Abstract
Allergic asthma is a major cause of morbidity in both pediatric and adult patients. Recent research has highlighted the role of hyaluronan (HA), an extracellular matrix glycosaminoglycan, in asthma pathogenesis. Experimental allergic airway inflammation and clinical asthma are associated with an increase of shorter fragments of HA (sHA), which complex with inter-α-inhibitor heavy chains (HCs) and induce inflammation and airway hyperresponsiveness (AHR). Importantly, the effects of sHA can be antagonized by the physiological counterpart high molecular weight HA (HMWHA). We used a mouse model of house dust mite-induced allergic airway inflammation and demonstrated that instilled HMWHA ameliorated allergic airway inflammation and AHR, even when given after the establishment of allergic sensitization and after challenge exposures. Furthermore, instilled HMWHA reduced the development of HA-HC complexes and the activation of Rho-associated, coiled-coil containing protein kinase 2. We conclude that airway application of HMWHA is a potential treatment for allergic airway inflammation.
Keywords: airway hyperresponsiveness, allergic asthma, hyaluronan, inflammation, treatment
INTRODUCTION
Chronic airway inflammation is a major contributor to morbidity and healthcare costs worldwide. A survey of the global prevalence of asthma in adults found that 2–6% have clinical or doctor-diagnosed asthma, whereas up to 10% report wheezing without an official asthma diagnosis, suggesting that the true prevalence of asthma may be higher than survey values (68). In the United States, 7 million children and 17.5 million adults suffered from asthma in 2009, and prevalence increased by over 10% in that decade (2, 3), leading to 10 million lost work days and 13 million lost school days, over 400,000 hospitalizations, and 1.5 million emergency department visits at an annual cost of $56 billion (18). Asthma is characterized by dynamic airway obstruction, airway inflammation, hyperresponsiveness, and remodeling.
Airway remodeling occurs through mesenchymal cell proliferation and deposition of extracellular matrix (ECM). An ECM component that is now increasingly recognized in asthma is the glycosaminoglycan hyaluronan (HA). HA is normally a large [molecular weight (MW) > 106] polysaccharide, comprised of N-acetyl-glucosamine-glucuronic acid disaccharides repeated up to 10,000 times (69). HA is the most abundant ECM glycosaminoglycan in the human body and is usually found in the peribronchial and perivascular space in the lungs (5). During lung injury, shorter fragments of HA (sHA) (MW, 100,000–400,000) are either produced de novo or released via enzymatic and nonenzymatic degradation of structural HA (6, 23, 43, 48, 61, 64, 69) and promote inflammation and airway hyperresponsiveness (AHR) (23).
Airway remodeling and AHR are closely related. Increased sHA has been documented in asthmatic airways (41, 45) and in models of allergic lung inflammation (16, 17, 41, 65). In chronic inflammation, sHA forms complexes with the heavy chains (HCs) from the serum proteoglycan inter-α-inhibitor (IαI) (57). These complexes have been termed “pathological HA matrix” (41, 65) and are responsible for the development of AHR in several models of inflammatory lung disease, including allergic airway inflammation. Deficiency in TNFα-stimulated gene 6 (TSG-6), which catalyzes the formation of the pathological HA matrix (sHA-HC complexes) leads to significant amelioration of AHR (63, 65).
An important aspect of the pathological HA matrix formation is that it is conceptually a reversible process because HCs can be transferred between different HA molecules (40, 41, 58). This has implications on inflammation and AHR since the effect of HA on these processes is dependent on its size: sHA is proinflammatory and pro-AHR, whereas high MW HA (HMWHA) (i.e., MW > 106) inhibits these effects (23, 43). This is of substantial translational interest, since HMWHA solutions for inhalation are on the market in Europe, being used as adjuncts in cystic fibrosis, rhinitis, and sinusitis treatment (13, 14, 21, 25, 47, 56). Anecdotal evidence suggests that inhaled HMWHA reduces AHR in inflammatory airway disease (4, 54). Thus, inhaled HMWHA offers itself as an attractive therapeutic option in allergic AHR.
In this work, we used a commercially available HMWHA preparation (Yabro, IBSA International, Switzerland) to test the efficacy of HMWHA in ameliorating allergic airway inflammation and AHR. We used the well-described, and environmentally relevant, house dust mite (HDM) asthma model. To approximate the human condition, we allowed the mice to be sensitized to HDM with two weekly nasal instillations without any intervention and then applied instilled HMWHA after inhaled HDM in the subsequent exposure phase only. We demonstrate that instilled HMWHA ameliorates allergic airway inflammation and AHR and is associated with a decrease in the formation of the pathological HA matrix and reduced activation of Rho-associated, coiled-coil containing protein kinase 2 (ROCK2), a kinase that mediates AHR in allergic airway inflammation. Thus, our results highlight the utility of HMWHA as an attractive treatment agent for inflammatory AHR.
MATERIALS AND METHODS
Mice.
All studies were approved by the Institutional Animal Care and Use Committee, National Institute of Environmental Health Sciences. C57Bl/6J mice (Jackson Laboratories, Bar Harbor, ME) were provided with chow and water ad libitum and kept in a 12-h light-dark cycle. Male mice were used for the in vivo experiments. Tissues and cells from male and female mice were utilized for the in vitro experiments.
HDM exposure.
Mice were sensitized with a 1-μg dose of Der p1 HDM (Greer, Lenoir, NC) in 50 μl of ultrapure PBS on day 1 and 7 via oropharyngeal aspiration. Control mice received ultrapure PBS vehicle instead. Mice were subsequently challenged via oropharyngeal aspiration for 3 days (days 14–16) with 50 μl of the same preparation of HDM. Control mice received PBS challenge. On the same days, at 30 min after HDM challenge, mice also received treatments of 50 μl of either ultrapure PBS or Yabro [pure, pharmaceutical grade HMWHA, MW, 800,000–1,200,000, at a concentration of 3 mg/ml (as provided by manufacturer in solution for nebulization)]. Thus, four groups of mice were compared, defined by the sensitization, exposure, and treatment they received: 1) PBS sensitization-PBS challenge-PBS treatment, 2) HDM sensitization-PBS challenge-PBS treatment, 3) HDM sensitization-HDM Challenge-PBS treatment, and 4) HDM sensitization-HDM Challenge-Yabro (HMWHA) treatment.
Phenotyping endpoints.
Airway physiology assessment by flexiVent occurred at 24 h following the final HDM challenge. Necropsy for lung lavage and tissue collection occurred at 4 h following the final HDM challenge. Different sets of mice were thus phenotyped for each endpoint.
Airway physiology measurements.
Airway responsiveness to 25–100 mg/ml methacholine (MCh) (Sigma) was measured 24 h following the last HDM challenge. Briefly, mice were anesthetized with urethane (2 g/kg; Sigma), tracheotomized with a tracheal cannula (Harvard Apparatus) with a Luer adapter, and mechanically ventilated on a 42°C water-heated pad at a rate of 150 breaths/min, a tidal volume of 10 ml/kg, and a positive end-expiratory pressure of 3 cmH2O with a computer-controlled small animal ventilator (FlexiVent, Scireq, Montreal, Canada). To block spontaneous breathing, mice were given pancuronium bromide (0.8 mg/kg ip; Sigma-Aldrich, St. Louis, MO) 5 min before assessment of airway responses. To measure airway responsiveness, a single-frequency forced oscillation waveform, followed by a broadband forced oscillation waveform (matched to the animal breathing frequency), was applied using the Flexiware 7.6 software default mouse inhaled dose-response script. The resulting pressure, volume, and flow signals were fit to either the single compartment or constant phase model of the lung to obtain total respiratory system resistance and elastance or Newtonian resistance, tissue damping, and tissue elastance, respectively (28). The peak response at each dose was averaged and graphed along with the average baseline measurement for each group.
Necropsy.
Mice were euthanized by pentobarbital injection, the trachea was cannulated, and lung lavage fluid was collected by instilling 1 ml saline three times. Right lungs were snap-frozen and used for RT-PCR and Western blot assays, whereas left lungs were inflated with formalin and processed for histology as detailed below.
ELISA.
Supernatants from the lung lavage fluid were assayed using DuoSet ELISA kits (R&D Systems, Minneapolis, MN).
Real-time RT-PCR.
Right lung lobes were pulverized with mortar and pestle, and RT-PCRs for TNFα, IL-4, IL-5, IL-13, IL-25, IL-33, TSLP (KiCqStart Primers, Sigma-Aldrich) were performed.
Histopathology.
The lung was inflated with neutral buffered formalin and then immersed in formalin for 24 h, embedded in paraffin, and sectioned at 5 μm. Slides were stained with hematoxylin-eosin. Histopathologic evaluation by light microscopy for inflammation was performed with an Olympus BX51 microscope. All grading was performed on a 4-grade scale as follows: 1 = minimal, 2 = mild, 3 = moderate, and 4 = marked. The inflammation score (0–8) was based upon the combination of perivenous (0–4) and peribronchial (0–4) inflammation. The reviewer was blinded as to the identity of the samples until the review was completed. Grading of the perivenous and peribronchial inflammation was based upon the number of vessels or bronchi involved, the average severity of the inflammation, and the severity of the inflammation around the most intensely inflamed area.
