Abstract
Pulmonary arterial hypertension (PAH) is a lethal disease characterized by progressive pulmonary vascular remodeling. The receptor for advanced glycation end products (RAGE) plays an important role in PAH by promoting proliferation of pulmonary vascular cells. RAGE is also known to mediate activation of Akt signaling, although the particular molecular mechanism remains unknown. This study aimed to identify the interacting partner of RAGE that could facilitate RAGE-mediated Akt activation and vascular remodeling in PAH. The progressive angioproliferative PAH was induced in 24 female Sprague-Dawley rats (n = 8/group) that were randomly assigned to develop PAH for 1, 2, or 5 wk [right ventricle systolic pressure (RVSP) 56.5 ± 3.2, 63.6 ± 1.6, and 111.1 ± 4.5 mmHg, respectively, vs. 22.9 ± 1.1 mmHg in controls]. PAH triggered early and late episodes of apoptosis in rat lungs accompanied by RAGE activation. Mass spectrometry analysis has identified IMPA1 as a novel PAH-specific interacting partner of RAGE. The proximity ligation assay (PLA) confirmed the formation of RAGE/IMPA1 complex in the pulmonary artery wall. Activation of IMPA1 in response to increased glucose 6-phosphate (G6P) is known to play a critical role in inositol synthesis and recycling. Indeed, we confirmed a threefold increase in G6P (P = 0.0005) levels in lungs of PAH rats starting from week 1 that correlated with accumulation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), membrane translocation of PI3K, and a threefold increase in membrane Akt levels (P = 0.02) and Akt phosphorylation. We conclude that the formation of the newly discovered RAGE-IMPA1 complex could be responsible for the stimulation of inositol pathways and activation of Akt signaling in PAH.
Keywords: glycolysis, inositol pathway, receptor for advanced glycation end products, proliferation, pulmonary hypertension
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a complex disease defined as a progressive increase in pulmonary vascular resistance that leads to right ventricle overload, dysfunction, and failure (45). The obliteration of small pulmonary arteries due to the thickening of intimal and medial layers and formation of angioproliferative lesions is the major contributor to the increased pulmonary pressure (58). Nevertheless, the current therapies predominantly target pulmonary vasoconstriction rather than vascular remodeling (55).
The insufficiency of the current PAH therapies is related to the complex nature of pathological events found at the stage of the developed disease. These include impaired cross talk between the dysfunctional endothelial cells and other components of the pulmonary vascular wall, dysregulated cellular energetics and metabolism, altered cell survival mechanisms, and activation of inflammatory and immune pathways (15, 37). Evaluation of the specific mechanisms responsible for the early transition of normal vascular cells to highly proliferative cells seems to be critical to develop effective treatment approaches. Unfortunately, diagnosis of PAH at the early stage is complicated due to the nonspecific nature of PAH symptoms (30). This limitation coupled with the insufficiency of the current screening tests to identify the early PAH prevents the scientific community from having a clear understanding of the pathways that predetermine PAH development. To address this need, we performed a time course evaluation of PAH progression using our recently published rat model that induces severe angioproliferative changes (42). We believe that better understanding of the pathogenic mechanisms that initiate vascular cell transition to highly proliferative phenotype could advance the earlier diagnostics and improve the treatment of PAH.
The Akt-mediated pathway is known to be responsible for cell survival, growth, proliferation, angiogenesis, and activation of glucose metabolism. It was confirmed to be directly associated with PAH pathogenesis (12, 54). Activation of this pathway depends on the adequate synthesis or recycling of myo-inositol, the central precursor of all phosphatidylinositols, including phosphatidylinositol 3,4,5-trisphosphate (PIP3) that binds to Akt and recruits it to the plasma membrane. Therefore, the molecular mechanisms involved in the control of myo-inositol synthesis or recycling are of high importance, as they may control the transition into highly proliferative phenotype. Inositol monophosphatase 1 (IMPA1) is the cytosolic enzyme that protects cells from the osmotic stress induced by the highly osmotic glucose metabolite glucose 6-phosphate (G6P) through its conversion to nonosmotic myo-inositol (13). Based on this property, IMPA1 was reported to be a critical contributor to inositol cycle, including both de novo inositol synthesis and recycling of inositol polyphosphates (10). Metabolic reprogramming of the pulmonary vascular cells with an increase in glucose uptake and metabolism is considered one of the most important hallmarks of the PAH. Because the activity of IMPA1 depends on the intracellular levels of G6P, the PAH that induces glycolytic shift could potentiate IMPA1 activity. This, in turn, would result in increased levels in myo-inositol production and stimulation of inositol-dependent pathways, including activation Akt. Nevertheless, the role of IMPA1 in this signaling and PAH pathogenesis in general has never been studied.
One of the early events confirmed to be a direct contributor to PAH initiation and progression is the vascular apoptosis (22, 48, 60). The particular trigger leading to the vascular damage in idiopathic PAH is unknown but may be multifactorial from a bacterial or viral infection, exposure to environmental toxins, reactive oxygen species, and shear stress to autoimmune mechanisms and impaired vascular repair (48). The critical role of this initial apoptosis is in a preselection of apoptosis-resistant highly proliferative vascular cells. The last suggests that there is a cross talk between dying and surviving cells that initiates a phenotypical alteration of the survived vascular cells. In particular, the augmented cell death induces activation of pattern recognition receptors (PRR) through increased binding of damage-associated molecular patterns (DAMPs) released from dying cells. The importance of this pathway in PAH has been confirmed recently by a study showing that one of the PRR, receptor for advanced glycosylation (RAGE), plays a critical role in PAH development in animals (32). In patients, a strong correlation between the levels of RAGE expression and disease severity has been reported (32).
RAGE is expressed in endothelial (EC) and smooth muscle cells (SMC) and was shown to be involved in inflammatory response mediated through NF-κB activation; however, the recent studies suggest that RAGE may attenuate or even resolve the inflammation (51). A number of previous publications have also highlighted the importance of RAGE-induced signaling in cell proliferation, survival, and migration. Thus, in cancer cells, RAGE was found to be responsible for apoptosis resistance, tumorogenesis, and metastasis development (23, 29). In vascular cells, activation of RAGE induces the neointima formation and accelerates vascular remodeling (27, 47, 57). In contrast, RAGE inhibition diminishes medial thickness of the pulmonary arteries (PA) and decreases pulmonary vascular cell proliferation in animal models of PAH (32).
Nevertheless, the particular molecular mechanisms responsible for RAGE-induced cell proliferation are not fully identified. It was reported that RAGE activation stimulates PI3K/Akt signaling and inhibition of PI3K/Akt abrogates RAGE-induced cell proliferation (3, 35). However, the specific molecular link that connects RAGE activation with stimulation of Akt is unclear. In this study, we report that PAH development is associated with the increased interaction of RAGE with IMPA1. Moreover, we propose that the formation of the RAGE/IMPA1 complex on a plasma membrane could potentiate phosphoinositol-required Akt signaling, thus contributing to the proliferative phenotype of the pulmonary vasculature.
METHODS
Rat model of pulmonary hypertension.
Thirty-two Sprague-Dawley female rats (200–250 g) were purchased from Charles River (Wilmington, MA). Animals were kept in a 12-h:12-h light-dark cycle and received standard rodent food and water ad libitum. All experimental procedures were approved by the University of Arizona’s Institutional Animal Care and Use Committee. To induce PAH, rats received either the vehicle or a single dose of the VEGF receptor 2 antagonist SU5416, 50 mg/kg sc, as previously described (42). On the day of injection the PAH rats were placed in a hypoxic chamber (BioSpherix, Redfield, NJ), and the oxygen was maintained at the level of 10 ± 0.5%. The O2 and CO2 concentrations were continuously monitored (PROOX 110 BioSpherix oxygen controller and LB-2 CO2 analyzer; Sensormedics).
The animals were analyzed after 1 or 2 wk of exposure to hypoxia or after 5 wk (3 wk of hypoxia and 2 wk of normoxia). The control rats were kept under normoxic conditions for the duration of the study (5 wk). At the end of each study period, animals were anesthetized (inactin, 100 mg/kg ip) and instrumented for measurement of right ventricle (RV) hemodynamics, as previously described (41). Briefly, a PE-240 polyethylene tube was inserted into the trachea to facilitate breathing. A customized pressure transducer catheter (SPR-513; Millar Instruments, Houston, TX), connected to a Millar Transducer Control Unit TC-510 and PL3504 PowerLab 4/35 data acquisition system (ADInstruments, Colorado Springs, CO) was inserted into RV via the right jugular vein and right atrium. A 30-min stabilization period was permitted before a 30-min recording of RV pressure. At the end of pressure recording, the trachea catheter was connected to a Harvard Rodent Ventilator (Model 683; Harvard Apparatus, South Natick, MA), the thorax was opened, the left atrium was cut, and the lungs were flushed with saline (0.9% sodium chloride) via a needle inserted into RV. Animals were euthanized by removing heart/lung block; lungs, RV, and left ventricle plus septum (LV + S) were dissected and weighed. The left lung was fixed in formalin and embedded in paraffin for histological examination. The right lung was quick-frozen and stored at −80°C for biochemical analysis.
