Abstract
Objective
The objective of this pilot study was to determine time point(s) at which maximum concentration of fluticasone propionate (Cmax) occurs in synovial fluid and plasma in Beagle dog knees after intra-articular injection of EP-104IAR.
Design
EP-104IAR is composed of fluticasone propionate drug crystals coated with heat-treated polyvinyl alcohol (PVA) to result in extended release properties. Thirty-two Beagle dogs had an injection of EP-104IAR into the knee joint at 2 different dose levels (0.6 mg and 12 mg). Outcome measures included plasma, synovial fluid, and articular cartilage fluticasone propionate concentrations as well as histological analysis of cartilage and synovium at a variety of time points up to 58 days postdosing.
Results
Intra-articular administration of 0.6 and 12 mg EP-104IAR was well tolerated. Early minor abnormalities found on microscopy resolved by the end of the study. There were no quantifiable concentrations of fluticasone propionate in plasma of animals administered 0.6 mg at any of the sampling time points. Highest concentrations in plasma following 12 mg administration occurred 1 day postdose and declined with a half-life of approximately 45 days. Highest concentrations of fluticasone propionate in synovial fluid and cartilage generally occurred 5 days postdose in both dose groups and declined with a half-life of approximately 11 to 14 days.
Conclusions
EP-104IAR is capable of providing a safe and prolonged local exposure to a corticosteroid in the synovial joint while minimizing systemic exposure, with peak exposures occurring within a matter of days after dosing before declining in all tissues in a predictable manner.
Keywords: EP-104IAR, fluticasone propionate, slow release, osteoarthritis, knee
Introduction
Osteoarthritis (OA) is the most prevalent worldwide chronic joint disease.1 Symptomatic knee OA affects about 6% of adults aged 30 years or older. Moreover, its prevalence increases with aging.2,3 OA results in significant morbidity, leading to pain and disability in more than 3.6% of the global population accounting for a significant health care challenge.4 Historically, OA was thought to be a mechanical disorder. However, it is increasingly recognized that local inflammation, in particular synovitis, is important in its pathophysiology.5-7 Synovitis is an important target for therapeutic intervention, because it is associated with both clinical symptoms and disease progression.6 Currently, medical management of OA focuses on symptom control, particularly of pain. Mild to moderate OA pain can be treated using nonsteroidal anti-inflammatory drugs (NSAIDs), with COX-2 inhibitors taking a significant market share from older members of this class.8 Unfortunately, they may fail to achieve a clinical response, and in these cases intra-articular (IA) corticosteroid injections can be considered as a treatment option.
The inflamed synovium is a site of action for IA corticosteroids and they have become one of the mainstays of treatment for osteoarthritic pain over the past 6 decades.9-11 A large survey in the United States revealed that more than 95% of rheumatologists use IA corticosteroid injections in knee OA treatment.12 It was estimated that in 2012, more than 3 million patients with knee osteoarthritis had received IA corticosteroids in the United States and their growth in use has been continuous over the past few years.13 In fact, the U.S. Food and Drug Administration (FDA) recommends corticosteroids for short-term treatment of pain.14-18
In clinical trials, IA corticosteroids demonstrate a large initial analgesic effect that wanes over 1 to 4 weeks with the rapid efflux of drug from the joint.19-22 Therefore, recent research focuses on IA injection of drug-encapsulating micro- or nanoparticles for slow release of drugs into the synovial fluid.23-25 In this way, EP-104IAR was developed to ensure a sustained release of fluticasone propionate over a longer time period while maintaining a minimum therapeutically effective concentration in the joint. Fluticasone propionate is a corticosteroid that is extensively used in the treatment of asthma, chronic obstructive pulmonary disease (COPD), allergic rhinitis, and nasal polyps.26-29
The objective of the present study was to determine the time point(s) at which the maximum concentration of fluticasone propionate (Cmax) occurs in synovial fluid and plasma in Beagle dog knees after an IA with EP-104IAR.
Methods
EP-104IAR
EP-104IAR is composed of fluticasone propionate drug crystals coated with heat-treated polyvinyl alcohol (PVA) to result in extended release properties intended for prolonged treatment of inflammation associated with osteoarthritis. Essentially, fluticasone propionate is coated with a semipermeable polymeric shell and injected into the joint. Water then diffuses through the polymer and dissolves the drug core creating a saturated solution inside the membrane. This concentration gradient drives a constant release of drug from the drug particle as long as there is some drug core remaining to maintain a saturated solution. The coated crystals have a mean diameter in a range of 50 to 100 μm.