Immunohistochemistry.
The staining methods for IαI and HA have been published (65). Briefly, formalin-fixed sections were incubated for 30 min with blocking buffer (1% BSA in PBS) then incubated with 5 μg/ml biotinylated hyaluronic acid binding peptide (no. 385911, EMD/Millipore, Billerica, MA) and a rabbit polyclonal antibody against IαI (1:100 dilution, A0301, Dako North America, Inc., Carpinteria, CA) for 1 h at room temperature or overnight at 4°C. Notably, the Dako IαI antibody does not recognize murine bikunin protein but selectively detects the HC protein (39). After washing, secondary reagents were added and incubated for 1 h at room temperature in the dark. Alexa Fluor 488-conjugated streptavidin (for hyaluronic acid binding peptide) and Alexa Fluor 594-conjugated (for anti-IαI) donkey anti-goat antibody were used as the secondary reagents. Each staining included a control of only the secondary reagent to demonstrate the specificity of staining. Sections were examined with a Leica DMIRB upright microscope equipped with a Retiga 2000R camera and QCapture Pro 6 software.
Detection of HA-HC complexes.
The protocol has been published before (65). Briefly, lung tissues of mice were transferred to dry ice prechilled Ependorf tubes to weigh and then transferred to dry ice prechilled Benchmark TriplePure M-Bio Grade tubes (no. D1032-15) prefilled with 1.5-mm diameter Zironium beads (≈100 beads/tube) to homogenize. Tubes were then placed on wet ice. Immediately after placement on wet ice, prechilled PBS was added to the tubes at 100 μl for every 33 mg of tissue. The tubes were inserted in the BeadBug Mini Homogenizer (no. D1030) at a 4°C operating environment, for 30–45 s mixing cycle. BeadBug Mini Homogenizer allows for three tubes to be inserted per mixing cycle. To prevent tissue degradation from sitting for too long on wet ice before treatments, and because of the number of hyaluronidase treatments, the samples were processed in groups of 8 per 1 wet ice incubation of 30 min. A 50-μl aliquot of the minced tissue suspension was transferred to 2 new prechilled 1.5-ml tubes, and Streptomyces hyaluronidase (5 μl of a 0.5 turbidity U/ml stock; no. 389561, EMD/Millipore) was added to 1 tube, whereas PBS (5 μl) was added to the other. The tubes were incubated on ice for 30 min and then centrifuged at 13,200 revolutions/min for 5 min at 4°C. The supernatants were transferred to new prechilled 1.5-ml tubes and incubated for another 30 min at 37°C. Then, after the addition of SDS sample buffer, 25 μl of the digests were added per lane on 4–15% Mini-PROTEAN TGX gels (Bio-Rad) and blotted using a Bio-Rad nitrocellulose and Trans-Blot Turbo system. The blots were blocked for 1 h with blocking buffer (catalog no. 927–40000, LI-COR, Lincoln, NE) and probed with a rabbit polyclonal antibody against IαI (1:8,000 dilution; A0301, Dako North America). The secondary antibody was IRDye 800CW anti-rabbit IgG (1:15,000 dilution; catalog no. 926–32211, LI-COR). The blots were washed and imaged using an Odyssey infrared imaging system (LI-COR). Images were analyzed for densitometry using the free Image J software (https://imagej.nih.gov/ij/). Briefly, a selection box was entered tightly around every band, and the integrated density was measured with the ImageJ software. An equal-size box was entered in a blank space of the blot, and the resulting background density was subtracted from all values to yield the corrected density values.
Isometric force transduction.
Isolated and cleaned mouse tracheas from C57BL/6 mice were cut into ~3-mm pieces and suspended in a 5-ml oxygenated Krebs’ solution bath and attached to an isometric transducer. After equilibration to 1 g of force contractility, maximum contractility was measured by dosing the bath with 80 mM KCl solution with subsequent washing of the tissue. The KCl dosing was repeated. After reestablishing baseline, tracheal rings were then dosed with sHA (0.5 mg/ml), Yabro (0.5 mg/ml), or both and incubated for 30 min before a contraction curve using MCh (1 nM to 3,000 nM)
RhoA activation assay.
The Rho activity assay was measured by using the RhoA Activation Assay Biochem Kit (Cytoskeleton) based on the Rhotekin pull-down assay per manufacturer’s instructions. In brief, mice lungs were homogenized in a radioimmunoprecipitation assay lysis buffer (Thermo Fisher Scientific, Rockford, IL) containing protease inhibitors. The samples were then sonicated for 10 s three times on ice in 1.5-ml Eppendorf tubes using an ultrasonic liquid processor and centrifuged at 14,000 g for 20 min at 4°C. Cleared supernatants were used to measure the protein concentration by the bicinchoninic acid assay. Equal amounts of protein were incubated with Rhotekin-RBD beads overnight at 4°C. After washing the beads with wash buffer (Cytoskeleton), proteins were removed from the beads with SDS-PAGE Laemmli buffer, then subjected to Western blot analysis.
Western blot analysis.
Mouse lungs were homogenized and sonicated in radioimmunoprecipitation assay lysis buffer (Thermo Fisher Scientific) containing protease inhibitors. Cleared supernatants were used to measure the protein concentration by the bicinchoninic acid assay. Equal amounts of protein were loaded in 10% Tris·HCl Criterion precast gels, and the proteins were transferred to PVDF membranes. Membranes were probed with phospho ROCK1 (Abcam, Cambridge, MA) and phospho ROCK2 (Sigma) and total ROCK1 or ROCK2 (Cell Signaling Technology) antibodies (1:1,000 dilution). Bands were detected by the chemiluminescent horseradish peroxidase substrate.
In vitro dendritic cell activation assay.
Bone marrow cells were cultured with IL-4 (2 ng/ml) and granulocyte-macrophage colony-stimulating factor (10 ng/ml) for 7 days and then washed and plated in 24-well plates coated with 3% low MWHA (LMWHA) or HMWHA or controls. After 4 h in culture, cells were exposed to UltraPure LPS (List Biologicals, Campbell, CA) at 1 μg/ml for 24 h before harvesting and phenotyping. Supernatants were analyzed using ELISA as described above. Other cells were fixed in the wells for 10 min with 4% paraformaldehyde, washed 3 times in 1 × PBS, then stored in PBS at 4°C until stained.
Immunofluorescence staining.
Cells were permeabilized in 0.3% TBST for 10 min, washed twice in 1 × TBST, then blocked for 2 h at room temperature in 10% normal donkey serum and 1% BSA in 1 × TBST. Cells were incubated overnight at 4°C in either rat anti-mouse CD62L (Abcam; 1:100) or rat anti-mouse IA/IE (MHCII) (Biolegends, San Diego, CA; 1:100). After washing twice with 1 × TBST, secondary antibodies were applied (donkey anti-rat Alexa Fluor-594 for CD26L and donkey anti-rat Alexa Fluor-488 for IA/IE, both at 1:100; antibodies from Molecular Probes, Eugene, OR) for 1 h at room temperature. Wells were washed 2 × 10 min with 1 × PBS, then Prolong Gold plus DAPI applied to cells for imaging. Three ×200 images were collected per well using an automated Zeiss Epifluorescent microscope (Carl Zeiss USA, San Diego, CA) for analysis.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 7 software. Results were analyzed using ANOVA with multiple comparison post hoc correction, as appropriate. Results in the figures are depicted as means ± SE.
RESULTS
Instilled HMWHA reduces allergic airway inflammation.