Histological analysis.
For the morphometric assessment of pulmonary vessels, 5-μm tissue sections were dewaxed and stained with hematoxylin and eosin by HistoWiz (histowiz.com) using standard operating procedures and fully automated workflow. Twenty transversely sectioned pulmonary arteries per animal (from 6 to 8 rats/group) were randomly selected from the whole-slide ×40 digitized image created by HistoWiz using Aperio AT2 scanner (Leica Biosystems). The morphometric analysis was done by an investigator blinded for the animal group, as previously described (18). The pulmonary artery wall thickness was measured perpendicular to the circumference of the vessel from the endothelium to the outer edge of the smooth muscle layer using ImageJ software. The average of four measurements at a different location of the circumference of each pulmonary artery was quantified. The pulmonary arteries were divided into two groups, small (an external diameter of <150 μm) and larger (an external diameter of ≥150 μm) vessels, and the data were presented separately for each group.
In situ apoptosis detection.
Apoptosis of pulmonary arteries was visualized using Click-iT Plus terminal deoxynucleotidyl transferase-dUTP nick end labeling (TUNEL) assay (ThermoFisher Scientific) according to the manufacturer’s protocol, with some modifications. Briefly, sections were deparaffinized in two changes of xylene for 5 min each, hydrated with two changes of 100, 95, 70, and 50% ethanol, 0.85% NaCl for 5 min, and wash in PBS for 5 min. Tissue sections were fixed with 4% paraformaldehyde for 15 min at room temperature and treated with proteinase K-ready solution for 2 min. Fixative solution (4% paraformaldehyde) was applied for 5 min, followed by incubation in 100 μl of TdT reaction buffer for 10 min at 37°C and Td reaction mixture for 15 min at 37°C. After washing, the slides were incubated in Click-iT TUNEL Colorimetric Reaction cocktail for 30 min at 37°C, washed again, and incubated in 100 µl of Streptavidin-Peroxidase Conjugate at room temperature for 30 min in a humidified chamber in the dark. After the next serial washes, the slides were covered with 100 μl of the DAB Reaction Mixture, washed, counterstained with hematoxylin for 5 s, rinsed, and dehydrated. Coverslips were applied with the xylene-based mounting medium.
Duolink in situ proximity ligation assay.
The formation of RAGE/IMPA1 complex was visualized using proximity ligation assay according to a standard protocol (Sigma-Aldrich, St. Louis, MO). Briefly, slides were deparaffinized, and antigen retrieval was performed in 10 mM sodium citrate, pH 6.0, at 125°C for 5 min and incubated with anti-RAGE rabbit polyclonal (Abcam, Cambridge, MA) and anti-IMPA1 mouse monoclonal (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies diluted 1:100. Incubation with Far-Red PLA probes, followed by ligation and amplification, was performed according to the manufacturer’s manual. Slides were dried and mounted with Prolong Diamond antifading agent with DAPI (Invitrogen). Microscopy was performed using a Leica 6000 inverted microscope, Y5 (Cy5) filter cube for PLA probe, and D cube for DAPI at ×40 and ×100 magnification. This technology allows a pair of oligonucleotide-labeled antibodies (PLA probes) attached to the secondary antibody to generate an amplified signal only when the probes are in close proximity (<40 nm). Six pulmonary arteries per animal (from 4 lung preparations per experimental group) were randomly selected and imaged.
Western blot and immunoprecipitation.
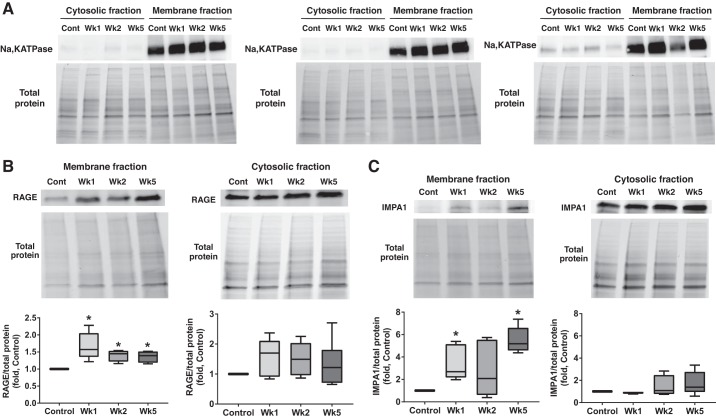
For an analysis of the total lung proteins, lung tissues were lysed as previously described (40). Isolation of membrane proteins was performed as previously published (28) using Mem-PER Plus Membrane Protein Extraction Kit (ThermoFisher Scientific, Rockford, IL) according to the manufacturer’s protocol (53). Briefly, 20–40 mg of lung tissue was quickly washed in Cell Wash Solution to remove any blood components, transferred to permeabilization buffer mixed with protease and phosphatase inhibitor cocktails, homogenized using Fisher Homogenizer 850 for 1 min, and shaken in UltraCruz Shaker at 0°C for 10 min. The homogenate was centrifuged at 16,000 g for 15 min to pellet permeabilized cells. The supernatant containing cytosolic proteins was carefully transferred in a separate tube. The pellet was suspended in solubilization buffer with protease and phosphatase inhibitor cocktails and shaken at 4°C for 30 min. The lysate was centrifuged at 16,000 g for 15 min, and the supernatant containing solubilized membrane-associated proteins was transferred to the separate tubes and used for the protein analysis as published (42). Briefly, the samples were incubated with 6× Laemmli sample buffer (Boston Bioproducts, Ashland, MA), for 5 min at 95°C, loaded on the 4–20% Mini-PROTEAN TGX Stain-Free gels (Bio-Rad Laboratories, Hercules, CA), and electrophoretically separated and transferred using PowerPac Universal power supply and Trans-Blot Turbo transferring system (Bio-Rad Laboratories). Membranes were probed using antibodies against cleaved caspase 3, Akt1, pS473-Akt, Na,K-ATPase (Cell Signaling Technology, Danvers, MA), RAGE, GLUT1, GLUT4, p110γ, p110α (Santa Cruz Biotechnology, Santa Cruz, CA), MyD88, and IMPA1 (Abcam, Cambridge, MA). The reactive bands were visualized using chemiluminescent protocol, recorded with the ChemiDoc MP Imaging System (Bio-Rad Laboratories, Hercules, CA), and analyzed using Image Laboratory software. The protein loading was normalized per total sample protein using free stain gels, as previously described (44). The efficiency of tissue fractionation was validated by using the antibodies against plasma membrane marker Na,K-ATPase (Fig. 5A). Some membranes were stripped and reprobed for more than one protein.
Fig. 5.
Pulmonary arterial hypertension (PAH) induces inositol monophosphatase 1 (IMPA1) translocation on a plasma membrane. A: to validate our method of separating the plasma membrane fraction and cytosolic fraction, samples were probed with plasma membrane protein sodium-potassium adenosine triphosphatase (Na,K ATPase). The 3 different presented sets of pulmonary lysates are collected from different animals. All sets show a strong signal from Na,K ATPase in the membrane but not a cytosolic fraction. B and C: both receptor for advanced glycation end products (RAGE; B) and IMPA1 (C) accumulate in the membrane fraction isolated from pulmonary tissue of PAH rats. No significant changes were found in the levels of these proteins measures in the cytosolic fraction. The protein loading was normalized per total sample protein using stain-free imaging technology; n = 6 for RAGE in the cytosolic fraction (B), and n = 5 for all the rest groups. Results are expressed as box and whisker plots (boxes: 25th to 75th percentile of the data; whiskers: minimum to maximum; line represents the median value). *P < 0.05 vs. control group. Statistical analysis was performed using Bonferroni’s multiple comparisons test for selected columns.
For immunoprecipitation (IP), RAGE antibodies conjugated to agarose beads were used according to the manufacturer’s (Santa Cruz Biotechnology) protocol. Lung tissue lysate (300 µg of total protein) were rotated at room temperature with 20 µl of bead solution for 2 h. Beads were collected by brief centrifugation (30 s at 1,000 g), washed four times with lysis buffer and PBS, resuspended, with 30 µl of 2× Laemmli sample buffer, and boiled for 5 min at 95°C. The supernatant collected by centrifugation was used for Western Blot analysis as described.
Antibody validation.
Antibodies used in this study have undergone previous validation. The summary of validation is presented in the Supplemental Table S1 (Supplemental Material for this article can be found on the AJP-Lung Cellular and Molecular Physiology website).
