Abstract
Objective
To characterize the graft survivorship and clinical outcomes of osteochondral allograft transplantation (OCA) of the knee in patients with an elevated body mass index (BMI).
Design
Prospective data on 38 consecutive patients with a BMI ≥30 kg/m2 treated with OCA from 2000 to 2015 were reviewed. Complications, reoperations, and patient responses to validated outcome measures were examined. Failures were defined by any removal/revision of the allograft or conversion to arthroplasty.
Results
Thirty-one knees in 31 patients (mean age, 35.4 years [range, 17-61 years]; 87% male) met the inclusion criteria. Mean BMI was 32.9 kg/m2 (range, 30-39 kg/m2). Mean chondral defect size was 6.4 cm2 (range, 1.0-15.3 cm2). Prior to OCA, 23 patients (74%) had undergone previous surgery to the ipsilateral knee. Mean duration of follow-up was 4.1 years (range, 2-11 years). After OCA, 5 knees (13%) underwent conversion to unicompartmental (1) or total (4) knee arthroplasty. Two- and 5-year graft survivorship were 87% and 83%, respectively. At final follow-up, clinically significant improvements were noted in the pain (49.3-72.6) and physical functioning (52.9-81.3) subscales of the Short Form–36 (P ≤ 0.001), International Knee Documentation Committee subjective form (43.5-67.0; P = 0.002), Knee Outcome Survey–Activities of Daily Living (58.2-80.4; P = 0.002), and overall condition subscale of the Cincinnati Knee Rating System (4.7-6.9; P = 0.046).
Conclusions
OCA can be a successful midterm treatment option for focal cartilage defects of the knee in select patients with a BMI ≥30 kg/m2.
Keywords: osteochondral allograft, BMI, body mass index, cartilage, obesity
Introduction
Obesity has generally been considered a contraindication to cartilage repair procedures in the knee. In addition to being a risk factor for more rapid osteoarthritis progression and cartilage turnover in the knee,1 obesity leads to greater joint reactive forces that increase rim cartilage deformation, opposing tibia cartilage incursion, and force transmission at the base of chondral defects.2-4 Lacy et al.2 investigated the effect of loads simulating a body mass index (BMI) of ≥30 kg/m2 on isolated full-thickness medial femoral condyle defects and reported a decreased area of containment and increased force at the base of defects for those 16 mm or larger in diameter.2 As a result, any reparative cartilage in the knees of obese patients may be highly prone to breakdown and failure. Previous studies have demonstrated poor functional outcomes, lower activities of daily living scores, and reduced cartilage in-fill after microfracture in patients with a BMI >30 kg/m2.5,6 Although a correlation between elevated BMI and poor clinical outcomes has not been specifically studied for other forms of cartilage surgery, a BMI threshold of 30 kg/m2 has been used a criterion for some insurance companies to deny coverage for other cartilage restoration procedures.7
However, in contrast to microfracture, which requires stable clot formation and biologic maturation of the reparative cartilage tissue, fresh osteochondral allograft transplantation (OCA) transfers viable, structurally stable, and mature hyaline cartilage into large chondral defects at the time of implantation. Therefore, compared to other types of reparative cartilage, OCA has much greater initial stability and may be more resistant to the increased joint reactive forces seen in heavier patients. Biomechanical studies have demonstrated that osteochondral grafts inserted flush to the host chondral surfaces restore rim stresses to near-intact conditions at the time of implantation.8,9 Long-term studies of OCA have demonstrated 5- and 10-year survival rates of 95% and 85%, respectively, for femoral condyle lesions with high patient satisfaction.10-12 Nevertheless, it is unknown whether graft survivorship and clinical improvement remain high after OCA in obese patients.
The purpose of this study was to characterize the clinical results of OCA in patients with an elevated BMI (≥30 kg/m2). We hypothesized that OCA in patients with a BMI ≥30 kg/m2 would demonstrate a low rate of complications, high survivorship, and clinically significant improvement in patient-reported outcomes at midterm follow-up.
Methods
In 1999, our institution implemented a prospective registry dedicated to the tracking of patient outcomes after articular cartilage restoration procedures. An institutional review board approved the registry, and all patients sign an informed consent form before participation. Patients included in the registry were evaluated preoperatively and were prospectively followed at 6 months and 1, 2, 3, 4, 5, and 10 years postoperatively.
