Abstract
Objective
To analyze the topography of the opposite condyle to treat focal femoral condyle articular defects with an osteochondral allograft (OCA).
Design
Three groups were created: Group 1, same condyle with same width; Group 2, opposite condyle with same width; Group 3, opposite condyle with different width. Computed tomography (CT) of 22 cadaveric femoral hemi-condyles was used to create 3-dimensional CT models that were exported into point-cloud models. Three zones of the donor condyle (anterior, middle, and posterior) were quantified. Four defect sizes were created (15, 18, 23, 25 mm) at the weight-bearing region. The defect was moved throughout each donor condyle zone and the least distance was calculated, defined as the shortest distance between the defect and the donor condyle.
Results
The mean least distance increased with larger defect size in all groups, yet there was a less than 0.2 mm difference in the least distance among defect sizes. The 15, 18, and 23 mm defect models in Group 1 exhibited greater least distances at the anterior than middle and posterior zones. The 15 mm defect model exhibited greater least distance at the anterior zone than posterior zone in Group 3. However, there was a less than 0.05 mm difference in the mean least distance between zones. There was no significant difference in the least distance between groups.
Conclusion
OCAs from opposite condyles yield similar topographic matching to OCAs from the same condyles, suggesting that opposite condyles can be utilized. Clinical correlation and outcomes are necessary.
Keywords: distal femoral condyle, osteochondral allograft, topography
Introduction
Focal chondral defects of the knee are prevalent and are a significant source of pain and morbidity in the young, active population. Studies have reported localized, full-thickness chondral lesions in 5% to 20% of all patients undergoing arthroscopic evaluation.1-3 Treatment options for focal chondral defects in younger patients include debridement, microfracture, osteochondral autograft transplantation, autologous chondrocyte implantation, and osteochondral allograft (OCA) transplantation.4-8 OCA transplantation has been performed for the past few decades, with a goal of restoring the articular surface topography. The grafts can be fashioned in one or more plugs to cover the entire defect using a press fit technique or a shell allograft can be employed. Its popularity has increased as a result of its clinical success and expanding indications for the procedure. However, limited tissue availability has slowed the widespread use of fresh OCA.9,10
When OCAs are indicated, size-matched donor condyles are ordered based on posteroanterior flexion weight-bearing radiographs of the knee. Other measurement methods exist as well including magnetic resonance imaging (MRI) data in the sagittal plane. This graft is typically from the same side (right or left), same condyle (medial or lateral), and similar size (width of the affected condyle). As obtaining a donor can take from months to even a year, OCA availability is a major limitation to more widespread utilization of this technique. Therefore, allowing for the donor condyle to be from the opposite condyle and vary in width may allow increased access for patients.
Matching native topographic anatomy is paramount when performing osteochondral graft transplantation. Failure to anatomically match the articular surfaces may lead to poor clinical results.11 While work has been done to analyze the best location of osteochondral autograft harvests based on topographic matching,8,12-17 few studies have been applied to OCAs.18-20 Mologne et al. investigated surface matching of opposite condyle OCAs for a 20 mm condylar defect utilizing micro-computed tomography.20 However, no study to date investigated the topographic matching of OCAs based on the condylar size and the graft location. The purpose of this study was to analyze the topographic matching of the opposite condyle to treat focal distal femoral condyle articular defects with a circular OCA plug. We hypothesized that OCA grafts obtained from opposite condyles would maintain precise surface topographic matching despite different sizes and locations.
Materials and Methods
Specimen Preparation
Cadaveric tissue was procured from a donor tissue bank (AlloSource, Denver, Colorado) after institutional review board exemption was approved. Twenty-two femoral hemi-condyles with intact articular cartilage surfaces from 22 donors were prepared according to the company’s standard protocol for planned implantation ( Table 1 ). Of these, 11 were medial femoral hemi-condyles (MFCs) and 11 were lateral femoral hemi-condyles (LFCs). Condylar width was measured with a digital micrometer 10 mm distal to the most superior aspect of the notch, which is the same method used by donor tissue suppliers ( Fig. 1 ). Samples that were within 0.5 mm in condylar width were considered to be same size. Three groups were created from combinations of the 22 hemi-condyles on the basis of the difference between condylar width: Group 1 (n = 12), combinations of same condyle with same size (MFC donor matched to MFC recipient and LFC donor matched to LFC recipient); Group 2 (n = 8), combinations of opposite condyle with same size (LFC donor matched to MFC recipient and MFC donor matched to LFC recipient); and Group 3 (n = 8), combinations of opposite condyle with different size ( Table 2 ). The recipient and donor condyles were matched by ipsilateral side (right and left).
Table 1.
Demographic Data and Characteristics of Specimens.
| Specimens |
||
|---|---|---|
| MFC | LFC | |
| N | 11 | 11 |
| Age, years | 24.4 ± 5.3 (14-33) | 23.6 ± 8.1 (12-33) |
| Side, right:left | 5:6 | 5:6 |
| Condylar width, mm | 24.8 ± 1.5 (22.5-27.0) | 28.9 ± 2.5 (24.1-33.0) |
MFC = medial distal femoral condyle; LFC = lateral distal femoral condyle. Results are expressed as mean ± standard deviation.
Figure 1.