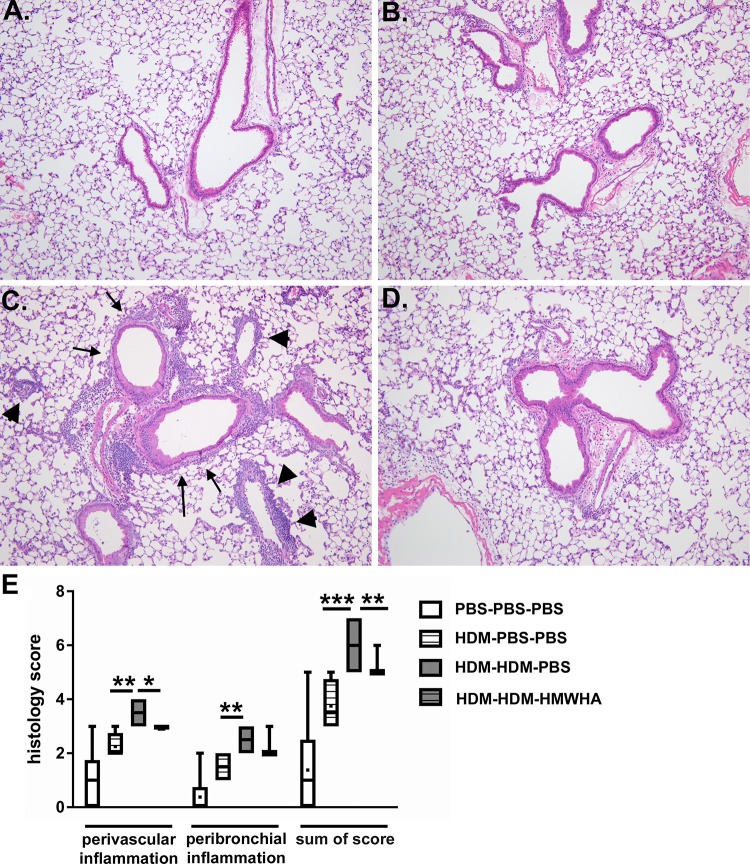
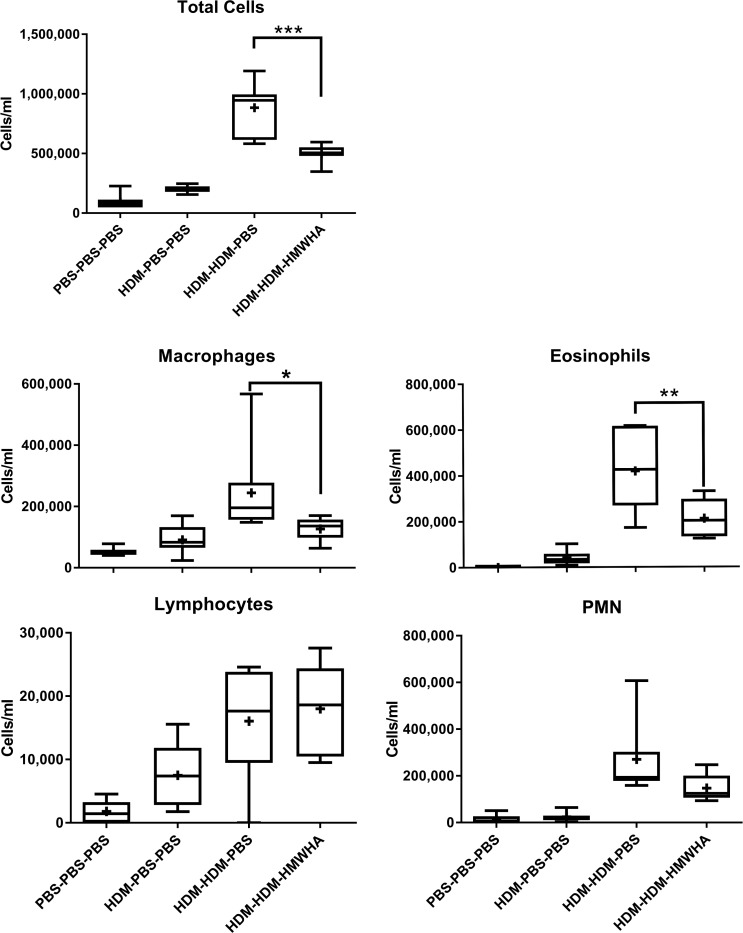
We compared four groups of mice, defined by the sensitization, exposure, and treatment they received: 1) PBS sensitization-PBS challenge-PBS treatment, 2) HDM sensitization-PBS challenge-PBS treatment, 3) HDM sensitization-HDM challenge-PBS treatment, and 4) HDM sensitization-HDM challenge-Yabro (HMWHA) treatment. We first evaluated histological evidence of airway inflammation (Fig. 1). HDM exposure induced significant lung inflammation (Fig. 1, A–C), and HMWHA treatment significantly reduced histological inflammation (Fig. 1D). While both peribronchial and perivascular inflammation were apparently reduced after HMWHA treatment, only the perivascular inflammation score was significantly lower in HMWHA-treated mice in semiquantitative scoring (Fig. 1E). The significance was higher for the total inflammation score. There was no difference in mucous metaplasia and goblet cell numbers or location regardless of HMWHA treatment (not shown). This histological difference in inflammation was also confirmed when we assessed cellular inflammation in the airway compartment (lung lavage fluid). We found significantly reduced cellular inflammation in HDM-sensitized/exposed, HMWHA-treated mice, which was due to a reduced influx of eosinophils and macrophages, whereas there was a nonsignificant trend for reduced polymorphonuclear cells (Fig. 2).
Fig. 1.
Histological inflammation after house dust mite (HDM) exposure. Sham-treated mice show no inflammation (A), and mice that were sensitized to HDM but not challenged have only mildly increased inflammation (B). HDM sensitization and exposure leads to significant perivascular (arrowheads) and peribronchial (arrows) cell infiltration (C), but treatment with high molecular weight hyaluronan (HMWHA) after HDM exposure reduces this infiltration (D). E: semiquantitative assessment of the perivascular and peribronchial inflammation confirms a significant decrease of inflammation in the HDM-HDM-HMWHA group compared with HDM-HDM-PBS. All groups have significantly increased scores compared with the PBS-PBS-PBS group (associations not depicted to avoid crowding). n = 8 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001; ANOVA with Holm-Sidak multiple comparison adjustment.
Fig. 2.
Cellular inflammation in whole lung lavage fluid. Treatment with high molecular weight hyaluronan (HMWHA) significantly reduces cell influx into the airway compartment. HMWHA reduces eosinophils and macrophages significantly, whereas there is a nonsignificant trend for the reduction of polymorphonuclear cells. There is no obvious effect on lymphocyte influx into the airway compartment. n = 8 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001; ANOVA with Tukey’s multiple comparison adjustment. PMN, polymorphonuclear neutrophils.
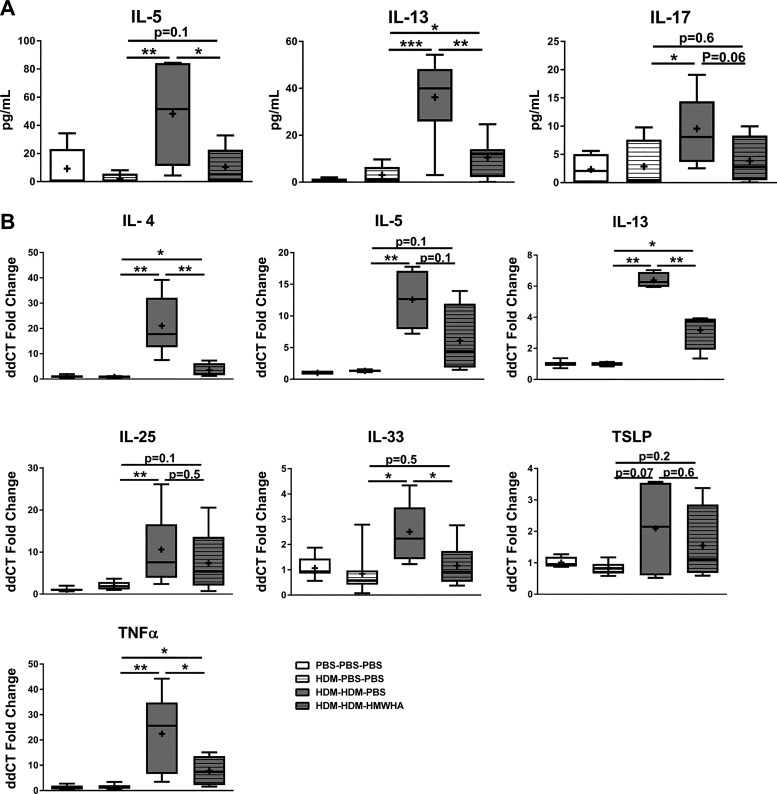
We then assayed inflammatory cytokine expression in the airways and lung tissue. There were significant decreases in relevant proinflammatory cytokines (IL-5, IL-13) in the HDM-sensitized/exposed, HMWHA-treated mice and a trend approaching significance (P = 0.06) for reduction in IL-17 (Fig. 3A). We could not detect IL-4 protein in the lavage fluid. To have a more comprehensive analysis of cytokine expression, we evaluated gene expression by real-time RT-PCR and confirmed a reduction in the transcription of inflammatory cytokine genes in mice that received HMWHA treatment after HDM sensitization and exposure (Fig. 3B).
Fig. 3.
Proinflammatory cytokines are reduced after high molecular weight hyaluronan (HMWHA) treatment in house dust mite (HDM)-sensitized/exposed mice. There are significant reductions in lung lavage proteins (A) and lung tissue gene expression assayed by real-time RT-PCR (B). n = 8 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001; ANOVA with Tukey’s multiple comparison adjustment.
Instilled HMWHA reduces allergic AHR.