Study Population
Thirty-two Beagle dogs (Canis familiaris) (16 males, 16 females) were received from Marshall Bioresources (North Rose, NY 14516, USA) or Covance Research Products Inc. (Cumberland, VA, USA). At the onset of dosing, the age of the animals was 10 to 11 months. The body weights ranged from 9.1 to 11.0 kg and from 7.9 to 10.2 kg for males and females, respectively. An acclimation period of 10 to 11 days was allowed between receipt of the animals and the start of dosing to accustom the animals to the laboratory environment. An attending veterinarian was on site or on call during the live phase of the study. No concurrent medications were given. Housing of the animals throughout the study conformed to the guidelines cited in the Guide for the Care and Use of Laboratory Animals and the applicable standard operating procedures of CiToxLAB North America, Inc.
Injection Scheme
EP-104IAR was administered at 2 dosage sizes: a low dose of 0.6 mg (group 1) and a high dose of 12.0 mg (group 2). These doses were selected based on extrapolation of proposed future clinical doses and knee cartilage volume considerations, as described below. Because of concentration and dose volume limitations of EP-104IAR, dosing of group 2 was performed over 2 days; animals received 6 mg on day 1 and 6 mg on day 2 to result in a total of 12.0 mg. Sample collections began following the second dosing. Group 1 animals received 0.6 mg on day 2 only in order to synchronize sample collections with group 2. Injection volume was 1 mL for all animals ( Table 1 ).
Table 1.
Injection Scheme.
| Treatment Groups | Dosing Regimen | Target Dose Level (mg) | Dose Concentration/Injection (mg/mL) | Dose Volume/Injection (mL) | No. of Animals |
|
|---|---|---|---|---|---|---|
| Male | Female | |||||
| 1 | Day 2 | 0.6 | 0.6 | 1 | 8 | 8 |
| 2 | Days 1 and 2 | 12 | 6.0 | 1 | 8 | 8 |
Dose Rationale
The cartilage volume of the human knee is 23.3 cm3 (16.6-31.4 cm3) per joint.30 The best estimate for the volume of cartilage in the dog knee is 1.1 ± 0.7 cm3 and was determined based on animals that weighed 25.0 kg.31 Because the measured weight of the dogs in the current study was on average 10.0 kg and because it was assumed that the cartilage volume varies approximately with body mass, we conservatively estimated a cartilage volume of 0.5 cm3, resulting in a human cartilage volume of approximately 40 times that of the beagle dog used in this study. The high dose of 12 mg in this dog study was chosen because, when matched for cartilage volume, it is approximately 2 orders of magnitude higher than the highest dose of 6 mg proposed for a future clinical study. The low dose of 0.6 mg in this dog study was approximately one log unit higher, when matched for cartilage volume, than the likely clinical dose of 2 mg. It is also the lowest dose for which an accurate weight and dose administration can be obtained for the test item.
Terminal Procedures
One male and 1 female animal from each group were euthanized on days 7, 29, 46, and 60. Euthanasia was performed by intravenous overdose of sodium pentobarbital, followed by exsanguinations and macroscopic evaluation. The animals were previously fasted overnight and sedated with propofol.
Injection Efficiency
To determine the approximate amount of drug that was administered to the dogs, the residual fluticasone in the vial, syringe, and needle was measured. Samples were received in individual, labeled bags containing all syringes/needles/vials associated with each animal (i.e., one bag per animal). Each individual item was removed from sample bag (sample tube, cap, syringe, syringe plunger, needle, and needle guard) and, using a Pasteur pipette, thoroughly rinsed with 70% EtOH into a 50 mL Falcon tube. All rinsed items were placed into 50 mL Falcon tube to dissolve any remaining fluticasone. The 50 mL Falcon tube was sealed with Parafilm and stored for 8 days at ambient temperature with shaking at 90 rpm. Each tube was vortexed for 3 seconds every 2 days until the 8-day soak was completed. One hundred microliter aliquots were transferred from each Falcon tube into 1 mL microcentrifuge tube. Nine hundred microliter mobile phase (0.1% NH4OH in 50:50 CH3CN:Milli-Q H2O) was added to each sample and mixed by vortex agitation. Standards were prepared and analyzed by liquid chromatography–mass spectrometry (LC-MS) (Agilent Series 1100 LC system in tandem with AB Sciex API 3000 MS) with estradiol as the internal standard according to the following method: Agilent Zorbax XDB-C8 column (2.1 × 50 mm, 3.5 µm); isocratic run using 0.1% NH4OH in 50:50 CH3CN:Milli-Q H2O as the mobile phase; run time 3.5 minutes.