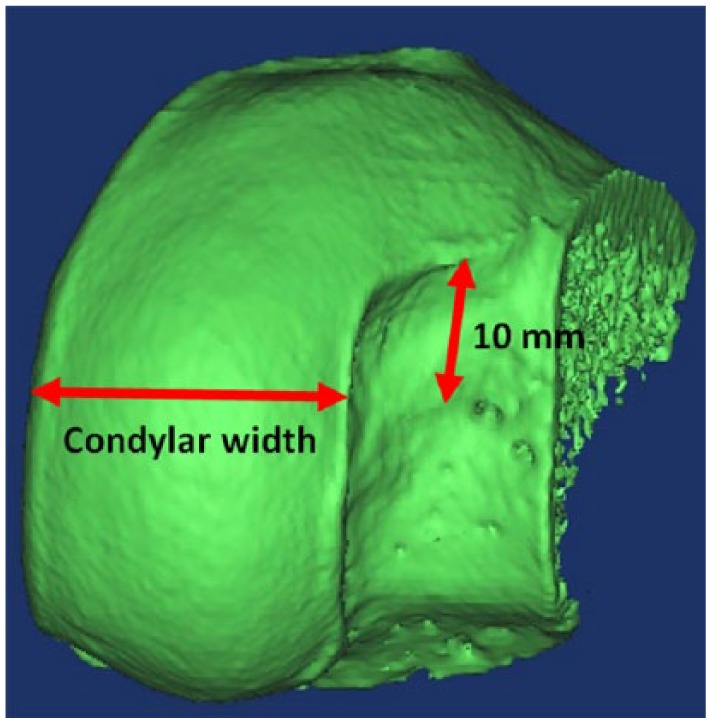
Condylar width was measured with a digital micrometer 10 mm distal of the most superior aspect of the notch.
Table 2.
Characteristics in Each Group.
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Condyle | Same | Opposite | Opposite |
| Condylar width | Same | Same | Different |
| N | 12 | 8 | 8 |
| Difference of width, mm | 0.21 ± 0.14 (0.10-0.40) | 0.18 ± 0.20 (0-0.49) | 6.78 ± 3.40 (2.1-10.5) |
Results are expressed as mean ± standard deviation.
Three-Dimensional CT Computer Model Creation of the Femoral Articular Surfaces
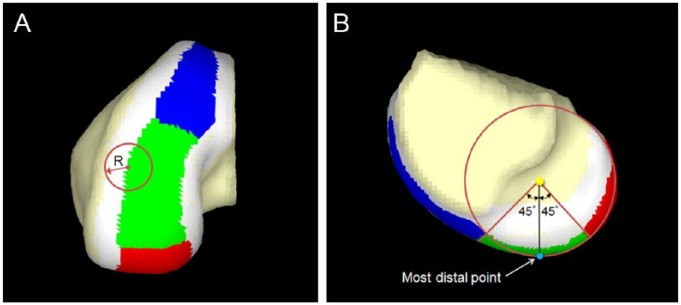
Computed tomography (CT) (BrightSpeed, GE Healthcare, Wauwatosa, Wisconsin) images were acquired in the coronal or sagittal planes using 0.625 mm contiguous slices (120 kV, 437 mA, 96 mm field of view, 512 × 512 matrices) ( Fig. 2 ). Three-dimensional (3D) CT models of the femoral hemi-condyles were then created and exported into point-cloud models using a 3D reconstruction software program (Mimics, Materialise Inc., Leuven, Belgium). The femoral hemi-condyle articular surface model was created by segmentation of the area covered by the articular cartilage. A local coordinate system of the articular surface was created as follows. A para-coronal plane including the most distal point was defined as a coronal plane (blue plane in Fig. 3 ). A para-transverse plane including the most posterior point was defined as a transverse plane (red plane in Fig. 3 ). A para-sagittal plane including the centroid of the articular surface mode was defined as a sagittal plane (green plane in Fig. 3 ). An intersection of these planes was defined as an origin of the local coordinate system. The Cartesian coordinate system was further transferred to a spherical coordinate system with the most distal point as the “South Pole.”
Figure 2.
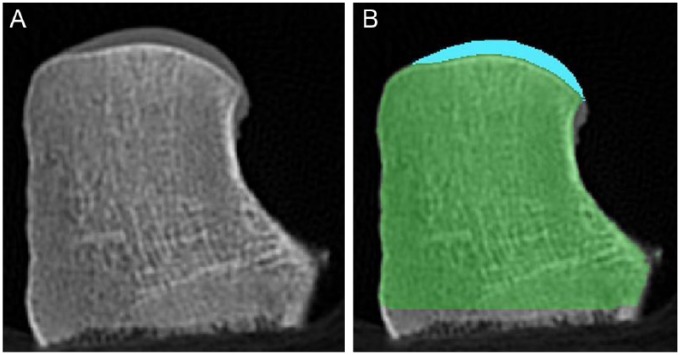
(A) Coronal CT image of the femoral hemi-condyle used for 3D reconstruction. (B) Same coronal CT image with blue and green representing the articular cartilage and underlying bone, respectively.
Figure 3.
