Abstract
The natriuretic peptides (NPs) B-type NP (BNP) and urodilatin (URO) exert renal protective properties via the particulate guanylyl cyclase A receptor (pGC-A). As a potential renal-enhancing strategy, we engineered a novel designer peptide that we call CRRL269. CRRL269 was investigated in human cell lines and in normal canines to define potential cardiorenal enhancing actions. The mechanism of its cardiorenal selective properties was also investigated. In vitro NP receptor activity was quantified with guanosine 3′,5′-cyclic monophosphate generation. In vivo effects were determined in normal canine acute infusion studies. We observed that CRRL269 demonstrated enhanced pGC-A activity in renal compared with nonrenal cell lines. CRRL269 exerted enhanced resistance to neprilysin compared with URO. Importantly, CRRL269 exhibited significant and greater increases in urinary sodium excretion and diuresis, with less blood pressure reduction, than BNP or URO in normal canines. CRRL269 retained potent renin-angiotensin-aldosterone system (RAAS) suppressing properties shared by URO and BNP. Also, CRRL269 exerted less arterial relaxation and higher cAMP cardiomyocytes generation than BNP. CRRL269 possessed superior renal and pGC-A activating properties compared with BNP or URO in vitro. CRRL269 exerted enhanced renal actions while suppressing RAAS in vivo and with less hypotension compared with URO or BNP. Together, our study suggests that CRRL269 is a promising innovative renal-enhancing drug, with favorable protective actions targeting cardiorenal disease states through the pGC-A receptor.
Keywords: cGMP, natriuretic peptide, NEP, pGC-A, renal selective
INTRODUCTION
The heart synthesizes two structurally similar but genetically distinct peptides, atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), which have broad diagnostic and therapeutic importance in cardiovascular, renal, and metabolic disease (14, 16, 39). Studies have established that ANP and BNP are potent activators of the particulate guanylyl cyclase A receptor (pGC-A), which is widely expressed in multiple tissues, especially in the kidney (4, 35, 38). Importantly, both cardiac hormones mediate their biological actions through pGC-A generation of the second messenger guanosine 3′,5′-cyclic monophosphate (cGMP). To date, it is well established that the natriuretic peptide (NP)/pGC-A/cGMP system mediates a variety of favorable biological actions, including glomerular filtration rate (GFR) enhancing, natriuretic, diuretic, anti-renin-angiotensin-aldosterone system (RAAS), vasodilating and antifibrotic effects (9, 12, 14, 18, 24, 25, 29, 30). Notably, when compared with ANP, BNP is more resistant to degradation to the enzyme neprilysin (NEP), which is highly expressed in the proximal tubular brush border membranes in the kidney (3).
Urodilatin (URO) is a 32 amino acid (AA) NP that is of renal origin and processed from proANP within the kidney (7). Its structure contains the 28 AA sequence of ANP, but with 4 additional AAs at the end of the NH2 terminal. URO has been shown to have greater renal-enhancing properties than ANP, also through the pGC-A/cGMP pathway (31). With reports of URO being produced in the kidney, others have advanced the concept that URO may function as the renal component of a novel cardiorenal hormonal cGMP-mediated system (1, 11).
Advances in peptide engineering have led to novel designer NPs that possess actions that go beyond the native NPs. Such designer peptides contain unique AA sequences that provide attractive biological properties making them potential innovative peptide therapeutics (20). One major goal for NP therapeutics is to develop more renal-selective and/or potent peptides that possess enhanced natriuretic and diuretic properties with preservation or enhancement of GFR, while retaining RAAS-suppressing actions. Optimally, such peptides would also possess less hypotensive properties, which has limited the therapeutic use of NPs such as BNP (i.e., nesiritide). Thus the present study was undertaken to develop a novel cardiorenal therapeutic designer NP that we call CRRL269. Our goal was to engineer an innovative peptide that possesses more renal-enhancing properties compared with BNP and URO, but retain RAAS-suppressing properties with less hypotension. Specifically, we hypothesized that integrating key AA sequences of BNP (a cardiac derived peptide), with key AAs of URO (a renal-derived peptide), would generate superior renal pGC-A activation in vitro and mediate renal-enhancing properties in vivo compared with the two respective native NPs.
After successful synthesis, we first defined the ability of CRRL269 to activate pGC-A and cGMP in human embryonic kidney 293 (HEK293) cells overexpressing human pGC-A receptors and in primary human renal (proximal tubular cells) and nonrenal cell-type (endothelial) cells in vitro. We also investigated its degradation by NEP to define the potential renal selectivity of CRRL269. Furthermore, we compared CRRL269 renal, hemodynamic, and neurohormonal actions to the native peptides BNP and URO. After the discovery of its novel cardiorenal properties, we investigated CRRL269-mediated vasorelaxation of canine arterial rings compared with BNP and compared cAMP production in cultured human cardiomyocytes.
METHODS
Studies were performed in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Institutional Animal Care and Use Committee.
Natriuretic peptide synthesis.
BNP, URO, and CRRL269 were synthesized at Phoenix Pharmaceuticals (Burlingame, CA) by solid-phase peptide synthesis method. The Cys-Cys disulfide bond was formed by oxidation after synthesis, and all peptides had 32 AAs.
The BNP AA sequence was SPKMVQGSGCFGRKMDRISSSSGLGCKVLRRH, URO AA sequence was TAPRSLRRSSCFGGRMDRIGAQSGLGCNSFRY, and CRRL269 AA sequence was TAPRSLRRSSCFGRKMDRISSSSGLGCNSFRY. Structures were confirmed by mass spectrometry, and high-performance liquid chromatography analysis confirmed purity to be >95%.
In vitro cGMP activation in HEK293 human pGC-A and pGC-B transfection cells.
HEK293 cells were stably transfected with either human pGC-A or pGC-B (cDNA clones from Origene, Rockville, MD) using Lipofectamine (Invitrogen, Grand Island, NY). Particulate GC-B, another receptor that selectively binds CNP, but not BNP or URO, was used as a control for ligand-receptor selectivity. Transfected cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 U/ml streptomycin, and 250 µg/ml G418.
Cells were plated in six-well plates and cultured for 2 days (80–90% confluency) before treatment. The treatment buffer included 0.5 mM 3-isobutyl-1-methylzanthine (Sigma, St. Louis, MO). HEK293 pGC-A cells received treatment buffer (negative control), 10−11, 10−10, 10−9 10−8 M, or 10−7 M ANP/BNP/URO/CRRL269 for 10 min, and HEK293 pGC-B cells received treatment buffer (negative control), 10−8 M CRRL269, or 10−8 M CNP for 10 min. Cells were lysed and sonicated, and cell lysates were then centrifuged; the supernatants were ether extracted, dried, and reconstituted in 300 µl cGMP assay buffer. The samples were assayed using a radioimmunoassay (RIA) cGMP kit (PerkinElmer, Boston, MA).
In vitro cGMP activation in human primary renal and nonrenal cells.