Plasma Pharmacokinetics
Blood samples (1.5-2 mL each) were collected from all animals at predose; on days 3, 5, and 7; and twice weekly thereafter until necropsy (including the day of necropsy). Each blood sample was collected by venipuncture (jugular vein) into tubes containing K2 EDTA as anticoagulant and kept on wet ice pending centrifugation. Samples were centrifuged under refrigeration (set to +4°C at 1500 × g RCF [relative centrifugal force]) for targeted 10 minutes. Plasma was transferred into 2 separate tubes, placed on dry ice pending storage in a freezer set to 70°C until pending analysis. A qualified bionanalytical LC-MS method was used for analysis of fluticasone propionate in plasma. The lower and upper limits of quantitation were 0.2 and 100.0 ng/mL, respectively.
Synovial Fluid Pharmacokinetics
Prior to necropsy, a synovial fluid collection was performed under intravenous propofol sedation. A synovial fluid sample (0.01-0.3 mL) was collected by puncture. Synovial fluid (target 0.1 mL measured precisely using a pipette) was diluted into 0.9 mL of type 1 water, mixed by vortexing and transferred into 2 separate tubes. When the volume of synovial fluid was <0.1 mL, the amount of type 1 water was adjusted to result in a 1/10 dilution (i.e., 1 part synovial fluid, 9 parts type 1 water ratio). Tubes were then placed on dry ice pending storage in a freezer set to 70°C, pending analysis. A qualified bionanalytical LC-MS method was used for analysis of fluticasone propionate in synovial fluid. The lower and upper limits of quantitation were 1 and 1000 ng/mL, respectively.
Tissue Bioanalysis
Femorotibial cartilage was delicately collected from the bones avoiding as much as possible to also collect bone tissue and surrounding flesh, muscle, and fatty material.
The cartilage was harvested from the treated knee joint sections of all animals and from the untreated knee joint sections of 4 randomly selected group 2 (high dose) animals (1 animal/sacrifice time point). The cartilage was crushed with a tissue grinder (Polytron), weighed, and brought to a 100 mg/ml concentration in water, in polypropylene collection tubes. This homogenate was kept frozen at a nominal temperature of −20°C, until analysis. A qualified bionanalytical LC-MS method was used for analysis of fluticasone propionate in femorotibial cartilage. The lower limit of quantitation (LLoQ) was 100 pg/mL.
Histopathology
Histopathological examination was performed by a certified veterinary pathologist on the untreated and treated knee sections from all animals. The untreated and treated knee joint sections intended for histological examination (preserved in neutral buffered 10% formalin) were prepared by embedding in paraffin wax and sectioning, and stained with hematoxylin and eosin–phloxin. The knee joints were then more specifically evaluated for changes of chondrocytes morphology.
Statistical Methods
To aid in the understanding of the pharmacokinetics, data that were below the lower limit of quantitation were used to supplement those that were within the quantitation ranges. Data were used as far as available; where data were not detectable, an imputation was made of half the lowest detectable measured concentration for that system (plasma/synovial fluid/cartilage). Only descriptive statistics are reported; no inferential testing was performed. Half-life calculations in the synovial fluid and cartilage were calculated by fitting the entirety of the log-concentration data to a linear model containing a single factor for group, and a continuous day term. Half-life calculations for the plasma data were calculated using only data from those animals that provided a useable amount of data, and were calculated using a linear mixed effects model allowing a per-animal intercept and gradient relating log-concentration to day, to share information across animals. All statistics and graphics were produced using the R software, in particular packages ggplot2, xtable, and lme4.32-35
Results
No significant adverse events were noted throughout the duration of the study. A number of dogs were noted to have a small patch of redness at the injection site but were not deemed to be significant issues.
Injection Efficiency
Based on results, the actual percentage of fluticasone propionate injected was 0.0% to 68.0% in animals from the 0.6 mg target dose group (group 1) and 29.0% to 60.0% in animals from the 12 mg target dose group (group 2). However, errors in weighing of test article into vials (estimated 10.0% error), loss of test article during the injection and extraction from dosing materials (estimated as much as 20.0% error), and analytical errors (estimated 5% error) in the measurements may have contributed to the residual drug analysis results, as animals reported as 0.0% injected did have nonzero levels of fluticasone propionate. For the second 6.0 mg dose on day 2, changing the body position of each dog and hence injection direction from vertical to horizontal seemed to improve injectability of the particles; in high-dose animals the amount injected on day 2 was generally higher than on day 1.
Results and interpretation in this study are expressed based on the target doses of 0.6 and 12.0 mg, and it should be taken into consideration that based on results from the residual drug analyses, the actual amount of fluticasone propionate injected into each knee was below target doses and highly variable between animals.
Assessment of Plasma Concentrations of Fluticasone Propionate
There were no concentrations above the LLoQ of 0.2 ng/mL of fluticasone propionate in the plasma of the low-dose animals at any of the sampling occasions. Quantifiable but very low concentrations of fluticasone propionate occurred in the plasma of 8 high-dose animals on day 3; in 4 of the animals on day 5; and in 2 of the animals on day 7. The highest concentrations occurred on day 3, with concentrations ranging from 0.2 to 0.5 ng/mL. Concentrations decreased by day 5 and were quantifiable in only 1 of the males and 3 of the females. On day 7, only 1 male and 1 female had quantifiable concentrations of 0.2 and 0.3 ng/mL ( Fig. 1 ). These results suggest that low concentrations of fluticasone propionate may reach blood circulation within a single day of IA injection.
