Abstract
Background: Lyme borreliosis (LB) is the most frequent vector-borne disease in France. Since 2009, surveillance of LB is conducted by a sentinel network of general practitioners (GPs). This system, in conjunction with the national hospitalisation database was used to estimate the incidence and describe the characteristics of LB in France.
Aim: To describe the estimated incidence and trends in GP consultations and hospital admissions for LB in France and identify risk groups and high-incidence regions.
Results: From 2011 to 2016, the mean yearly incidence rate of LB cases was 53 per 100,000 inhabitants (95% CI: 41–65) ranging from 41 in 2011 to 84 per 100 000 in 2016. A mean of 799 cases per year were hospitalised with LB associated diagnoses 2005–16. The hospitalisation incidence rate (HIR) ranged from 1.1 cases per 100,000 inhabitants in 2005 to 1.5 in 2011 with no statistically significant trend. We observed seasonality with a peak during the summer, important inter-regional variations and a bimodal age distribution in LB incidence and HIR with higher incidence between 5 and 9 year olds and those aged 60 years. Erythema migrans affected 633/667 (95%) of the patients at primary care level. Among hospitalised cases, the most common manifestation was neuroborreliosis 4,906/9,594 (51%).
Conclusion: Public health strategies should focus on high-incidence age groups and regions during the months with the highest incidences and should emphasise prevention measures such as regular tick checks after exposure and prompt removal to avoid infection.
Keywords: Lyme borreliosis, neuroborreliosis, general practitioners, hospitalisation, incidence, surveillance
Introduction
Lyme borreliosis (LB) is caused by spirochaetes of the Borrelia burgdorferi sensu lato species complex, which are transmitted by different Ixodes spp. ticks [1]. The most common clinical manifestation is erythema migrans (EM). However, in the absence of antibiotic treatment the infection can spread and cause severe manifestations affecting a patient's skin, nervous system, joints, or heart [1].
LB is the most common tick-borne infectious disease in North America [2,3] and countries with temperate climates within Europe and Asia [4,5]. Incidence of LB has been increasing in some European countries [6-12] and it has been suggested that LB will become a more prominent health concern with predicted climate changes potentially impacting tick density and geographical distribution [4]. Therefore, knowledge of epidemiological characteristics of LB is important to decide on allocation of resources and to target prevention measures [13].
Since 2009, Lyme disease has been monitored by the general practitioners of the Sentinelles network. This network is a sentinel network of general practitioners (GPs) and operates as routine, systematic and standardised surveillance system allowing for trend analyses of LB incidence and the estimation of national and regional LB incidence rates. Between 2009 and 2012, the national estimated incidence rate was stable [14]. Between 2001 and 2012, LB incidence estimated by regional studies varied considerably on a regional level from 24 cases per 100,000 inhabitants in Aquitaine (south-western France) to 232 cases per 100,000 inhabitants in Alsace (eastern France) [15]. Another source of LB data in France is the national hospital discharge base. Between 2004 and 2009, the average annual LB-associated hospitalisation rate was estimated as 1.55 cases per 100,000 inhabitants; important regional variations in hospitalisation rates were also observed [14].
In the present study, we estimated the annual incidence of LB cases diagnosed at primary care level between 2011 and 2016 in mainland France and describe the characteristics of these cases. We also estimated the incidence of hospitalised LB cases between 2005 and 2016, with a particular focus on Lyme neuroborreliosis (LNB).
Methods
The Sentinelles network
The Sentinelles network, established in 1984 is a real-time epidemiologic surveillance system comprised of a sample of GPs located throughout mainland France, who participate on a voluntary basis [14,16,17]. The sentinel general practitioners (SGPs) report new LB diagnoses on a weekly basis as part of the Sentinelles surveillance system since 2009. A comparison between SGPs and GPs found that they are similar in terms of age, but SGPs have slightly more consultations per week; the impact of this difference on incidence estimates is small [17].
All reported LB cases were validated by an expert group constituted by clinicians, microbiologists and epidemiologists applying the European Union Concerted Action on Lyme Borreliosis (EUCALB) case definitions (Box 1) [18].
Box 1. Sentinelles network case definition for Lyme borreliosis.
(i) Presence of EM
(ii) Arthritis, cutaneous (other than EM) or heart manifestations associated with LB confirmed by ELISA and Western blot, or
(iii) Neurological manifestations associated with LB confirmed by ELISA and Western blot, associated with the presence of antibodies in the cerebrospinal fluid (CSF)
In the presence of meningoradiculitis or unilateral facial paralysis, cases were validated even in the absence of CSF fluid analysis, if clinically suggestive (consensus agreement) in patients with serological confirmation (ELISA and Western blot) and who reported a history of EM less than 2 months before the onset of neurological manifestations.
EM: Erythema migrans.
Information was collected from cases by the SGP during the medical consultation using a standardised questionnaire developed by the Sentinelles network (Box 2).
Box 2. Information collected from standardised questionnaire, Sentinelles network, France, 2009–2016.
• Age
• Sex
• Date of diagnosis
• History and dates of tick bites
• Presence and description of EM, i.e. solitary or multiple, central clearing, centrifugal expansion and, since 2011, diameter in centimetre
• Other cutaneous manifestations, i.e. cutaneous lymphocytoma, acrodermatitis chronica atrophicans
• Arthritis (affected joint(s))
• Cardiac manifestations such as atrioventricular block, pericarditis, myocarditis, other
• Presence of neurological manifestations such as meningoradiculitis, clinical signs of meningitis, meningoencephalitis, radiculitis, facial paralysis, events related to another cranial nerve
• Date and results of serological tests and/or CSF analyses and reason(s) for hospitalisation
CSF: cerebrospinal fluid; EM: Erythema migrans.
Data analysis
Data on LB cases reported to the Sentinelles network 1 January 2011–31 December 2016 were analysed. Estimated LB incidence rates were calculated as follows: the average number of cases notified by SGPs (adjusted for participation and geographic distribution) multiplied by the total number of GPs practicing in France (or in a given region for regional incidence rates) [17] divided by the total French population [19]. Confidence intervals (CI) were estimated under the assumption that the number of reported cases followed a Poisson distribution. In addition, we estimated annual incidence rates for the following four subgroups of cases: EM regardless of diameter, EM greater or equal to 5 cm, early and late disseminated LB and all cases except EM smaller than 5 cm (corresponding to EM ≥ 5 cm or disseminated LB).