Inclusion and Exclusion Criteria
Inclusion criteria included (1) BMI of ≥30 kg/m2, (2) symptomatic focal cartilage lesions in the knee that were classified as Outerbridge grade III or IV lesions at the time of arthroscopic surgery and did not involve substantial bone loss requiring additional bone grafting, (3) treatment with fresh osteochondral allograft, and (4) a minimum of 2 years of follow-up. Exclusion criteria for this cartilage procedure were advanced osteoarthritis (OA) exceeding a Kellgren-Lawrence grade of 2, simultaneous multiligamentous reconstruction, inflammatory arthritis or autoimmune conditions, and inability to comply with the postoperative rehabilitation protocol.
Patients
Demographic, preoperative, intraoperative, and postoperative data were collected for all patients. Demographic data included age, sex, and BMI. Preoperative data included the number and type of previous ipsilateral knee surgical procedures and baseline patient-reported outcome scores. Lower leg alignment was assessed and recorded during the preoperative office visit. For the majority of patients, long-leg radiographs were only obtained if gross malalignment was detected and an osteotomy was being considered. Intraoperative data included laterality, exam under anesthesia (range of motion, ligamentous stability), location, size, and depth of the chondral defect(s), concomitant procedures performed, and postoperative rehabilitation protocol. Postoperative data included complications, reoperations, and patient-reported outcome scores at a minimum of 2 years after surgery.
Surgical Technique
All surgical procedures were performed by fellowship-trained orthopedic surgeons at a single institution with extensive experience in OCA transplantation. After induction of neuraxial anesthesia and examination of the knee under anesthesia, patients were treated with an initial diagnostic arthroscopy of the joint for assessment of the chondral lesion as well as the other articular surfaces, menisci, and ligaments. Any meniscus tears were addressed with partial meniscectomy or repair.
Fresh cold-stored osteochondral allografts were obtained from commercially available sources. Donor tissue was screened and processed according to American Association of Tissue Banks standards.13 Preoperatively, donor and recipient were matched on the basis of size using standard anteroposterior radiographs. Grafts were transplanted between 16 and 28 days after harvest depending on serologic testing and patient availability. After the arthroscopic portion of the procedure, OCA was performed via the dowel technique described by Williams et al.14 Briefly, chondral lesions were exposed via a small parapatellar arthrotomy and debrided to a stable rim. Lesions were then sized and reamed to a bed of normal bone, and an appropriate graft was taken from the corresponding region of the osteochondral allograft. Lesion depth was carefully measured at 3 to 4 points around the lesion, marked, and matched on the donor tissue. Grafts were then gently impacted into place for press-fit fixation. Grafts were a single or dual circular dowel shape, depending on lesion shape.
Postoperatively, patients remained touchdown or nonweightbearing for a minimum of 1 to 2 weeks. Those treated with concomitant meniscus allograft transplantation or realignment osteotomy were kept touchdown weightbearing for a minimum of 6 weeks. Full range of motion was permitted immediately and encouraged with the use of a continuous passive motion device. Brace wear was discontinued at 2 to 6 weeks. A supervised physical therapy program was undertaken postoperatively in all cases. The duration of the postoperative physical therapy program depended on the restoration of normal gait, return of quadriceps function, and performance of sport-specific skills. Return to athletics was initiated on an individual patient basis, typically starting with a running program at 6 months. Higher level activities were then progressed thereafter depending on return of lower extremity strength.
Assessment of Clinical Outcomes
All complications and reoperations after the index OCA were documented. A reoperation was defined as any subsequent surgery on the ipsilateral knee, including arthroscopic chondroplasty, removal of loose bodies, lysis of adhesions, and hardware removal. Failure of the allograft was defined as any procedure that involved removal or revision of the allograft, unicompartmental knee arthroplasty (UKA), or total knee arthroplasty (TKA).