Figure 1.
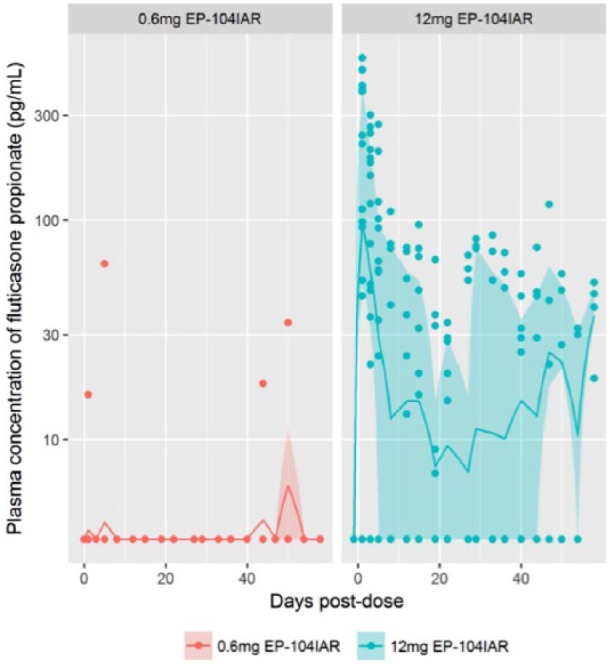
Plasma concentrations of fluticasone propionate. Individual plasma concentrations of fluticasone propionate are presented. In addition, the per-collection day interquartile range and geometric mean are presented. For plasma concentrations that are below limit of detection (BLD), half the lowest reported concentration, or 3.5 pg/mL, was used as an imputation. The below limit of quantitation (BLQ) was 200.0 pg/mL.
By including the below limit of quantitation (BLQ) data, crude estimates of the terminal half-life of fluticasone propionate in plasma are calculable, as described in the statistical methods section above. Seven animals, all from the high-dose group, contributed to this analysis. From these data, the average half-life of fluticasone propionate in plasma was approximately 45 days.
Assessment of Synovial Fluid Concentrations of Fluticasone Propionate
Measurable concentrations of fluticasone propionate occurred in the synovial fluid of at least some of the low-dose animals up to day 46. The highest concentrations were generally observed on day 7, the first assessment time, with concentrations ranging from 3.7 to 24.7 ng/mL. Concentrations then fell through day 46 where only 2 animals had quantifiable concentrations of 1.9 and 14.3 ng/mL. There were no quantifiable concentrations on day 60.
Quantifiable concentrations of fluticasone propionate occurred in the synovial fluid of all but one of the high-dose animals through day 60. The highest concentrations occurred on day 7, the first assessment time, with concentrations ranging from 179.2 to 855.2 ng/mL. Concentrations then fell through day 46, where quantifiable concentrations ranged from 1.8 to 50.6 ng/mL; however, concentrations were higher on day 60, where concentrations of 97.1 and 209.7 ng/mL occurred in the 2 females ( Table 2 ).
Table 2.
Summary of Synovial Fluid Concentrations of Fluticasone Propionate.a
| Synovial Fluid Concentration of Fluticasone Proprionate (ng/mL) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Gmean | SD | CV (%) | Min | Q1 | Median | Q3 | Max | |
| 0.6 mg EP-104IAR | ||||||||||
| Day 7 | 4 | 13.3 | 10.8 | 8.8 | 80 | 3.7 | 8.7 | 12.5 | 17.2 | 24.7 |
| Day 29 | 3 | 9.6 | 1.9 | 15.2 | 256 | 0.2 | 0.8 | 1.5 | 14.3 | 27.1 |
| Day 46 | 4 | 4.2 | 1.2 | 6.8 | 191 | 0.2 | 0.4 | 1.2 | 5.0 | 14.3 |
| Day 60 | 4 | 0.2 | 0.2 | 0.1 | 35 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 |
| 12 mg EP-104IAR | ||||||||||
| Day 7 | 4 | 450.4 | 368.5 | 316.2 | 74 | 179.2 | 209.7 | 383.6 | 624.4 | 855.2 |
| Day 29 | 4 | 59.6 | 20.9 | 69.1 | 226 | 0.8 | 22.1 | 39.3 | 76.8 | 158.9 |
| Day 46 | 3 | 18.2 | 5.9 | 28.1 | 187 | 1.8 | 2.0 | 2.2 | 26.4 | 50.6 |
| Day 60 | 2 | 153.4 | 142.7 | 79.6 | 54 | 97.1 | 125.3 | 153.4 | 181.5 | 209.7 |
CV = coefficient of variation; Q1 = first quartile; Q3 = third quartile.