Lyme borreliosis hospitalisations, 2005–2016
The French national hospital discharge database (Programme de Médicalisation des Systèmes d’Information – PMSI) collects information on every hospital stay in France [20]. Each hospital discharge report, corresponding to a hospital stay, is described according to the following items: reasons of hospitalisation (principal diagnosis) and related medical conditions (associated diagnoses) coded with the tenth revision of the International Classification of Diseases (ICD-10) [21], length of hospital stay and characteristics of the patient (age, sex, place of hospitalisation and residence). A unique patient identifier allows the identification of multiple hospital stays for the same patient.
Hospital discharge reports with a principal or associated diagnosis of LB and admitted to hospital in France 1 January 2005–31 December 2016 were extracted from the PMSI. LB diagnoses were identified using the ICD10 codes: A69.2 for Lyme disease, M01.2 for arthritis in Lyme disease and L90.4 for acrodermatitis chronica atrophicans (ACA).
As described in the previous study [14] and due to the poor predictive value of the LB codes in the PMSI [22], we included discharge reports which met the following criteria: (i) a LB specific diagnosis (M01.2 or L90.4); (ii) a A69.2 code in the absence of any other diagnosis, or (iii) a A69.2 code associated with code(s) compatible with LB symptoms (neurological, cardiac, articular and ocular disorders) (Table 1). Hospital discharge reports with no patient identifier and those of patients living outside mainland France were excluded.
Table 1. ICD-10 codes of clinical disorders that may be related to Lyme borreliosis.
| Chapter VI: Diseases of the nervous system | ICD-10 code concerned |
|---|---|
| Meningitis | G00, G00.9, G01, G02, G03,G03.0, G03.1, G03.8, G03.9 |
| Encephalitis, myelitis and encephalomyelitis | G04, G04.2, G04.8, G04.9, G05, G05.0, G05.2, G05.8 |
| Disorders of trigeminal nerve | G50.8, G50.9 |
| Facial nerve disorders | G51, G51.0,G51.8, G51.9 |
| Disorders of other cranial nerves | G52, G52.0–3, G52.7–9 |
| Cranial nerve disorders in diseases classified elsewhere | G53, G53.1, G53.8 |
| Nerve root and plexus disorders | G54, G54.0–5, G54.8–9 |
| Other polyneuropathies | G62, G62.8–9 |
| Polyneuropathy in diseases classified elsewhere | G63, G63.0 |
| Other disorders of peripheral nervous system | G64 |
| Chapter VII: Diseases of the eye and adnexa | |
| Iridocyclitis | H20, H20.0–1, H20.8–9 |
| Other disorders of iris and ciliary body | H21, H21.8–9 |
| Disorders of iris and ciliary body in diseases classified elsewhere | H22, H22.0, H22.1, H22.8 |
| Chorioretinal inflammation | H30, H30.0–9 |
| Other disorders of choroid | H31, H31.8–9 |
| Chorioretinal disorders in diseases classified elsewhere | H32, H32.0, H32.08, H32.8 |
| Chapter IX: Diseases of the circulatory system | |
| Acute pericarditis | I30, I30.0–9 |
| Pericarditis in diseases classified elsewhere | I32, I32.0–8 |
| Acute myocarditis | I40, I40.0–9 |
| Myocarditis in diseases classified elsewhere | I41, I41.0, I41.2, I41.8 |
| Cardiomyopathy | I42, I42.9 |
| Cardiomyopathy in diseases classified elsewhere | I43, I43.0 |
| Atrioventricular and left bundle-branch block | I44, I44.0–7 |
| Other conduction disorders | I45, I45.0–5, I45.8–9 |
| Other heart disorders in diseases classified elsewhere | I52, I52.0–8 |
| Chapter XIII: Diseases of the musculoskeletal system and connective tissue | |
| Arthritis and polyarthritis due to other specified bacterial agents | M00.8, M00.80–9 |
| Direct infections of joint in infectious and parasitic diseases classified elsewherea | M01, M01.30–9, M01.80–9 |
| Other arthritis | M13, M13.0–9 |
| Arthropathies in other diseases classified elsewhere | M14, M148 |
ICD10: tenth revision of the International Classification of Diseases.
a Other than M01.20–9 codes: Arthritis in Lyme disease.
ICD-10 codes identified from [21].
We defined a case of LB as a person hospitalised in mainland France for LB (following the criteria above) at the first stay during the time period 2005–16. We defined a case of LNB as a person hospitalised with at least one of the specific neurological disorders listed in Table 1, associated with a LB disease code. We described the annual number of cases (patients) and the number of hospital stays and geographical distribution of patients according to place of residence, or if unknown, place of hospitalisation.
In a given geographical area, the hospitalisation incidence rate (HIR) was calculated by dividing the number of hospitalised cases (excluding repeat admissions) observed in the defined time period by the number of inhabitants of the geographical area (estimated by the National Institute of Statistic’s and Economic Studies [19] and then multiplied by 100,000. We also estimated hospitalisation rates per age group. To assess statistically significant changes in hospitalisation rates over the study period we performed a negative binomial regression with the number of cases per year as a dependent variable and annual population when hospitalisation occurred as exposure.
Results
The Sentinelles network (2011–2016)
The total number of SGPs that participated between 2011 and 2016 was 723, which is 1.17% of the total number of GPs in France in 2016 (n = 61,789). The number of SGPs who participated varied year to year, with the lowest number participating in 2013 and the highest in 2015 (332 and 455, respectively).
Over the study period, 932 LB cases were reported by SGPs of which, 265 were excluded by the expert group for the following reasons: did not meet the case definition (n = 61), clinical manifestation not described (n = 146), absence of serology confirmation for disseminated LB (n = 44), no lumbar puncture for neurological manifestations, except for meningoradiculitis or unilateral facial paralysis (n = 14). A total of 667 LB cases were included (94 in 2011, 85 in 2012, 113 in 2013, 76 in 2014, 105 in 2015 and 194 in 2016).
Among LB cases, 340 (53%) were female ranging from 46% to 57% depending on the year (Table 2). The median age was 54 years.