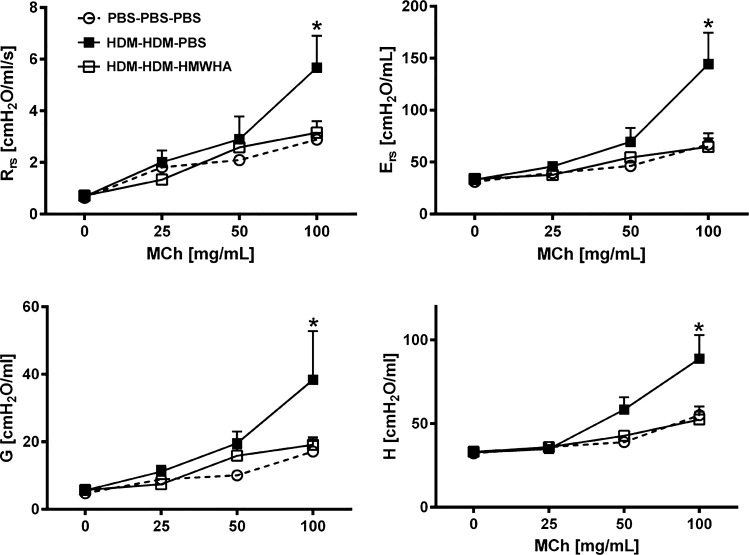
AHR is a hallmark of asthma and allergic airway inflammation models and underlies many of the symptoms found in patients with asthma. We therefore investigated the effect of HMWHA on inflammatory AHR. HDM sensitization/challenge resulted in significantly elevated respiratory system resistance and elastance, whereas HMWHA treatment abolished this AHR (Fig. 4). HMWHA treatment affected global resistance and elastance, and this was mainly a result of reductions in peripheral resistance and elastance, as assessed in the constant phase model, whereas Newtonian resistance was not significantly affected by either HDM exposure or HMWHA treatment (not shown).
Fig. 4.
Inflammatory airway hyperresponsiveness is reduced after treatment with high molecular weight hyaluronan (HMWHA). HMWHA leads to total lung resistance (Rrs) and elastance (Ers) values that are as low as those of sham-treated mice. The difference is driven by reductions in peripheral tissue resistance (G) and elastance (H). n = 8 mice per group. *P < 0.05; ANOVA with Tukey’s multiple comparison adjustment. MCh, methacholine.
Instilled HMWHA reduces formation of HA-HC complexes.
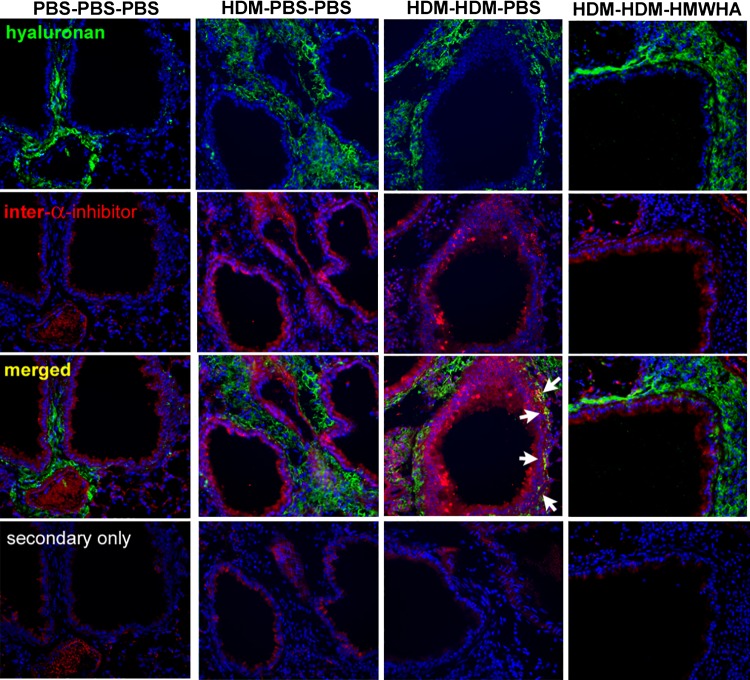
Chronic allergic airway inflammation upregulates TSG-6, which transfers the HCs from the serum proteoglycan IαI to form HA-HC complexes, which are involved in the development of allergic inflammatory AHR (41, 65). However, the process of HA-HC complex formation is dynamic, and HCs can be transferred between HA molecules (58). We therefore reasoned that treatment with HMWHA may reverse or inhibit the formation of pathological HA-HC complexes. Immunohistochemical staining for HA and HC showed that HDM exposure leads to increased deposition of HA (Fig. 5) and detection of HA-HC complexes in the peribronchial and perivascular spaces (Fig. 5, arrows), confirming previous reports in other models of allergic lung disease (17, 41, 65). HA-HC formation was increased, as shown by Western blotting (Fig. 6A). Densitometry analysis confirmed that there was a decrease in free HCs released by hyaluronidase in the HDM-HDM-HMWHA treatment compared with the HDM-HDM-PBS treatment, indicating the ability of HMWHA treatment to diminish the formation of HA-HC complexes (Fig. 6, A and B). Interestingly, increases in free HC and pre-α-inhibitor were observed, even before hyaluronidase treatment, indicating the presence of IαI elements that were not attached to HA, and that may have extravasated or been produced by resident lung cells (1). To ensure that we captured the true abundance of HA-HC complexes, we subtracted free HC before hyaluronidase treatment from free HC after hyaluronidase treatment and confirmed that HCs freed from HA-HC by hyaluronidase were reduced to within PBS control levels after treatment with HMWHA (Fig. 6C).
Fig. 5.
Immunohistochemistry staining for hyaluronan (HA) and inter-α-inhibitor increased peribronchial deposition of HA-heavy chain complexes after house dust mite (HDM) sensitization and exposure (arrows), which is reduced after high molecular weight HA (HMWHA) treatment. Note that peribronchial HA deposition is still present.
Fig. 6.
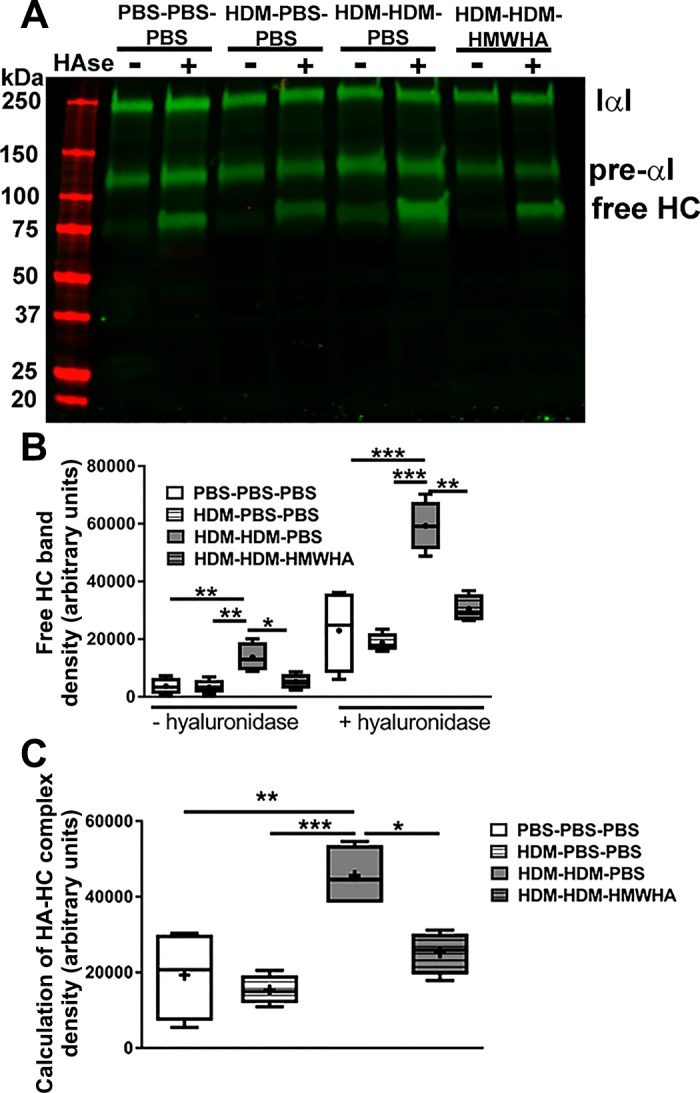
High molecular weight hyaluronan (HMWHA) reduces HA-heavy chain (HC) complexes after HDM exposure. A: semiquantitative assessment of HA-HC abundance by detection of free HC after treatment with Streptomyces hyaluronidase. HCs previously bound to HA (which cannot enter the gel because of HA size) are now detectable as free HC. Note the presence of free HC before hyaluronidase treatment in tissue of mice sensitized and exposed to house dust mites (HDMs). HMW treatment of these mice leads to a reduction in free HCs. B: densitometry demonstrates a reduction in free HCs in HMWHA-treated mice, before and after hyaluronidase treatment. C: calculation of HA-HC complexes in each sample was done by subtracting the density values of the [−hyaluronidase] free HC in B from the density values of the [+hyaluronidase] free HC in B. These calculations show that HA-HC complexes are reduced in HMWHA-treated mice. n = 4 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001; ANOVA with Tukey’s multiple comparison adjustment.