Human renal proximal tubular epithelial cells (HRPTCs) (ScienCell Research Laboratories, Carlsbad, CA) and human aortic endothelial cells (HAECs) (Lonza, Walkersville, MD) were maintained and subcultured according to the manufacturer’s protocols. Endothelial cells were chosen as a nonrenal cell type for the purposes of quantification and comparison of cGMP activating properties by different NPs. Cells were treated with buffer (negative control), 10−9 M, 10−8 M, or 10−7 M BNP, URO, or CRRL269 for 10 min, and then cell lysates were collected. Cellular cGMP measurement by RIA is described above.
In vitro neprilysin degradation assay.
In vitro NEP resistance contributes to enhanced renal actions. Thus we assessed CRRL269 degradation by NEP in vitro. BNP, URO, or CRRL269 degradation by NEP (R&D Systems, Minneapolis, MN) were determined by cGMP generation in HEK293 pGC-A cells (6). One micromolar of peptide was incubated with 50 ng recombinant NEP in Tris-0.1% BSA buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 0.1% bovine serum albumin). The incubation temperature was 37°C. At each time point, equal volume of perchloric acid (0.5 N) was added to the reaction aliquot to inactivate the enzyme and stop degradation, while 2.5 N NaOH was used to neutralize the reaction. Residual peptide after degradation was added to HEK293 human pGC-A cells to determine the capability to generate cGMP (as described above).
Ex vivo arterial ring relaxations.
To assess vasorelaxation, femoral arteries were harvested from normal mongrel canines, flushed with Krebs solution, and stored in Krebs solution (composition in mM: NaCl 119, KCl 5.4, CaCl2 2.5, KH2PO4 0.6, MgSO4 1.2, NaHCO3 25, and glucose 11.7) at 4°C. Vessels were cleaned from the surrounding connective tissue, and 2-mm rings of vessel were dissected and were transferred to organ chambers with 3 ml Krebs solution (37°C; pH 7.4) and oxygenated with 95% O2-5% CO2. The rings were suspended between two stirrups and connected to a strain gauge for continuous recording of isometric tension. After equilibration for 1 h at a resting tension, all vessels were examined for viability by a contractile response to 20 mM KCl at baseline and at 2, 4, and 6 g each time after the KCl had been washed out. The vessels were washed with fresh Krebs solution and incubated for an additional 30 min. Next, all vessels were contracted with 10−5 M phenylepherine followed by relaxation with cumulative concentrations (10−10 to 10−6 M) of BNP or CRRL269 (28).
In vitro cAMP generation in cardiomyocytes.
As cAMP mediates enhanced inotropic effects in cardiomyocytes, we determined cAMP generation in human cardiomyocytes (HCMs). HCMs (ScienCell Research Laboratories, Carlsbad, CA) were plated in 96-well plates and were treated with 10−8, 10−6 M BNP, or CRRL269 for 10 min, and cell lysates were collected for cAMP measurements. The cAMP Glo assay (Promega, Madison, WI) was performed according to manufacturer’s instructions.
In vivo studies in normal canines.
Studies were performed in normal male mongrel canines (21 to 30 kg, n = 5 for each group). The in vivo protocol has been described previously (19). Briefly, dogs were anesthetized, intubated, and mechanically ventilated on the day of experiment. A flow-directed balloon-tipped thermodilution catheter was advanced to the pulmonary artery through the external jugular vein for measurement of cardiac filling pressures and cardiac output (CO). The femoral artery was cannulated for mean arterial pressure (MAP) monitoring and blood sampling. The femoral vein was cannulated for inulin and saline/peptide infusion. A calibrated electromagnetic flow probe (Carolina Medical Electronics, East Bend, NC) was placed around the renal artery to measure renal blood flow. The study protocol started with the administration of a weight-adjusted inulin bolus. Continuous inulin and saline infusions at a rate of 1 ml/min each were started. After 60 min of equilibrium, a baseline clearance was performed. All clearances lasted 30 min and consisted of urine collection over 30 min. Hemodynamic measurements were measured at each clearance, and arterial blood was drawn. After the baseline clearance, the saline infusion was replaced by low-dose NP (BNP, URO, or CRRL269, 2 pmol·kg−1·min−1) and infused for 45 min (15 min lead in period followed by 30 min clearance). After low-dose NP infusion, canines were infused with high-dose NP (BNP, URO or CRRL269, 33 pmol·kg−1·min−1) for 45 min. Peptide infusion was then discontinued, and three 30-min clearances were performed (washout, recovery 1, and recovery 2). Systemic vascular resistance (SVR) was calculated as (MAP minus right atrial pressure) divided by CO. GFR was measured by inulin clearance. Urinary excretion of cGMP or sodium was determined by cGMP/sodium concentration × urine volume rate (ml/min).
Hormone measurements.
Plasma and urinary samples for cGMP were measured by RIA after extraction (PerkinElmer, Waltham, MA). Plasma renin activity was determined by RIA (Diasorin, Stillwater, MN), and plasma angiotensin II was measured by RIA (Phoenix Pharmaceuticals). Plasma aldosterone was determined by ELISA kits (DRG International, Springfield, NJ). All measurements followed the manufacturer’s instructions. Inulin concentrations were measured using the anthrone method for GFR analysis. Electrolytes, including sodium and potassium, were measured by flame photometry (IL943, Instrumentation Laboratory, London, UK).
Statistical analysis.
Data are expressed as means ± SE. Unpaired t-test was performed for comparison between groups. Comparisons within a group were made by one-way analysis of variance (ANOVA) for repeated measures followed by Dunnett’s posttests. Two-way ANOVA was used to compare the main group effects of BNP, URO, and CRRL269, followed by Bonferroni posttests. GraphPad Prism 5 (GraphPad Software, La Jolla, CA) was used for the above calculations, and statistical significance was accepted as P < 0.05.
RESULTS
CRRL269 selective pGC-A activity in HEK293 cells transfected with human pGC-A and pGC-B receptors.
CRRL269 possessed the highest potency in activating pGC-A and generating significantly higher cGMP levels than BNP or URO (Fig. 1). All peptides increased cGMP levels compared with no treatment group. Importantly, at the low concentrations (10−11, 10−10, and 10−9 M), CRRL269 generated significantly higher cGMP values compared with ANP, BNP, or URO. At 10−8 M, both CRRL269 and URO activated cGMP greater than BNP. At a higher concentration than 10−7 M, all peptides induced comparable high amount of cGMP. Also, we observed that in HEK293 cells overexpressing pGC-B receptors, 10−8M CRRL269 induced minimal cGMP levels, comparable to the vehicle treatment (1.2 ± 0.003 vs. 0.5 ± 0.002 pmol/well) and was markedly lower in value compared with 10−8M CNP (40.1 ± 1.79 pmol/well). Together, our in vitro HEK293 cell studies demonstrated that CRRL269 is a selective and potent pGC-A activator that possesses greater cGMP-generating properties than BNP or URO at low concentrations and mimics URO at higher doses.
Fig. 1.