The lower limit of quantitation of the assay was 1 ng/mL; all values below this level have been reported at their assayed value.
By including the BLQ data, crude estimates of the terminal half-life of fluticasone propionate in synovial fluid are calculable, as described in the statistical methods section above. This analysis showed an estimated half-life of approximately 11 days ( Fig. 2 ).
Figure 2.
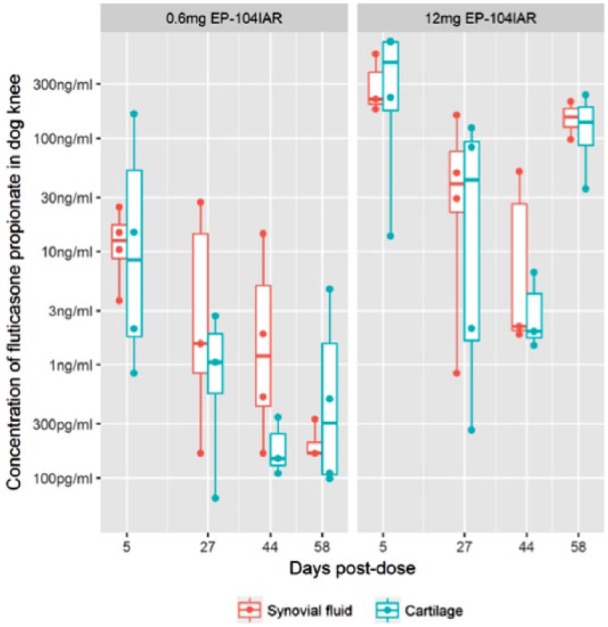
Comparison of synovial fluid and cartilage concentrations of fluticasone propionate. Both raw observations (as points) and a summary boxplot are shown; the boxplot shows the range, quartiles and median of the samples. The lower limit of quantitation (LLoQ) of the synovial fluid assay was 1 ng/mL. The LLoQ of the cartilage assay was 100 pg/mL. All values below the LLoQ have been reported at their assayed value.
Assessment of Cartilage Concentrations of Fluticasone Propionate
Quantifiable concentrations of fluticasone propionate occurred in the dog knee cartilage samples of at least some of the low-dose animals through day 60. The highest concentrations generally occurred on day 7, the first assessment time, with concentrations ranging from 0.8 to 164.0 ng/mL. Concentrations then generally fell through day 60.
Quantifiable concentrations of fluticasone propionate occurred in the cartilage samples of high-dose animals through day 60. The highest concentrations occurred on day 7, with concentrations ranging from 13.7 to 713.0 ng/mL. Concentrations then generally fell through day 46, where measurable concentrations ranged from 0.8 to 6.5 ng/mL. However, concentrations were higher on day 60 and ranged from 26.5 to 239.0 ng/mL. These results indicate that fluticasone propionate was detectable in cartilage tissue ( Table 3 ), and at levels that were approximately 20.0% of those measured in the synovial fluid.
Table 3.
Summary of Cartilage Concentrations of Fluticasone Propionate.a
| Cartilage Concentration of Fluticasone Proprionate (ng/mL) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Gmean | SD | CV (%) | Min | Q1 | Median | Q3 | Max | |
| 0.6 mg EP-104IAR | ||||||||||
| Day 7 | 4 | 45.4 | 8.0 | 79.3 | 234 | 0.8 | 1.8 | 8.4 | 52.0 | 164.0 |
| Day 29 | 4 | 1.0 | 0.4 | 1.2 | 167 | 0.1 | 0.2 | 0.6 | 1.5 | 2.7 |
| Day 46 | 4 | 0.2 | 0.1 | 0.1 | 97 | 0.0 | 0.1 | 0.1 | 0.2 | 0.3 |
| Day 60 | 4 | 1.3 | 0.4 | 2.2 | 180 | 0.1 | 0.1 | 0.3 | 1.5 | 4.6 |
| 12 mg EP-104IAR | ||||||||||
| Day 7 | 4 | 413.9 | 198.9 | 349.1 | 186 | 13.7 | 175.2 | 464.5 | 703.2 | 713.0 |
| Day 29 | 4 | 52.2 | 8.7 | 61.1 | 296 | 0.3 | 1.6 | 42.7 | 93.2 | 123.0 |
| Day 46 | 4 | 2.7 | 2.0 | 2.6 | 89 | 0.8 | 1.3 | 1.7 | 3.1 | 6.5 |
| Day 60 | 4 | 84.7 | 53.9 | 103.0 | 100 | 26.5 | 33.3 | 36.5 | 87.9 | 239.0 |
CV = coefficient of variation; Q1 = first quartile; Q3 = third quartile.