The general health outcome for each patient was assessed with use of the Short Form–36 (SF-36) (version 1.0),15 which has the ability to evaluate 8 domains of general well-being. Only the general health, pain, physical functioning, and role limitations due to physical health domains were reported in this study. Knee function was assessed with use of the International Knee Documentation Committee subjective form (IKDC) and the Knee Outcome Survey–Activities of Daily Living (KOS-ADL). The IKDC score is a reliable and valid knee-specific measure of symptoms and function and has been shown to provide a good overall measure of knee-related disability in patients who have undergone a cartilage restoration procedure.16,17 Similarly, the KOS-ADL has been shown to have high reliability, validity, and responsiveness in athletic patients with various knee conditions.18 Patient activity level was assessed with use of the Marx activity rating scale.19 Finally, the overall condition of the knee was assessed using the patient perception component of the Cincinnati Knee Rating System.20 This is a single item that asks “Rate the overall condition of your knee at the present time” on a numeric 1 to 10 rating scale, with 2 indicating “poor—I have significant limitations that affect activities of daily living,” 4 indicating “fair—I have moderate limitations that affect activities of daily living, no sports possible,” 6 indicating “good—I have some limitations with sports but I can participate; I compensate,” and 10 indicating “normal/excellent—I am able to do whatever I wish (any sport) with no problems.”20 An independent observer performed postoperative data collection for all clinical outcome instruments. All these knee-specific outcome instruments have been used previously to prospectively evaluate articular cartilage repair procedures in the knee.18,21-25
Statistical Analysis
Kaplan-Meier survivorship analysis was performed for graft failures, with comparisons between groups conducted using the log-rank test. Comparisons between factors were performed using the Mann-Whitney tests for binary characteristics and chi-square or Fisher exact tests for discrete variables. Changes in subjective patient outcome scores (SF-36, IKDC, KOS-ADL, Marx Activity Rating Scale, and overall condition) between preoperative and postoperative time points were assessed using the Wilcoxon signed rank test. Two-tailed tests were used for all statistical analyses with a critical P value set to 0.05 to indicate significance.
Results
A total of 1902 registry patients were screened, and 38 consecutive patients treated between 2000 and 2014 met the inclusion and exclusion criteria. Seven patients were lost to follow-up. As a result, a total of 31 knees in 31 patients were analyzed (82% follow-up). The mean age was 35.4 years (range, 17-61 years), and the mean BMI was 32.9 kg/m2 (range, 30-39 kg/m2). Eight patients had a BMI of ≥35 kg/m2 at the time of surgery. Mean duration of follow-up was 4.1 years (range, 2-11 years). Patient demographics, chondral lesion characteristics, and concomitant procedures are shown in Table 1 .
Table 1.
Patient Demographics and Concomitant Surgeries.a
| All Knees (n = 31) | Failures (n = 5) | |
|---|---|---|
| Patient characteristics | ||
| Age, y | 35.4 (17-61) | 45.6 (37-61) |
| Body mass index, kg/m2 | 32.9 (30-39) | 34.5 (31-39) |
| Sex (male/female), n | 27/4 | 5/0 |
| Laterality (right/left), n | 14/17 | 1/4 |
| Preoperative Marx activity rating scale | 4.0 (0-14) | 3.7 (0-11) |
| No. of prior ipsilateral knee surgeries | 2.2 (0-10) | 4.8 (1-10) |
| Follow-up, y | 4.1 (2.0-10.9) | 2.9 (2.0-4.4) |
| Lesion characteristics | ||
| OCA location, nb | ||
| Medial femoral condyle | 13 | 3 |
| Lateral femoral condyle | 15 | 2 |
| Trochlea | 10 | 1 |
| Total chondral defect area, cm2 | 6.4 (1.0-15.3) | 6.1 (1.0-10.0) |
| No. of plugs used | 1.7 (1-4) | 2.0 (1-3) |
| Concomitant procedures, n | ||
| ACL reconstruction | 3 | 1 |
| Meniscus allograft transplantation | 3 | 0 |
| Realignment osteotomy | 3 | 1 |
ACL = anterior cruciate ligament; OCA = osteochondral allograft transplantation.
Data are reported as mean with the range in parentheses unless otherwise indicated.
Seven knees had multiple locations with chondral defects treated with OCA.
