221-11.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lian H et al. NFkappaB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 85, 101–115, doi: 10.1016/j.neuron.2014.11.018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lian H et al. Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. Journal of Neuroscience 36, 577–589, doi: 10.1523/JNEUROSCI.2117-15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Q & Barres BA Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol, doi: 10.1038/nri.2017.125 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Varvel NH et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci U S A 109, 18150–18155, doi: 10.1073/pnas.1210150109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prokop S et al. Impact of peripheral myeloid cells on amyloid-beta pathology in Alzheimer’s disease-like mice. J Exp Med 212, 1811–1818, doi: 10.1084/jem.20150479 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Varvel NH et al. Replacement of brain-resident myeloid cells does not alter cerebral amyloid-beta deposition in mouse models of Alzheimer’s disease. J Exp Med 212, 1803–1809, doi: 10.1084/jem.20150478 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Michaud JP, Bellavance MA, Prefontaine P & Rivest S Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep 5, 646–653, doi: 10.1016/j.celrep.2013.10.010 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Baik SH et al. Migration of neutrophils targeting amyloid plaques in Alzheimer’s disease mouse model. Neurobiol Aging 35, 1286–1292, doi: 10.1016/j.neurobiolaging.2014.01.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zenaro E et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 21, 880–886, doi: 10.1038/nm.3913 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Kumar DK et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 8, 340ra372, doi: 10.1126/scitranslmed.aaf1059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holtzman DM, Herz J & Bu G Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harbor perspectives in medicine 2, doi: 10.1101/cshperspect.a006312 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bu G Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nature reviews. Neuroscience 10, 333–344, doi: 10.1038/nrn2620 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boyles JK, Pitas RE, Wilson E, Mahley RW & Taylor JM Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest 76, 1501–1513, doi: 10.1172/jci112130 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uchihara T et al. ApoE immunoreactivity and microglial cells in Alzheimer’s disease brain. Neurosci Lett 195, 5–8 (1995). [DOI] [PubMed] [Google Scholar]
- 92.Corder EH et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261 (1993). [DOI] [PubMed] [Google Scholar]
- 93.Schmechel DE et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America 90, 9649–9653, doi: 10.1073/pnas.90.20.9649 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agosta F et al. Apolipoprotein E ε4 is associated with disease-specific effects on brain atrophy in Alzheimer’s disease and frontotemporal dementia. Proceedings of the National Academy of Sciences of the United States of America 106, 2018–2022, doi: 10.1073/pnas.0812697106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strittmatter WJ et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America 90, 1977–1981, doi: 10.1073/pnas.90.5.1977 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bales KR et al. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet 17, 263–264, doi: 10.1038/ng1197-263 (1997). [DOI] [PubMed] [Google Scholar]
- 97.Kim J et al. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-beta amyloidosis. J Neurosci 31, 18007–18012, doi: 10.1523/jneurosci.3773-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holtzman DM et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America 97, 2892–2897, doi: 10.1073/pnas.050004797 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fagan AM et al. Human and murine ApoE markedly alters A beta metabolism before and after plaque formation in a mouse model of Alzheimer’s disease. Neurobiol Dis 9, 305–318, doi: 10.1006/nbdi.2002.0483 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Castellano JM et al. Human apoE Isoforms Differentially Regulate Brain AmyloidPeptide Clearance. Science Translational Medicine 3, 89ra57–89ra57, doi: 10.1126/scitranslmed.3002156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huynh TV, Davis AA, Ulrich JD & Holtzman DM Apolipoprotein E and Alzheimer’s disease: the influence of apolipoprotein E on amyloid-beta and other amyloidogenic proteins. J Lipid Res 58, 824–836, doi: 10.1194/jlr.R075481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strittmatter WJ et al. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America 91, 11183–11186, doi: 10.1073/pnas.91.23.11183 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fleming LM, Weisgraber KH, Strittmatter WJ, Troncoso JC & Johnson GVW Differential Binding of Apolipoprotein E Isoforms to Tau and Other Cytoskeletal Proteins. Experimental Neurology 138, 252–260, doi: 10.1006/exnr.1996.0064 (1996). [DOI] [PubMed] [Google Scholar]
- 104.Mishra A et al. Gene-based association studies report genetic links for clinical subtypes of frontotemporal dementia. Brain, doi: 10.1093/brain/awx066 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Tsuang D et al. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol 70, 223–228, doi: 10.1001/jamaneurol.2013.600 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gallardo G, Schluter OM & Sudhof TC A molecular pathway of neurodegeneration linking alpha-synuclein to ApoE and Abeta peptides. Nat Neurosci 11, 301–308, doi: 10.1038/nn2058 (2008). [DOI] [PubMed] [Google Scholar]