Proteomic analysis of RAGE Co-IP.
The lungs lysates from the control and week 5 groups were subjected to IP, as described above. Proteins were separated on 4–20% Mini-PROTEAN TGX Stain-Free gel and stained with Bio-Safe Coomassie G-250 Stain (Bio-Rad, Hercules, CA). For the proteome analysis, each lane of the gel was cut into three slices to target proteins in the 10- to 50-kDa range. Destaining, in-gel digestion, and peptide extraction steps were done as previously published (25). The dried peptides were resuspended in 6 µl of 0.1% formic acid, followed by sonication for 2 min. HPLC-ESI-MS/MS was performed in positive ion mode on a Thermo Scientific Orbitrap Fusion Lumos tribrid mass spectrometer fitted with an EASY-Spray Source (Thermo Scientific, San Jose, CA). NanoLC was performed using a Thermo Scientific UltiMate 3000 RSLCnano System with an EASY Spray C18 LC column (75 cm × 75 μm inner diameter, packed with PepMap RSLC C18 material, 2 µm, cat. no. ES805; Thermo Scientific), loading phase for 15 min at 0.300 µl/min, mobile phase, and linear gradient of 1–34% Solvent B in 119 min at 0.220 µl/min, followed by a step to 95% buffer B over 4 min at 0.220 µl/min, hold for 5 min at 0.250 µl/min, and then a step to 1% buffer B over 5 min at 0.250 µl/min, and a final hold for 10 min (total run 159 min): buffer A = 100% H2O in 0.1% FA, buffer B = 80% ACN in 0.1% FA. All solvents were liquid chromatography mass spectrometry grade. Spectra were acquired using XCalibur version 2.3 (Thermo Scientific). A “top speed” data-dependent MS/MS analysis was performed. Dynamic exclusion was enabled with a repeat count of 1, a repeat duration of 30 s, and an exclusion duration of 60 s. Tandem mass spectra were extracted from Xcalibur “RAW” files, and charge states were assigned using the ProteoWizard 3.0 msConvert script using the default parameters. The fragment mass spectra were then searched against the rat SwissProt_2016_10 database (7983 entries) using Mascot (version 2.6.0; Matrix Science, London, UK) using the default probability cutoff score. The search variables that were used were 10 ppm mass tolerance for precursor ion masses and 0.5 Da for product ion masses, digestion with trypsin, a maximum of two missed tryptic cleavages, and variable modifications of oxidation of methionine and phosphorylation of serine, threonine, and tyrosine. Cross correlation of Mascot search results with X! Tandem was accomplished with Scaffold (version Scaffold_4.7.5; Proteome Software, Portland, OR). Probability assessments of peptide assignments and protein identifications were made through the use of Scaffold. To enrich peptide coverage, threshold was set to ≥0% probability, and protein threshold was set to P > 99%.
Homology modeling and docking.
To identify the interaction sites between IMPA1 and RAGE, we built the homology model of IMPA1. The available structure of IMPA1 (PDB ID: 2BJI) was used. To model the structure, we utilized the Yasara Structure software package (24). The geometry of the reconstructed region of human IMPA1 was automatically optimized using the steepest descent energy minimization algorithm in the solvent implicit model. In the refined structure of IMPA1, we analyzed electrostatic potential on the surface and the RAGE peptide docking to IMPA1 using embedded in Yarasa algorithms.
Glucose 6-phosphate quantification.
Glucose 6-phosphate (G6P) fluorometric assay from Abnova (Littleton, CO) was used to detect the levels of G6P according to the manufacturer’s protocol. Briefly, 100 mg of lung tissue was rapidly homogenized in ice-cold assay buffer. 10K cutoff filters (Amicon) were used for deproteinization of the samples. After incubation with enzymes, measurement of fluorescence using Ex/Em = 535/587 nm was done on the Biotek Synergy H1m plate reader.
Phosphatidylinositol 3,4,5-trisphosphate quantification.
Phosphatidylinositol 3,4,5-trisphosphate (PIP3) assay kit (Echelon Biosciences) was used for quantification of PIP3 in tissue samples. Lipid fraction was extracted from 100 mg of lung tissue and subjected to manufacturer-supplied protocol. Absorbance at 450 nm was measured on the Biotek Synergy H1m multi-plate reader.
Cell culture.
Human pulmonary artery endothelial cells (HPAEC; cat. no. 3100, lot no. 3904) and human pulmonary artery smooth muscle cells (HPASMC; cat. no. 3110, lot no.0294) were purchased from ScienCell (Carlsbad, CA), one donor for each cell type. These primary vascular cells are nontransformed, nonimmortalized human cells isolated directly from lung tissue. HPAEC (passages 4–6) were cultured in ECM growth media (cat. no.1001; ScienCell) supplemented with 5% FBS (cat. no. 25-514H; Genesee Scientific) and penicillin-streptomycin (cat. no. 15140-122; Gibco) in a humidified incubator (21% O2, 5% CO2) at 37°C. To induce apoptosis, the medium was changed to serum-free ECM for 48–72 h. HPASMC were cultured in DMEM supplemented with 10% FBS and 4.5g/l glucose. Apoptosis of HPASMC was induced by changing the media to DMEM with 5% FBS and 0.1 g/l glucose for 48–72 h. The level of apoptosis was quantified using Apoptosis and Necrosis Quantification Kit (Biotum, Fremont, CA) according to the manufacturer’s protocol. Briefly, cells were collected with trypsin, washed with PBS, and resuspended in 50 µl of reaction mix containing a buffer, FITC-Annexin V, and Ethidium homodimer. After 25 min of incubation in the dark at room temperature, samples were diluted with 200 µl of the kit 1× buffer. The analysis was performed using NovoCyte Flow Cytometer (ACEA Biosciences, San Diego, CA). The conditioned medium was collected from apoptotic and untreated cells and centrifuged at 12,000 g for 10 min at 4°C. The medium collected from apoptotic HPASMC was supplemented back with glucose (4.5 g/l), and medium collected from apoptotic HPAEC was supplemented with 2% FBS. The naïve HPASMC were treated with conditioned media or media premixed with selective IMPA1 inhibitor (cat. no. sc-202685AL-690,330, 500µM; Santa Cruz Biotechnology) or RAGE antagonist peptide (RAP; 50 µM, EMD Millipore, Burlington, MA, cat no. 553031). After 24 h of incubation, the medium was removed, and cells were washed with PBS and frozen for the future Western blot (WB) analysis as, described above. Alternatively, the naïve HPASMC treated by control and apoptotic conditioned media were used for RAGE immunoprecipitation, as described above.
Statistical analysis.
Statistical calculations were performed using the GraphPad Prism software version 7.04. The mean value (±SE) was calculated for all samples, and significance was determined by either the unpaired t-test or analysis of variance (ANOVA). For ANOVA, Newman-Keuls or Bonferroni multiple comparison tests to compare the selected pairs of columns were used. A value of P < 0.05 was considered significant. The Grubbs test (extreme studentized deviate) was used to determine the significant outliers. This criterion was predetermined before the initiation of the data analysis.
RESULTS
Time course of pulmonary hypertension development.
In this study, we used an experimental model of severe angio-proliferative PAH (1), as previously described (42). Previously published research showed that stimulation of RAGE signaling induces PAH only in females (7). Therefore, our study aimed at addressing the role of RAGE-mediated signaling in PAH was performed in females. By week 5, our model produced a marked increase of right ventricle systolic pressure (RVSP; Fig. 1A), RV hypertrophy (Fig. 1B), and pulmonary artery remodeling (Fig. 2). However, to evaluate the contribution of the early pathological events in PAH development and progression, the rats were also analyzed at two additional time points: week 1 (early stage of PAH) and week 2 (middle stage). We found that the combination of SU5416 and hypoxia initiates a rapid increase in RVSP (Fig. 1A), which corresponded to the significant RV remodeling (Fig. 1B), and changes in RV function-increased RV contractility (Fig. 1C) and RV relaxation (Fig. 1D) as early as 1 wk after PAH initiation. Interestingly, by week 2, the progression of PAH slowed down, and none of the physiological parameters analyzed were found to be significantly different between weeks 1 and 2. Nevertheless, at week 5 the disease accelerated again and resulted in pronounced changes that were found to be significantly different compared with both week 1 and week 2.
Fig. 1.