Seven knees (23%) had previously undergone a cartilage restoration procedure, including microfracture (n = 5), autologous chondrocyte implantation (ACI; n = 1) and OCA at another institution (n = 1). Five patients (16%) had previously underwent anterior cruciate ligament reconstruction (ACLR). Documented Lachman and pivot shift grades at the time of exam under anesthesia were 1A and 0, respectively, for 3 of these patients who did not have any symptoms or signs of ACL graft failure. The other 2 patients had positive signs of ACL graft failure preoperatively—one was treated with combined OCA, revision ACLR, and high tibial osteotomy (HTO), and the other was treated with combined OCA, revision ACLR, and posterolateral corner repair. Three knees (10%) were treated with a combined OCA and meniscus allograft transplantation. Two knees had varus malalignment preoperatively and were treated with concomitant valgus-producing HTO, and one knee had valgus malalignment preoperatively and was treated with a concomitant varus-producing distal femoral osteotomy. For all other patients, alignment was normal or the mechanical axis did not fall through the region of the planned cartilage restoration procedure. Three knees (10%) were treated with both medial and lateral condylar OCAs. There were no patellar or tibial OCAs in this series.
Complications, Reoperations, and Failures
No superficial or deep infections were observed in the initial postoperative period after OCA. Three knees (10%) developed arthrofibrosis postoperatively and were treated with lysis of adhesions/scar excision. No other perioperative complications were reported.
At final follow-up, 11 patients (35%) had undergone a reoperation to the ipsilateral knee after OCA ( Table 2 ). Four patients (13%) underwent arthroscopic chondroplasty and/or loose body removal. One patient had a prior subtotal meniscectomy on the side of the treated chondral defect and was treated with meniscus allograft transplantation 29 months after the index OCA. At the time of meniscus allograft transplantation, the previously implanted osteochondral allograft was intact on second look. This patient did not have any subsequent surgery after the meniscus allograft transplantation. Failures were documented in 5 patients (16%) as defined by subsequent UKA (n = 1), and TKA (n = 4). The mean time to failure was 19.6 months (range, 14-27 months), and the average age of the patient at the time of failure was 47.4 years (range, 38-63 years). Kaplan-Meier survival analysis demonstrated 87% and 83% survivorship at 2 and 5 years, respectively ( Fig. 1 ). There was no significant difference in failure rate between patients with a BMI of ≥35 kg/m2 and patients with a BMI 30 to 34 kg/m2 (P = 0.417).
Table 2.
Reoperations After OCA in Patients with BMI ≥30 kg/m2.
| Procedure | No.a |
|---|---|
| Arthroscopic chondroplasty/loose body removal | 4 |
| Arthroscopic meniscectomy | 1 |
| Lysis of adhesions and manipulation under anesthesia | 2 |
| Meniscus allograft transplantation | 1 |
| Hardware removal | 2 |
| Unicompartmental knee arthroplastyb | 1 |
| Total knee arthroplastyb | 4 |
BMI = body mass index; OCA = osteochondral allograft transplantation.
Some knees had more than one procedure during reoperation or more than one reoperation.
Failures.
Figure 1.
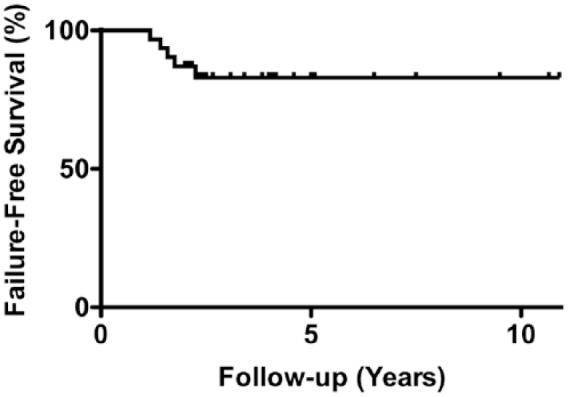
Survivorship of osteochondral allograft transplantation in patients with a body mass index (BMI) of ≥30 kg/m2.