- 107.Shin S et al. Apolipoprotein E mediation of neuro-inflammation in a murine model of multiple sclerosis. J Neuroimmunol 271, 8–17, doi: 10.1016/j.jneuroim.2014.03.010(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gale SC et al. APOE4 is associated with enhanced in vivo innate immune responses in human subjects. The Journal of allergy and clinical immunology 134, 127–134, doi: 10.1016/j.jaci.2014.01.032 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vitek MP, Brown CM & Colton CA APOE genotype-specific differences in the innate immune response. Neurobiology of Aging 30, 1350–1360, doi: 10.1016/j.neurobiolaging.2007.11.014 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu Y et al. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia 60, 559–569, doi: 10.1002/glia.22289 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fessler MB & Parks JS Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol 187, 1529–1535, doi: 10.4049/jimmunol.1100253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yvan-Charvet L et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328, 1689–1693, doi: 10.1126/science.1189731 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murphy AJ et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest 121, 4138–4149, doi: 10.1172/jci57559 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Okoro EU et al. Apolipoprotein E4 is deficient in inducing macrophage ABCA1 expression and stimulating the Sp1 signaling pathway. PLoS One 7, e44430, doi: 10.1371/journal.pone.0044430 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lynch JR et al. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem 278, 48529–48533, doi: 10.1074/jbc.M306923200 (2003). [DOI] [PubMed] [Google Scholar]
- 116.Atagi Y et al. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2). J Biol Chem 290, 26043–26050, doi: 10.1074/jbc.M115.679043 (2015).This work demonstrates that ApoE is a receptor for TREM2, and shows that ApoE binds to apoptotic neuronal cell surface and increases TREM2-mediated phagocytosis by microglia in vitro, supporting the ApoE oponization hypothesis.
- 117.Bailey CC, DeVaux LB & Farzan M The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E. J Biol Chem 290, 26033–26042, doi: 10.1074/jbc.M115.677286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jendresen C, Arskog V, Daws MR & Nilsson LN The Alzheimer’s disease risk factors apolipoprotein E and TREM2 are linked in a receptor signaling pathway. J Neuroinflammation 14, 59, doi: 10.1186/s12974-017-0835-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yeh FL, Wang Y, Tom I, Gonzalez LC & Sheng M TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron 91, 328–340, doi: 10.1016/j.neuron.2016.06.015 (2016). [DOI] [PubMed] [Google Scholar]
- 120.Ulrich JD et al. ApoE facilitates the microglial response to amyloid plaque pathology. J Exp Med, doi: 10.1084/jem.20171265 (2018).This work demonstrates that ApoE is required for microglial association with plaques to perform plaque-trimming function similar to that of TREM2, supporting an ApoE-TREM2 axis in mediating microglial function.
- 121.Huynh TV et al. Age-Dependent Effects of apoE Reduction Using Antisense Oligonucleotides in a Model of beta-amyloidosis. Neuron 96, 1013–1023.e 1014, doi: 10.1016/j.neuron.2017.11.014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu CC et al. ApoE4 Accelerates Early Seeding of Amyloid Pathology. Neuron 96, 1024–1032.e1023, doi: 10.1016/j.neuron.2017.11.013 (2017).References 121 and 122 show that ApoE is essential for plaque formation during the initial seeding stage.
- 123.Namba Y, Tomonaga M, Kawasaki H, Otomo E & Ikeda K Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res 541, 163–166 (1991). [DOI] [PubMed] [Google Scholar]
- 124.Wisniewski T & Frangione B Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett 135, 235–238 (1992). [DOI] [PubMed] [Google Scholar]
- 125.Rodriguez G. a., Tai LM, LaDu MJ & Rebeck GW Human APOE4 increases microglia reactivity at Abeta plaques in a mouse model of Abeta deposition. Journal of neuroinflammation 11, 111–111, doi: 10.1186/1742-2094-11-111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang Y et al. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci U S A 98, 8838–8843, doi: 10.1073/pnas.151254698 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Farrer LA et al. Allele epsilon 4 of apolipoprotein E shows a dose effect on age at onset of Pick disease. Exp Neurol 136, 162–170 (1995). [DOI] [PubMed] [Google Scholar]
- 128.Brecht WJ Neuron-Specific Apolipoprotein E4 Proteolysis Is Associated with Increased Tau Phosphorylation in Brains of Transgenic Mice. Journal of Neuroscience 24, 2527–2534, doi: 10.1523/JNEUROSCI.4315-03.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harris FM, Brecht WJ, Xu Q, Mahley RW & Huang Y Increased tau phosphorylation in apolipoprotein E4 transgenic mice is associated with activation of extracellular signal-regulated kinase: modulation by zinc. J Biol Chem 279, 44795–44801, doi: 10.1074/jbc.M408127200 (2004). [DOI] [PubMed] [Google Scholar]
- 130.Zhou M et al. APOE4 Induces Site-Specific Tau Phosphorylation Through Calpain CDK5 Signaling Pathway in EFAD-Tg Mice. Curr Alzheimer Res 13, 1048–1055 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Pericak-Vance MA et al. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. American journal of human genetics 48, 1034–1050 (1991). [PMC free article] [PubMed] [Google Scholar]
- 132.Neyen CD & Gordon S in eLS (John Wiley & Sons, Ltd, 2001).