Angioproliferative model of pulmonary arterial hypertension (PAH) induces severe changes in right ventricle (RV) pressure, RV hypertrophy, and RV function. Injection of Sprague-Dawley female rats with SU5416 followed by 3 wk of hypoxia and 2 wk of normoxia induced a progressive increase of right ventricle pressure (A), RV hypertrophy measured as a wet weight ratio of RV free wall normalized on left ventricle-Fulton index (B), and changes in RV contractility (C) and RV relaxation (D) evaluated by measuring RV maximal (dP/dtmax) and minimal rate of RV pressure (dP/dtmin). PAH progression was especially evident at the early stage (week 1) and late stage (week 5) of PAH. Results are expressed as box whisker plots (boxes: 25–75% percentile of the data; whiskers: minimum to maximum; line represents the median value); n = 8 rats in each group. *P < 0.05 vs. control group; #P < 0.05 vs. week 1; †P < 0.05 vs. week 2. Statistical analysis was performed by Newman-Keuls multiple-comparisons test. RVSP, right ventricle systolic pressure; RV/LV + S, right ventricle/left ventricle plus septum ratio.
Fig. 2.
Small pulmonary arteries become progressively remodeled in pulmonary arterial hypertension (PAH). A: representative images from hematoxylin and eosin-stained pulmonary arteries (PA) of control and PAH rats at different stages of the disease. B: quantitative analysis of the vascular wall thickness. Twenty random PA per animal were analyzed. Vascular thickness was significantly higher in PAH rats compared with controls in both categories of PA examined (<150 and ≥150 μm). The remodeling of the smaller vessels progressed throughout the study, whereas hypertrophy of larger vessels showed an early increase and then stayed preserved. Results are expressed as box whisker plots (boxes: 25–75% percentile of the data; whiskers: minimum to maximum; line represents the median value); n = 8 rats for control and week 1 groups, n = 7 for week 2 group, and n = 6 for week 5 group. *P < 0.05 vs. control group; #P < 0.05 vs. week 1 group. Statistical analysis was performed by Newman-Keuls multiple-comparisons test. Open bars correspond to 100 μm.
The histological evaluation of lungs revealed that PAH induced a strong and progressive pulmonary vascular remodeling. However, the hypertrophy was found to be different for the small (<150 uM) and larger (≥150 uM) pulmonary arteries (PA). Thus, the small PAs continued to develop angioproliferative changes through the course of the study, repeating the overall pattern of the disease development with quick progression at the early and late stages, but not at the middle stage. In contrast, the larger vessels responded only during the 1st week of the disease and were preserved during the rest of the study, thus confirming that the vasculopathy of small but not larger PAs is the primary contributor to PAH progression.
Apoptosis and RAGE activation in PAH.
The initial apoptosis of pulmonary vasculature plays an essential role in the selection of phenotypically altered apoptosis-resistant vascular cells and subsequent vascular remodeling (16, 48). By measuring the level of cleaved caspase 3 in pulmonary tissue, we confirmed the activation of early apoptosis in the lungs (Fig. 3A). However, by week 2 there was a resolution from apoptosis, which corresponded to the deceleration of the disease progression at this midpoint (Figs. 1 and 2). Finally, at the late stage of PAH (week 5), the apoptosis was evidenced again, suggesting that there is also a second delayed episode of PAH-induced damage that could contribute to the disease progression. To evaluate the particular source of apoptotic cells in PAH lungs, we performed a TUNEL staining. At the early stage (week 1), apoptosis was evident in endothelium and adventitia of pulmonary arteries (Fig. 3B), confirming that not only initial endothelial apoptosis but also damage that occurs in the adventitial layer is an important modulator of vascular remodeling, as previously reported (52). At the middle stage of PAH (week 2), the apoptosis positive cells were not found, suggesting that the initially apoptotic endothelial cells and fibroblasts have later transformed into apoptosis-resistant cells (9). By week 5, apoptosis manifested again and was visualized in all layers of the pulmonary vascular wall.
Fig. 3.
Pulmonary apoptosis and receptor for advanced glycation end product (RAGE) activation occur in the early and late stages of pulmonary arterial hypertension (PAH). The development of PAH was associated with early (week 1) and late (week 5) episodes of apoptosis in pulmonary tissue and pulmonary vascular wall. A: the total pulmonary apoptosis was evaluated at different time points of PAH progression by measuring the levels of the proapoptotic marker cleaved caspase 3 in total lung lysate. The protein loading was normalized per total sample protein using stain-free imaging technology; n = 5 rats in each group. B: the level of apoptosis in the pulmonary artery vascular wall was visualized by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL). Images are representative of 6 to 10 pulmonary arteries per rat with n = 4 rats/group. Black arrowheads, TUNEL-positive endothelial cells; open arrowheads, TUNEL-positive smooth muscle cells; black arrows, TUNEL-positive adventitial cells. Black bar corresponds to 100 µm. The increase in pulmonary vascular apoptosis correlated with RAGE activation assessed by measuring the level of RAGE interaction with its adaptor protein myeloid differentiation primary response 88 (MyD88; C); n = 4 rats in each group. Graphs are expressed as box whisker plots (boxes: 25–75th percentile of the data; whiskers: minimum to maximum; line represents the median value). *P < 0.05 vs. control group. Statistical analysis was performed by Newman-Keuls multiple-comparisons test. Significance between control and week 5 was confirmed using unpaired t-test. RAGE indicates advanced glycation end products.
The factors released from dying cells are known to bind to RAGE and activate it. By measuring the level of RAGE interaction with its adaptor protein myeloid differentiation primary response 88 (MyD88), we evaluated the level of RAGE activation in pulmonary tissues of control and PAH rats. There was a strongly augmented interaction of RAGE and MyD88 during the early and late stages of PAH (Fig. 3C), confirming activation of RAGE signaling specifically in weeks 1 and 5 of the disease.
Pulmonary hypertension mediates a formation of RAGE-IMPA1 complex.
To understand the particular downstream signaling induced by RAGE activation, we performed a mass spectrometry analysis of RAGE interactome. IMPA1 was found to be one of RAGE interacting partners that bind to RAGE in PAH but not in control samples (Fig. 4A). Although the threshold was set to P > 0, at least four peptides were found with a probability of >89%. The immunoprecipitation/immunoblotting analysis confirmed the absence of RAGE/IMPA 1 interaction in control animals and revealed that the formation of this complex starts already at week 1 and is maintained throughout the duration of the disease (Fig. 4B).
Fig. 4.
Receptor for advanced glycation end products (RAGE) interacts with inositol monophosphatase 1 (IMPA1) in pulmonary arterial hypertension (PAH). A: mass spectrometry analysis of proteins coimmunoprecipitated (co-IP) with RAGE from lungs identified IMPA1 as a novel RAGE interacting partner. RAGE-IMPA1 complex was discovered in both samples from PAH animals (week 5) but not in control (peptide probability P > 0; 4 peptides with P > 89%). B: time course of RAGE-IMPA1 interaction was investigated by co-IP. There was no interaction between RAGE and IMPA1 in controls from week 1 RAGE/IMPA1 were efficiently co-IP (n = 4 for all groups). Results are expressed as box and whisker plots (boxes: 25th to 75th percentile of the data; whiskers: minimum to maximum; line represents the median value). *P < 0.05 vs. control. Statistical analysis was performed by Newman-Keuls multiple-comparisons test. C: the formation of the RAGE-IMPA1 complex in the pulmonary vascular wall was visualized using the proximity ligation assay (PLA) method. Images are representative of 6 pulmonary arteries/at n = 4 rats/group. Gray images were taken using the light microscopy (×20) to visualize the tissue structure; red fluorescent signal indicates RAGE-IMPA1 interaction; blue fluorescent signal indicates nuclei stained by DAPI. Yellow square represents the area of magnification (×100). The white marker corresponds to 25 μm. D: the amplified signal from the RAGE-IMPA1 complex (red) was registered in the media of hypertrophied pulmonary arteries, as seen on enlarged images from weeks 1 (Wk1) and 5 (Wk5) (D). The yellow dotted line is traced following the external lamina of the vessel. Gray arrowheads point on the endothelial cells that have no red PLA signal; white arrows point on the cells in the pulmonary artery media and adventitia cells that have bright red PLA signal. IB, immunoblot.
The previous study showed that PAH induces RAGE expression in PA smooth muscle cells (32). Therefore, it could be expected that the formation of the RAGE-IMPA1 complex also takes place in the media layer of PAs. To visualize the localization of complex RAGE-IMPA1 in pulmonary tissue, we performed Duolink proximity ligation assay (PLA). This technology generates an amplified signal only when the probes attached to each protein of interest are in close proximity (<40 nm). The results of PLA have additionally confirmed that PAH initiates a direct interaction of RAGE and IMPA1 starting from week 1. The PLA signal was especially apparent in week 1 and week 5 (Fig. 4C). Importantly, the signal was visualized in the adventitial and medial layers of hypertrophied pulmonary arteries (Fig. 4D), supporting our hypothesis that RAGE and IMPA1 have an increased interaction in the pulmonary vascular wall, although formation of RAGE-IMPA1 complex was also seen in pulmonary parenchyma.