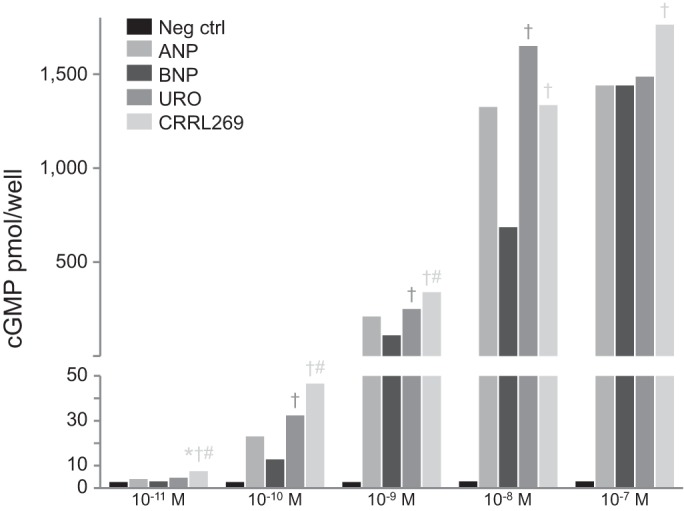
Generation of guanosine 3′,5′-cyclic monophosphate (cGMP) in human embryonic kidney 293 (HEK293) cells overexpressing particulate guanylyl cyclase A (pGC-A) receptors stimulated by atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), urodilatin (URO), and CRRL269. Five doses including 10−11, 10−10, 10−9, 10−8, and 10−7 M were used. *P < 0.05 vs. negative control, †P < 0.05 vs. BNP, #P < 0.05 vs. URO.
CRRL269 cGMP activation in human renal tubular and endothelial cells.
We observed an incremental cGMP generation dose response with increasing concentrations of CRRL269 in HRPTCs and HAECs (Fig. 2). Specifically, as observed in HEK 293 pGC-A overexpressing cells, CRRL269 was the only NP to activate cGMP in HRPTCs at a lower dose (10−9 M) compared with BNP or URO. At the highest dose (10−7 M), CRRL269 remained the most potent activator of cGMP (Fig. 2). A similar pattern was observed in HAECs, although cGMP generation was markedly less with all NPs compared with the robust generation of cGMP observed in HRPTCs.
Fig. 2.
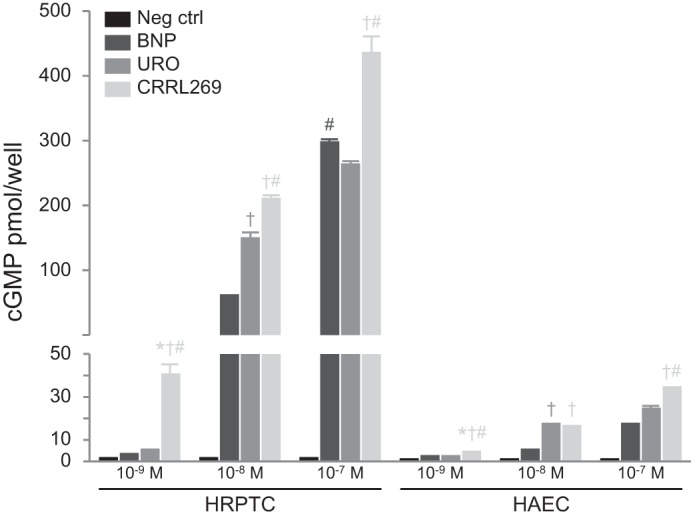
Generation of cGMP in human renal proximal tubular epithelial cells (HRPTCs) and human aortic endothelial cells (HAECs) stimulated by BNP, URO, and CRRL269. Three doses including 10−9, 10−8, and 10−7 M were used. *P < 0.05 vs. negative control, †P < 0.05 vs. BNP, #P < 0.05 vs. URO.
CRRL269 NEP degradation in vitro.
We then assessed BNP, URO, and CRRL269 degradation by NEP in vitro (Fig. 3). CRRL269 exerted prolonged activity and improved ability to resist degradation compared with URO. CRRL269 lost its activity at 240 min compared with URO whose degradation was complete at 120 min. BNP was the most resistant of the three peptides to NEP degradation.
Fig. 3.
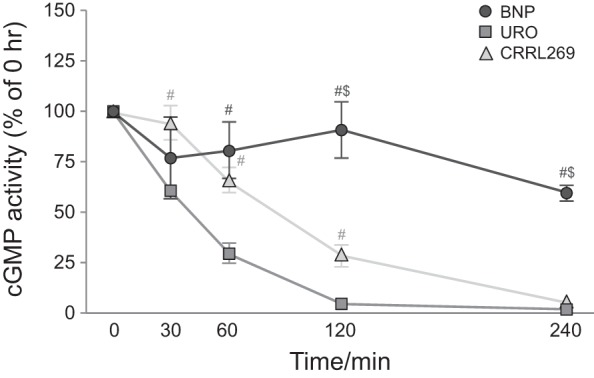
In vitro neprilysin (NEP) degradation curve of BNP, URO, and CRRL269 quantified by cGMP-generating activity. #P < 0.05 vs. URO, $P < 0.05 vs. CRRL269.
CRRL269 renal actions in normal canines.
In normal canine studies, we evaluated the acute renal responses to CRRL269 compared with BNP and URO infusion at low and high doses. We observed that CRRL269 is biologically active in vivo. Specifically, CRRL269 produced a significant increase in urine flow (UV) and urinary sodium excretion (UNaV), which was similar to URO, but greater than BNP (Fig. 4, A and B). Importantly, CRRL269 produced a more sustained increase in both UV and UNaV during the washout and recovery periods, which supports its greater diuretic and natriuretic actions than native BNP and URO. Variability was observed with CRRL269 in GFR, with an increase observed in GFR during high-dose infusion and during the last washout, which may reflect the lower reduction in blood pressure with CRRL296 compared with BNP or URO (Fig. 5A). Less variability was observed in renal blood flow, which increased from baseline during high-dose infusion of all three peptides and remained increased during washout (Fig. 5B). Together, our in vivo data suggests that CRRL269 was the most potent in enhancing renal actions of the three peptides, with renal actions most similar to URO.
Fig. 4.
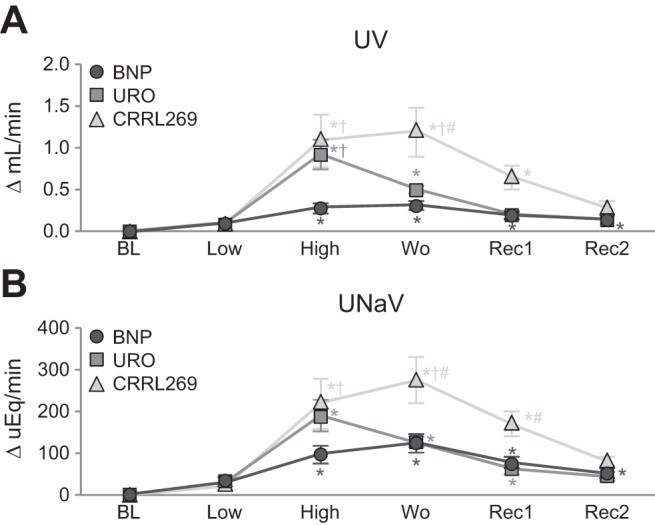
Urinary flow (UV) (A) and urinary sodium excretion (UNaV) (B) after infusion of low- 2 pmol·kg−1·min−1 and high-dose 33 pmol·kg−1·min−1 BNP, URO, or CRRL269 in normal canines. Data are calculated from the difference from baseline. BL, baseline; Low, infusion of low-dose 2 pmol·kg−1·min−1 BNP, URO, or CRRL269; High, infusion of high-dose 33 pmol·kg−1·min−1; Wo, washout (0–30 min postinfusion); Rec 1, recovery 1, 30–60 min postinfusion; Rec 2, recovery 2, 60–90 min postinfusion. *P < 0.05 vs. baseline (one-way ANOVA and Dunnett posttests), †P < 0.05 vs. BNP, #P < 0.05 vs. URO (two-way ANOVA and Bonferroni post hoc tests).