Table 2. Demographic and clinical characteristics of Lyme borreliosis cases, Sentinelles Network, mainland France, 2011–2016 (n = 667).
| Characteristic | N | % | Median (range) |
|---|---|---|---|
| Sex (md = 23) | |||
| Female | 340 | 53 | |
| Male | 304 | 47 | |
| Age | NA | NA | 54 (1–91) |
| Clinical characteristics | |||
| Erythema migransa | 633 | 95 | |
| ≥ 5cm (md = 14) | 465 | 75 | |
| Solitary lesion (md = 16) | 591 | 96 | |
| Central clearing (md = 52) | 393 | 68 | |
| Centrifugal extension (md = 61) | 537 | 94 | |
| Disseminated Lyme borreliosis | 34 | 5 | |
| Acrodermatitis | 6 | 17 | |
| Lymphocytoma | 4 | 12 | |
| Arthritis | 17 | 50 | |
| Radiculitis | 4 | 12 | |
| Facial paralysis | 2 | 6 | |
| Radiculitis and meningoradiculitis | 1 | 3 | |
| Tick exposure | |||
| Tick bite (md = 83) | 414 | 71 | |
| Days between bite and diagnosis (md = 93) | NA | NA | 11 (1–250) |
| In mainland France (md = 9) | 403 | 99 | |
| Hospitalisation (md = 103) | 4 | 1 | |
md: missing data; NA: not applicable.
a For individuals with Erythema migrans multiple clinical characteristics could be reported.
Of 667 diagnoses, 633 were EM diagnoses (95%), 591 (96%) were solitary lesions, 465 (75%) were equal or greater than 5 cm, 537 (94%) had a centrifugal extension and 393 (68%) had a central clearing (Table 2). A total of 34 cases presented with disseminated LB (5%), corresponding to arthritis (n = 17), acrodermatitis (n = 6), lymphocytoma (n = 4), radiculitis (n = 4), facial paralysis (n = 2) and both meningoradiculitis and radiculitis (n = 1).
A tick bite was reported in 414 cases (71%), among which 403 (97%) were diagnosed with EM. The median time between tick bite and diagnosis for the 414 cases was 10 days for all LB cases (range 1–250) (Table 2).
The estimated annual incidence rate of LB over the period 2011–16 averaged 53 cases per 100,000 inhabitants (95% CI: 41–65). This rate varied between 41 cases per 100,000 in 2011 to 84 in 2016, when the incidence increased significantly compared with previous years (Table 3). The estimated average annual incidence rate per 100,000 inhabitants was 50 cases (95% CI: 38–62) for EM (regardless of size), 37 cases (95% CI: 27–47) for EM ≥ 5 cm, three cases (95% CI: 0–5) for disseminated LB and 40 (95% CI: 30–51) for EM ≥ 5 cm or disseminated LB (Table 3). In 2016, only LB cases diagnosed with EM increased significantly, as opposed to cases diagnosed with disseminated LB.
Table 3. Yearly incidence rates of Lyme borreliosis by clinical manifestation, in general medicine, Sentinelles network, mainland France, 2011–2016.
| Cases | Incidence rate per 100,000 inhabitants (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | Average | |
| Number of cases included | 94 | 85 | 113 | 76 | 105 | 194 | NR |
| Lyme borreliosis | 41 (31–51) | 44 (32–56) | 55 (43–67) | 41 (30–52) | 51 (38–64) | 84 (70–98) | 53 (41–65) |
| Erythema migransa | 37 (27–46) | 41 (29–53) | 54 (42–66) | 39 (29–49) | 50 (38–63) | 80 (66–93) | 50 (38–62) |
| Erythema migrans ≥ 5 cm | 30 (22–38) | 28 (18–38) | 45 (35–56) | 25 (16–33) | 37 (25–48) | 59 (47–71) | 37 (27–47) |
| Disseminated Lyme borreliosis | 5 (1–8) | 3 (0–6) | 1 (0–3) | 2 (0–4) | 1 (0–3) | 4 (1–7) | 3 (0–5) |
| Erythema migrans ≥ 5 cm or disseminated Lyme borreliosis | 35 (26–44) | 31 (21–41) | 47 (36–58) | 26 (18–35) | 38 (26–49) | 63 (51–75) | 40 (30–51) |
CI: confidence interval; NR: not reported.
a Regardless of the size.
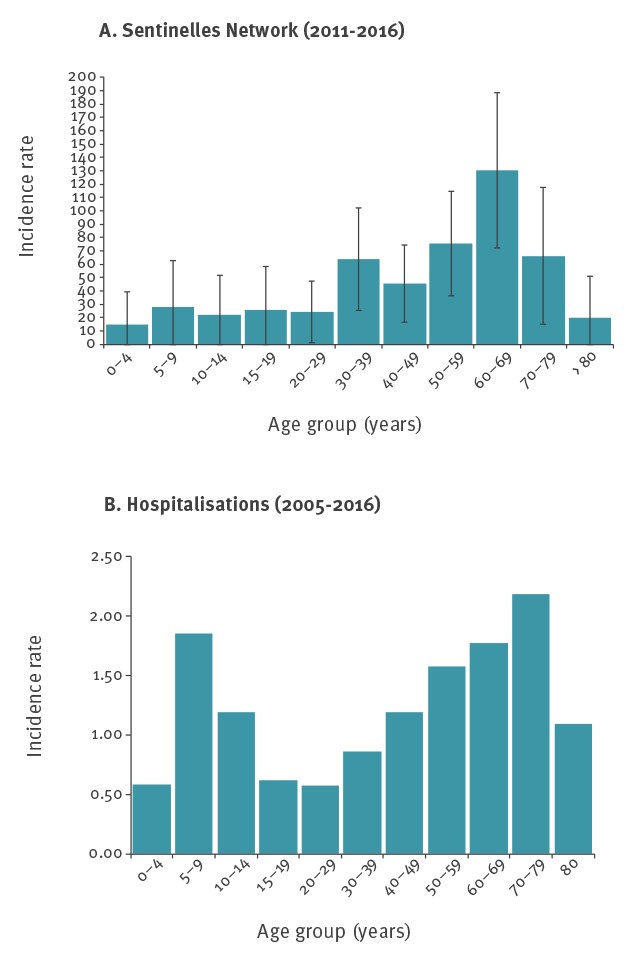
The highest estimated incidence rate was seen in the 60–69 age group (131 cases per 100,000 (95% CI: 73–188)), followed by the 50–59 age group (76 per 100,000 (95% CI: 36–115)), and the 70–79 age group (66 cases per 100,000 (95% CI: 15–118)) (Figure 1).
Figure 1.
Average yearly rates of Lyme borreliosis by age group in mainland France (A) estimated incidence in general practice, 2011–2016 and (B) hospitalisations, 2005–2016
Incidence rate is calculated per 100,000 inhabitants.
LB cases were diagnosed mainly from May to October (n = 518, 78%) over the 2011–16 period, with a peak in July (Figure 2).
Figure 2.