To evaluate whether the interaction happens due to the translocation of IMPA1, known to be a cytosolic protein, on a plasma membrane, we evaluated the distribution of RAGE and IMPA1 between cytosolic and membrane fraction in control and diseased animals. By using the Na,K-ATPase as a plasma membrane marker, we first validated the efficiency of lung tissue fractionation in different animal sets (Fig. 5A). We confirmed that the membrane fraction had a strong Na,K-ATPase signal, whereas the cytosol was almost lacking the Na,K-ATPase signal. These validated samples were used to analyze the plasma membrane/cytosol distribution of RAGE, IMPA1, and other proteins. There was a significant accumulation of RAGE in the membrane fraction of samples from PAH rats (Fig. 5B) and a nonsignificant increase of RAGE in the cytosol fraction, possibly due to RAGE internalization and recycling (34). IMPA1 has also accumulated in the membrane but not in a cytosolic fraction (Fig. 5C). This increase was significant in week 1 and week 5, which corresponds with the profile of apoptosis-induced RAGE activation (Fig. 3C).
Previous studies have shown the importance of positively charged patch at aa364–370 in the cytosolic tail of RAGE for binding to adaptor proteins (31, 43). Because we have observed the negatively charged COOH-terminus in the IMPA1 structure, we propose that the same positively charged region of RAGE could also be responsible for an electrostatic interactions between RAGE and IMPA1. Therefore, we have utilized computational docking to model binding of RAGE region aa365–376 to the IMPA1 molecule. Docking of the RAGE-originated peptide 365-RRQRRGEERKAP-376 to an IMPA1 structure utilizing molecular modeling algorithm confirmed a possible interaction between the intracellular positively charged arginine-rich region of RAGE and negatively charged COOH-terminus of IMPA1. Interestingly, this region of IMPA1 has a cavity that is stretching into the active IMPA1 site, and our model predicted that one of the arginine residues from RAGE-peptide interacts with Mg2+ inside the active site of IMPA1 (Fig. 6A). However, binding of RAGE peptide does not induce any sterical hindrance to the main entrance of the IMPA1 active site on the opposite side of the molecule (Fig. 6B). Analysis of electrostatic map of IMPA1 showed additional, positively charged regions that can increase docking of IMPA1 to membrane due to electrostatic interactions. The negatively charged RAGE binding region of IMPA1 will be neutralized by positively charged RAGE residues (Fig. 6C), and this will additionally facilitate IMPA1-membrane interaction (Fig. 6D). Based on this analysis, we propose that interaction between IMPA1 and RAGE includes 1) docking of the negatively charged COOH-terminus of IMPA1 to positively charged RAGE residues just below the intermembrane region of RAGE, 2) neutralization of the negative charge on the surface of IMPA1, and 3) binding the positively charged region of IMPA1 to the membrane (Fig. 6E).
Fig. 6.
Molecular modeling of receptor for advanced glycation end products (RAGE)-inositol monophosphatase 1 (IMPA1) interaction. A: docking of RAGE-originated peptide 365-RRQRRGEERKAP-376 to IMPA1 structure revealed interaction with negatively charged COOH terminus of IMPA1. RAGE peptide binds into the cavity in IMPA1. Arginine (Arg) residue from RAGE peptide interacts with magnesium (Mg) ion at the active site. B: RAGE peptide binds to IMPA1 from the side that is opposite of the entrance into an active site. C: analysis of surface electrostatic potential of IMPA1 showed the negatively charged region that binds RAGE peptide as well as the positively charged surface that can bind to the membrane. D: illustration of the IMPA1 binding to membrane based on the electrostatic map. E: schematic mechanism of RAGE-IMPA1 interaction. The negatively charged IMPA1 pocket interacts with a positively charged loop of the intracellular domain of RAGE. In this configuration, the positively charged surface of IMPA1 maintains IMPA1 attachment to the inner side of the plasma membrane.
Pulmonary hypertension promotes an increase in pulmonary glucose uptake and metabolism.
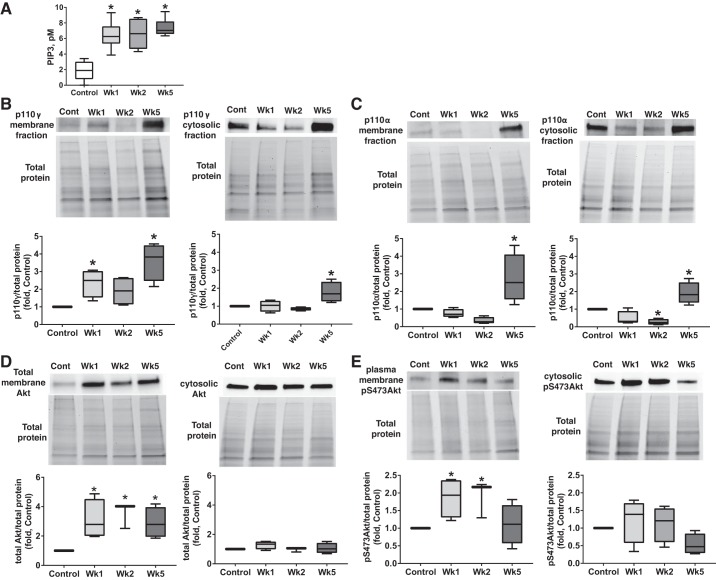
The shift of the pulmonary metabolism from mitochondrial oxidative phosphorylation to aerobic glycolysis is a hallmark of PAH that contributes to the hyperproliferative profile of pulmonary vascular cells. IMPA1 is known to be sensitive to increased levels of glucose metabolite G6P and catalyzes a critical step of G6P conversion to myo-inositol (4). Thus, the elevated glucose uptake and metabolism provides the circumstantial background for IMPA1 activation. To confirm that in our model there was a metabolic shift and to evaluate the timing of the changes in metabolic profile, we measured the membrane translocation of two main glucose transporters, GLUT1 and GLUT4, in lung tissue from control and diseased animals. PAH induced a significant, almost fourfold increase in the amount of membrane GLUT4 at an early stage (week 1), which remained elevated through the course of the study (Fig. 7A). This early membrane translocation of GLUT4 was not due to an increase in its expression, as the GLUT4 cytosolic levels were not significantly changed in week 1 or week 5 and only slightly increased in week 2. In contrast, GLUT1 showed a significant increase only by week 5 and was found to be upregulated in both membrane and cytosolic fractions (Fig. 7B). There was also a strong accumulation of G6P in pulmonary hypertensive rats starting from week 1 (Fig. 7C) that confirmed the presence of a PAH-induced glycolytic shift in pulmonary tissue of these animals and a rational demand for IMPA1 activation.
Fig. 7.
Increased glucose uptake in lungs starting from the early stage of pulmonary arterial hypertension (PAH). A and B: time course of membrane translocation of 2 major glucose transporters, glucose transporter type 4 (GLUT4; A) and glucose transporter type 1 (GLUT1; B), revealed an early (week 1) significant accumulation of GLUT4 in the membrane fraction that was maintained throughout the study and accompanied by only mild changes in the cytosol. GLUT1 levels were found to be progressively increased during the study and became significant by week 5 (Wk5) in both the membrane and cytosolic fractions. Protein loading was normalized per total sample protein using stain-free imaging technology; n = 5 rats in each group. C: increased pulmonary levels of glucose 6-phosphate (G6P) confirmed an upregulated lung glucose uptake starting from week 1 and provided the background for inositol monophosphatase 1 (IMPA1) activation; n = 4 for control; n = 8 for weeks 1 (Wk1) and 2 (Wk2); n = 6 for the Wk5 group. Results are expressed as box and whisker plots (boxes: 25th to 75th percentile of the data; whiskers: minimum to maximum; line represents the median value). *P < 0.05 vs. control group. Statistical analysis was performed using Bonferroni’s multiple-comparisons test for selected columns (A and B) and by Newman-Keuls multiple-comparisons test (C).
Activation of PIP3-Akt axis is a characteristic of not only a developed but an incipient PAH.
Myo-inositol generated by IMPA1 is a precursor of phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3, in turn, ensures Akt binding to the membrane and allosterically activates Akt kinase by relieving an intramolecular autoinhibitory mechanism and permitting the binding of substrate (8). Our results confirm that the formation of PIP3 in the lipid fraction extracted from rat lungs is strongly increased upon PAH development (>3-fold greater in PAH rats compared with controls) starting from the earliest stage (week 1) (Fig. 8A). Because the synthesis of PIP3 from phosphatidylinositol 4,5-bisphosphate (PIP2) depends directly on the activity of phosphatidylinositol-3-kinase (PI3K), we have also analyzed the time course of PI3K accumulation in the membrane fraction. According to our results, PAH induced an increased translocation of PI3K catalytic subunit p110γ to the membrane in week 1 and week 5 (Fig. 8B). Importantly, the early accumulation of p110γ in membrane fraction was not due to p110γ overexpression, since the cytosolic portion of p110γ had not been altered until week 5, when it was mildly increased. Another catalytic subunit of PI3K, p110α, was also upregulated, but only by week 5, and found to be elevated in both membrane and cytosolic fractions (Fig. 8C). This finding suggests that p110γ may be the primary subunit responsible for the early synthesis of PIP3.