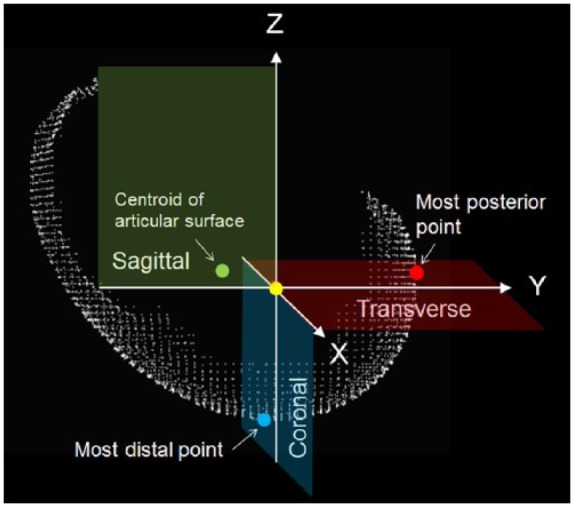
An orthogonal local coordinate system of the articular cartilage surface of femoral hemi-condyle. A para-coronal plane (blue plane), a para-transverse plane (red plane), and a para-sagittal plane (green plane) were defined based on the most distal point, the most posterior point, and the centroid of the articular cartilage surface. An intersection of these planes was defined as an origin of the local coordinate system (yellow dot).
Three-Dimensional CT Computer Model Creation of the Femoral Hemi-Condyle Articular Surface Defect
Circular articular surface defect models were created in each recipient condyle with 4 different diameters (15.0 mm, 18.0 mm, 23.0 mm, and 25.0 mm) at the most distal region in each femoral hemi-condyle ( Fig. 4 ). The point-cloud data within a distance of radius of each defect (i.e., 7.5 mm, 9.0 mm, 11.5 mm, and 12.5 mm) from the most distal point of the hemi-condyle were defined as the dataset of the circular articular surface defect model.
Figure 4.
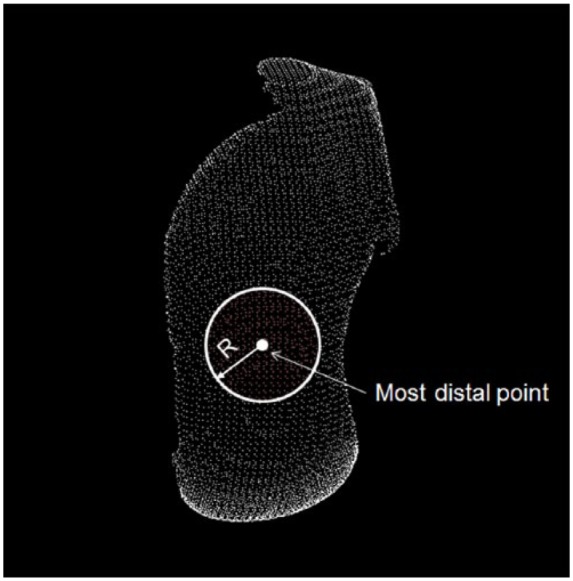
A circular articular cartilage surface defect model was created in the femoral hemi-condyle with 4 different diameters (15 mm, 18 mm, 23 mm, and 25 mm). A center of the circle was defined as the most distal point. Each radius (R) was 7.5 mm, 9.0 mm, 11.5 mm, or 12.5 mm. Points within the circle were defined as the articular cartilage surface points (red points).
Zoning of the Femoral Condyle Articular Surfaces
The articular surface was first divided into the peripheral zone and the central zone. The margin of the central zone was defined such that its shape was concentric to the outer margin. The distance between the central margin and the outer margin was the radius of the defect model to be analyzed ( Fig. 5A ). Therefore, a defect centered beyond the central zone that is at least partially outside the articular cartilage surface was not included in this study. The central zone was further divided into 3 zones defined by an angular parameter in the spherical coordinate of each point of the articular surface model ( Fig. 5A and B ): anterior zone (blue zone), over 45° anteriorly; middle zone, within 45°; and posterior zone (red zone), over 45° posteriorly.
Figure 5.
(A) The articular cartilage surface was divided into the peripheral zone and the central zone. The margin between the central zone and the peripheral zone was defined as the radius (R) of the defect model. (B) The central zone was then divided into 3 zones: anterior zone (blue zone), over 45° anteriorly; middle zone (green zone), within 45°; and posterior zone (red zone), over 45° posteriorly.
Defect Model-Donor Condyle 3D Articular Surface Topography Matching
Three-dimensional surface topography was compared between the defect model and the femoral hemi-condyle articular surface for 112 defect-femoral hemi-condyle comparative combinations (4 sizes of defect models × 28 defect-femoral condyle models combinations). The defect articular surface model was virtually placed on the articular cartilage surface of the donor condyle so that the centroid of the defect merged at a point in the central zone (anterior, middle, and posterior zones) of the femoral condyle ( Fig. 7 ). Defect model-donor condyle 3D articular cartilage surface topography matching was performed using the previous procedures.21 The “footprint” underneath the defect model was defined as a circle with the radius of the defect. Orientation of the defect model was adjusted so that its axis matched that of the footprint area on the femoral condyle. Distances between the defect model and the donor surface were calculated in 3D space. Least distance was defined as the shortest distance from the point in question to the footprint, where a perfect congruent match would equal a least distance of 0 mm for the given data points on the simulated articular cartilage surfaces. A mean value of the least distances was calculated for each position and each orientation of the defect model ( Fig. 6 ).