- 133.de Jong A Activation of human T cells by CD1 and self-lipids. Immunol Rev 267, 16–29, doi: 10.1111/imr.12322 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Pablo MA & Alvarez de Cienfuegos G Modulatory effects of dietary lipids on immune system functions. Immunol Cell Biol 78, 31–39, doi: 10.1046/j.14401711.2000.00875.x (2000). [DOI] [PubMed] [Google Scholar]
- 135.Jonsson T et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368, 107–116, doi: 10.1056/NEJMoa1211103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guerreiro R et al. TREM2 variants in Alzheimer’s disease. NEngl J Med 368, 117–127, doi: 10.1056/NEJMoa1211851 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Colonna M & Wang Y TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci 17, 201–207, doi: 10.1038/nrn.2016.7 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Ulland TK et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 170, 649–663.e613, doi: 10.1016/j.cell.2017.07.023 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071, doi: 10.1016/j.cell.2015.01.049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Poliani PL et al. TREM2 sustains microglial expansion during aging and response to demyelination. J Clin Invest 125, 2161–2170, doi: 10.1172/jci77983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takahashi K, Rochford CD & Neumann H Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 201, 647–657, doi: 10.1084/jem.20041611 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yeh FL, Hansen DV & Sheng M TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol Med 23, 512–533, doi: 10.1016/j.molmed.2017.03.008 (2017). [DOI] [PubMed] [Google Scholar]
- 143.Ulrich JD, Ulland TK, Colonna M & Holtzman DM Elucidating the Role of TREM2 in Alzheimer’s Disease. Neuron 94, 237–248, doi: 10.1016/j.neuron.2017.02.042 (2017). [DOI] [PubMed] [Google Scholar]
- 144.Zheng H et al. Opposing roles of the triggering receptor expressed on myeloid cells 2 and triggering receptor expressed on myeloid cells-like transcript 2 in microglia activation. Neurobiol Aging 42, 132–141, doi: 10.1016/j.neurobiolaging.2016.03.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Benitez BA et al. Missense variant in TREML2 protects against Alzheimer’s disease. Neurobiol Aging 35, 1510.e1519–1526, doi: 10.1016/j.neurobiolaging.2013.12.010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jehle AW et al. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J Cell Biol 174, 547–556, doi: 10.1083/jcb.200601030 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim WS et al. Deletion of Abca7 increases cerebral amyloid-beta accumulation in the J20 mouse model of Alzheimer’s disease. J Neurosci 33, 4387–4394, doi: 10.1523/jneurosci.4165-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sakae N et al. ABCA7 Deficiency Accelerates Amyloid-beta Generation and Alzheimer’s Neuronal Pathology. J Neurosci 36, 3848–3859, doi: 10.1523/jneurosci.3757-15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Crehan H et al. Complement receptor 1 (CR1) and Alzheimer’s disease. Immunobiology 217, 244–250, doi: 10.1016/j.imbio.2011.07.017 (2012). [DOI] [PubMed] [Google Scholar]
- 150.Cunin P et al. Clusterin facilitates apoptotic cell clearance and prevents apoptotic cell-induced autoimmune responses. Cell Death Dis 7, e2215, doi: 10.1038/cddis.2016.113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang KL et al. A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat Neurosci 20, 1052–1061, doi: 10.1038/nn.4587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bradshaw EM et al. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat Neurosci 16, 848–850, doi: 10.1038/nn.3435 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Griciuc A et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78, 631–643, doi: 10.1016/j.neuron.2013.04.014 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Halle A et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9, 857–865, doi: 10.1038/ni.1636 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He Y, Hara H & Nunez G Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci 41, 1012–1021, doi: 10.1016/j.tibs.2016.09.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Walsh JG, Muruve DA & Power C Inflammasomes in the CNS. Nature Publishing Group 15, 1–14, doi: 10.1038/nrn3638 (2014). [DOI] [PubMed] [Google Scholar]