The lower limit of quantitation of the assay was 100 pg/mL; all values below this level have been reported at their assayed value.
By including the BLQ data, crude estimates of the terminal half-life of fluticasone propionate in cartilage are calculable, as described in the Statistical Methods section above. This analysis showed an estimated half-life of approximately 14 days ( Fig. 2 ).
Assessment of Joint Structure
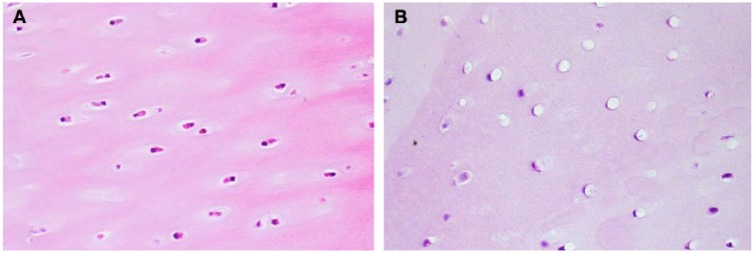
Minimal vacuolation of the articular cartilage chondrocytes was noted on day 7 in 3 of 4 dogs from group 1. Vacuolation observed in the femur, tibia, and/or patella articular cartilage was characterized by the presence of a single large clear vacuole compressing and flattening the chondrocyte cytoplasm and nucleus against the surrounding lacunae wall ( Fig. 3 ). The chondrocyte vacuolation was not observed in dogs on days 29, 46, or 60, indicating complete reversal of this finding that was noted on day 7.
Figure 3.
Example of chondrocyte vacuolation in cartilage (40× objective). All pictures (A untreated and B treated knees) were taken from animal euthanized on day 7. For the treated knee image, all were taken from animals dosed with the highest dose (12 mg).
Mild to moderate vacuolation of the articular cartilage chondrocytes was noted on day 7 in 4 of 4 dogs from group 2. Chondrocyte vacuolation was associated with concurrent mild vacuolation (4 of 4 dogs) and minimal foreign material (4 of 4 dogs) in the synovial intima. The synovial intima vacuolation was characterized by the presence of numerous small clear vacuoles distending the cytoplasm of intima cells with or without concurrent presence of yellow amorphous or fibrillar foreign material. Minimal chondrocyte vacuolation was noted in 3 of 4 dogs on day 29 with concurrent presence of minimal foreign material (3 of 4 dogs). This foreign material may have been residual PVA coating material from the EP-104IAR particles. Synovial intima vacuolation noted on day 7 was not present on day 29. Chondrocyte vacuolation was not observed in dogs on days 46 and 60, indicating complete reversal. The vacuolation did not lead to degeneration or necrosis of the chondrocytes and did not lead to any structural changes in the cartilage matrix. Foreign material was noted in the majority of dogs on days 46 and 60; however, there was no observed immune response to the foreign material.
Discussion
The most important finding from this study was that an IA administration of nominal doses of 0.6 and 12 mg EP-104IAR to male and female Beagle dogs was well tolerated with no EP-104IAR related clinical signs other than redness at injection site after the first 6.0 mg injection to the high-dose animals, although it should be noted that the actual dose delivered appears to be below these nominal doses; ongoing efforts are in place to improve this delivery. Microscopic changes to the femorotibial knee joint were limited to dose-related mild to moderate vacuolation of the articular cartilage chondrocytes observed in the femur, tibia, and/or patella articular cartilage in the 0.6 and 12.0 mg groups and to mild vacuolation and minimal foreign material in the synovial intima in the 12.0 mg group. All findings resolved by the end of the study; however, foreign material in the synovial intima was still visible on days 46 and 60 in the majority of dogs given 12 mg. There were no quantifiable concentrations of free fluticasone propionate in plasma of animals administered 0.6 mg at any of the sampling time points. Highest concentrations in plasma following the 12.0 mg administration occurred on day 3, 1 day after completion of dosing and the first sample time; and disappeared from the plasma with a half-life of approximately 45 days. Highest concentrations of fluticasone propionate in synovial fluid generally occurred in both dose groups on day 7, 5 days after conclusion of dosing and the time of first sample; and disappeared from the synovial fluid with a half-life of approximately 11 days.