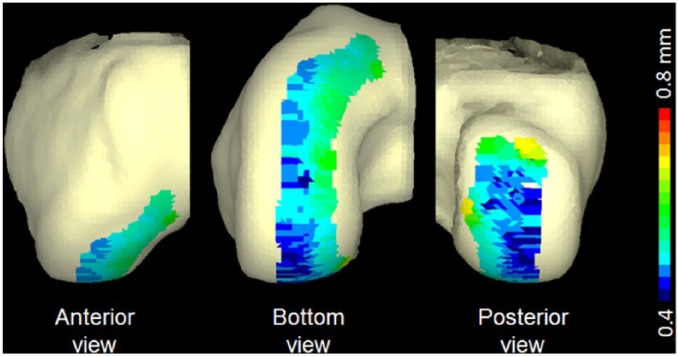
Figure 7.
A 3-dimensional representation of the distance distribution of the18 mm defect model in Group 3. Gradient color code represents from 0.4 mm to 0.8 mm. Blue represents penetration into the femoral condyle surface, whereas red indicates prominence with respect to the femoral condyle surface.
Figure 6.
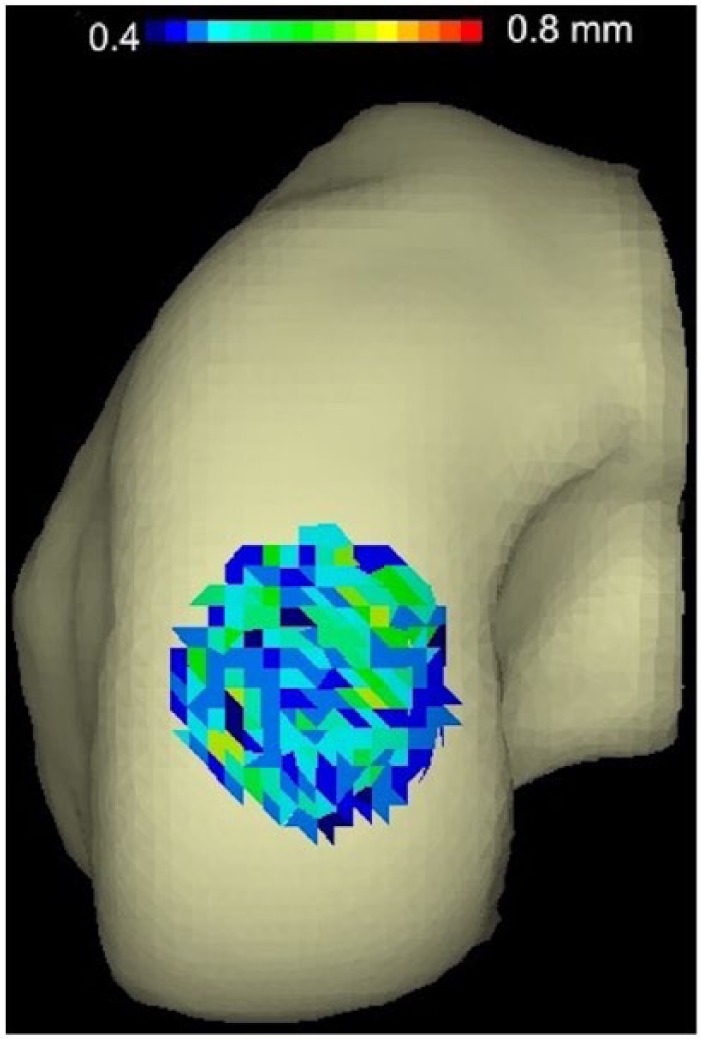
Example of articular cartilage topography mismatch between an 18 mm defect and graft from the same position on the femoral hemi-condyle. Gradient color code represents from 0.4 mm to 0.8 mm. Blue represents penetration into the femoral condyle surface, whereas red indicates prominence with respect to the femoral condyle surface.
The defect model was then rotated 360° around the axis perpendicular to the articular cartilage surface in 1° increments and the least distance was calculated at each rotating angle. The position of the defect model was then moved throughout the entire femoral condyle articular cartilage surface model and the least distance was calculated at each position ( Fig. 7 ). In this procedure, the centroid of the defect model was located within the central zone. For each position that the simulated graft was moved, the articular surfaces were compared for match with respect to all data points.
Statistical Analysis
The data were analyzed using Excel 2010 (Microsoft Corp, Redmond, Washington) and JMP software (v12.0, SAS Institute, Cary, NC). Statistical analysis proceeded with analysis of variance (ANOVA) to compare the condylar width among groups and the effect of defect size within each group followed by Fisher post hoc analysis. Group 1 was then used as a control group to compare mismatch when looking at the opposite condyle (Group 2) or different size of condyle (Group 3) as the variable. This analysis was performed with unpaired Student’s t test. The data were presented as mean ± standard deviation, and the level of significance for all analysis was set at P < 0.05.
Results
The mean condylar width of the MFCs measured 24.8 ± 1.5 mm (range = 22.5-27.0 mm; mean age = 24.4 ± 5.3 years), whereas the LFCs measured 28.9 ± 2.5 mm (range = 24.1-33.0 mm; mean age, 23.6 ± 8.1 years) ( Table 1 ).
The mean differences in the condylar width were 0.21 ± 0.14 mm (range = 0.10-0.40 mm) in Group 1, 0.18 ± 0.20 mm (range = 0-0.49 mm) in Group 2, and 6.78 ± 3.40 mm (range = 2.1-10.5 mm) in Group 3 ( Table 2 ). There was no significant difference between Groups 1 and 2 (P = 0.968), whereas the mean difference in the condylar width in Group 3 was significantly greater than that in Group 1 (P < 0.01) and Group 2 (P < 0.01).