Outcome Scores
Of the 27 patients with surviving allografts at a minimum of two years after OCA, 23 had complete responses to outcome measures (85%). Postoperatively, statistically significant improvements were noted in the pain, physical functioning, and role limitations due to physical health subscales of the SF-36 (∆ = 23.3, P = 0.001 for pain; ∆ = 28.1, P ≤ 0.001 for physical functioning; ∆ = 36.5, P = 0.035 for role limitations due to physical health), IKDC (∆ = 22.5, P = 0.002), KOS-ADL (∆ = 21.1, P = 0.002), and overall condition scores (∆ = 2.1, P = 0.046) ( Table 3 ). These score improvements are larger than published thresholds indicative of a minimally clinically important difference.26-28 In contrast, no statistically significant changes were noted in the SF-36 general health subscale or Marx activity rating scale.
Table 3.
Preoperative and Postoperative Outcome Scores at Final Follow-up in Patients with BMI ≥30 kg/m2.
| Measure | Preoperative | Postoperative | P |
|---|---|---|---|
| SF-36 | |||
| General health | 71.9 ± 20.3 | 75.5 ± 18.8 | 0.250 |
| Pain | 49.3 ± 22.7 | 72.6 ± 23.7 | 0.001 |
| Physical functioning | 52.9 ± 21.6 | 81.0 ± 14.9 | <0.001 |
| Role limitations due to physical health | 44.8 ± 46.0 | 81.3 ± 30.2 | 0.035 |
| IKDC | 43.5 ± 13.9 | 65.9 ± 18.2 | 0.003 |
| KOS-ADL | 58.2 ± 12.3 | 79.3 ± 15.6 | 0.002 |
| Marx activity rating scale | 4.0 ± 5.7 | 3.6 ± 4.8 | 1.000 |
| Overall condition | 4.7 ± 1.7 | 6.9 ± 1.9 | 0.046 |
IKDC = International Knee Documentation Committee Subjective Form; KOS-ADL = Knee Outcome Survey–Activities of Daily Living Scale; SF-36 = Short Form–36.
Values are presented as mean ± standard deviation, in points.
Comparison of outcome scores between patients with a BMI of ≥35 kg/m2 and those with a BMI 30 to 34 kg/m2 demonstrated no significant differences in postoperative and change scores at final follow-up.
Discussion
In this present study of OCA in patients with a BMI of ≥30 kg/m2, there were no perioperative complications other than arthrofibrosis. Two- and 5-year graft survivorship was 87% and 83%, respectively, with a mean time to failure of 19.6 months. Patients with surviving allografts at a minimum of 2 years after OCA reported clinically significant improvements in the pain, physical functioning, and role limitations due to physical health subscales of the SF-36, IKDC, KOS-ADL, and overall condition scores. These favorable results suggest that a patient BMI of ≥30 kg/m2 should not be an absolute contraindication to this procedure for select patients.
A threshold BMI of 30 kg/m2 as a contraindication for cartilage repair in the knee appears to be partly derived from the definition for obesity (BMI ≥30 kg/m2), its association with higher rates of knee osteoarthritis and need for TKA, and its correlation with worse outcomes after microfracture and knee arthroplasty.5,6, 29-31 Among all types of surgeries, obese patients have a significantly higher risk of perioperative complications, including myocardial infarction, wound infection, nerve injury, urinary tract infection, and death.32 Within the cartilage repair literature, an elevated BMI has been negatively correlated with clinical outcomes after microfracture, ACI, and matrix-induced autologous chondrocyte implantation (MACI).5,6,33 Mithoefer et al.5,6 first examined the correlation between an elevated BMI and outcomes after microfracture treatment of isolated chondral defects of the femur and reported that patients with an elevated BMI had poor repair fill on magnetic resonance imaging and a decreased improvement in SF-36 physical component, KOS-ADL, and subjective rating scores. Specifically, those with a BMI of >30 kg/m2 demonstrated the least postoperative improvement and poorest outcome scores. Jaiswal et al.33 examined 60 patients treated with ACI and 62 treated with MACI. Patients with an ideal weight demonstrated sustained improvement in modified Cincinnati scores throughout the 2-year study period, while obese patients (defined by the authors as a BMI of >30 kg/m2) demonstrated no improvement in scores at 2 years. These results are not surprising, given that the reparative cartilage tissue created by these techniques is immature at the time of implantation and typically requires 12 to 36 months before complete biologic maturation.34,35 Because full weightbearing is initiated well before the completion of maturation, the immature repair tissue would be prone to early breakdown as a result of the greater loading conditions in the knees of heavier patients, compromising its functionality and longevity.