Synovitis plays an important role in the pathology and joint degeneration of OA. Currently, OA is considered as a disease of the “whole joint.” Proinflammatory mediators can be produced by both synoviocytes and chondrocytes, with dysregulation of complement also having been shown.36,37 IA corticosteroids can inhibit synovial inflammation and thus also suppress cytokine production and metalloproteinase synthesis. As such they have theoretically the capacity to diminish structural damage and limit OA progression in humans.38,39 This maintained suppression of synovitis could not only lead to prolonged pain relief and functional improvement but also may limit structural progression in OA.24
Recently, there has been an increasing interest in developing IA drug-encapsulating micro- or nanoparticles with the intention to obtain a prolonged release of a low-dose corticosteroid into the joint.24 To our knowledge, Zilretta (FX006) is the only long-acting corticosteroid that has already been tested in humans to date,40 providing an extended and predictable release of triamcinolone acetonide (TCA).
In the present study, a new product was introduced. EP-104IAR is composed of fluticasone propionate drug crystals coated with heat-treated PVA to result in extended release properties intended for prolonged treatment of inflammation associated with osteoarthritis. It must be stated that the actual percentage of fluticasone propionate injected was 0% to 68% in animals from the 0.6 mg target dose group (group 1) and 29% to 60% in animals from the 12 mg target dose group (group 2). In this way, the actual amount of fluticasone propionate injected into each knee was below target doses and highly variable between animals. This can account for the high variability in synovial concentrations of EP-104IAR observed in this pilot study across the dogs in the same group that were given the same dose of EP-104IAR. Currently, efforts are being made to improve the homogeneity of EP-104IAR.
Another drawback of this pilot study is the fact that it did not use an accepted score for cartilage histopathology. However, this safety aspect of EP-104IAR will be studied more in detail in a future project.
This study shows that EP-104IAR can provide a safe and prolonged local exposure to a corticosteroid in the synovial joint while minimizing systemic exposure. As such, it forms the basis for future nonclinical and clinical work with this product.
Footnotes
Acknowledgments and Funding: The authors wish to acknowledge the staff at CiToxLAB North America Inc. for helping to run the study. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Eupraxia Pharmaceuticals.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Amanda Malone, James Price, and James Helliwell are employees of Eupraxia Pharmaceuticals. Amanda Malone and James Helliwell have stock options in Eupraxia Pharmaceuticals. The funding source was involved in the study design, interpretation of the data, and writing the manuscript.
Ethical Approval: This study was approved by the Institutional Animal Care and Use Committee (IACUC) of CitoxLab North America.
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
References
- 1. Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343-55. doi: [DOI] [PubMed] [Google Scholar]
- 3. Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207-13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163-96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263-7. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625-35. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 7. Wenham CY, Conaghan PG. The role of synovitis in osteoarthritis. Ther Adv Musculoskelet Dis. 2010;2:349-59. doi: 10.1177/1759720X10378373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morgen M, Tung D, Boras B, Miller W, Malfait AM, Tortorella M. Nanoparticles for improved local retention after intra-articular injection into the knee joint. Pharm Res. 2013;30:257-68. doi: 10.1007/s11095-012-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dieppe PA, Sathapatayavongs B, Jones HE, Bacon PA, Ring EF. Intra-articular steroids in osteoarthritis. Rheumatol Rehabil. 1980;9:212-7. [DOI] [PubMed] [Google Scholar]
- 10. Creamer P. Intra-articular corticosteroid injections in osteoarthritis: do they work and if so, how? Ann Rheum Dis. 1997;56:634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Creamer P. Intra-articular corticosteroid treatment in osteoarthritis. Curr Opin Rheumatol. 1999;11:417-21. [DOI] [PubMed] [Google Scholar]
- 12. Hochberg MC, Perlmutter DL, Hudson JI, Altman RD. Preferences in the management of osteoarthritis of the hip and knee: results of a survey of community-based rheumatologists in the United States. Arthritis Care Res. 1996;9:170-6. [DOI] [PubMed] [Google Scholar]
- 13. Insurance Management System, PharMetrics Plus database, 2010-2012. [Google Scholar]
- 14. Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, et al. American College of Rheumatology. Guidelines for the medical management of osteoarthritis. Part I. Osteoarthritis of the hip. Arthritis Rheum. 1995;38:1535-40. [DOI] [PubMed] [Google Scholar]
- 15. Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, et al. American College of Rheumatology. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. Arthritis Rheum. 1995;38:1541-6. [DOI] [PubMed] [Google Scholar]