The mean least distances in the topographic articular cartilage surface matching are summarized in Table 3 . Overall, the mean least distance of each combination was less than 1 mm in all groups. However, the mean least distance increased with increased defect size within each group. Within Group 1, the mean least distance significantly increased with increased defect size when comparing defect size 15 mm to 18 mm to 23 and 25 mm (F[3, 44] = 50.011, P < 0.001), while there was no significant difference between defects sized 23 mm and 25 mm (P = 0.696). Within Groups 2 and 3, similarly, the mean least distance significantly increased with increased defect size (Group 2: F[3, 28] = 54.048, P < 0.001; Group 3: F[3, 28] = 25.617, P < 0.001).
Table 3.
The Mean Least Distance between the Graft Model and the Donor Condyle.
| Zone of Donor Condyle | The Mean Least Distance in Each Defect Size |
||||
|---|---|---|---|---|---|
| 15 mm | 18 mm | 23 mm | 25 mm | ||
| Group 1 | Overall | 0.455 ± 0.018 | 0.508 ± 0.029 | 0.583 ± 0.041 | 0.588 ± 0.033 |
| P value | <0.001 | <0.001 | 0.696 | ||
| Anterior | 0.468 ± 0.027 | 0.532 ± 0.034 | 0.603 ± 0.059 | 0.616 ± 0.050 | |
| Middle | 0.452 ± 0.011* | 0.494 ± 0.026* | 0.571 ± 0.029* | 0.586 ± 0.045 | |
| Posterior | 0.446 ± 0.012* | 0.485 ± 0.021* | 0.559 ± 0.040* | 0.576 ± 0.037 | |
| Group 2 | Overall | 0.450 ± 0.013 | 0.499 ± 0.024 | 0.561 ± 0.031 | 0.590 ± 0.025 |
| P value | <0.001 | <0.001 | 0.025 | ||
| Anterior | 0.460 ± 0.032 | 0.519 ± 0.041 | 0.588 ± 0.072 | 0.616 ± 0.052 | |
| Middle | 0.461 ± 0.019 | 0.496 ± 0.024 | 0.563 ± 0.030 | 0.578 ± 0.016 | |
| Posterior | 0.435 ± 0.017 | 0.481 ± 0.031 | 0.548 ± 0.037 | 0.584 ± 0.055 | |
| Group 3 | Overall | 0.459 ± 0.016 | 0.521 ± 0.046 | 0.569 ± 0.027 | 0.606 ± 0.044 |
| P value | 0.002 | <0.001 | 0.044 | ||
| Anterior | 0.471 ± 0.020 | 0.530 ± 0.052 | 0.594 ± 0.047 | 0.634 ± 0.069 | |
| Middle | 0.460 ± 0.021 | 0.516 ± 0.048 | 0.564 ± 0.029 | 0.616 ± 0.034 | |
| Posterior | 0.439 ± 0.026* | 0.517 ± 0.039 | 0.543 ± 0.035 | 0.586 ± 0.035 | |
Results are expressed as mean ± standard deviation.
Statistical significant compared with anterior zone (P < 0.05).
The mean least distance was compared among zones of donor condyles (anterior, middle, and posterior zones) within each group. Within Group 1, the 15 mm, 18 mm, and 23 mm defect models exhibited significantly greater mean least distance at the anterior zone than the posterior zone (15 mm, P < 0.01; 18 mm, P < 0.01; 23 mm, P < 0.01) and the middle zone (15 mm, P = 0.031; 18 mm, P < 0.01; 23 mm, P = 0.049). However, there was less than 0.05 mm difference in the mean least distance among zones of donor condyles. Within Groups 2 and 3, similarly, there was less than 0.05 mm difference in the mean least distance among zones of donor condyles for each defect size. Within Group 2, the mean least distance of all defect models exhibited no significant difference among zones of donor condyles. However, the 15 mm defect model exhibited significantly greater mean least distance at the anterior zone than the posterior zone within Group 3 (P = 0.02).
When comparing same and opposite donor hemi-condyles (Group 1 and Group 2), there was no significant difference in the mean least distance for any defect sizes or donor zones. When comparing same and different sized condyles (Group 1 and Group 3), there was no significant difference in the mean least distance for any defect sizes or donor zones.
Discussion
The findings of this study are that OCAs from opposite condyles provide similar topographic matching at the most distal region of the recipient condyle regardless of different sizes and different locations. Data from this study demonstrates that our “best efforts” when using the same condyle yields a fit of 0.45 to 0.62 mm. This mismatch increased with increasing defect size up to 25 mm. In terms of the graft harvest location, the best-fit hierarchy was as follows: the posterior zone was better than the middle zone, which was better than the anterior zone. However, the largest mismatch difference was between the posterior 15 mm defect (0.45 mm) and the anterior 25 mm defect (0.62 mm, difference 0.17 mm). The opposite condyle of the same or different size showed similar tendency of surface matching when comparing defect sizes or locations. However, most of the mismatch was not significantly different among locations. Importantly, there were no significant differences of mismatch in any sizes and any locations between the same condyle and the opposite condyle with the same or different size.