Fig. 8.
Upregulated inositol pathway and protein kinase B (Akt) activity in pulmonary arterial hypertension (PAH) rats. Activated in response to elevated glucose 6-phosphate (G6P) levels, inositol monophosphatase 1 (IMPA1) could stimulate inositol synthesis on a plasma membrane. A: indeed, the pulmonary phosphatidylinositol (3,4,5)-trisphosphate (PIP3) was found to be strongly increased similar to G6P levels (Fig. 6C); n = 6 rats in each group. B and C: the PAH has also induced an accumulation of catalytic subunits of phosphatidylinositol-3-kinase (PI3K) in the membrane fraction, although phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-γ (p100γ) was elevated at early and late stages (B), whereas phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-α (p100α) levels increased only by week 5 (C); n = 4 rats in each group. The binding of PIP3 to the PH domain of Akt is known to induce Akt translocation on a plasma membrane and phosphorylation. D: we confirmed that the increased formation of PIP3 correlated with the strong accumulation of Akt in the membrane fraction starting from the early stage of PAH, whereas the cytosolic levels of Akt were not affected. E: accumulation of Akt on the plasma membrane corresponded with the increased phosphorylation of Akt in the membrane but not a cytosolic fraction; n = 4 rats in each group. Protein loading was normalized per total sample protein using stain-free imaging technology. Results are expressed as box and whisker plots (boxes: 25th to 75th percentile of the data; whiskers: minimum to maximum; line represents the median value). *P < 0.05 vs. control group. Statistical analysis was performed by Newman-Keuls multiple-comparisons test or using Bonferroni’s multiple comparisons test for selected columns (for control vs. week 1 in B and E). pS473Akt, protein kinase B phosphorylated at Ser473.
As expected, the elevated levels of PIP3 formation were associated with a sustained threefold increase in Akt membrane translocation (Fig. 8D) and a twofold increase in membrane pSer473-Akt levels (Fig. 8E). As with RAGE, IMPA1, GLUT4, and p110γ, the cytosolic levels of total and phosphorylated Akt, were not altered, supporting the specific role of membrane microdomains in proliferation signal transduction.
Formation of RAGE-IMPA1 complex and RAGE/IMPA-dependent activation of Akt in vitro.
To additionally validate the role of apoptosis in RAGE-IMPA1 interaction, we have performed in vitro experiments in which we have initiated apoptosis of pulmonary artery vascular cells by either keeping the cells in serum-free media (for HPAEC) or placing the cells in a low-glucose media (for HPASMC). Both treatments induced a significant apoptotic cell death that was confirmed by flow cytometry (Fig. 9A). The conditioned media collected from untreated and apoptotic cells were used to stimulate the naïve HPASMC. We observed that treatment with apoptotic media initiated an interaction between RAGE and IMPA1 (Fig. 9B), confirming an important role of factors released from dying cells in the activation of RAGE and IMPA1. We also used the same conditioned media to evaluate the level of Akt activation in naïve HPASMC in the presence or absence of specific IMPA1 inhibitor L-690,330 and the RAGE antagonist RAP. Apoptotic factors stimulated activation of Akt that was significantly attenuated in the presence of IMPA1 inhibitor and was even further reduced by RAGE antagonist (Fig. 9C). These results confirm an important role of both proteins in the activation of Akt signaling.
Fig. 9.
Apoptosis induced formation of receptor for advanced glycation end products (RAGE)-inositol monophosphatase 1 (IMPA1) complex and protein kinase B (Akt) activation in vitro. A: apoptosis induced in pulmonary artery vascular cells was used to prepare conditioned media. B and C: cell media collected from apoptotic but not untreated control cells induced interaction between RAGE and IMPA1 (B) and activation of Akt (C) in naïve human pulmonary artery smooth muscle cells (HPASMC). Akt phosphorylation was significantly attenuated in the presence of selective IMPA1 inhibitor (L-690,330; 500 µM) and RAGE antagonist (RAP; 50 µM), confirming an important role of both proteins in activation of Akt signaling; n = 6/group (C) and n = 5/group (B). Protein loading was normalized per total sample protein using stain-free imaging technology. Results are expressed as box and whisker plots (boxes: 25th to 75th percentile of the data; whiskers: minimum to maximum; line represents the median value). Statistical analysis was performed using unpaired t-test (B) or Newman-Keuls multiple-comparisons test (C). Apo, media collected from apoptotic cells. *P < 0.05 vs. control group; #P < 0.05 vs. apoptosis group.
DISCUSSION
The pathogenesis of PAH is complex. It requires multiple “hits” on the background of genetic predisposition to initiate and drive the disease progression. Although the key pathogenic events contributing to PAH have been identified, the clear interconnection between these events, as well as the mechanisms that initiate the disease transition from one stage to another, are not completely understood. In this study, we identified that PAH initiates the interaction between RAGE and IMPA1 that is absent in the healthy animals. Although additional studies are required to fully understand the particular role of the RAGE-IMPA1 complex in PAH, we consider this discovery to be very important. Indeed, it delineates the potential molecular mechanism of RAGE-mediated PASMC proliferation and resistance to apoptosis. The direct interaction between RAGE and IMPA1 may also interconnect a few pathological events confirmed to play an important role in PAH onset and progression. These include initial pulmonary vascular apoptosis, which stimulates DAMPs receptors such as RAGE, increased glucose uptake with subsequent glycolytic shift and activation of IMPA1, and reprogramming of survived vascular cells toward overproliferative cells.
Pulmonary apoptosis, RAGE activation, and PAH progression.
The concept of initial pulmonary vascular cell injury playing a role in PAH initiation and progression is strongly supported by the literature (22, 48, 60). The studies showed that apoptosis inhibition prevents the development of PAH (17, 60), providing evidence for a causal role of vascular apoptosis in PAH pathogenesis. Nevertheless, the particular mechanisms that link this initial apoptosis with the later vascular remodeling are not fully established. Stressed, injured, or dying cells are known to be a source of DAMPs, such as HMGB1, SAP130, and S100 proteins. Besides, some of these alarmins are actively secreted by macrophage-engulfed apoptotic cells (36). Binding of these alarmins to RAGE induces its activation and may be the primary reason for upregulation of RAGE signaling in PAH (32). In complete agreement with these previous findings, we report here the presence of a strong apoptotic signal at the early stage of PAH associated with an increase in RAGE signaling.
Interestingly, by analyzing the particular source of apoptotic cells in the lungs, we have identified that apoptosis is localized mainly in the adventitia and endothelial cells of pulmonary arteries. The potential contribution of apoptosis that emerged from the adventitial layer in vascular remodeling has been previously observed and discussed (52). In response to the injurious stimuli, adventitial fibroblasts become activated and undergo transdifferentiation into myofibroblasts, which are capable of migrating from the adventitia to the media, thus contributing to the media hypertrophy (50). This ability of active fibroblasts to respond to the stress by differentiation into myofibroblasts, and ultimately to SMC, together with their ability to secrete proliferative factors, supports the idea of an “outside-in” mechanism of vascular remodeling. In addition, apoptosis localized in endothelium initiates a well-described vascular transformation from “inside out.”
The importance of the vascular damage in PAH progression could explain a significant deceleration of disease progression at the middle stage of PAH (week 2) found in our study. We observed that the changes in RVSP, RV hypertrophy, RV contractility, RV relaxation, and remodeling of small pulmonary arteries were significantly attenuated at the middle stage and correlated with apoptosis resolution and decreased the level of RAGE activity compared with week 1. Nevertheless, at the late stage of PAH (week 5), the apoptosis was evident again. At this stage, the damage was nonspecifically localized in all layers of the vascular wall. Importantly, the apoptosis at the developed stage of pulmonary hypertension has been described in patients. Thus, it was reported that the patient pulmonary arteries show the high expression of proapoptotic markers such as caspase-3 and p53 (26), whereas antagonizing of apoptosis reverses an established PAH (56). Therefore, we conclude that even a developed stage of PAH is associated with vascular damage, which could continue to contribute to the selection of apoptosis-resistant cells and disease progression. Although additional investigation is necessary to fully understand the mechanisms behind this late onset of the apoptotic cell death, we hypothesize that this second episode of apoptosis may be provoked by insufficient nutrient supply to the hypertrophied pulmonary vasculature. Indeed, when the vascular wall thickness exceeds the effective diffusion distance of oxygen, the nutrition of the vascular wall starts to fully depend on vasa vasorum (19). In PAH, the vasa vasorum was shown to undergo neovascularization and become dysfunctional due to the severe vascular leakage (6), and thus it could not adequately supply oxygen and nutrients to the hypertrophied pulmonary vessels. We found that this second hit of the pulmonary apoptosis induces the second episode of RAGE activation and further accelerates PAH.