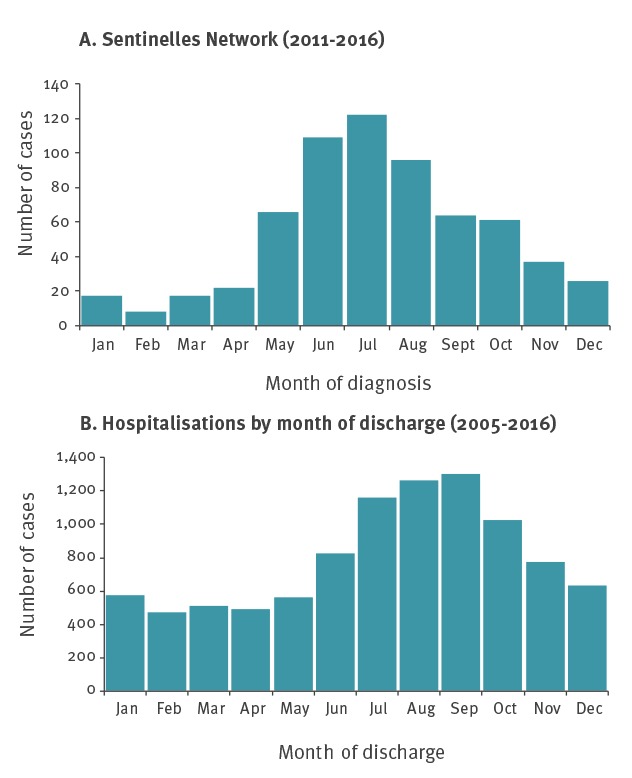
Distribution of Lyme borreliosis cases by month in mainland France (A) in general practice, 2011–2016 and (B) hospitalisations, 2005–2016
From 2011 to 2016, the regions with the highest average yearly estimated incidence rate per 100,000 inhabitants were Limousin and Alsace, with 239 cases (95% CI: 68–410) and 148 cases (95% CI: 45–251), respectively (Figure 3). The regions with the lowest average yearly incidence rates per 100,000 inhabitants were Pays de la Loire and Provence-Alpes-Côtes-d’Azur with five (95% CI: 0–25) and eight cases (95% CI: 0–20), respectively.
Figure 3.
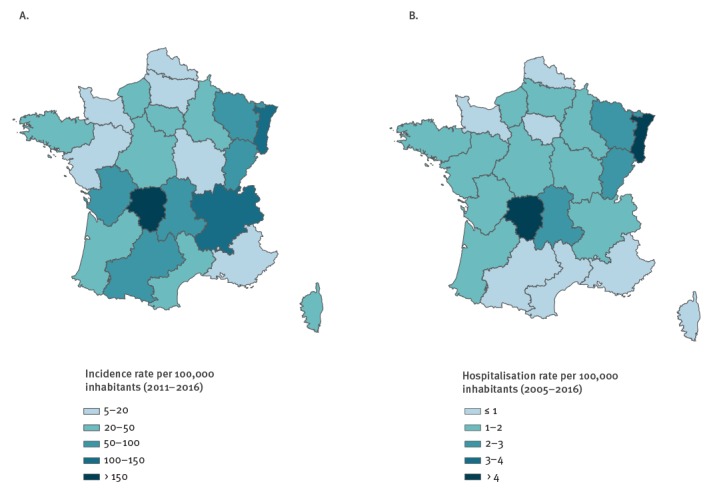
Estimated mean annual regional incidence rates of Lyme borreliosis (A) in general practice, 2011–2016 (B) hospitalisations, 2005–2016
Lyme borreliosis hospitalisations, 2005–2016
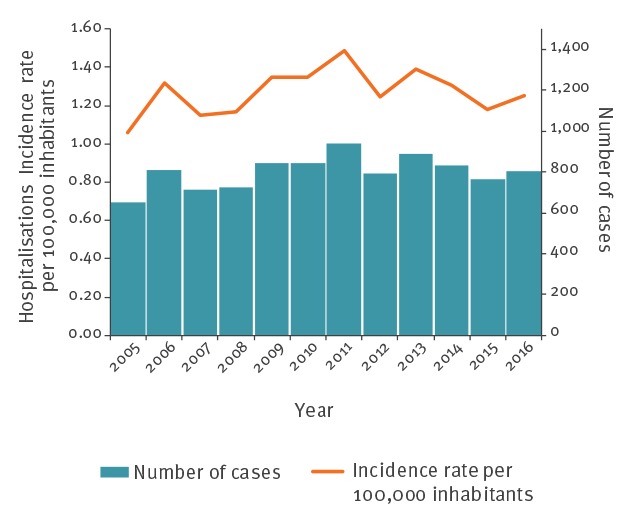
Among the 29,331 discharge reports extracted with a LB code, 11,551 met our inclusion criteria. Accounting for readmissions, 9,594 hospitalised LB cases were identified. Between 2005 and 2016, the mean annual number of hospital stays was 963 (range 846–1,129). The mean annual number of hospitalised cases was 799 (range 649–937). The median number of hospital stays by case was one (range 1–40).
The average estimated HIR was 1.3 per 100,000 inhabitants per year. The HIR fluctuated from 1.1 per 100 000 inhabitants in 2005 to 1.5 per 100,000 inhabitants in 2011 with no significant trend (p = 0.260) (Figure 4). Regions with the highest incidence rates of LB estimated by the Sentinelles network also had the highest hospitalisation rates (Figure 3). The HIR ranged from 4.2 cases per 100,000 inhabitants per year in Limousin to 0.3 in Corsica and Provence-Alpes-Côte d’Azur.
Figure 4.
Number of cases hospitalised for Lyme borreliosis and hospitalisation incidence rate per year, French national hospital discharge databasea, mainland France, 2005–2016
a Programme de Médicalisation des Systèmes d’Information (PMSI).
Reasons for hospitalisation were neurological disorders (n = 4,906; 51%), arthritis (n = 1,250; 13%), cardiac events (n = 639; 7%), ocular disorders (n = 177; 2%) and ACA (n = 231; 2%). For 27% (2,577/9,594) of the hospitalised cases a Lyme disease code (A69.2) was reported in the absence of any other diagnosis.