The goal of OCA transplantation is to restore normal biologic and biomechanical properties of intact articular cartilage with normal joint function. Many factors, such as patient age, chondrocyte viability of the recipient and the graft, and surgical procedures affect the outcome after OCA transplantation. The surface matching between the graft and the recipient is an important factor in achieving good clinical results after OCA transplantation.11 An ideal match for resurfacing of the articular cartilage would be a perfect congruous relationship between the graft and the recipient. Failure to anatomically match surfaces can lead to poor clinical results, which are caused by eccentric loading and altered contact pressure leading to early failure of the graft.
Topographic analysis is a crucial method to elucidate the surface geometry of the human body. Furthermore, topographic anatomy can help surgeons achieve successful osteochondral graft transplantation. Previous studies mostly focused on topographic anatomy for osteochondral autografting.12,13,15-17,20 However, there are few studies that evaluate topographic matching of the distal femoral condyle to find the optimal donor site for OCA transplantation.18-20 Typical clinical practice involves obtaining OCAs with the same side, condyle, and size, which can result in limited graft availability and increased patient wait time to find a matching graft. Opposite condyles for the affected lesions may be a good graft source, allowing for significantly increased graft availability for OCAs. However, previous studies showed that the anatomical morphologies of MFCs and LFCs differ in shape, curvature, and size.22-24 Therefore, topographic analysis of the distal femoral condyle needs to be clarified before potentially utilizing the opposite condyle as OCAs. Mologne et al. previously investigated OCA surface matching with an MFC or LFC graft into a 20 mm MFC defect model.20 This study showed that the overall articular cartilage mismatch of 0.63 mm for area and 0.47 mm for step-off, with no significant differences between the MFC and LFC graft. In addition, the original articular cartilage surface contour matched within ±1 mm in 87.4% of the MFC grafts and 87.7% of the LFC grafts. These results were consistent with our study. Furthermore, our study evaluated topography matching using different size of donor condyle because it is difficult to adjust the size of the opposite condyle. Our results suggest that OCAs from the opposite condyle can yield an acceptable articular cartilage surface matching regardless of the size of donor condyle.
The clinical relevance of the mismatch, which affects clinical results for OCA transplantation, might be analyzed in the setting of previous works. Nakagawa et al. evaluated clinical results and second look arthroscopy after transplantation.11 They showed that the proud plugs gave poor clinical results but recessing less than 1 mm promoted acceptable cartilage healing and lead to good clinical results. Therefore, while recessing plugs up to 1 mm may be tolerated, proud plugs are likely not as well tolerated. On the other hand, a study using a sheep model showed that the flush and 1 mm recessing of osteochondral grafting obtained a good smooth surface compared with 2 mm recessing.14 In addition, another study using a rabbit model showed that grafts sunk by 2 mm lead to cartilage necrosis and fibrous overgrowth.25 Therefore, incongruities ranging from flush to proud or recessing of less than 1 mm can be tolerated for grafting. Our results showed that OCAs from opposite condyle had less than 1 mm of mismatch in any defect sizes and any graft locations.
With regard to biomechanical properties of graft incongruity surrounding the recipient, D’Lima et al. showed that grafts proud by 0.5 mm increase peak contact stress by 2 times compared to intact cartilage.26 This study showed that a graft that is even 0.25 mm proud over the recipient surface increased stress and strain. In addition, recessing of the graft increased the stress and strain in the recipient area surrounding the graft. Koh et al. demonstrated that peak contact pressure significantly increased with plugs elevated 1.0 and 0.5 mm above the surrounding surface, and that plugs sunk 0.5 and 1.0 mm significantly increased the peak contact pressure in the intact area.27 The peak contact stress and peak compression strain approached levels that have been shown to induce cartilage damage and cell death.28,29 Our results therefore suggest that the contact pressure may increase the same as the mismatch with increasing defect size, though a direct comparison as such is subject to speculation. However, the contact pressure could be unchanged whether grafts are harvested from the same or opposite condyles.
The strengths of this study include the computer-based computational analysis, which is based on an established 3D point-cloud creation instrument, determination of the centroid of the femoral condyle articular cartilage surface, matching of the orientation of the articular cartilage surface point clouds, and cross-referencing of each femoral condyle cloud with each defect cloud to account for any variability that may be present between combinations. Another strength is that the specimens used in this study were actual materials for OCA transplantation. So, these specimens could retain the articular cartilage surface and provide actual results of topography mapping relevant to performing OCA transplantation.
There were several limitations in the current study. First, his study focused strictly on the articular cartilage surface congruence. However, a surgeon should consider several situations of chondral defects such as different size, depth, and locations. Second, the thickness of cartilage was not considered for this analysis. The thickness of cartilage is different for MFCs and LFCs, and the cartilage in contact regions is thicker than noncontact regions.22,30 From a biomechanical point of view, the thickness of graft cartilage should be equal to that of recipient cartilage. Based on these limitations, further studies are needed to increase the graft availability for OCA transplantation.
In conclusion, our results indicate that OCAs from opposite condyles can yield precise surface topographic matching in the direct weight-bearing region regardless of the different size and the different location of the donor condyle. These findings suggest that the utilization of the opposite condyle can increase the graft availability for OCAs’ transplantation while maintaining precise surface topographic. This is in agreement with recently published results reporting no significant difference in clinical outcomes when utilizing the opposite condyle for OCA transplantation.31 Future randomized trials and long-term studies are necessary to further understand the clinical effect of utilizing the opposite condyle.