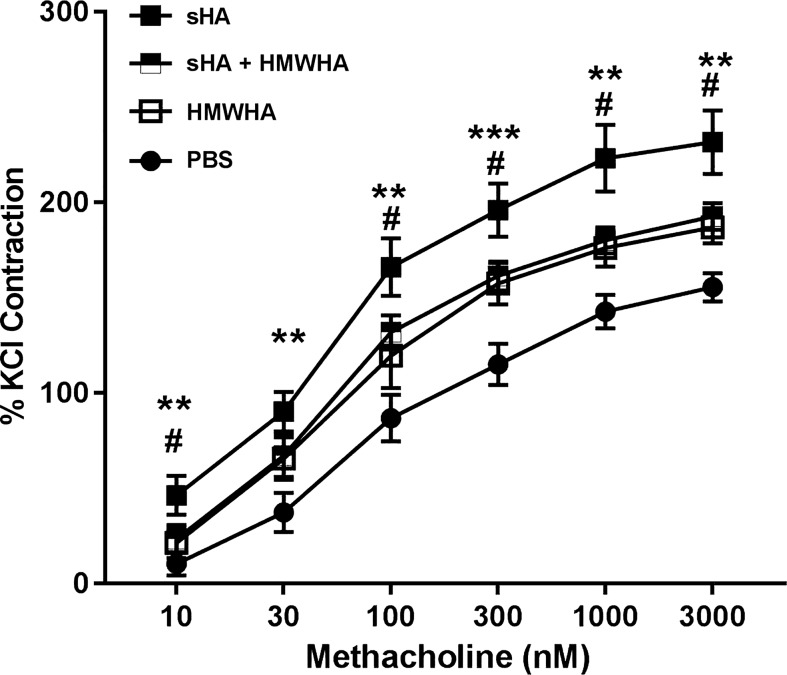
HMWHA ameliorates AHR to sHA in vitro.
We have previously shown that formation of HA-HC complexes supports the development of AHR (63). We therefore investigated whether HMWHA can inhibit the development of AHR induced by sHA in the tracheal ring model. Tracheal rings exposed to sHA had a significant increase in the responsiveness to MCh, as described previously (Fig. 7). HMWHA also induced a moderate increase in MCh responsiveness. However, sHA and HMWHA together were indistinguishable from the HMWHA alone response, suggesting that HMWHA reduces the effects of sHA on smooth muscle contractility.
Fig. 7.
Shorter fragments of hyaluronan (sHA)-induced airway hyperresponsiveness in airway smooth muscle (tracheal ring assay) is ameliorated by high molecular weight HA (HMWHA). n = 8–12 tracheal rings per group. **P < 0.01; ***P < 0.001, sHA compared with HMWHA or PBS; and #P < 0.05, sHA compared with sHA + HMWHA. ANOVA with Holm-Sidak multiple comparison adjustment.
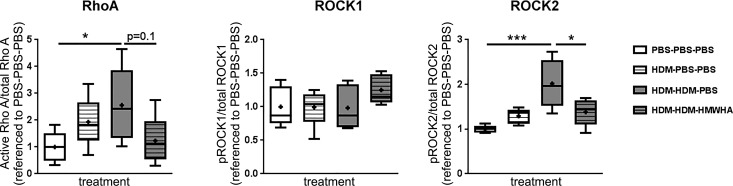
HMWHA ameliorates activation of ROCK2 in vivo.
Previous work has suggested a role for RhoA and Rho kinases in the development of AHR in allergic lung inflammation (32, 55, 70) and after ozone exposure (31). We therefore examined the activation of RhoA, ROCK1, and ROCK2 (i.e., phosphorylation) in lung tissue after HDM exposure with or without HMWHA treatment (Fig. 8). HMWHA did not significantly affect RhoA activity, although there appeared to be a trend toward a decrease in the HMWHA-treated group compared with the PBS-treated group (P = 0.1). There was no change in ROCK1 phosphorylation with HDM exposure and no difference between the HMWHA-treated and sham-treated groups. However, HMWHA treatment significantly ameliorated activation of ROCK2.
Fig. 8.
High molecular weight hyaluronan (HMWHA) treatment ameliorates Rho-associated, coiled-coil containing protein kinase (ROCK)2 activation in vivo. Quantitation of RhoA, ROCK1, and ROCK2 activation in whole lung lysates shows an increase of RhoA activation with house dust mite (HDM) treatment, which is partly ameliorated by HMWHA. ROCK1 is not affected by HDM or HMWHA treatment. In contrast, ROCK2 is significantly increased by HDM, and HMWHA significantly reduces this change. n = 5 mice per condition. *P < 0.05 and ***P < 0.001; ANOVA with Dunnett’s multiple comparison adjustment.
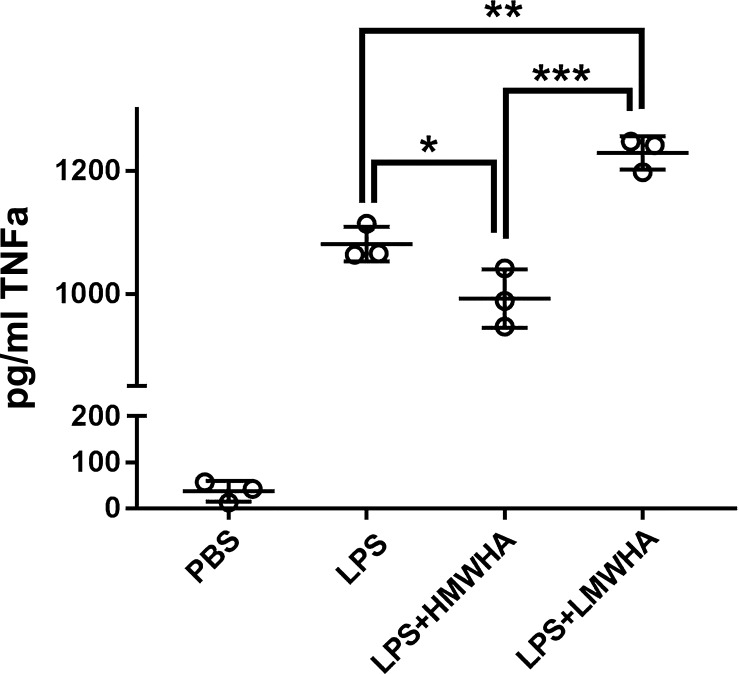
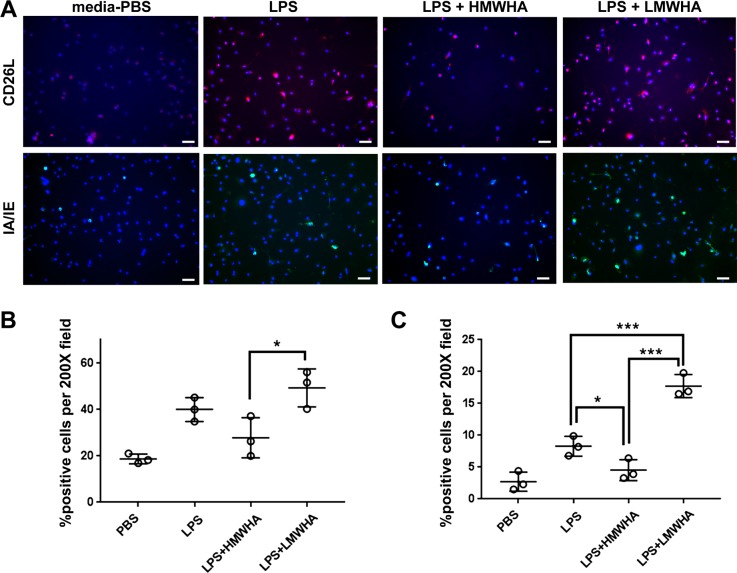
LMWHA promotes and HMWHA inhibits LPS-induced dendritic cell maturation in vitro.
We investigated whether HMWHA and LMWHA have direct effects on immune cell activation or whether the observed HMWHA effects are only indirectly mediated by effects on the HA-HC complex formation. We found that HMWHA and LMWHA had independent and opposite effects on LPS-induced activation of bone marrow derived dendritic cells (DCs), measured by TNFα expression (Fig. 9) and upregulation of DC cell surface markers CD62L and IA/IE. (Fig. 9, Fig. 10).
Fig. 9.
Low molecular weight hyaluronan (LMWHA) promotes, whereas high MWHA (HMWHA) inhibits, dendritic cell expression of TNFα after LPS exposure. n = 3 wells per experimental condition. * P < 0.05, **P < 0.01, and ***P < 0.001; ANOVA with Holm-Sidak multiple comparison adjustment.
Fig. 10.
Low molecular weight hyaluronan (LMWHA) promotes, whereas high MWHA (HMWHA) inhibits, dendritic cell expression of surface activation markers after LPS exposure. A: representative images from 3 wells per experimental condition. ×200 magnification. Scale bar = 50 µm length. B: quantification of CD26L-positive cells from 3 wells per experimental condition. C: quantification of IA/IE-positive cells from 3 wells per experimental condition. *P < 0.01 and ***P < 0.001; ANOVA with Holm-Sidak multiple comparison adjustment.