Of 9,594 cases, 57% were men. Two peaks were observed in the age distribution, in the age groups 5–9 and 70–79 years (Figure 1). The median age was 51 years (range 1–95). Among the 1,805 cases in the 0–15 age group, 62% were hospitalised for neurological disorders compared with 45% (2,058/4,601) in the 15–60 age group and 54% (1,725/3,188) in those aged 60 years and over (p < 0.001). Cases aged 60 years and older were more often hospitalised for cardiac manifestations (n = 320, 11%) than cases 0–15 years (n = 12, 0.7%) and 15–60 years (n = 304, 7%) (p < 0.001). The age and sex distribution are similar over the time period 2005–2016 (data not shown).
The highest numbers of LB hospitalised cases were observed in June–November with a peak every year in August or September (Figure 2). Hospitalised cases with neurological disorders were more often hospitalised during the summer with a peak in September (16%, p < 0.001). For hospitalised cases with cardiac manifestations a peak in August (14%) was observed. However, the incidence of hospitalised cases with cardiac manifestations peaked in August but did not differ significantly (p = 0.758) from the incidence in the other months. Cases with arthritis or ocular disorders were hospitalised evenly throughout the year.
The average length of stay was 6 days (range 0–239) overall, 9.6 days for cases with cardiac disorders, 7.6 days for neurological disorders and 2.7 days for cases with a LB code in the absence of any other associated diagnoses.
Lyme neuroborreliosis hospitalisations, 2005–2016
Among the 4,906 cases hospitalised with neurological manifestations, 58% were men and the median age was 52 years (range 1–94). The estimated mean annual HIR was 0.6 cases per 100,000 inhabitants ranging from 0.5–0.8 between 2005 and 2011, respectively. Alsace and Limousin were the regions with the highest mean incidence rates with 2.4 cases per 100,000 inhabitants.
Among LNB hospitalised cases, 37% presented facial nerve disorders (including 27% with unilateral facial nerve paralysis), 35% had meningitis, 9% polyneuropathy and 9% encephalitis, myelitis or encephalomyelitis.
HIR of LNB show a bimodal distribution of age with a first peak occurring in children aged 5–9 years old (1.3/100,000 of the age group) and a second peak in adults 70–79 years old (1.3 cases/100,000 of the age group). The proportion of hospitalised cases with LNB differed significantly by age (Figure 5). Among hospitalised cases, those age 5–9 and 70–79 years old were significantly more likely to have had LNB compared with other age groups (odds ratio (OR): 3.02; 95% CI: 2.41–3.78 and OR: 1.94; 95% CI: 1.58–2.39, respectively).
Figure 5.
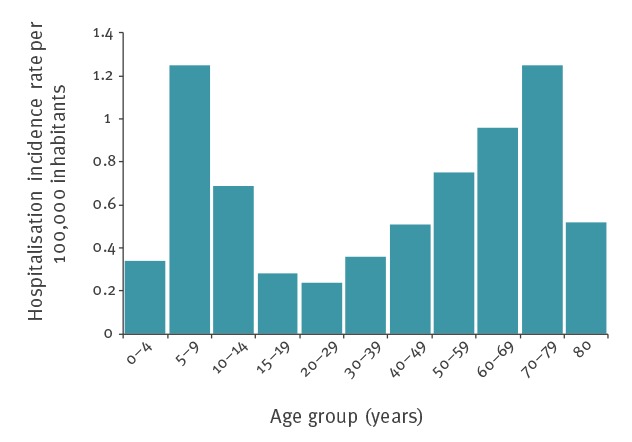
Yearly hospitalisation rates of Lyme neuroborreliosis by age group, mainland France, 2005–2016
Discussion
Our study on LB using two complementary data sources, the national Sentinelles GP network (2011–16) and the national hospital discharge database (2005–16), provides an updated overview of the epidemiology of LB in France and documents the trends in incidence of LNB in France. Our results are consistent with those described previously in France [14] and in Europe [6-12,23-27].
In France, between 2011 and 2015, the national incidence rates estimated by the Sentinelles network were stable. However, in 2016, an increase in incidence was noted. We found that yearly LB hospitalisation incidence rates fluctuated with no significant trend, whereas in general practice there was a significant increase in LB incidence during 2016 for patients diagnosed with EM. The most frequent clinical manifestation among cases presenting to GPs was EM and given that EM represents the early stage of LB and that antibiotic therapy is effective, this highlights the major role of GPs in secondary prevention of disseminated LB and late manifestations [28]. While there was a predominance of women among the cases consulting to GP, men were predominant among the hospitalised cases. In addition, our data supports the existing evidence that incidence of LNB is higher in children when compared with adults [12].
LB cases who consulted GPs were more often aged 50 years and older and had been bitten by ticks from late spring to early autumn. The numbers of LB cases consulting GPs increased in May and peaked in July, while LB hospital admissions peaked in September. This difference might be due to the delay between infected tick bite(s) and the development of disseminated LB requiring hospitalisation. For individual patients this delay might be difficult to estimate due to recall bias, particularly on the date of the potential infected tick bite(s).
An increase in LB over the last decade has been described in some European countries, but not in others [4]. Regarding countries bordering France, the incidence of LB positive tests reported by the sentinel laboratory network in Belgium (2003–12) and the incidence of GP consultation for EM (90/100,000 inhabitants in 2008–09 and 103/100,000 inhabitants in 2015) were both stable [23,29,30]. In nine states in Germany where LB is mandatory notifiable [27], annual fluctuations in reported numbers of LB cases were observed 2013–17 with no clear increasing or decreasing trend. In Switzerland [26], the incidence of reported LB cases was stable between 2008 and 2011 (mean annual incidence 131/100,000 inhabitants). In the Netherlands a continuous increase in incidence of GP consultations for EM was observed 1994–2014 when the incidence stabilised at 140/100,000 inhabitants [7,24]. In parallel, a decrease in tick bite consultations was observed in 2014, that may reflect the impact of public health education interventions (in particular body checking and prompt tick removal). No significant change in Ixodes ricinus abundance was reported in the Netherlands 2009–14 [24].
In European countries, surveillance of LB is based on different case definitions and surveillance systems: voluntary/compulsory reporting, laboratory reporting vs physician reporting, or hospital diagnoses [4,5]. The use of a common case definition developed by EUCALB is a first step towards harmonisation. Since 2011, the EUCALB case definition includes a criterion on the size of EM (greater or equal to 5 cm) and this definition can be used when comparing data from different countries.
However, comparisons between countries must be interpreted with caution. Heterogeneity among surveillance systems, difference in how healthcare is accessed and varying practices with regard to diagnostic investigations can all impact the estimates. These surveillance artefacts should be distinguished from differences in the genuine incidence rates that are conditioned by geographical, environmental (land use and density of animal reservoirs) and climatic factors, as well as the heterogeneous distribution of Borrelia burgdorferi sensu lato species, among others.