Metabolic shift and stimulation of inositol pathway.
The switch from mitochondrial respiration to glycolysis, known as the “Warburg effect” when it happens under normoxic conditions, is well described for cancer cells and has also been confirmed to occur in pulmonary vascular cells isolated from PAH patients and animals (59, 61). The pulmonary and RV glucose uptake measured by positron emission tomography (PET) was found to be elevated in PAH patients compared with healthy controls and correlated with the severity of PAH (11, 49, 62). In this study, we confirmed that the membrane translocation of two main glucose transporters, GLUT1 and GLUT4, was significantly increased in the lungs of animals with PAH, although the profile of this alteration for each transporter was different. In accordance with previous research, we found that GLUT1, known to be predominantly expressed in the perivascular macrophages, was upregulated only at the developed stage of PAH (62). The increase of GLUT1 levels in both membrane and cytosolic fractions suggests an upregulation of GLUT1 expression rather than its translocation. In contrast, the GLUT4 signal was specifically increased in the membrane fraction starting from week 1. Interestingly, although membrane translocation of GLUT4 in response to insulin is very well established, its ability to translocate in the absence of insulin stimulation is not well studied, especially in PAH. Our results suggest that GLUT4 could be the primary transporter responsible for the early glucose uptake in pulmonary hypertensive lungs.
In this study, we also confirmed that the levels of glucose 6-phosphate (G6P), the first glucose metabolite in the glycolytic pathway, were markedly elevated in the lungs of PAH rats, again starting from the early stage of the disease. This is consistent with our previous studies, which showed the glycolytic shift in the lungs of rats treated by monocrotaline at the early stage of PAH, which preceded the PAH manifestation (39). IMPA1 is known to protect the cells from the osmotic stress induced by highly osmotic G6P through its conversion into nonosmotic myo-inositol (46). Based on this knowledge, we propose that the upregulated levels of G6P in the lungs of PAH animals could be the primary mechanism responsible for IMPA1 activation. Indeed, we found that PAH triggers IMPA1 to translocate to the plasma membrane, where it directly interacts with RAGE. This translocation could be very important for the increased recycling and de novo synthesis of inositols close to the site of inositol action (Fig. 10). IMPA1, a critical modulator of intracellular signal transduction, catalyzes the rate-limiting step in inositol synthesis-inositol monophosphate dephosphorylation and generates free myo-inositol, a precursor of phosphatidylinositol (PI). PI gives the rise to all phosphatidylinositol phosphates. Because we were specifically interested in identifying the molecular mechanism that could be involved in RAGE-mediated Akt signaling, we evaluated whether the formation of RAGE/IMPA1 complex corresponds with the activation of PIP3 synthesis and found that the formation of PIP3 was significantly upregulated starting from week 1.
Fig. 10.
Schematic representation of the proposed protein kinase B (Akt) activation in response to receptor for advanced glycation end products (RAGE)-inositol monophosphatase 1 (IMPA1) interaction. Accumulation of glucose (G) transporter type 4 (GLUT4) on a plasma membrane at the early stage of pulmonary arterial hypertension (PAH) increases glucose uptake and formation of glucose 6-phosphate (G6P). The increased levels of G6P stimulate IMPA1 activation and membrane translocation. Simultaneous activation of RAGE occurs in response to its interaction with damage-associated molecular patterns (DAMPs) that are released from dying cells. The formation of the RAGE-IMPA1 complex on a plasma membrane accelerates inositol synthesis and recycling. Phosphatidylinositol-3-kinase (PI3K) converts phosphatidylinositol 4,5-bisphosphate (PIP2) into phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which mediates Akt membrane translocation and activation. The active Akt not only stimulates activation of proliferative pathways but also ensures GLUT4 translocation on a plasma membrane, thus maintaining the feedforward stimulation of proliferative mechanisms. Thus, we propose that the co-occurrence of the early vascular damage and the glycolytic shift could be responsible for the persistent activation of uncontrolled growth in pulmonary vascular cells. Ins, inositol; IP, inositol monophosphate; IP3, inositol 1,4,5-trisphosphate; p85, regulatory subunit of phosphatidylinositol-4,5-bisphosphate 3-kinase; p100, catalytic subunit of PIP3K; pS473, phosphorylated Ser473 of Akt.
Although the direct role of RAGE/IMPA1 interaction in Akt activation could be validated only by a currently unavailable approach that would disrupt the formation of this complex, we demonstrated that inhibition of either RAGE or IMPA1 in vitro is sufficient to attenuate apoptosis-induced Akt activation. Besides, we have confirmed that in vivo, all critical steps required for Akt activation are preset and ready. Under these conditions, the emergence of IMPA1 on the plasma membrane and its anchoring to RAGE and membrane, as suggested by our computational modeling analysis, would be expected to boost the production of PIP3, Akt translocation, and activation, all of which have been observed in our study. Notably, the time course of these events corresponds with the RAGE/IMPA1 complex formation and disease progression.
Activation of proliferative signaling.
There is a large body of experimental evidence confirming that initial vascular apoptosis triggers angioproliferative changes in the pulmonary arteries (22). The current hypothesis of PAH pathogenesis proposes that the massive pulmonary vascular stress and severe endothelial loss provide the conditions for the subsequent emergence of abnormal hyperproliferative vascular cells. The characterization of these apoptosis-resistant cells uncovered an upregulation of many prosurvival and proliferative pathways. However, the clear understanding of the mechanisms responsible for this transition into a highly proliferation phenotype was not gained. RAGE is one of the receptors that become activated in response to damage (51). A recently discovered ability of RAGE to regulate the proliferation of pulmonary EC and SMC cells in PAH (32) suggests that RAGE-mediated signaling could be interconnected with initial vascular damage and subsequent vascular proliferation.
It has also been described that there is a direct relationship between the increased glucose uptake and cell proliferation (5). Thus, the high concentration of glucose in cell media induces proliferation and increases the growth of aortic SMC (33). Importantly, overexpression of GLUT1 does not induce vascular remodeling in healthy mice but promotes medial hypertrophy in response to vascular damage (2). These previously published results show a solid connection between vascular injury, glycolytic shift, and proliferation. The direct interaction between RAGE and IMPA1 reported here provides a missing mechanistic link connecting these events (Fig. 10). Although in our study GLUT1 translocation at the early stage of PAH did not reach statistical significance, GLUT4 was found to be activated. Known to be regulated by Akt, translocation of GLUT4 could occur as a result of nitration-mediated Akt activation. Indeed, we have previously confirmed that Akt nitration increases its activity (38). Therefore, the oxidative/nitrative stress developed at the early stages of PAH could activate Akt and mediate accumulation of GLUT4 on the plasma membrane. The appearance of GLUT4 on the membrane will, in turn, trigger a feedforward loop and induce Akt overactivation (Fig. 10).
The importance of Akt-mediated signaling in cell proliferation and migration is well established. Numerous studies have confirmed that Akt controls the proliferation of cancer cells, stem and progenitor cells, and smooth muscle cells, including PASMC (54). Inhibition of Akt signaling suppresses PASMC proliferation in pulmonary hypertension (14, 21). Contrary to this, inactivation of natural Akt inhibitor, phosphatase, and tensin homolog deleted on chromosome 10 (PTEN) promotes vascular remodeling and severe pulmonary hypertension (20). Finally, the recent study indicated that Akt, and specifically isoform Akt1, has a dominant role in pulmonary vascular remodeling (54).
CONCLUSIONS
Our results provide compelling evidence of a direct interaction between RAGE and IMPA1 in PAH. Based on the known properties of IMPA1 to regulate inositol metabolism, we propose a straightforward hypothesis that appearance of IMPA1 on the plasma membrane would boost inositol-dependent Akt pathway and promote vascular remodeling. The ability of RAGE to interact with IMPA1 also interconnects the early pathological events such as initial vascular damage and metabolic reprogramming that together can serve as multifactorial “hit” and predispose pulmonary vasculature to remodeling. We believe that this knowledge would help to better understand the molecular mechanisms that control PAH development and creates a background for the effective identification of the biomarkers of early PAH.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-133085 (O. Rafikova) and R01-HL-132918 (R. Rafikov) and Scientist Development Grant 14SDG20480354 (R. Rafikov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.R. and O.R. conceived and designed research; R.R., M.L.M., M.Z., S.K., N.M., M.N., and P.R.L. performed experiments; R.R., M.L.M., M.Z., S.K., N.M., P.R.L., and O.R. analyzed data; R.R. and O.R. interpreted results of experiments; R.R., S.K., and O.R. prepared figures; R.R. and O.R. drafted manuscript; R.R. and M.L.M. edited and revised manuscript; O.R. approved final version of manuscript.