DISCUSSION
While asthma management has undoubtedly improved with the recognition of well-defined endotypes and the advent of precision treatment directed to specific patient populations, there is still need for adjunct therapies that support anti-inflammatory and AHR-suppressive effects of existing drugs. Current treatment is directed toward cellular or humoral components of the asthmatic airway by providing smooth muscle relaxation, global immune suppression, or targeted suppression of inflammatory components. Notably, the ECM is an untapped treatment target, even though ECM deposition is a defining characteristic of chronic airway inflammation (29). The present work suggests that modulation of the ECM through the application of HMWHA in the airways is a viable treatment option for allergic airway inflammation.
The role of HA in airway inflammation and remodeling is increasingly highlighted. HA is not only a structural component of the lung parenchyma (5) but also a highly dynamic molecule with rapid turnover. Lung HA levels are elevated in virtually every chronic lung disease (38), including asthma (45). HA mediates multiple processes that are present in the ailing airway, like inflammation, angiogenesis, AHR, and bacterial biofilm formation (22). sHA is generated during lung injury (66), activates adaptive (20, 37, 67) and innate (50, 51, 53) immunity, and promotes AHR (23, 43), whereas HMWHA inhibits these effects (9–11, 23, 43). Furthermore, sHA accumulates in the asthmatic ECM (16, 17, 41, 45), and the HA receptor CD44 is necessary for the development of allergic inflammation (33, 34). In aggregate, these data strongly suggest a central role of sHA in the process of allergic sensitization and inflammation.
Recently, it has become clear that HA effects in lung inflammation depend on HA interactions with ECM proteins like TSG-6 and the HC of IαI (42, 49, 65). IαI is produced in the liver and secreted in the blood at concentrations of 0.2–0.6 mg/ml (7, 15, 19, 46). From the circulation, IαI extravasates into inflammatory sites but can also be produced locally by injured lung cells (1). IαI availability is therefore amplified in the asthmatic lung, and pathologic HA-HC complexes are abundant in asthmatic lungs and animal models of asthma (41, 65). Thus, interventions that block the formation of the pathological HA-HC matrix may also disrupt airway inflammation and AHR. In this work, we took advantage of the observation that HA-HC interactions can be reversible (40, 58), i.e., HCs binding to sHA (sHA-HC) can be removed by TSG-6 and transferred to HMWHA, which has notable anti-inflammatory properties, thereby diminishing the proinflammatory properties of sHA-HC (10, 11, 23, 30, 43). Thus, the pathological HA-HC matrix may be neutralized by providing a benign substrate (HMWHA), which becomes a recipient for the HCs that are dynamically removed from the sHA molecules. The translational benefit of this concept is that HMWHA preparations for inhalation are already on the market for human patients and can be readily utilized for clinical trials.
Patients with asthma are treated well after airway inflammation and AHR are established. To approximate this scenario, we designed this study so that HDM sensitization was completed without intervention (HDM dosing on days 0 and 7), and HMWHA was given 30 min after each subsequent HDM exposure (HDM dosing on days 14–16). Even so, HMWHA treatment had a significant effect on mitigating inflammation and AHR. This suggests that HMWHA can reverse, to some extent, cellular activation in allergic airway inflammation. ECM acts as a reservoir for cytokines (62), and disruption of the HA-HC pathological matrix formation may also impact cytokine availability and engagement with receptors. Furthermore, HMWHA may directly compete with sHA, which has intrinsic proinflammatory activity (53). Since our model had a relatively short-term HDM exposure, it remains to be seen whether HMWHA effects could be sustained in a longer-term model. Elegant studies have demonstrated that HMWHA activates regulatory T cells and promotes expression of anti-inflammatory cytokines, suggesting its effect may be long-lived (9–12). In our study, there was a significant decrease in eosinophil and macrophage counts after HMWHA treatment but no difference in total lymphocyte counts, although we did not investigate lymphocyte subpopulations. This is in agreement with previously published studies where inhibition of LMWHA signaling predominantly affected eosinophil and macrophage influx with minor effects in lymphocytes (33, 34). We also saw a broad effect in inflammatory cytokines. We investigated cytokines in different pathways associated with allergic airway inflammation, i.e., Th2 axis (IL-4, IL-5, IL-13) (35), Th17 axis (IL-17, TNFα) (8), and epithelial-derived (IL-33, IL-25, TSLP) (52), to differentiate potential target pathways of HMWHA in allergic asthma. Our results suggest that HMWHA does not have a specific target pathway but rather has broad anti-inflammatory activity. The HA matrix surrounds all cells in the lung, and the CD44-HA axis is an adjunct activator of inflammation for the majority of immune cells found in the lung (44). In aggregate, our results suggest that HMWHA may be a broadly effective anti-inflammatory agent in allergic lung inflammation.
A related question would be whether HMWHA can modify allergen sensitization. A recent study found that thiolated (i.e., degradation-resistant) HMWHA coupled to antigens ameliorated lung inflammation by reducing the inflammatory response to antigens upon exposure (24). In that paper, thiolated HMWHA worked by inducing anti-inflammatory IL-10 (24), which, in our study, did not significantly increase (although there was a trend; P = 0.07, not shown). Thus, the mechanisms of thiolated and unmodified HMWHA may diverge slightly, although it is likely that the basic biology remains the same. In aggregate, our work and published literature (24) suggest that HMWHA is a plausible treatment agent for allergic lung inflammation and support that modifications of HMWHA may allow for improved pharmacological efficacy. We also confirmed the findings of the Gebe et al. study (24) that HMWHA directly inhibits, whereas LMWHA promotes, DC activation. Our results were comparatively somewhat more pronounced, perhaps because we used 3% HMWHA (the strength of the commercial HA preparation) as opposed to 1% HA solutions. Thus, our results support a multifaceted role for HMWHA in the inhibition of allergic inflammation.
Activation of the RhoA-Rho kinase (ROCK) axis has been repeatedly shown to promote inflammation and AHR in allergic asthma (26, 27, 32, 55, 59, 60, 70). We have previously found that sHA-exposed airway myocytes induce RhoA activation, which is abolished by HMWHA (43). We also recently showed that sHA, released after acid-induced lung injury, mediates the activation of ROCK2, which HMWHA treatment ameliorates (71). We now show that HMWHA treatment reduces ROCK2 activation in allergic lung inflammation as well and that it ameliorates AHR in an ex vivo tracheal ring assay. Our results from this and previous studies (43, 71) support a role for the RhoA-ROCK axis in the development of inflammatory AHR and suggest that HA contributes to the activation of this axis. Interestingly, our work suggests that ROCK2 is preferentially affected by allergic inflammation and HA treatment since ROCK1 phosphorylation was not changed by either HDM exposure or HMWHA. However, others have shown a role for ROCK1 in allergic asthma (32), so it is possible that our results were influenced by the model or timing of sampling. Previous work on the role of ROCK in allergic airway inflammation provides conflicting assessments on the mechanisms of action; some papers suggest that ROCK inhibition affects AHR but not inflammation (32), whereas others show an effect on both inflammation and AHR (26, 27, 60). Differences in allergens and examined species may account for some of these inconsistencies. Our research supports that HMWHA may partly counteract sHA effects at the airway myocyte level. Inability to form HA-HC complexes (through TSG-6 deficiency) ameliorated AHR without influencing cytokine expression in airway inflammation models (63, 65), suggesting that HA effects on inflammation and AHR may be diverging. Thus, HMWHA may have multiple targets: immune cells (9, 11, 24); epithelial cells, as has been shown in fibrotic lung injury (30); and airway myocytes (43, 63). Together, the HMWHA effects contribute toward ameliorated inflammation and AHR.
Generation of proinflammatory sHA is thought to occur, at least partly, through breakdown of already existing HMWHA. Thus, a reasonable concern in the use of HMWHA as a treatment for airway inflammation would be its safety: could HMWHA become a source for additional sHA, thus adding fuel to the fire? It should be noted that HMWHA has been used for several years in human patients with upper and lower airway disease [reviewed in (22)] without any observed untoward effects. We recently measured proinflammatory cytokines in the induced sputum of patients with cystic fibrosis before and after initiation of treatment with HMWHA and did not see any increase after inhaled HMWHA was introduced (36). The results of the present study support this finding, as we did not observe a breakdown of HA when we performed HA sizing via agarose gel electrophoresis of the lavage fluid (not shown). Interestingly, in our tracheal ring experiment, HMWHA had a moderate pro-AHR effect but inhibited sHA-induced AHR in vitro and allergic AHR in vivo. It is thus possible that HMWHA effects differ in naïve mice/tissues and in tissues with preexisting inflammation. In aggregate, existing literature suggests that HMWHA is a safe treatment of inflammatory airway disease.
In conclusion, this report shows that HMWHA ameliorates allergic inflammation and AHR, reduces the formation of pathological HA-HC complexes, and ameliorates sHA-induced AHR and ROCK2 activation. Our results thus support the use of HMWHA as an adjunct treatment in asthma.