Fig. 5.

Glomerular filtration rate (GFR) (A) and renal blood flow (RBF) (B) after infusion of low-dose 2 pmol·kg−1·min−1 and high-dose 33 pmol·kg−1·min−1 BNP, URO, or CRRL269 in normal canines; data analysis and abbreviations as in Fig. 4.
CRRL269 hemodynamics and neurohormonal properties.
MAP and pulmonary capillary wedge pressure (PCWP) are illustrated in Fig. 6, A and B. At low dose only BNP reduced MAP. At high dose all three peptides reduced MAP; however, CRRL269 was less hypotensive than either URO or BNP. Furthermore, all three peptides reduced PCWP. These results demonstrated that CRRL269 unloaded the heart with less hypotension compared with BNP or URO. At high dose, CRRL269 was similar to BNP in increasing CO compared with URO (Fig. 7A). All three peptides reduced SVR compared with baseline (Fig. 7B). Although CRRL269 was less hypotensive than BNP or URO, its CO action was similar to BNP.
Fig. 6.

Mean arterial pressure (MAP) (A) and pulmonary capillary wedge pressure (PCWP) (B) after infusion of low- and high-dose peptides in normal canines; data analysis and abbreviations as in Fig. 4.
Fig. 7.
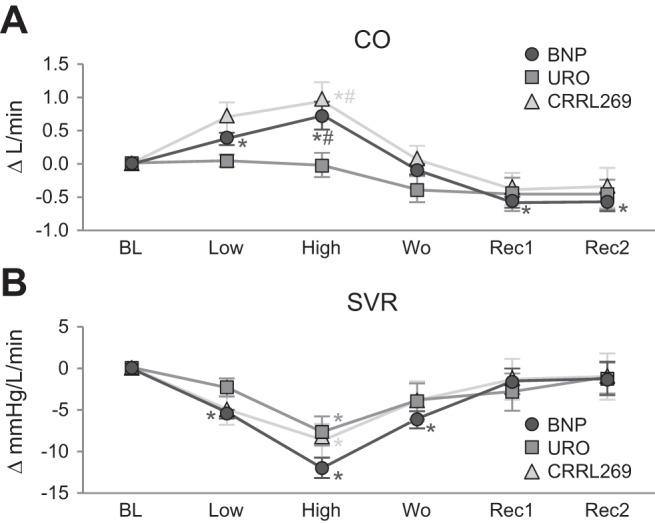
Cardiac output (CO) (A) and systemic vascular resistance (SVR) (B) after infusion of low-dose 2 pmol·kg−1·min−1 and high-dose 33 pmol·kg−1·min−1 BNP, URO, or CRRL269 in normal canines; data analysis and abbreviations as in Fig. 4.
Plasma and urinary cGMP and RAAS parameters are summarized in Table 1 (absolute values of renal and hemodynamic parameters are summarized in Table 2). There was a trend for higher plasma cGMP (PcGMP) with CRRL269 than with the other two peptides. CRRL269 resulted in a greater and more sustained increase in urinary cGMP excretion rate than URO, although it was lower than BNP. RAAS parameters were similar among the three peptide groups with suppression of plasma renin activity and aldosterone. Of note, aldosterone was significantly reduced in all timed phases during BNP and CRRL269 infusion compared with baseline.
Table 1.
cGMP and neurohormonal function with BNP, URO, and CRRL269
| Natriuretic Peptide Infusion |
|||||||
|---|---|---|---|---|---|---|---|
| Variable | Peptides | Baseline | Low dose | High dose | Washout | Recovery 1 | Recovery 2 |
| Plasma cGMP, nmol/ml | BNP | 5.7 ± 1.1 | 13.0 ± 2.4* | 37.8 ± 3.7* | 30.9 ± 3.1* | 20.9 ± 1.2* | 15.1 ± 1.2* |
| URO | 7.4 ± 1.2 | 9.8 ± 1.6 | 42.9 ± 7.9* | 25.5 ± 3.0* | 13.0 ± 2.0 | 9.7 ± 2.4 | |
| CRRL269 | 5.3 ± 1.0 | 16.9 ± 2.8 | 49.0 ± 7.6* | 37.3 ± 4.5* | 20.5 ± 0.8* | 12.8 ± 0.9 | |
| Urinary cGMP, pmol/min | BNP | 904.3 ± 99.9 | 2,285.5 ± 453.4 | 15,343.6 ± 1,110.3#* | 15,028.0 ± 2,635.6#* | 11,429.2 ± 1,578.4#* | 4,844.7 ± 873.4 |
| URO | 914.7 ± 136.2 | 1,300.4 ± 201.4 | 6,022.8 ± 1,060.1* | 5,646.7 ± 1,056.6* | 2,770.5 ± 443.6 | 1,497.0 ± 185.4 | |
| CRRL269 | 816.2 ± 84.3 | 1,899.2 ± 284.3 | 11,330.9 ± 1,597.9#* | 11,482.3 ± 1,634.9#* | 5,491.2 ± 659.1* | 2,997.6 ± 298.7 | |
| Plasma renin activity, pg·ml−1·h−1 | BNP | 4.7 ± 1.2 | 1.9 ± 0.7* | 2.2 ± 0.7* | 1.9 ± 0.7* | 3.3 ± 1.1 | 5.7 ± 1.7 |
| URO | 3.7 ± 0.9 | 1.7 ± 0.7 | 0.8 ± 0.02* | 1.4 ± 0.4* | 3.6 ± 0.6 | 5.8 ± 1.1* | |
| CRRL269 | 4.9 ± 0.8 | 2.1 ± 0.2* | 1.2 ± 0.4* | 0.9 ± 0.1* | 2.4 ± 0.5* | 5.0 ± 1.4 | |
| Plasma Aldo, ng/dl | BNP | 17.6 ± 5.5 | 7.3 ± 1.9* | 5.4 ± 1.6* | 3.6 ± 0.3* | 4.7 ± 1.5* | 8.0 ± 3.7* |
| URO | 13.0 ± 4.4 | 9.0 ± 4.3 | 5.1 ± 1.4* | 4.4 ± 0.9* | 6.7 ± 0.90 | 11.2 ± 2.0 | |
| CRRL269 | 18.1 ± 3.9 | 7.4 ± 1.0* | 5.5 ± 0.5* | 5.5 ± 0.5* | 4.0 ± 0.4* | 5.5 ± 1.0* | |
Data are expressed as means ± SE. Plasma and urinary guanosine 3′,5′-cyclic monophosphate (cGMP), plasma renin activity, and aldosterone levels were measured after infusion of low-dose 2 pmol·kg−1·min−1 and high-dose 33 pmol·kg−1·min−1 B-type natriuretic peptide (BNP), urodilatin (URO), or CRRL269 in normal canines. Low dose, infusion of low-dose 2 pmol·kg−1·min−1 BNP, URO, or CRRL269; High dose, infusion of high-dose 33 pmol·kg−1·min−1; Washout, 0–30 min postinfusion; Recovery 1 = 30–60 min postinfusion; Recovery 2 = 60–90 min postinfusion. Aldo, aldosterone.