In contrast, osteochondral grafts are structurally stable 2-phase constructs consisting of a mature osteochondral interface, conferring superior stability and resistance to joint loads at the time of implantation. Additionally, with its viable and mature chondral layer, osteochondral grafts are able to absorb loads as well as distribute rim stresses at the graft-recipient interface when inserted flush to the host surface.8,9 Although a few studies have examined the association between BMI and outcomes after OCA, none have specifically characterized the mid- to long-term clinical outcomes of OCA in obese patients.10,36 In a case series of 224 patients treated with OCA, of which a large portion (36%) underwent concomitant meniscus allograft transplantation, Frank et al.10 identified elevated BMI as an independent risk factor for failure. However, the difference in BMI between failures and nonfailures was only 3.4 (29.4 ± 5.3 and 26.0 ± 5.0 kg/m2, respectively), and the odds ratio in their logistic regression was only 1.165. Nuelle et al.36 reviewed 75 patients with an average BMI of 29.6 kg/m2 (range, 19.1-54.0 kg/m2) treated with OCA. The authors defined a successful outcome as a visual analog scale (VAS) of 0 or improvement in score (decrease) of 2 or more at final follow-up. Patients with a BMI of >35 kg/m2 were 4 times more likely to be associated with nonsuccessful outcomes. However, among the patients with a minimum 2-year follow-up (31 patients), there was no difference in success rates between patients with a BMI of >35 kg/m2 and those with a BMI of <35 kg/m2.
Certainly, there appears to be a trend toward higher risk of graft failure and less successful outcomes in heavier patients treated with OCA; however, our results suggest that OCA in obese patients can still provide good clinical outcomes with low complication rates, high graft survivorship, and high patient satisfaction. The 2- and 5-year graft survivorship of 87% and 83% in this series is comparable to that reported in recently published OCA studies of patients with normal BMIs using fresh press-fit grafts.10,14,37,38 It is important to note that our series of patients consisted of a younger, athletic population without significant medical comorbidities, such as diabetes or cardiovascular disease, that would increase the risk of perioperative complications. Additionally, neuraxial anesthesia is almost always used for this procedure at our institution and has been shown to reduce the risk of blood loss, deep venous thrombosis, and pulmonary compromise compared with general anesthesia, particularly in the obese patient.39,40 Another potential reason for the good outcomes found in this study include the absence of patellar or bipolar OCAs, which have been shown to have higher failure rates and inferior outcomes.41,42 Furthermore, obese patients with knee osteoarthritis are more likely to have joint loads concentrated in the medial compartment due to varus malalignment.43 However, more patients in this series were treated with lateral condylar OCAs (48%) than medial condylar OCAs (42%). Therefore, the osteochondral grafts implanted in our series may not have experienced the higher loading conditions thought to increase the risk of failure in heavier patients.
There are several limitations of this study. Mean follow-up was only 4.1 years, allowing us to only make conclusions regarding the intermediate-term following OCA. Longer follow-up is needed to more accurately assess graft longevity and allow for longitudinal tracking of outcome scores to assess for any potential deterioration in symptoms and function. Additionally, the lack of long-term imaging follow-up precluded additional investigation into the healing and integrity of the grafts as well as any progression of osteoarthritis. Comparison with a control group with a BMI of <30 kg/m2 was not performed; however, failure of OCA depends on a multitude of factors (e.g., number of previous surgeries, chondral defect location and size) that can be difficult to control for without a large number of patients in each group. The majority of patients did not have preoperative long-leg standing radiographs (ordered based on the surgeon’s discretion), which precluded a more accurate quantification of lower leg alignment in this study. The decision to perform a concomitant osteotomy or meniscus allograft transplantation was mostly dependent on surgeon and patient discretion, rather than predetermined thresholds of malalignment and meniscal deficiency, respectively. Finally, this study consisted of patients treated by surgeons at a single institution who perform a high volume of OCAs, potentially introducing performance bias.