Our results suggest that mainland France has an estimated LB incidence rate similar to that of bordering countries with comparable environmental conditions (Germany, Belgium and Switzerland) but higher than countries such as Spain and Italy where environmental conditions do not favour the presence of Ixodes ticks [4,31].
The increase in incidence of LB cases in 2016 in general practice was not observed in hospitals neither at the national level nor in high incidence regions. This difference in trends could be explained by several hypotheses. In 2016, following the launch of the national plan against Lyme disease and tick-borne diseases [32], information and training activities were conducted for the general public and health professionals to increase awareness, specifically in regard to better detection of tick bites and skin lesions, possibly leading to increased consultations in general medicine. In addition, the media have largely covered this subject. The increase in incidence observed in 2016 by the Sentinelles network may therefore be the result of a surveillance bias due to better case detection and the incidence might have been underestimated in previous years. It is also possible that the incidence of EM actually increased in 2016, but because of appropriate care at the primary care level, this increase in incidence did not translate in an increase in the number of hospitalised cases. Hypothetically, this increase might be also due to special climate conditions, such as a mild winter followed by a warm and wet summer, as was observed in Sweden between 1999 and 2000 [10]. However, according to the 2016 weather report by Météo France, climatic conditions were not particularly favourable for the tick activity. Indeed, rainfall between July and September 2016 was one of the lowest in this period since 1959 [33].
Only a few European countries have published hospital discharge data related to LB. In Germany, a study analysing a large nationwide health insurance database, estimated the yearly LB hospitalisation incidence rate for the period 2008–11 to be nine per 100,000 inhabitants [34]. In Finland a study of the national hospital database estimated this incidence in 2014 at 19 per 100,000 [25]. These estimations are higher than the estimate in France (1.3/100,000). In the German and Finnish studies, the case definition was based only on the ICD-10 code A69.2. In our study, the hospitalisation incidence rate of patients with the A69.2 code (without other criteria), was 2.8 cases per 100,000 inhabitants. In Sweden [35], the incidence of LNB determined using the Swedish hospital discharge database, was estimated at 5.2 cases per 100,000 inhabitants, higher than in France (0.6/100,000 inhabitants).
The highest estimated incidence rate and the highest hospitalisation rate over the study period were in eastern and central regions of mainland France. It should be noted that these estimates were based on place of residence or hospitalisation and not place of infection.
The vector, the tick Ixodes ricinus is present in most parts of mainland France, except above 1,200 m and in the dry Mediterranean areas [36]. Ixodes ricinus ticks, the primary vector in Europe, are usually found in vegetation types with deciduous or mixed woodland that maintain high humidity (requiring a relative humidity of at least 80%) and in areas of moderate to high rainfall, such as in eastern and central regions of mainland France [4,36-38]. Since infection is correlated with tick abundance and human to tick exposure, this variability in incidence rates could be explained by differences in geographical and climate characteristics, in types of exposure (recreational and occupational exposure to ticks and outdoor activities) and presence of competent reservoir hosts. Tick nymphs are mainly responsible for transmitting Borrelia to humans and quest most actively from spring to autumn [4,36,37]. Diagnoses of acute LB peak in summer in many northern and central countries of Europe [4]. These findings were also confirmed in our study with higher incidences of LB from July to September.
In the absence of further information about distribution and density of ticks in France, surveillance data can be used to guide future studies such as research studies on ecology of the vector and its reservoir or the prevalence of infection in ticks by geographical areas and to target public health actions such as health communication campaigns to the most affected populations.
Importantly, over the study period, there was no change in the validation protocol, case definitions, SGPs participation and methods used to estimate regional and national incidences, strengthening the reliability of our results in terms of trends and LB incidence estimates. Incidences estimated by the Sentinelles network account only for patients consulting GPs. If it is assumed that the proportion of cases not presenting to GP remained stable over the study period, this method is suitable to follow the trend in incidence of LB. EM is the most discriminating sign enabling a reliable clinical diagnosis at the primary level care and is therefore a key indicator for LB surveillance [39]. These data permit to target public health communication about preventive measures for the general population including prevention of tick bites and LB and elaborate guidelines on the prevention and diagnosis for health professionals [40].
Limitations
There are important limitations in using data from the national hospital discharge database, including diagnosis and coding errors, involuntary omissions and reporting of pre-existing conditions not related to the stay, as well as lack of information about clinical symptoms and laboratory diagnostic results which could help to validate the diagnosis. Therefore, it is possible that we overestimated the hospitalisation rate when LB was coded but not directly related to the cause of hospitalisation. It is also possible that diagnoses of LB are undercoded or underdiagnosed and so the incidence may be underestimated. These biases are inherent to the use of the hospital discharge database [20] since this database was initially created as a tool for resource allocation [20].
The algorithm of codes we used to define LB, developed by a team of clinicians and epidemiologists, also has its limitations. We can overestimate the number of LB cases if the retained case definitions have low specificity. Because an earlier French study of hospital files estimated that the positive predictive value of a case definition based on the presence of at least one specific ICD Lyme code (A69.2 or M01.2) was only 65%, we decided to have a more specific case definition. By doing so, we aimed to reduce the background noise of inclusion of ‘false positive cases’ which could hinder the interpretation of the trends. However, we may have still included non-confirmed cases but it is also likely that our case definitions lack sensitivity and that we underestimate the true incidence. Existing guidelines for the diagnosis of LNB in Europe are based on clinical symptoms and laboratory analysis, particularly intrathecal specific antibody production [39,41]. It will be important to study in more depth how ICD Codes compare to laboratory data and to further validate the algorithms.
Meanwhile, in spite of the limitations, the hospitalisation database provides useful data and allows for monitoring of trends over time. In addition, it can be used to determine seasonality, high-risk regions and characteristics of hospitalised patients. The method and the database used are stable which are essential attributes required for trend analysis. Furthermore, the PMSI database has coding rules to minimise errors and variations between institutes and is a comprehensive national system providing opportunities to implement national level studies [42].
Conclusion
The combination of a sentinel network of GPs and the hospitalisation discharge database permits monitoring of two key indicators: EM and LNB and therefore provides a more comprehensive understanding of the epidemiology of LB in France. Furthermore, these data sources provide information at a regional level allowing the analysis of the geographical distribution and potential expansion of LB across the country.
Public health strategies should focus on age groups and regions with a high incidence of LB (particularly during the months with the highest incidence) and should emphasise prevention measures such as regular tick checks after exposure and prompt removal to avoid infection.