GRANTS
This work was supported partly through funds from the Division of Intramural Research, National Institute of Environmental Health Sciences (ZIA ES102605) and by Award No. P01-HL-107147 from the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.G. conceived and designed research; C.G.J., V.P.S., J.M.C.-D., C.S.T., V.C., I.A., R.J.M., and M.A.A. performed experiments; C.G.J., V.P.S., J.M.C.-D., C.S.T., G.P.F., V.C., I.A., R.J.M., M.A.A., S.M., and S.G. analyzed data; C.G.J., V.P.S., J.M.C.-D., C.S.T., G.P.F., V.C., I.A., R.J.M., M.A.A., S.M., and S.G. interpreted results of experiments; S.G. prepared figures; S.G. drafted manuscript; C.G.J., V.P.S., J.M.C.-D., C.S.T., G.P.F., V.C., I.A., R.J.M., M.A.A., S.M., and S.G. edited and revised manuscript; C.G.J., V.P.S., J.M.C.-D., C.S.T., G.P.F., V.C., I.A., R.J.M., M.A.A., S.M., and S.G. approved final version of manuscript.
REFERENCES
- 1.Adair JE, Stober V, Sobhany M, Zhuo L, Roberts JD, Negishi M, Kimata K, Garantziotis S. Inter-alpha-trypsin inhibitor promotes bronchial epithelial repair after injury through vitronectin binding. J Biol Chem 284: 16922–16930, 2009. doi: 10.1074/jbc.M808560200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief 94: 1–8, 2012. [PubMed] [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. Natl Health Stat Report 32: 1–14, 2011. [PubMed] [Google Scholar]
- 4.Allegra L, Abraham WM, Fasano V, Petrigni G. Methacholine challenge in asthmatics is protected by aerosolised hyaluronan at high (1,000 kDa) but not low (300 kDa) molecular weight. G Ital Mal Torace 62: 297–301, 2008. [Google Scholar]
- 5.Allegra L, Della Patrona S, Petrigni G. Hyaluronic acid: perspectives in lung diseases. Handb Exp Pharmacol 207: 385–401, 2012. doi: 10.1007/978-3-642-23056-1_17. [DOI] [PubMed] [Google Scholar]
- 6.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, Erzurum SC, Dweik RA. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 295: L789–L799, 2008. doi: 10.1152/ajplung.90306.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baek YW, Brokat S, Padbury JF, Pinar H, Hixson DC, Lim YP. Inter-alpha inhibitor proteins in infants and decreased levels in neonatal sepsis. J Pediatr 143: 11–15, 2003. doi: 10.1016/S0022-3476(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 8.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature 453: 1051–1057, 2008. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, Evanko SP, Buckner JH, Wight TN, Nepom GT. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-beta. J Immunol 183: 2232–2241, 2009. doi: 10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol 86: 567–572, 2009. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol 179: 744–747, 2007. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- 12.Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, Teng B, Holt GE, Standifer NE, Braun KR, Xie CF, Samuels PL, Vernon RB, Gebe JA, Wight TN, Nepom GT. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci USA 108: 7938–7943, 2011. doi: 10.1073/pnas.1017360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buonpensiero P, De Gregorio F, Sepe A, Di Pasqua A, Ferri P, Siano M, Terlizzi V, Raia V. Hyaluronic acid improves “pleasantness” and tolerability of nebulized hypertonic saline in a cohort of patients with cystic fibrosis. Adv Ther 27: 870–878, 2010. doi: 10.1007/s12325-010-0076-8. [DOI] [PubMed] [Google Scholar]
- 14.Casale M, Sabatino L, Frari V, Mazzola F, Dell’Aquila R, Baptista P, Mladina R, Salvinelli F. The potential role of hyaluronan in minimizing symptoms and preventing exacerbations of chronic rhinosinusitis. Am J Rhinol Allergy 28: 345–348, 2014. doi: 10.2500/ajra.2014.28.4045. [DOI] [PubMed] [Google Scholar]
- 15.Chaaban H, Singh K, Huang J, Siryaporn E, Lim YP, Padbury JF. The role of inter-alpha inhibitor proteins in the diagnosis of neonatal sepsis. J Pediatr 154: 620–622.e1, 2009. doi: 10.1016/j.jpeds.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Correlation of hyaluronan deposition with infiltration of eosinophils and lymphocytes in a cockroach-induced murine model of asthma. Glycobiology 23: 43–58, 2013. doi: 10.1093/glycob/cws122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol 30: 126–134, 2011. doi: 10.1016/j.matbio.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croisant S. Epidemiology of asthma: prevalence and burden of disease. Adv Exp Med Biol 795: 17–29, 2014. doi: 10.1007/978-1-4614-8603-9_2. [DOI] [PubMed] [Google Scholar]
- 19.Degraeuwe PL, Nieman FH. The diagnostic value of inter-alpha inhibitor proteins for neonatal sepsis. J Pediatr 156: 341, 2010. doi: 10.1016/j.jpeds.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Do Y, Nagarkatti PS, Nagarkatti M. Role of CD44 and hyaluronic acid (HA) in activation of alloreactive and antigen-specific T cells by bone marrow-derived dendritic cells. J Immunother 27: 1–12, 2004. doi: 10.1097/00002371-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Furnari ML, Termini L, Traverso G, Barrale S, Bonaccorso MR, Damiani G, Piparo CL, Collura M. Nebulized hypertonic saline containing hyaluronic acid improves tolerability in patients with cystic fibrosis and lung disease compared with nebulized hypertonic saline alone: a prospective, randomized, double-blind, controlled study. Ther Adv Respir Dis 6: 315–322, 2012. doi: 10.1177/1753465812458984. [DOI] [PubMed] [Google Scholar]
- 22.Garantziotis S, Brezina M, Castelnuovo P, Drago L. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am J Physiol Lung Cell Mol Physiol 310: L785–L795, 2016. doi: 10.1152/ajplung.00168.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009. [Erratum in J Biol Chem 291: 19257–19259, 2016.] doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 24.Gebe JA, Yadava K, Ruppert SM, Marshall P, Hill P, Falk BA, Sweere JM, Han H, Kaber G, Medina C, Mikecz K, Ziegler SF, Balaji S, Keswani SG, de Jesus Perez VA, Butte MJ, Nadeau K, Altemeier WA, Fanger N, Bollyky PL. Modified high molecular weight hyaluronan promotes allergen-specific immune tolerance. Am J Respir Cell Mol Biol 56: 109–120, 2017. doi: 10.1165/rcmb.2016-0111OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelardi M, Guglielmi AV, De Candia N, Maffezzoni E, Berardi P, Quaranta N. Effect of sodium hyaluronate on mucociliary clearance after functional endoscopic sinus surgery. Eur Ann Allergy Clin Immunol 45: 103–108, 2013. [PubMed] [Google Scholar]
- 26.Hashimoto K, Peebles RS Jr, Sheller JR, Jarzecka K, Furlong J, Mitchell DB, Hartert TV, Graham BS. Suppression of airway hyperresponsiveness induced by ovalbumin sensitisation and RSV infection with Y-27632, a Rho kinase inhibitor. Thorax 57: 524–527, 2002. doi: 10.1136/thorax.57.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry PJ, Mann TS, Goldie RG. A rho kinase inhibitor, Y-27632 inhibits pulmonary eosinophilia, bronchoconstriction and airways hyperresponsiveness in allergic mice. Pulm Pharmacol Ther 18: 67–74, 2005. doi: 10.1016/j.pupt.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Irvin CG, Bates JH. Measuring the lung function in the mouse: the challenge of size. Respir Res 4: 1, 2003. doi: 10.1186/rr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 164, suppl 2: S28–S38, 2001. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 30.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 31.Kasahara DI, Mathews JA, Park CY, Cho Y, Hunt G, Wurmbrand AP, Liao JK, Shore SA. ROCK insufficiency attenuates ozone-induced airway hyperresponsiveness in mice. Am J Physiol Lung Cell Mol Physiol 309: L736–L746, 2015. doi: 10.1152/ajplung.00372.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasahara DI, Ninin FM, Wurmbrand AP, Liao JK, Shore SA. Abrogation of airway hyperresponsiveness but not inflammation by rho kinase insufficiency. Clin Exp Allergy 45: 457–470, 2015. doi: 10.1111/cea.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katoh S, Kaminuma O, Hiroi T, Mori A, Ohtomo T, Maeda S, Shimizu H, Obase Y, Oka M. CD44 is critical for airway accumulation of antigen-specific Th2, but not Th1, cells induced by antigen challenge in mice. Eur J Immunol 41: 3198–3207, 2011. doi: 10.1002/eji.201141521. [DOI] [PubMed] [Google Scholar]
- 34.Katoh S, Matsumoto N, Kawakita K, Tominaga A, Kincade PW, Matsukura S. A role for CD44 in an antigen-induced murine model of pulmonary eosinophilia. J Clin Invest 111: 1563–1570, 2003. doi: 10.1172/JCI16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubo M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol Rev 278: 162–172, 2017. doi: 10.1111/imr.12557. [DOI] [PubMed] [Google Scholar]