P < 0.05 vs. URO (two-way ANOVA and Bonferroni post hoc tests),
P < 0.05 vs. baseline (one-way ANOVA and Dunnett posttests).
Table 2.
Renal function and hemodynamics with BNP, URO, and CRRL269
| Natriuretic Peptide Infusion |
|||||||
|---|---|---|---|---|---|---|---|
| Variable | Peptides | Baseline | Low dose | High dose | Washout | Recovery 1 | Recovery 2 |
| UV, ml/min | BNP | 0.13 ± 0.01 | 0.23 ± 0.04 | 0.41 ± 0.06* | 0.45 ± 0.05* | 0.32 ± 0.03* | 0.27 ± 0.03* |
| URO | 0.15 ± 0.01 | 0.24 ± 0.01 | 1.07 ± 0.17*† | 0.65 ± 0.04* | 0.35 ± 0.05 | 0.28 ± 0.03 | |
| CRRL269 | 0.11 ± 0.01 | 0.18 ± 0.03 | 1.19 ± 0.32*† | 1.30 ± 0.29*†# | 0.75 ± 0.14* | 0.39 ± 0.17 | |
| UNaV, µEq/min | BNP | 16.3 ± 6.0 | 45.6 ± 10.8 | 112.9 ± 20.9* | 140.2 ± 21.9* | 93.5 ± 13.4* | 63.4 ± 12.0* |
| URO | 14.2 ± 3.9 | 45.4 ± 12.9 | 203.0 ± 38.6* | 140.6 ± 14.0* | 75.8 ± 10.8* | 57.4 ± 9.5 | |
| CRRL269 | 11.5 ± 3.8 | 31.0 ± 1.81 | 230.2 ± 59.5*† | 283.9 ± 55.7*†# | 180.2 ± 28.9*# | 90.5 ± 9.7 | |
| GFR, ml/min | BNP | 54.5 ± 14.3 | 61.6 ± 18.9 | 55.5 ± 12.7 | 48.0 ± 7.4 | 47.2 ± 6.9 | 57.5 ± 5.5 |
| URO | 38.1 ± 8.9 | 60.6 ± 8.8 | 45.3 ± 8.4 | 43.7 ± 9.7 | 60.8 ± 10.9 | 51.4 ± 7.5 | |
| CRRL269 | 28.1 ± 4.3 | 41.5 ± 11.4 | 69.5 ± 9.5* | 53.1 ± 5.8 | 53.1 ± 8.7 | 65.9 ± 10.8* | |
| RBF, ml/min | BNP | 252.7 ± 26.3 | 254.5 ± 24.4 | 336.7 ± 14.4* | 335.0 ± 16.0* | 305.0 ± 17.4* | 302.4 ± 18.5 |
| URO | 235.0 ± 26.0 | 256.5 ± 30.8 | 306.1 ± 36.6* | 308.8 ± 32.1* | 310.4 ± 36.0* | 293.5 ± 39.8* | |
| CRRL269 | 254.1 ± 34.2 | 258.1 ± 36.1 | 319.0 ± 56.3* | 364.8 ± 58.0* | 355.1 ± 50.9* | 349.5 ± 43.6* | |
| MAP, mmHg | BNP | 124.9 ± 4.4 | 113.8 ± 3.4* | 92.3 ± 2.5* | 97.2 ± 2.7* | 99.3 ± 5.0* | 99.4 ± 5.5* |
| URO | 114.8 ± 4.5 | 107.2 ± 2.7 | 84.8 ± 4.2* | 87.6 ± 3.1* | 89.4 ± 4.1* | 95.7 ± 4.4* | |
| CRRL269 | 118.1 ± 3.8 | 116.8 ± 8.6 | 103.8 ± 9.1* | 101.4 ± 7.8* | 103.3 ± 6.0* | 105.5 ± 6.7* | |
| PCWP, mmHg | BNP | 6.5 ± 0.6 | 5.5 ± 0.6* | 3.4 ± 0.3* | 2.9 ± 0.04* | 3.2 ± 0.5* | 3.9 ± 0.5* |
| URO | 5.7 ± 0.9 | 4.7 ± 1.1 | 3.0 ± 0.7* | 2.7 ± 0.6* | 3.2 ± 0.7* | 3.5 ± 0.9* | |
| CRRL269 | 6.6 ± 0.3 | 5.5 ± 0.6* | 3.0 ± 0.4* | 2.8 ± 0.4* | 3.2 ± 0.4* | 3.6 ± 0.5* | |
| CO, l/min | BNP | 3.8 ± 0.1 | 4.2 ± 0.2* | 4.5 ± 0.2* | 3.7 ± 0.1 | 3.2 ± 0.1* | 3.2 ± 0.1* |
| URO | 3.7 ± 0.3 | 3.7 ± 0.2 | 3.7 ± 0.3 | 3.3 ± 0.3 | 3.2 ± 0.3 | 3.2 ± 0.2 | |
| CRRL269 | 4.1 ± 0.4 | 4.8 ± 0.4 | 5.0 ± 0.5*# | 4.1 ± 0.5 | 3.7 ± 0.3 | 3.7 ± 0.2 | |
| SVR, mmHg·l−1·min−1 | BNP | 32.0 ± 1.5 | 26.6 ± 1.4* | 20.0 ± 1.0* | 25.8 ± 1.0* | 30.5 ± 1.5 | 30.8 ± 1.7 |
| URO | 30.5 ± 1.7 | 28.1 ± 1.0 | 23.0 ± 1.4* | 26.5 ± 1.7 | 27.6 ± 1.7 | 29.4 ± 1.3 | |
| CRRL269 | 29.2 ± 2.9 | 24.1 ± 2.0 | 20.6 ± 1.7* | 25.3 ± 3.2 | 27.9 ± 2.4 | 28.3 ± 2.6 | |
Data are expressed as means ± SE. Absolute values of key parameters after infusion of low-dose 2 pmol·kg−1·min−1 and high-dose 33 pmol·kg−1·min−1 B-type natriuretic peptide (BNP), urodilatin (URO), or CRRL269 in normal canines. Low dose, infusion of low-dose 2 pmol·kg−1·min−1 BNP, URO, or CRRL269; High dose, infusion of high-dose 33 pmol·kg−1·min−1; Washout, 0–30 min postinfusion; Recovery 1 = 30–60 min postinfusion; Recovery 2 = 60–90 min postinfusion. UV, urinary flow; UNaV, urinary sodium excretion; GFR, glomerular filtration rate; RBF, renal blood flow; MAP, mean arterial pressure; PCWP, pulmonary capillary wedge pressure; CO, cardiac output; SVR, systemic vascular resistance.