Acknowledgements
The authors wish to thank all the Sentinelles network GPs, Thomas Hanslik for his advice and discussion about the design of this work and Julien Durand and Marjorie Boussac for their help with the hospital discharge database. We would like to thank also Eve Robinson for her helpful comments on this manuscript and for reviewing the English.
Conflict of interest: None declared.
Authors’ contributions: All authors participated in the conceptualisation and writing of this manuscript. AS, TG, BJ, SG, SD, VR, HDV, TB and EC participated to the expert group, validated cases reported by the Sentinelles network. AS, TG, BJ, SG, SD, VR, HDV, TB, EC and JF participated in the discussion to define the criteria for a LB hospitalisation. AS analysed hospitalisation data and drafted the manuscript. TG and TL analysed the Sentinelles data and TG drafted the manuscript. BJ, SG, SD, VR, LF, HDV, TB, EC and JF revised the manuscript critically for important intellectual content.
References
- 1. Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461-73. 10.1016/S0140-6736(11)60103-7 [DOI] [PubMed] [Google Scholar]
- 2. Bacon RM, Kugeler KJ, Mead PS, Centers for Disease Control and Prevention (CDC) Surveillance for Lyme disease--United States, 1992-2006. MMWR Surveill Summ. 2008;57(10):1-9. [PubMed] [Google Scholar]
- 3. Applegren ND, Kraus CK. Lyme Disease: Emergency Department Considerations. J Emerg Med. 2017;52(6):815-24. 10.1016/j.jemermed.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 4. Rizzoli A, Hauffe H, Carpi G, Vourc H G, Neteler M, Rosa R. Lyme borreliosis in Europe. Euro Surveill. 2011;16(27):19906. [PubMed] [Google Scholar]
- 5. Sykes RA, Makiello P. An estimate of Lyme borreliosis incidence in Western Europe. J Public Health (Oxf). 2017;39(1):74-81. [DOI] [PubMed] [Google Scholar]
- 6. Sprong H, Hofhuis A, Gassner F, Takken W, Jacobs F, van Vliet AJ, et al. Circumstantial evidence for an increase in the total number and activity of Borrelia-infected Ixodes ricinus in the Netherlands. Parasit Vectors. 2012;5(1):294. 10.1186/1756-3305-5-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hofhuis A, van der Giessen JW, Borgsteede FH, Wielinga PR, Notermans DW, van Pelt W. Lyme borreliosis in the Netherlands: strong increase in GP consultations and hospital admissions in past 10 years. Euro Surveill. 2006;11(6):E060622.2. [DOI] [PubMed] [Google Scholar]
- 8. Dubrey SW, Bhatia A, Woodham S, Rakowicz W. Lyme disease in the United Kingdom. Postgrad Med J. 2014;90(1059):33-42. 10.1136/postgradmedj-2012-131522 [DOI] [PubMed] [Google Scholar]
- 9. Trájer A, Bobvos J, Páldy A, Krisztalovics K. Association between incidence of Lyme disease and spring-early summer season temperature changes in Hungary--1998-2010. Ann Agric Environ Med. 2013;20(2):245-51. [PubMed] [Google Scholar]
- 10. Bennet L, Halling A, Berglund J. Increased incidence of Lyme borreliosis in southern Sweden following mild winters and during warm, humid summers. Eur J Clin Microbiol Infect Dis. 2006;25(7):426-32. 10.1007/s10096-006-0167-2 [DOI] [PubMed] [Google Scholar]
- 11. Fülöp B, Poggensee G. Epidemiological situation of Lyme borreliosis in Germany: surveillance data from six Eastern German States, 2002 to 2006. Parasitol Res. 2008;103(S1) Suppl 1;S117-20. 10.1007/s00436-008-1060-y [DOI] [PubMed] [Google Scholar]
- 12. Wilking H, Stark K. Trends in surveillance data of human Lyme borreliosis from six federal states in eastern Germany, 2009-2012. Ticks Tick Borne Dis. 2014;5(3):219-24. 10.1016/j.ttbdis.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 13. Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348(24):2424-30. 10.1056/NEJMra021397 [DOI] [PubMed] [Google Scholar]
- 14. Vandenesch A, Turbelin C, Couturier E, Arena C, Jaulhac B, Ferquel E, et al. Incidence and hospitalisation rates of Lyme borreliosis, France, 2004 to 2012. Euro Surveill. 2014;19(34):20883. 10.2807/1560-7917.ES2014.19.34.20883 [DOI] [PubMed] [Google Scholar]
- 15.Sante publique France. Données épidémiologiques Borreliose de Lyme. [Data and surveillance. Lyme Borreliosis]. Paris: Sante publique France; [Accessed: 2 Mar 2019]. French. Available from: http://invs.santepubliquefrance.fr/Dossiers-hematiques/Maladies-infectieuses/Maladies-a-transmission-vectorielle/Borreliose-de-lyme/Donnees-epidemiologiques
- 16. Flahault A, Blanchon T, Dorléans Y, Toubiana L, Vibert JF, Valleron AJ. Virtual surveillance of communicable diseases: a 20-year experience in France. Stat Methods Med Res. 2006;15(5):413-21. 10.1177/0962280206071639 [DOI] [PubMed] [Google Scholar]
- 17. Souty C, Turbelin C, Blanchon T, Hanslik T, Le Strat Y, Boëlle PY. Improving disease incidence estimates in primary care surveillance systems. Popul Health Metr. 2014;12(1):19. 10.1186/s12963-014-0019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, Kaiser R, Krause A, et al. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 2011;17(1):69-79. 10.1111/j.1469-0691.2010.03175.x [DOI] [PubMed] [Google Scholar]
- 19.Instit national de la statistique et des études éconmiques (INSEE). Evolution et structure de la population. [Population structure and its evolution]. Paris: INSEE; 2019. French. Available from: https://www.insee.fr/fr/statistiques/1893198
- 20. Boudemaghe T, Belhadj I. Data Resource Profile: The French National Uniform Hospital Discharge Data Set Database (PMSI). Int J Epidemiol. 2017;46(2):392-392d. 10.1093/ije/dyw359 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO). International statistical classification of diseases and related health problems. 10th Revision. Volume 3. Fifth edition. Geneva: WHO; 2016. Available from: https://icd.who.int/browse10/2016/en