In conclusion, OCA can be a successful midterm treatment option for focal cartilage defects of the knee in select obese patients. Compared with microfracture and ACI/MACI, structural osteochondral grafts have superior time zero stability, are more capable of bearing and sharing loads, and are more resistant to the increased joint reactive forces seen in heavier patients. These findings may help expand the clinical indications for OCA of the knee in patients with an elevated BMI; however, further long-term outcomes should be studied.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Institutional review board approval (#2013-024) was obtained for the prospective registry used in this study.
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable
ORCID iD: Dean Wang  https://orcid.org/0000-0002-3005-1154
https://orcid.org/0000-0002-3005-1154
References
- 1. Mouritzen U, Christgau S, Lehmann HJ, Tanko LB, Christiansen C. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis. 2003;62(4):332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lacy KW, Cracchiolo A, Yu S, Goitz H. Medial femoral condyle cartilage defect biomechanics: effect of obesity, defect size, and cartilage thickness. Am J Sports Med. 2016;44(2):409-16. [DOI] [PubMed] [Google Scholar]
- 3. Braman JP, Bruckner JD, Clark JM, Norman AG, Chansky HA. Articular cartilage adjacent to experimental defects is subject to atypical strains. Clin Orthop Relat Res. 2005;(430):202-7. [DOI] [PubMed] [Google Scholar]
- 4. Messier SP, Pater M, Beavers DP, Legault C, Loeser RF, Hunter DJ, et al. Influences of alignment and obesity on knee joint loading in osteoarthritic gait. Osteoarthritis Cartilage. 2014;22(7):912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. Chondral resurfacing of articular cartilage defects in the knee with the microfracture technique. Surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 pt 2):294-304. [DOI] [PubMed] [Google Scholar]
- 6. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911-20. [DOI] [PubMed] [Google Scholar]
- 7. Bugbee W. The real life problem: getting your cartilage procedure approved—dealing with insurers. http://drbugbee.com/resources/gettingprocedureapproved.pdf. Accessed January 5, 2018.
- 8. Kock NB, Smolders JM, van Susante JL, Buma P, van Kampen A, Verdonschot N. A cadaveric analysis of contact stress restoration after osteochondral transplantation of a cylindrical cartilage defect. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D’Lima DD, Chen PC, Colwell CW., Jr. Osteochondral grafting: effect of graft alignment, material properties, and articular geometry. Open Orthop J. 2009;3:61-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frank RM, Lee S, Levy D, Poland S, Smith M, Scalise N, et al. Osteochondral allograft transplantation of the knee: analysis of failures at 5 years. Am J Sports Med. 2017;45(4):864-74. [DOI] [PubMed] [Google Scholar]
- 11. Gross AE, Kim W, Las Heras F, Backstein D, Safir O, Pritzker KP. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466(8):1863-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levy YD, Gortz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471(1):231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McAllister DR, Joyce MJ, Mann BJ, Vangsness CT., Jr. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148-58. [DOI] [PubMed] [Google Scholar]
- 14. Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint surg Am. 2007;89(4):718-26. [DOI] [PubMed] [Google Scholar]
- 15. McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247-63. [DOI] [PubMed] [Google Scholar]
- 16. Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-13. [DOI] [PubMed] [Google Scholar]
- 17. Hambly K, Griva K. IKDC or KOOS? Which measures symptoms and disabilities most important to postoperative articular cartilage repair patients? Am J Sports Med. 2008;36(9):1695-704. [DOI] [PubMed] [Google Scholar]
- 18. Marx RG, Jones EC, Allen AA, Altchek DW, O’Brien SJ. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83-A(10):1459-69. [DOI] [PubMed] [Google Scholar]
- 19. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213-8. [DOI] [PubMed] [Google Scholar]