P < 0.05, vs. baseline (one-way ANOVA and Dunnett posttests),
P < 0.05, vs. BNP,
P < 0.05, vs. URO (two-way ANOVA and Bonferroni post hoc tests).
CRRL269 arterial ring relaxation ex vivo.
We also measured arterial relaxation in precontracted canine femoral arteries. From concentrations 10−10 to 10−6 M, CRRL269 induced less arterial relaxation compared with BNP, as shown in Fig. 8A.
Fig. 8.
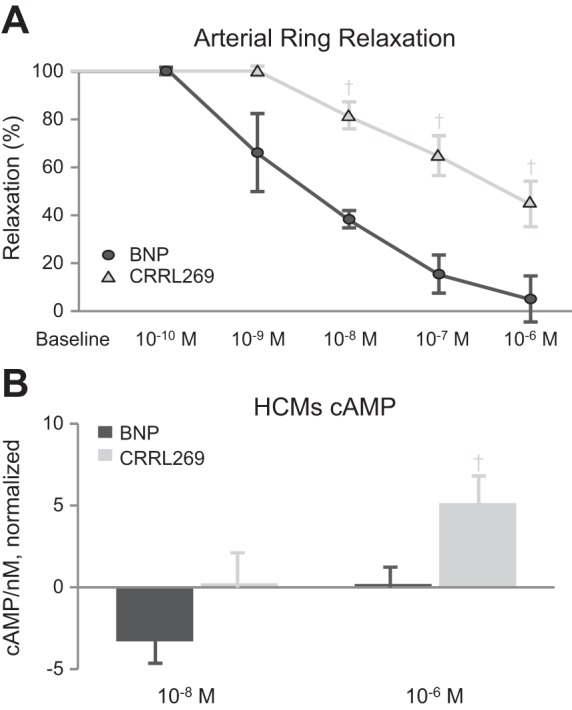
A: ex vivo arterial relaxation by BNP and CRRL269 in normal canine femoral arterial rings. B: in vitro cAMP levels of BNP and CRRL269 in human cardiomyocytes (HCMs). †P < 0.05 vs. BNP.
CRRL269 cAMP generation in cardiomyocytes.
We investigated BNP and CRRL269 generation of cAMP, an important second messenger for inotropic function in HCMs in vitro. At 10−8 M, there was a trend that CRRL269 generated more cAMP than BNP (Fig. 8B). At 10−6 M, CRRL269 generated higher cAMP levels than BNP.
DISCUSSION
In this study a novel renal-selective pGC-A activator, CRRL269, was engineered, synthesized, and tested in vitro and in vivo and compared with two native pGC-A agonists, BNP and URO. We report that CRRL269 possesses the most potent cGMP activating properties in HEK293 cells overexpressing human pGC-A receptors compared with BNP and URO. Importantly, CRRL269 exerted more renal-selective receptor activity as it generated markedly higher cGMP in HRPTCs compared with nonrenal endothelial cells. We demonstrated that CRRL269 exerted resistance to NEP although less than BNP. Furthermore, CRRL269 induced the strongest diuretic and natriuretic actions, with less blood pressure (BP) reduction, in normal canines compared with BNP or URO. CRRL269 also had sustained aldosterone-suppressing and GFR-preserving actions. Finally, CRRL269 generated higher cAMP than BNP in vitro and exerted less arterial ring relaxation ex vivo. Our results support the conclusion that CRRL269 may represent a potential renal-enhancing therapeutic for cardiorenal disease states going beyond native pGC-A activators.
It is known that the pGC-A/cGMP pathway is a critical driver of water and sodium excretion with increases in GFR. Studies have also demonstrated that cGMP mediates its renal actions through cGMP-gated ion channels such as amiloride-sensitive cation channel and through protein kinase G (17, 32). Sodium retention observed with HS-142-1 (a pGC-A receptor antagonist) in canine studies clearly supports the role of pGC-A/cGMP in the renal actions of NPs (34). The enhanced cGMP-generating properties observed in renal cells and the trend of higher plasma cGMP generation by CRRL269 supports the critical role of cGMP in its enhanced renal actions. Importantly, during the infusion of all three peptides, the increase in diuresis was similar with CRRL269 and URO and greater for both compared with BNP. A similar pattern was observed for natriuresis. CRRL269 natriuresis and diuresis was, however, more sustained and persistent well into the washout and recovery periods compared with BNP or URO. Renal blood flow increased with all three peptides. Notably, GFR increased only with CRRL269 compared with baseline that was also observed at the end of the recovery period. The greater maintenance of BP and thus renal perfusion pressure may importantly be a key mechanism by which CRRL269 mediates the increase of GFR. Further studies are needed, such as the use of isolated glomeruli, to provide greater insights into the GFR-enhancing actions of CRRL269. We did not observe enhanced urinary cGMP levels in CRRL269 infusion compared with BNP. It is likely that the kidney cells and tissues efficiently utilized renal cGMP generated by CRRL269, thus less cGMP was excreted to the urine. Of note, additional cGMP-independent pathways may also contribute to natriuresis and diuresis (10), as reported by studies that demonstrate that novel ANP molecular forms may mediate natriuresis through inhibition of Na+-K+-ATPase channels and generation of prostaglandin E2 independent of cGMP (10).
A key property of pGC-A activation is the inhibition of both renin and aldosterone (5, 13). The mechanism(s) may involve direct activation of pGC-A in the adrenal gland and in the kidney, but also may involve a macula densa mechanism for renin suppression. CRRL269 retained RAAS-suppressing properties observed with URO and BNP. Thus the unique design of CRRL269 possesses favorable renal and adrenal properties beyond native BNP and URO, with more sustained natriuresis and diuresis together with GFR-enhancing and RAAS-suppressing actions.
NEP is a major NP-degrading enzyme and is highly expressed in the kidney (3). Previous studies reported that BNP is highly resistant to NEP degradation (6). Studies of URO and ANP also documented that the enhanced renal actions of URO compared with ANP were attributed, at least partly, to the improved resistance to NEP (1, 31). It is plausible to assume that CRRL269 retained the resistance to NEP degradation from the core AA sequences of BNP and/or URO. Here we report that CRRL269 possessed improved resistance to NEP compared with URO, but less than BNP, which also may a mechanism that contributes to CRRL269’s enhanced renal actions in vitro and in vivo.
The maintenance of BP by CRRL269 was somewhat unexpected and may be due to increased CO and less decline in SVR. It is plausible that enhanced inotropic function through cAMP generation and less pronounced vasorelaxation rendered CRRL269 less hypotensive than the other two native peptides. Indeed, one could speculate that the crosstalk between cGMP and cAMP may play a role in CO elevation by NPs. In addition to the hydrolysis by PDEs, cGMP also could modulate PDE activities (26). NPs were demonstrated to increase cardiac cAMP levels through a cGMP-PDE3-cAMP pathway (37). The cardiac cAMP levels generated by NPs thus could boost cardiac inotropic function and increase BP, and it is likely that CRRL269 maintained BP partially through cGMP-PDE3-cAMP pathway. Here we observed greater cAMP levels generated by CRRL269 compared with BNP supporting its BP-preserving effects. Additionally, we observed less of a decrease in SVR in the CRRL269 and URO infusion groups compared with BNP in vivo, which could be due to their more renal-selective properties. Our ex vivo study provided direct evidence of less vasorelaxation in isolated canine femoral arteries with CRRL269 compared with BNP.