Footnotes
Acknowledgments and Funding: The authors thank Brett T. Madden, BS, for his assistance with this study.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Gregory L. Cvetanovich, Erin Moran, and Atsuhi Urita, have nothing to disclose. Adam B. Yanke reports research support from Arthrex, Inc. and NuTech Medical. He is also a paid consultant for JRF Ortho. Nozomue Inoue reports research support from the National Institutes of Health (NCCIH). Jason J. Shin reports publishing royalties, financial or material support from SLACK incorporated. Brian J. Cole reports the following relationships: Aesculap/B. Braun: research support; American Journal of Orthopedics: editorial or governing board; American Journal of Sports Medicine: editorial or governing board; Aqua Boom: stock or stock options; Arthrex, Inc: IP royalties, paid consultant, research support; Arthroscopy: editorial or governing board; Arthroscopy Association of North America: board or committee member; Athletico: other financial or material support; Biomerix: stock or stock options; Cartilage: editorial or governing board; DJ Orthopaedics: IP royalties; Elsevier Publishing: IP royalties; Flexion: paid consultant; Geistlich: research support; Giteliscope: stock or stock options; International Cartilage Repair Society: board or committee member; Journal of Bone and Joint Surgery–American: Editor only: editorial or governing board; Journal of Shoulder and Elbow Surgery: Editor only: editorial or governing board; Journal of the American Academy of Orthopaedic Surgeons: Editor only: editorial or governing board; JRF Ortho: other financial or material support; Medipost: research support; National Institutes of Health (NIAMS & NICHD): research support; Norvartis: research support; Operative Techniques in Sports Medicine: publishing royalties, financial or material support; Ossio: stock or stock options; Regentis: paid consultant, stock or stock options; Sanofi-Aventis: research support; Saunders/Mosby-Elsevier: publishing royalties, financial or material support; Smith & Nephew: other financial or material support, paid consultant; Tornier: other financial or material support; Zimmer: paid consultant, research support. Bernard R. Bach reports research support from Arthrex, Inc., CONMED Linvatec, DJ Orthopaedics, Ossur, Smith & Nephew, and Tornier as well as publishing royalties, financial or material support from SLACK Incorporated. Nikhil N. Verma reports the following disclosures: American Orthopaedic Society for Sports Medicine: board or committee member; American Shoulder and Elbow Surgeons: board or committee member; Arthrex, Inc.: research support; Arthroscopy: editorial or governing board, publishing royalties, financial or material support; Arthroscopy Association Learning Center Committee: board or committee member; Arthrosurface: research support; Cymedica: stock or stock Options; DJ Orthopaedics: research support; Journal of Knee Surgery: editorial or governing board; Minivasive: paid consultant, stock or stock options; Omeros: stock or stock options; Orthospace: paid consultant; Ossur: research support; SLACK Incorporated: editorial or governing board; Smith & Nephew: IP royalties, paid consultant, research support; Athletico: research support; ConMed Linvatec: research support; Miomed: research support; Mitek: research support; Vindico Medical-Orthopedics Hyperguide: publishing royalties, financial or material support.
Ethical Approval: Ethical approval for this study was waived by Rush University Institutional Review Board because it did not involve human subjects, only non-identifiable hemi-condyle allografts.
Informed Consent: Informed consent was not sought for the present study because it did not involve human subjects.
Trial Registration: Not applicable.
References
- 1. Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211-5. [DOI] [PubMed] [Google Scholar]
- 2. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-60. [DOI] [PubMed] [Google Scholar]
- 3. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730-4. [DOI] [PubMed] [Google Scholar]
- 4. Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91:1778-90. [PubMed] [Google Scholar]
- 5. Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907-14. doi: 10.1177/0363546507299932 [DOI] [PubMed] [Google Scholar]
- 6. Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468:1269-78. doi: 10.1007/s11999-010-1250-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levy YD, Gortz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471:231-7. doi: 10.1007/s11999-012-2556-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishizawa Y, Matsumoto T, Araki D, Nagamune K, Matsushita T, Kurosaka M, et al. Matching articular surfaces of selected donor and recipient sites for cylindrical osteochondral grafts of the femur: quantitative evaluation using a 3-dimensional laser scanner. Am J Sports Med. 2014;42:658-64. doi: 10.1177/0363546513518005 [DOI] [PubMed] [Google Scholar]
- 9. McAllister DR, Joyce MJ, Mann BJ, Vangsness CT., Jr. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35:2148-58. doi: 10.1177/0363546507308936 [DOI] [PubMed] [Google Scholar]
- 10. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22:121-33. doi: 10.5435/jaaos-22-02-121 [DOI] [PubMed] [Google Scholar]