- 36.Lamas A, Marshburn J, Stober VP, Donaldson SH, Garantziotis S. Effects of inhaled high-molecular weight hyaluronan in inflammatory airway disease. Respir Res 17: 123, 2016. doi: 10.1186/s12931-016-0442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larkin J, Renukaradhya GJ, Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. CD44 differentially activates mouse NK T cells and conventional T cells. J Immunol 177: 268–279, 2006. doi: 10.4049/jimmunol.177.1.268. [DOI] [PubMed] [Google Scholar]
- 38.Lauer ME, Dweik RA, Garantziotis S, Aronica MA. The rise and fall of hyaluronan in respiratory diseases. Int J Cell Biol 2015: 712507, 2015. doi: 10.1155/2015/712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauer ME, Fulop C, Mukhopadhyay D, Comhair S, Erzurum SC, Hascall VC. Airway smooth muscle cells synthesize hyaluronan cable structures independent of inter-alpha-inhibitor heavy chain attachment. J Biol Chem 284: 5313–5323, 2009. doi: 10.1074/jbc.M807979200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lauer ME, Glant TT, Mikecz K, DeAngelis PL, Haller FM, Husni ME, Hascall VC, Calabro A. Irreversible heavy chain transfer to hyaluronan oligosaccharides by tumor necrosis factor-stimulated gene-6. J Biol Chem 288: 205–214, 2013. doi: 10.1074/jbc.M112.403998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lauer ME, Majors AK, Comhair S, Ruple LM, Matuska B, Subramanian A, Farver C, Dworski R, Grandon D, Laskowski D, Dweik RA, Erzurum SC, Hascall VC, Aronica MA. Hyaluronan and its heavy chain modification in asthma severity and experimental asthma exacerbation. J Biol Chem 290: 23124–23134, 2015. doi: 10.1074/jbc.M115.663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauer ME, Mukhopadhyay D, Fulop C, de la Motte CA, Majors AK, Hascall VC. Primary murine airway smooth muscle cells exposed to poly(I,C) or tunicamycin synthesize a leukocyte-adhesive hyaluronan matrix. J Biol Chem 284: 5299–5312, 2009. doi: 10.1074/jbc.M807965200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee-Sayer SS, Dong Y, Arif AA, Olsson M, Brown KL, Johnson P. The where, when, how, and why of hyaluronan binding by immune cells. Front Immunol 6: 150, 2015. doi: 10.3389/fimmu.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, Degan S, Leonard M, Kraft M, Noble PW. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol 128: 403–411.e3, 2011. doi: 10.1016/j.jaci.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, Palardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis 188: 919–926, 2003. doi: 10.1086/377642. [DOI] [PubMed] [Google Scholar]
- 47.Macchi A, Terranova P, Digilio E, Castelnuovo P. Hyaluronan plus saline nasal washes in the treatment of rhino-sinusal symptoms in patients undergoing functional endoscopic sinus surgery for rhino-sinusal remodeling. Int J Immunopathol Pharmacol 26: 137–145, 2013. doi: 10.1177/039463201302600113. [DOI] [PubMed] [Google Scholar]
- 48.Maile R, Jones S, Pan Y, Zhou H, Jaspers I, Peden DB, Cairns BA, Noah TL. Association between early airway damage-associated molecular patterns and subsequent bacterial infection in patients with inhalational and burn injury. Am J Physiol Lung Cell Mol Physiol 308: L855–L860, 2015. doi: 10.1152/ajplung.00321.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem 278: 47223–47231, 2003. doi: 10.1074/jbc.M304871200. [DOI] [PubMed] [Google Scholar]
- 50.McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin BY, Choi AM, Noble PW. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor kappaB-dependent mechanism. J Biol Chem 272: 8013–8018, 1997. doi: 10.1074/jbc.272.12.8013. [DOI] [PubMed] [Google Scholar]
- 51.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest 98: 2403–2413, 1996. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell PD, O’Byrne PM. Epithelial-Derived Cytokines in Asthma. Chest 151: 1338–1344, 2017. doi: 10.1016/j.chest.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 53.Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med 183: 2373–2378, 1996. doi: 10.1084/jem.183.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrigni G, Allegra L. Aerosolised hyaluronic acid prevents exercise-induced bronchoconstriction, suggesting novel hypotheses on the correction of matrix defects in asthma. Pulm Pharmacol Ther 19: 166–171, 2006. doi: 10.1016/j.pupt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Possa SS, Charafeddine HT, Righetti RF, da Silva PA, Almeida-Reis R, Saraiva-Romanholo BM, Perini A, Prado CM, Leick-Maldonado EA, Martins MA, Tibério IF. Rho-kinase inhibition attenuates airway responsiveness, inflammation, matrix remodeling, and oxidative stress activation induced by chronic inflammation. Am J Physiol Lung Cell Mol Physiol 303: L939–L952, 2012. doi: 10.1152/ajplung.00034.2012. [DOI] [PubMed] [Google Scholar]
- 56.Ros M, Casciaro R, Lucca F, Troiani P, Salonini E, Favilli F, Quattrucci S, Sher D, Assael BM. Hyaluronic acid improves the tolerability of hypertonic saline in the chronic treatment of cystic fibrosis patients: a multicenter, randomized, controlled clinical trial. J Aerosol Med Pulm Drug Deliv 27: 133–137, 2014. doi: 10.1089/jamp.2012.1034. [DOI] [PubMed] [Google Scholar]
- 57.Salier JP, Rouet P, Raguenez G, Daveau M. The inter-alpha-inhibitor family: from structure to regulation. Biochem J 315: 1–9, 1996. doi: 10.1042/bj3150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanggaard KW, Scavenius C, Rasmussen AJ, Wisniewski HG, Thøgersen IB, Enghild JJ. The TSG-6/HC2-mediated transfer is a dynamic process shuffling heavy chains between glycosaminoglycans. J Biol Chem 285: 21988–21993, 2010. doi: 10.1074/jbc.M109.041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaafsma D, Bos IS, Zuidhof AB, Zaagsma J, Meurs H. Inhalation of the Rho-kinase inhibitor Y-27632 reverses allergen-induced airway hyperresponsiveness after the early and late asthmatic reaction. Respir Res 7: 121, 2006. doi: 10.1186/1465-9921-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaafsma D, Bos IS, Zuidhof AB, Zaagsma J, Meurs H. The inhaled Rho kinase inhibitor Y-27632 protects against allergen-induced acute bronchoconstriction, airway hyperresponsiveness, and inflammation. Am J Physiol Lung Cell Mol Physiol 295: L214–L219, 2008. doi: 10.1152/ajplung.00498.2007. [DOI] [PubMed] [Google Scholar]
- 61.Song W, Yu Z, Doran SF, Ambalavanan N, Steele C, Garantziotis S, Matalon S. Respiratory syncytial virus infection increases chlorine induced airway hyper-responsiveness. Am J Physiol Lung Cell Mol Physiol 309: L205–L210, 2015. doi: 10.1152/ajplung.00159.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol 10: 712–723, 2010. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 63.Stober VP, Johnson CG, Majors A, Lauer ME, Cali V, Midura RJ, Wisniewski HG, Aronica MA, Garantziotis S. TNF-stimulated gene 6 promotes formation of hyaluronan-inter-α-inhibitor heavy chain complexes necessary for ozone-induced airway hyperresponsiveness. J Biol Chem 292: 20845–20858, 2017. doi: 10.1074/jbc.M116.756627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stober VP, Szczesniak C, Childress Q, Heise RL, Bortner C, Hollingsworth JW, Neuringer IP, Palmer SM, Garantziotis S. Bronchial epithelial injury in the context of alloimmunity promotes lymphocytic bronchiolitis through hyaluronan expression. Am J Physiol Lung Cell Mol Physiol 306: L1045–L1055, 2014. doi: 10.1152/ajplung.00353.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swaidani S, Cheng G, Lauer ME, Sharma M, Mikecz K, Hascall VC, Aronica MA. TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J Biol Chem 288: 412–422, 2013. doi: 10.1074/jbc.M112.389874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Puré E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 296: 155–158, 2002. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 67.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant 6: 2622–2635, 2006. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 68.To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet LP. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 12: 204, 2012. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4: 528–539, 2004. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 70.Xie T, Luo G, Zhang Y, Wang X, Wang X, Wu M, Li G. Rho-kinase inhibitor fasudil reduces allergic airway inflammation and mucus hypersecretion by regulating STAT6 and NFκB. Clin Exp Allergy 45: 1812–1822, 2015. doi: 10.1111/cea.12606. [DOI] [PubMed] [Google Scholar]
- 71.Zhou T, Yu Z, Jian MY, Ahmad I, Trempus C, Wagener BM, Pittet JF, Aggarwal S, Garantziotis S, Song W, Matalon S. Instillation of hyaluronan reverses acid instillation injury to the mammalian blood gas barrier. Am J Physiol Lung Cell Mol Physiol 314: L808–L821, 2018. doi: 10.1152/ajplung.00510.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]