- 20. Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27(4):402-16. [DOI] [PubMed] [Google Scholar]
- 21. Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16(2):83-6. [PubMed] [Google Scholar]
- 22. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-84. [DOI] [PubMed] [Google Scholar]
- 23. Krych AJ, Harnly HW, Rodeo SA, Williams RJ., 3rd Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94(11):971-8. [DOI] [PubMed] [Google Scholar]
- 24. Krych AJ, Robertson CM, Williams RJ, 3rd; Cartilage Study Group. Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40(5):1053-9. [DOI] [PubMed] [Google Scholar]
- 25. Krych AJ, Robertson CM, Williams RJ., 3rd Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40(5):1053-9. [DOI] [PubMed] [Google Scholar]
- 26. Escobar A, Quintana JM, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cartilage. 2007;15(3):273-80. [DOI] [PubMed] [Google Scholar]
- 27. Greco NJ, Anderson AF, Mann BJ, Cole BJ, Farr J, Nissen CW, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891-902. [DOI] [PubMed] [Google Scholar]
- 28. Williams VJ, Piva SR, Irrgang JJ, Crossley C, Fitzgerald GK. Comparison of reliability and responsiveness of patient-reported clinical outcome measures in knee osteoarthritis rehabilitation. J Orthop Sports Phys Ther. 2012;42(8):716-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bourne R, Mukhi S, Zhu N, Keresteci M, Marin M. Role of obesity on the risk for total hip or knee arthroplasty. Clin Orthop Relat Res. 2007;465:185-8. [DOI] [PubMed] [Google Scholar]
- 30. Berend ME, Ritter MA, Meding JB, Faris PM, Keating EM, Redelman R, et al. Tibial component failure mechanisms in total knee arthroplasty. Clin Orthop Relat Res. 2004(428):26-34. [DOI] [PubMed] [Google Scholar]
- 31. Berend KR, Lombardi AV, Jr, Mallory TH, Adams JB, Groseth KL. Early failure of minimally invasive unicompartmental knee arthroplasty is associated with obesity. Clin Orthop Relat Res. 2005;440:60-6. [DOI] [PubMed] [Google Scholar]
- 32. Bamgbade OA, Rutter TW, Nafiu OO, Dorje P. Postoperative complications in obese and nonobese patients. World J Surg. 2007;31(3):556-60. [DOI] [PubMed] [Google Scholar]
- 33. Jaiswal PK, Bentley G, Carrington RW, Skinner JA, Briggs TW. The adverse effect of elevated body mass index on outcome after autologous chondrocyte implantation. J Bone Joint Surg Br. 2012;94(10):1377-81. [DOI] [PubMed] [Google Scholar]
- 34. Niethammer TR, Safi E, Ficklscherer A, Horng A, Feist M, Feist-Pagenstert I, et al. Graft maturation of autologous chondrocyte implantation: magnetic resonance investigation with T2 mapping. Am J Sports Med. 2014;42(9):2199-204. [DOI] [PubMed] [Google Scholar]
- 35. Fortier LA, Cole BJ, McIlwraith CW. Science and animal models of marrow stimulation for cartilage repair. J Knee Surg. 2012;25(1):3-8. [DOI] [PubMed] [Google Scholar]
- 36. Nuelle CW, Nuelle JA, Cook JL, Stannard JP. Patient factors, donor age, and graft storage duration affect osteochondral allograft outcomes in knees with or without comorbidities. J Knee Surg. 2017;30(2):179-84. [DOI] [PubMed] [Google Scholar]
- 37. Murphy RT, Pennock AT, Bugbee WD. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42(3):635-40. [DOI] [PubMed] [Google Scholar]
- 38. Gracitelli GC, Meric G, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation for knee lesions after failure of cartilage repair surgery. Cartilage. 2015;6(2):98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Memtsoudis SG, Sun X, Chiu YL, Stundner O, Liu SS, Banerjee S, et al. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients. Anesthesiology. 2013;118(5):1046-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91(7):935-42. [DOI] [PubMed] [Google Scholar]
- 41. Gracitelli GC, Meric G, Pulido PA, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for isolated patellar cartilage injury. Am J Sports Med. 2015;43(4):879-84. [DOI] [PubMed] [Google Scholar]
- 42. Meric G, Gracitelli GC, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-14. [DOI] [PubMed] [Google Scholar]
- 43. Sharma L, Lou C, Cahue S, Dunlop DD. The mechanism of the effect of obesity in knee osteoarthritis: the mediating role of malalignment. Arthritis Rheum. 2000;43(3):568-75. [DOI] [PubMed] [Google Scholar]