- 11. Nakagawa Y, Suzuki T, Kuroki H, Kobayashi M, Okamoto Y, Nakamura T. The effect of surface incongruity of grafted plugs in osteochondral grafting: a report of five cases. Knee Surg Sports Traumatol Arthrosc. 2007;15:591-6. doi: 10.1007/s00167-006-0253-0 [DOI] [PubMed] [Google Scholar]
- 12. Agneskirchner JD, Brucker P, Burkart A, Imhoff AB. Large osteochondral defects of the femoral condyle: press-fit transplantation of the posterior femoral condyle (MEGA-OATS). Knee Surg Sports Traumatol Arthrosc. 2002;10:160-8. doi: 10.1007/s00167-001-0259-6 [DOI] [PubMed] [Google Scholar]
- 13. Ahmad CS, Cohen ZA, Levine WN, Ateshian GA, Mow VC. Biomechanical and topographic considerations for autologous osteochondral grafting in the knee. Am J Sports Med. 2001;29:201-6. [DOI] [PubMed] [Google Scholar]
- 14. Huang FS, Simonian PT, Norman AG, Clark JM. Effects of small incongruities in a sheep model of osteochondral autografting. Am J Sports Med. 2004;32:1842-8. [DOI] [PubMed] [Google Scholar]
- 15. Shin JJ, Haro M, Yanke AB, Mascarenhas R, Romeo AA, Cole BJ, et al. Topographic analysis of the capitellum and distal femoral condyle: finding the best match for treating osteochondral defects of the humeral capitellum. Arthroscopy. 2015;31:843-9. doi: 10.1016/j.arthro.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 16. Thaunat M, Couchon S, Lunn J, Charrois O, Fallet L, Beaufils P. Cartilage thickness matching of selected donor and recipient sites for osteochondral autografting of the medial femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2007;15:381-6. doi: 10.1007/s00167-006-0222-7 [DOI] [PubMed] [Google Scholar]
- 17. Bartz RL, Kamaric E, Noble PC, Lintner D, Bocell J. Topographic matching of selected donor and recipient sites for osteochondral autografting of the articular surface of the femoral condyles. Am J Sports Med. 2001;29:207-12. [DOI] [PubMed] [Google Scholar]
- 18. Bernstein DT, O’Neill CA, Kim RS, Jones HL, Noble PC, Harris JD, et al. Osteochondral allograft donor-host matching by the femoral condyle radius of curvature. Am J Sports Med. 2017;45:403-9. doi: 10.1177/0363546516671519 [DOI] [PubMed] [Google Scholar]
- 19. Birman MV, Le Dan T, Ismaily SK, Miller BS. The humeral head as a potential donor source for osteochondral allograft transfer to the knee. J Knee Surg. 2009;22:99-105. [DOI] [PubMed] [Google Scholar]
- 20. Mologne TS, Cory E, Hansen BC, Naso AN, Chang N, Murphy MM, et al. Osteochondral allograft transplant to the medial femoral condyle using a medial or lateral femoral condyle allograft: is there a difference in graft sources? Am J Sports Med. 2014;42:2205-13. doi: 10.1177/0363546514540446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta AK, Forsythe B, Lee AS, Harris JD, McCormick F, Abrams GD, et al. Topographic analysis of the glenoid and proximal medial tibial articular surfaces: a search for the ideal match for glenoid resurfacing. Am J Sports Med. 2013;41:1893-9. doi: 10.1177/0363546513484126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eckstein F, Winzheimer M, Hohe J, Englmeier KH, Reiser M. Interindividual variability and correlation among morphological parameters of knee joint cartilage plates: analysis with three-dimensional MR imaging. Osteoarthritis Cartilage. 2001;9:101-11. doi: 10.1053/joca.2000.0365 [DOI] [PubMed] [Google Scholar]
- 23. Nuno N, Ahmed AM. Three-dimensional morphometry of the femoral condyles. Clin Biomech (Bristol, Avon). 2003;18:924-32. [DOI] [PubMed] [Google Scholar]
- 24. Rehder U. Morphometrical studies on the symmetry of the human knee joint: femoral condyles. J Biomech. 1983;16:351-61. [DOI] [PubMed] [Google Scholar]
- 25. Kuroki H, Nakagawa Y, Mori K, Ohba M, Suzuki T, Mizuno Y, et al. Acoustic stiffness and change in plug cartilage over time after autologous osteochondral grafting: correlation between ultrasound signal intensity and histological score in a rabbit model. Arthritis Res Ther. 2004;6:R492-R504. doi: 10.1186/ar1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D’Lima DD, Chen PC, Colwell CW., Jr. Osteochondral grafting: effect of graft alignment, material properties, and articular geometry. Open Orthop J. 2009;3:61-8. doi: 10.2174/1874325000903010061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koh JL, Wirsing K, Lautenschlager E, Zhang LO. The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am J Sports Med. 2004;32:317-20. [DOI] [PubMed] [Google Scholar]
- 28. D’Lima DD, Hashimoto S, Chen PC, Colwell CW, Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9:712-9. doi: 10.1053/joca.2001.0468 [DOI] [PubMed] [Google Scholar]
- 29. D’Lima DD, Hashimoto S, Chen PC, Lotz MK, Colwell CW., Jr. In vitro and in vivo models of cartilage injury. J Bone Joint Surg Am. 2001;83(Suppl. 2):22-4. [DOI] [PubMed] [Google Scholar]
- 30. Li G, Park SE, DeFrate LE, Schutzer ME, Ji L, Gill TJ, et al. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clin Biomech (Bristol, Avon). 2005;20:736-44. doi: 10.1016/j.clinbiomech.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 31. Wang D, Jones KJ, Eliasberg CD, Pais MD, Rodeo SA, Williams RJ. Condyle-specific matching does not improve midterm clinical outcomes of osteochondral allograft transplantation in the knee. J Bone Joint Surg Am. 2017; 99:1614-20. [DOI] [PubMed] [Google Scholar]