Importantly, the greater and sustained renal actions of CRRL269 underscore its potential as a renal therapeutic drug. This is further supported by the RAAS-suppressing actions with less hypotension than BNP or URO. Indeed, the combination of renal-enhancing and RAAS-inhibiting actions possessed by CRRL269 may render it a potentially promising drug for acute kidney injury (AKI) therapeutics as well as in states of avid sodium and water retention used with or without a diuretic agent like furosemide. AKI is characterized by reduced GFR and urine output (2), and new drugs to enhance renal function are an unmet need. In human clinical trials, Sezai et al. (33) and Mentzer et al. (21) documented that ANP and BNP infusion exerted sustained renal benefits in patients undergoing cardiac surgery. Nevertheless, both ANP and BNP infusion were associated with unwanted arterial hypotension. On the basis of the superior renal-selective properties in vitro and in vivo of CRRL269 and less of a BP lowering action compared with BNP or URO, it is reasonable to suggest that CRRL269 may represent a novel drug for renoprotection in states of potential AKI; studies in relevant animal models are needed to confirm.
Recombinant BNP (nesiritide) was approved for acute HF (AHF) treatment in 2001, but in the pivotal ASCEND-HF Trial, nesiritide demonstrated no significant additive beneficial effects compared with standard therapy (23). One important reason is that the clinical benefits with nesiritide in ASCEND-HF may be limited because of excessive hypotension. This reduction in BP may result in reduced renal perfusion pressure, thus worsening kidney function and reinforcing the concept that BP preservation is critical during HF treatment (36). As a renal-derived NP, URO has less BP lowering effects compared with BNP. Most recently it was reported that in human AHF, ularitide exerted favorable physiological effects, but short-term treatment did not improve clinical composite end points or reduce long-term cardiovascular mortality (27). In the present study, CRRL269 has enhanced natriuresis and diuresis, which was more sustained than URO or BNP with less BP reductions compared with BNP or URO. Importantly, it also retained important anti-RAAS properties. These favorable properties suggest that CRRL269 may represent a novel drug for AHF, but perhaps long-term administration may be required to improve adverse outcomes (22).
As stated by Gardner (8), intriguing possibilities may exist for designing novel NPs with attractive therapeutic profiles based solely on the selection and synthesis of specific structural motifs from native NPs. Such designer NPs could result in profiles that are not available in natural-occurring NPs. Indeed, CRRL269 represents a novel new designer NP that possesses a more robust renal profile of URO or BNP, being a more natriuretic and diuretic peptide than either BNP or URO, both of which bind to pGC-A. It represents an engineered pGC-A agonist that possesses key renal-enhancing properties with less hypotension that go beyond URO or BNP alone.
The present study does have limitations. In vivo studies were performed in normal canines and not in models of renal or cardiovascular disease. The mechanism of the greater activation of cGMP in vitro requires future studies to measure pGC-A guanylyl cyclase activity (catalytic activation of pGC-A) and direct receptor binding. Also, immunogenicity of CRRL269 in humans was not determined. For peptide or protein therapeutics, immune responses such as specific antibody generation should be avoided, and our previous experience with cenderitide in our laboratory (15) suggests CRRL269 may also lack immunogenicity in humans. However, investigations of CRRL269 immunogenicity issues in humans should be performed in the future.
Perspectives and Significance
A novel renal-selective pGC-A activator, CRRL269, was designed and tested in vitro and in vivo. CRRL269 demonstrated enhanced renal actions such as natriuresis, diuresis, and increases in GFR, with less hypotension than BNP (nesiritide) and urodilatin (ularitide). The underlying mechanisms for its enhanced selective renal activity were due to enhanced resistance to NEP degradation, reduced vasorelaxation, and greater cardiac cAMP generation, as well as RAAS suppression. CRRL269 may represent a promising medicine for cardiorenal disease states, and future studies to investigate CRRL269 cardiorenal actions in animal disease models such as AKI and HF are warranted.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants PO1 HL-76611 and RO1 HL-36634, a Mayo Clinic Graduate School of Biological Sciences Fellowship, and American Heart Association Predoctoral Fellowship 16PRE30770009.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.C., S.J.S., T.I., and J.C.B. conceived and designed research; Y.C., G.J.H., B.K.H., S.R.I., D.M.H., G.E.H., L.M.M., S.P., and T.I. performed experiments; Y.C., S.P., S.J.S., and J.C.B. analyzed data; Y.C., B.K.H., S.J.S., T.I., and J.C.B. interpreted results of experiments; Y.C. and J.C.B. prepared figures; Y.C. and J.C.B. drafted manuscript; Y.C. and J.C.B. edited and revised manuscript; Y.C., G.J.H., B.K.H., S.R.I., D.M.H., G.E.H., L.M.M., S.P., S.J.S., T.I., and J.C.B. approved final version of manuscript.