- 16. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2005;2:CD005321. doi: 10.1002/14651858.CD005321. [DOI] [PubMed] [Google Scholar]
- 17. Jevsevar DS, Brown GA, Jones DL, Matzkin EG, Manner PA, Mooar P, et al. American Academy of Orthopaedic Surgeons. The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am. 2013;95:1885-6. [DOI] [PubMed] [Google Scholar]
- 18. Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. Standing Committee for International Clinical Studies Including Therapeutic Trials ESCISIT. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bjordal JM, Johnson MI, Lopes-Martins RA, Bogen B, Chow R, Ljunggren AE. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord. 2007;8:51. doi: 10.1186/1471-2474-8-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Godwin M, Dawes M. Intra-articular steroid injections for painful knees. Systematic review with meta-analysis. Can Fam Physician. 2004;50:241-8. [PMC free article] [PubMed] [Google Scholar]
- 21. Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;18:351-61. doi: 10.5312/wjo.v5.i3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Derendorf H, Möllmann H, Grüner A, Haack D, Gyselby G. Pharmacokinetics and pharmacodynamics of glucocorticoid suspensions after intra-articular administration. Clin Pharmacol Ther. 1986;39:313-7. [DOI] [PubMed] [Google Scholar]
- 23. Morgen M, Tung D, Boras B, Miller W, Malfait AM, Tortorella M. Nanoparticles for improved local retention after intra-articular injection into the knee joint. Pharm Res. 2013;30:257-68. doi: 10.1007/s11095-012-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar A, Bendele AM, Blanks RC, Bodick N. Sustained efficacy of a single intra-articular dose of FX006 in a rat model of repeated localized knee arthritis. Osteoarthritis Cartilage. 2015;23:151-60. doi: 10.1016/j.joca.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 25. Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, et al. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: sustained release of a local antiinflammatory therapeutic. Arthritis Rheum. 2007;56:3650-61. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- 26. Latorre M, Paggiaro P, Canonica W, Foschino MP, Papi A. A valid option for asthma control: clinical evidence on efficacy and safety of fluticasone propionate/formoterol combination in a single inhaler. Pulm Pharmacol Ther. 2015;34:31-6. doi: 10.1016/j.pupt.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 27. Bender BG, Hernandez Vecino RA, McGrath K, Jones S. Comparative analysis of persistence to treatment among patients with asthma or COPD receiving AirFluSal Forspiro or Seretide Diskus salmeterol/fluticasone propionate combination therapy. J Allergy Clin Immunol Pract. 2016;4:884-9. doi: 10.1016/j.jaip.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 28. Prenner BM. A review of the clinical efficacy and safety of MP-AzeFlu, a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate, in clinical studies conducted during different allergy seasons in the US. J Asthma Allergy. 2016;9:135-43. doi: 10.2147/JAA.S98172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nordin S, Olsson P, Hedén Blomqvist E, Stjärne P, Ehnhage A. NAF2S2 Study Group. Effects of FESS and additional fluticasone propionate nasal drops on psychological well-being in nasal polyposis with asthma. Acta Otolaryngol. 2013;133:939-43. doi: 10.3109/00016489.2013.783715. [DOI] [PubMed] [Google Scholar]
- 30. Eckstein F, Winzheimer M, Hohe J, Englmeier K-H, Reiser M. Interindividual variability and correlation among morphological parameters of knee joint cartilage plates: analysis with three-dimensional MR imaging. Osteoarthritis Cartilage. 2001;9:101-11. doi: 10.1053/joca.2000.0365. [DOI] [PubMed] [Google Scholar]
- 31. Boileau C, Martel-Pelletier J, Abram F, Raynauld J-P, Troncy E, D’Anjou M-A, et al. Magnetic resonance imaging can accurately assess the long-term progression of knee structural changes in experimental dog osteoarthritis. Ann Rheum Dis. 2008;67:926-32. doi: 10.1136/ard.2007.077297. [DOI] [PubMed] [Google Scholar]
- 32. Wickham H. Ggplot2: elegant graphics for data analysis. New York, NY: Springer-Verlag; 2009. [Google Scholar]
- 33. Dahl DB. Xtable: export tables to LaTeX or HTML. R package version. 1:8-2. 2016. https://CRAN.R-project.org/package=xtable. [Google Scholar]
- 34. Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1-48. [Google Scholar]
- 35. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. https://www.R-project.org/.
- 36. Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11:227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674-9. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DiBattista JA, Martel-Pelletier J, Wosu LO, Sandor T, Antakly T, Pelletier JP. Glucocorticoid receptor mediated inhibition of interleukin-1 stimulated neutral metalloprotease synthesis in normal human chondrocytes. J Clin Endocrinol Metab. 1991;72:316-26. doi: 10.1210/jcem-72-2-316. [DOI] [PubMed] [Google Scholar]
- 39. Pelletier JP, Cloutier JM, Martel-Pelletier J. In vitro effects of NSAIDs and corticosteroids on the synthesis and secretion of interleukin 1 by human osteoarthritic synovial membranes. Agents Actions Suppl. 1993;39:181-93. [DOI] [PubMed] [Google Scholar]
- 40. Bodick N, Lufkin J, Willwerth C, Kumar A, Bolognese J, Schoonmaker C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877-88. doi: 10.2106/JBJS.N.00918. [DOI] [PubMed] [Google Scholar]