- 22. Gueorguiev Penev D, Laurent E, Baron S, Diot E, Bastides F, de Gialluly C, et al. Borréliose de Lyme : recensement des cas adultes hospitalisés en Indre-et-Loire, à partir du PMSI (1999–2006) [Lyme borreliosis: census of adult patients hospitalized in Indre-et-Loire (France), from the Hospital Discharge Data (1999-2006)]. Rev Epidemiol Sante Publique. 2010;58(5):339-47. 10.1016/j.respe.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 23. Bleyenheuft C, Lernout T, Berger N, Rebolledo J, Leroy M, Robert A, et al. Epidemiological situation of Lyme borreliosis in Belgium, 2003 to 2012. Arch Public Health. 2015;73(1):33. 10.1186/s13690-015-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hofhuis A, Bennema S, Harms M, van Vliet AJ, Takken W, van den Wijngaard CC, et al. Decrease in tick bite consultations and stabilization of early Lyme borreliosis in the Netherlands in 2014 after 15 years of continuous increase. BMC Public Health. 2016;16(1):425. 10.1186/s12889-016-3105-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sajanti E, Vrtanen M, Helve O, Kuusi M, Lvvtikäinen O. Hvtönen, Sane Jussi. Lyme borreliosis in Finland, 1995-2014: a nationwide register-based study. Poster presentation at the European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE), 28-30 November 2016, Stockholm. [Google Scholar]
- 26. Altpeter E, Zimmermann H, Oberreich J, Péter O, Dvořák C, Swiss S. Tick related diseases in Switzerland, 2008 to 2011. Swiss Med Wkly. 2013;143:w13725. 10.4414/smw.2013.13725 [DOI] [PubMed] [Google Scholar]
- 27. Enkelmann J, Böhmer M, Fingerle V, Siffczyk C, Werber D, Littmann M, et al. Incidence of notified Lyme borreliosis in Germany, 2013-2017. Sci Rep. 2018;8(1):14976. 10.1038/s41598-018-33136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vandererven C, Bellanger AP, Faucher JF, Marguet P. Primary care physician management of tick bites in the Franche-Comté region (Eastern France, 2013). Med Mal Infect. 2017;47(4):261-5. 10.1016/j.medmal.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 29.Institut scientifique de Santé publique (WIV-ISP). Zoonoses et Maladies à Transmission Vectorielle. [Zoonoses and vector borne diseases]. Brussels: WIV-ISP; 2017. French. Available from: https://epidemio.wiv-isp.be/ID/reports/Zoonoses%20et%20maladies%20%C3%A0%20transmission%20vectorielle.%20Rapport%20annuel%202015%20et%202016.pdf
- 30. Vanthomme K, Bossuyt N, Boffin N, Van Casteren V. Incidence and management of presumption of Lyme borreliosis in Belgium: recent data from the sentinel network of general practitioners. Eur J Clin Microbiol Infect Dis. 2012;31(9):2385-90. 10.1007/s10096-012-1580-3 [DOI] [PubMed] [Google Scholar]
- 31.Lindgren E, Jaenson TGT. Lyme Borreliosis in Europe: Influences of Climate and Climate Change, Epidemiology, Ecology and Adaptation Measures (report no.: EUR/04/5046250). Copenhagen: World Health Organization; 2006. Available from: http://www.euro.who.int/__data/assets/pdf_file/0006/96819/E89522.pdf [Google Scholar]
- 32.Ministère des affaires sociales et de la santé. Plan national de lutte contre la maladie de Lyme et les maladies transmissibles par les tiques 2016. [National Plan against Lyme disease and tick-borne diseases 2016]. Paris: Ministère des affaires sociales et de la santé; 2016. French. Available from: https://solidarites-sante.gouv.fr/IMG/pdf/plan_lyme_281216_aes_-_2.pdf
- 33.Meteo France. Bilan climatique de l'année 2016. Météo France. [Climate report. Annual 2016. France]. Paris: Meteo France; 2016. French. Available from: http://www.meteofrance.fr/climat-passe-et-futur/bilans-climatiques/bilan-2016/bilan-climatique-de-l-annee-2016
- 34. Lohr B, Müller I, Mai M, Norris DE, Schöffski O, Hunfeld KP. Epidemiology and cost of hospital care for Lyme borreliosis in Germany: lessons from a health care utilization database analysis. Ticks Tick Borne Dis. 2015;6(1):56-62. 10.1016/j.ttbdis.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 35.Dahl V, Tegmark-Wisell K, Tegnell A, Wallensten A. The national hospital discharge diagnosis registry is the most suitable data source for surveillance of Lyme borreliosis in Sweden. Poster presentation at the European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE), 11-13 November 2015, Stockholm. [Google Scholar]
- 36.Claudine Pérez‐Eid. Les tiques. Identification, biologie, importance médicale et vétérinaire. [Ticks - Identification, biology, medical and veterinary importance]. Monographie de microbiologie, Tec & Doc EMinter‐Lavoisier ; 2007. Available from: https://www.unitheque.com/Livre/tec_et_doc_-_em_inter_-_lavoisier/Monographies_de_microbiologie/Les_tiques-12028.html
- 37.European Centre for Disease Prevention and Control (ECDC). Ixodes ricinus - current known distribution: October 2017. Stockholm: ECDC; 2017. Available from: https://ecdc.europa.eu/en/publications-data/ixodes-ricinus-current-known-distribution-october-2017
- 38. Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George JC, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6(1):1. 10.1186/1756-3305-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van den Wijngaard CC, Hofhuis A, Simões M, Rood E, van Pelt W, Zeller H, et al. Surveillance perspective on Lyme borreliosis across the European Union and European Economic Area. Euro Surveill. 2017;22(27):30569. 10.2807/1560-7917.ES.2017.22.27.30569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sante publique France – Dossier thématique Borreliose de Lyme. Saint-Maurice: Sante publique France; [Cited 2018]. French. Available from: http://invs.santepubliquefrance.fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-transmission-vectorielle/Borreliose-de-lyme/Points-sur-les-connaissances
- 41. Mygland A, Ljøstad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I, European Federation of Neurological Societies EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol. 2010;17(1):8-16, e1-4. 10.1111/j.1468-1331.2009.02862.x [DOI] [PubMed] [Google Scholar]
- 42. Fonteneau L, Le Meur N, Cohen-Akenine A, Pessel C, Brouard C, Delon F, et al. Apport des bases médico-administratives en épidémiologie et santé publique des maladies infectieuses [The use of administrative health databases in infectious disease epidemiology and public health]. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S174-82. 10.1016/j.respe.2017.03.131 [DOI] [PubMed] [Google Scholar]