REFERENCES
- 1.Abassi ZA, Golomb E, Agbaria R, Roller PP, Tate J, Keiser HR. Hydrolysis of iodine labelled urodilatin and ANP by recombinant neutral endopeptidase EC. 3.4.24.11. Br J Pharmacol 113: 204–208, 1994. doi: 10.1111/j.1476-5381.1994.tb16194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 380: 756–766, 2012. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Burnett JC Jr. Biochemistry, therapeutics, and biomarker implications of neprilysin in cardiorenal disease. Clin Chem 63: 108–115, 2017. doi: 10.1373/clinchem.2016.262907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28: 89–94, 1981. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 5.Demerath T, Staffel J, Schreiber A, Valletta D, Schweda F. Natriuretic peptides buffer renin-dependent hypertension. Am J Physiol Renal Physiol 306: F1489–F1498, 2014. doi: 10.1152/ajprenal.00668.2013. [DOI] [PubMed] [Google Scholar]
- 6.Dickey DM, Potter LR. Human B-type natriuretic peptide is not degraded by meprin A. Biochem Pharmacol 80: 1007–1011, 2010. doi: 10.1016/j.bcp.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feller SM, Gagelmann M, Forssmann WG. Urodilatin: a newly described member of the ANP family. Trends Pharmacol Sci 10: 93–94, 1989. doi: 10.1016/0165-6147(89)90199-5. [DOI] [PubMed] [Google Scholar]
- 8.Gardner DG. Designer natriuretic peptides. J Clin Invest 92: 1606–1607, 1993. doi: 10.1172/JCI116744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George I, Morrow B, Xu K, Yi GH, Holmes J, Wu EX, Li Z, Protter AA, Oz MC, Wang J. Prolonged effects of B-type natriuretic peptide infusion on cardiac remodeling after sustained myocardial injury. Am J Physiol Heart Circ Physiol 297: H708–H717, 2009. doi: 10.1152/ajpheart.00661.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunning ME, Brady HR, Otuechere G, Brenner BM, Zeidel ML. Atrial natriuretic peptide(31-67) inhibits Na+ transport in rabbit inner medullary collecting duct cells. Role of prostaglandin E2. J Clin Invest 89: 1411–1417, 1992. doi: 10.1172/JCI115730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch JR, Meyer M, Forssmann WG. ANP and urodilatin: who is who in the kidney. Eur J Med Res 11: 447–454, 2006. [PubMed] [Google Scholar]
- 12.Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest 111: 1399–1407, 2003. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito T, Yoshimura M, Nakamura S, Nakayama M, Shimasaki Y, Harada E, Mizuno Y, Yamamuro M, Harada M, Saito Y, Nakao K, Kurihara H, Yasue H, Ogawa H. Inhibitory effect of natriuretic peptides on aldosterone synthase gene expression in cultured neonatal rat cardiocytes. Circulation 107: 807–810, 2003. doi: 10.1161/01.CIR.0000057794.29667.08. [DOI] [PubMed] [Google Scholar]
- 14.Lee CY, Burnett JC Jr. Natriuretic peptides and therapeutic applications. Heart Fail Rev 12: 131–142, 2007. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee CY, Chen HH, Lisy O, Swan S, Cannon C, Lieu HD, Burnett JC Jr. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol 49: 668–673, 2009. doi: 10.1177/0091270009336233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 339: 321–328, 1998. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 17.Light DB, Corbin JD, Stanton BA. Dual ion-channel regulation by cyclic GMP and cyclic GMP-dependent protein kinase. Nature 344: 336–339, 1990. doi: 10.1038/344336a0. [DOI] [PubMed] [Google Scholar]
- 18.Martin FL, Sangaralingham SJ, Huntley BK, McKie PM, Ichiki T, Chen HH, Korinek J, Harders GE, Burnett JC Jr. CD-NP: a novel engineered dual guanylyl cyclase activator with anti-fibrotic actions in the heart. PLoS One 7: e52422, 2012. doi: 10.1371/journal.pone.0052422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKie PM, Cataliotti A, Huntley BK, Martin FL, Olson TM, Burnett JC Jr. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure-lowering, renal-enhancing, and aldosterone-suppressing actions. J Am Coll Cardiol 54: 1024–1032, 2009. doi: 10.1016/j.jacc.2009.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meems LMG, Burnett JC Jr. Innovative therapeutics: designer natriuretic peptides. JACC Basic Transl Sci 1: 557–567, 2016. doi: 10.1016/j.jacbts.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mentzer RM Jr, Oz MC, Sladen RN, Graeve AH, Hebeler RF Jr, Luber JM Jr, Smedira NG; NAPA Investigators . Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA Trial. J Am Coll Cardiol 49: 716–726, 2007. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor CM. Development of acute decompensated heart failure therapies: is the journey over? JACC Heart Fail 5: 464–465, 2017. doi: 10.1016/j.jchf.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 365: 32–43, 2011. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa Y, Mukoyama M, Yokoi H, Kasahara M, Mori K, Kato Y, Kuwabara T, Imamaki H, Kawanishi T, Koga K, Ishii A, Tokudome T, Kishimoto I, Sugawara A, Nakao K. Natriuretic peptide receptor guanylyl cyclase-A protects podocytes from aldosterone-induced glomerular injury. J Am Soc Nephrol 23: 1198–1209, 2012. doi: 10.1681/ASN.2011100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver PM, John SW, Purdy KE, Kim R, Maeda N, Goy MF, Smithies O. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci USA 95: 2547–2551, 1998. doi: 10.1073/pnas.95.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res 100: 309–327, 2007. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 27.Packer M, O’Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J; TRUE-AHF Investigators . Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 376: 1956–1964, 2017. doi: 10.1056/NEJMoa1601895. [DOI] [PubMed] [Google Scholar]
- 28.Pan S, Chen HH, Dickey DM, Boerrigter G, Lee C, Kleppe LS, Hall JL, Lerman A, Redfield MM, Potter LR, Burnett JC Jr, Simari RD. Biodesign of a renal-protective peptide based on alternative splicing of B-type natriuretic peptide. Proc Natl Acad Sci USA 106: 11282–11287, 2009. doi: 10.1073/pnas.0811851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandey KN. The functional genomics of guanylyl cyclase/natriuretic peptide receptor-A: perspectives and paradigms. FEBS J 278: 1792–1807, 2011. doi: 10.1111/j.1742-4658.2011.08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito H, Ogihara T, Nakamaru M, Hara H, Higaki J, Rakugi H, Tateyama H, Minamino T, Iinuma K, Kumahara Y. Hemodynamic, renal, and hormonal responses to alpha-human atrial natriuretic peptide in patients with congestive heart failure. Clin Pharmacol Ther 42: 142–147, 1987. doi: 10.1038/clpt.1987.124. [DOI] [PubMed] [Google Scholar]
- 31.Saxenhofer H, Raselli A, Weidmann P, Forssmann WG, Bub A, Ferrari P, Shaw SG. Urodilatin, a natriuretic factor from kidneys, can modify renal and cardiovascular function in men. Am J Physiol Renal Fluid Electrolyte Physiol 259: F832–F838, 1990. doi: 10.1152/ajprenal.1990.259.5.F832. [DOI] [PubMed] [Google Scholar]
- 32.Scavone C, Scanlon C, McKee M, Nathanson JA. Atrial natriuretic peptide modulates sodium and potassium-activated adenosine triphosphatase through a mechanism involving cyclic GMP and cyclic GMP-dependent protein kinase. J Pharmacol Exp Ther 272: 1036–1043, 1995. [PubMed] [Google Scholar]
- 33.Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, Wakui S, Kimura H, Shiono M, Takayama T, Hirayama A. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP Infusion Therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 58: 897–903, 2011. doi: 10.1016/j.jacc.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 34.Stevens TL, Burnett JC Jr, Kinoshita M, Matsuda Y, Redfield MM. A functional role for endogenous atrial natriuretic peptide in a canine model of early left ventricular dysfunction. J Clin Invest 95: 1101–1108, 1995. doi: 10.1172/JCI117757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature 332: 78–81, 1988. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 36.Vaduganathan M, Butler J, Pitt B, Gheorghiade M. Contemporary drug development in heart failure: call for hemodynamically neutral therapies. Circ Heart Fail 8: 826–831, 2015. doi: 10.1161/CIRCHEARTFAILURE.115.002271. [DOI] [PubMed] [Google Scholar]
- 37.Wen JF, Cui X, Jin JY, Kim SM, Kim SZ, Kim SH, Lee HS, Cho KW. High and low gain switches for regulation of cAMP efflux concentration: distinct roles for particulate GC- and soluble GC-cGMP-PDE3 signaling in rabbit atria. Circ Res 94: 936–943, 2004. doi: 10.1161/01.RES.0000123826.70125.4D. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox JN, Augustine A, Goeddel DV, Lowe DG. Differential regional expression of three natriuretic peptide receptor genes within primate tissues. Mol Cell Biol 11: 3454–3462, 1991. doi: 10.1128/MCB.11.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zois NE, Bartels ED, Hunter I, Kousholt BS, Olsen LH, Goetze JP. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol 11: 403–412, 2014. doi: 10.1038/nrcardio.2014.64. [DOI] [PubMed] [Google Scholar]