REFERENCES
- 1.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 2.Adhikari N, Basi DL, Carlson M, Mariash A, Hong Z, Lehman U, Mullegama S, Weir EK, Hall JL. Increase in GLUT1 in smooth muscle alters vascular contractility and increases inflammation in response to vascular injury. Arterioscler Thromb Vasc Biol 31: 86–94, 2011. doi: 10.1161/ATVBAHA.110.215004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao JM, He MY, Liu YW, Lu YJ, Hong YQ, Luo HH, Ren ZL, Zhao SC, Jiang Y. AGE/RAGE/Akt pathway contributes to prostate cancer cell proliferation by promoting Rb phosphorylation and degradation. Am J Cancer Res 5: 1741–1750, 2015. [PMC free article] [PubMed] [Google Scholar]
- 4.Chauvin TR, Griswold MD. Characterization of the expression and regulation of genes necessary for myo-inositol biosynthesis and transport in the seminiferous epithelium. Biol Reprod 70: 744–751, 2004. doi: 10.1095/biolreprod.103.022731. [DOI] [PubMed] [Google Scholar]
- 5.Chiong M, Morales P, Torres G, Gutiérrez T, García L, Ibacache M, Michea L. Influence of glucose metabolism on vascular smooth muscle cell proliferation. Vasa 42: 8–16, 2013. doi: 10.1024/0301-1526/a000243. [DOI] [PubMed] [Google Scholar]
- 6.Davie NJ, Crossno JT Jr, Frid MG, Hofmeister SE, Reeves JT, Hyde DM, Carpenter TC, Brunetti JA, McNiece IK, Stenmark KR. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol 286: L668–L678, 2004. doi: 10.1152/ajplung.00108.2003. [DOI] [PubMed] [Google Scholar]
- 7.Dempsie Y, Nilsen M, White K, Mair KM, Loughlin L, Ambartsumian N, Rabinovitch M, Maclean MR. Development of pulmonary arterial hypertension in mice over-expressing S100A4/Mts1 is specific to females. Respir Res 12: 159, 2011. doi: 10.1186/1465-9921-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebner M, Lučić I, Leonard TA, Yudushkin I. PI(3,4,5)P3 engagement restricts Akt activity to cellular membranes. Mol Cell 65: 416–431.e6, 2017. doi: 10.1016/j.molcel.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 9.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, Fini MA, Graham BB, Tuder RM, Friedman JE, Eltzschig HK, Sokol RJ, Stenmark KR. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol 193: 597–609, 2014. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueiredo T, Melo US, Pessoa AL, Nobrega PR, Kitajima JP, Rusch H, Vaz F, Lucato LT, Zatz M, Kok F, Santos S. A homozygous loss-of-function mutation in inositol monophosphatase 1 (IMPA1) causes severe intellectual disability. Mol Psychiatry 21: 1125–1129, 2016. doi: 10.1038/mp.2015.150. [DOI] [PubMed] [Google Scholar]
- 11.Frille A, Steinhoff KG, Hesse S, Grachtrup S, Wald A, Wirtz H, Sabri O, Seyfarth HJ. Thoracic [18F]fluorodeoxyglucose uptake measured by positron emission tomography/computed tomography in pulmonary hypertension. Medicine (Baltimore) 95: e3976, 2016. doi: 10.1097/MD.0000000000003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garat CV, Crossno JT Jr, Sullivan TM, Reusch JE, Klemm DJ. Inhibition of phosphatidylinositol 3-kinase/Akt signaling attenuates hypoxia-induced pulmonary artery remodeling and suppresses CREB depletion in arterial smooth muscle cells. J Cardiovasc Pharmacol 62: 539–548, 2013. doi: 10.1097/FJC.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardell AM, Yang J, Sacchi R, Fangue NA, Hammock BD, Kültz D. Tilapia (Oreochromis mossambicus) brain cells respond to hyperosmotic challenge by inducing myo-inositol biosynthesis. J Exp Biol 216: 4615–4625, 2013. doi: 10.1242/jeb.088906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation 129: 864–874, 2014. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guignabert C, Tu L, Girerd B, Ricard N, Huertas A, Montani D, Humbert M. New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: importance of endothelial communication. Chest 147: 529–537, 2015. doi: 10.1378/chest.14-0862. [DOI] [PubMed] [Google Scholar]
- 16.Gurbanov E, Shiliang X. The key role of apoptosis in the pathogenesis and treatment of pulmonary hypertension. Eur J Cardiothorac Surg 30: 499–507, 2006. doi: 10.1016/j.ejcts.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Hameed AG, Arnold ND, Chamberlain J, Pickworth JA, Paiva C, Dawson S, Cross S, Long L, Zhao L, Morrell NW, Crossman DC, Newman CM, Kiely DG, Francis SE, Lawrie A. Inhibition of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reverses experimental pulmonary hypertension. J Exp Med 209: 1919–1935, 2012. doi: 10.1084/jem.20112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasegawa J, Wagner KF, Karp D, Li D, Shibata J, Heringlake M, Bahlmann L, Depping R, Fandrey J, Schmucker P, Uhlig S. Altered pulmonary vascular reactivity in mice with excessive erythrocytosis. Am J Respir Crit Care Med 169: 829–835, 2004. doi: 10.1164/rccm.200308-1154OC. [DOI] [PubMed] [Google Scholar]
- 19.Heistad DD, Marcus ML. Role of vasa vasorum in nourishment of the aorta. Blood Vessels 16: 225–238, 1979. [DOI] [PubMed] [Google Scholar]
- 20.Horita H, Furgeson SB, Ostriker A, Olszewski KA, Sullivan T, Villegas LR, Levine M, Parr JE, Cool CD, Nemenoff RA, Weiser-Evans MC. Selective inactivation of PTEN in smooth muscle cells synergizes with hypoxia to induce severe pulmonary hypertension. J Am Heart Assoc 2: e000188, 2013. doi: 10.1161/JAHA.113.000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houssaini A, Abid S, Mouraret N, Wan F, Rideau D, Saker M, Marcos E, Tissot CM, Dubois-Randé JL, Amsellem V, Adnot S. Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am J Respir Cell Mol Biol 48: 568–577, 2013. doi: 10.1165/rcmb.2012-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther 126: 1–8, 2010. doi: 10.1016/j.pharmthera.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, Bierhaus A, Lotze MT, Zeh HJ. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ 17: 666–676, 2010. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger E, Darden T, Nabuurs SB, Finkelstein A, Vriend G. Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins 57: 678–683, 2004. doi: 10.1002/prot.20251. [DOI] [PubMed] [Google Scholar]
- 25.Kruse R, Krantz J, Barker N, Coletta R, Rafikov R, Luo M, Hoejlund K, Mandarino LJ, Langlais PR. The CLASP2 protein interaction network in adipocytes links CLIP2 to AGAP3, CLASP2 to G2L1, MARK2, and SOGA1, and identifies SOGA1 as a microtubule-associated protein. Mol Cell Proteomics 16: 1718–1735, 2017. doi: 10.1074/mcp.RA117.000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lévy M, Maurey C, Celermajer DS, Vouhé PR, Danel C, Bonnet D, Israël-Biet D. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol 49: 803–810, 2007. doi: 10.1016/j.jacc.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Liu S, Zhang Z, Xu Q, Xie F, Wang J, Ping S, Li C, Wang Z, Zhang M, Huang J, Chen D, Hu L, Li C. RAGE mediates accelerated diabetic vein graft atherosclerosis induced by combined mechanical stress and AGEs via synergistic ERK activation. PLoS One 7: e35016, 2012. doi: 10.1371/journal.pone.0035016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim J, Menon V, Bitzer M, Miller LM, Madrid-Aliste C, Weiss LM, Fiser A, Angeletti RH. Frozen tissue can provide reproducible proteomic results of subcellular fractionation. Anal Biochem 418: 78–84, 2011. doi: 10.1016/j.ab.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logsdon CD, Fuentes MK, Huang EH, Arumugam T. RAGE and RAGE ligands in cancer. Curr Mol Med 7: 777–789, 2007. doi: 10.2174/156652407783220697. [DOI] [PubMed] [Google Scholar]
- 30.Mandras SA, Gilkin RJ Jr, Pruett JA, Raspa S. Pulmonary arterial hypertension: progress and challenges in the modern treatment era. Am J Manag Care 20, Suppl: S191–S199, 2014. [PubMed] [Google Scholar]
- 31.Manigrasso MB, Pan J, Rai V, Zhang J, Reverdatto S, Quadri N, DeVita RJ, Ramasamy R, Shekhtman A, Schmidt AM. Small molecule inhibition of ligand-stimulated RAGE-DIAPH1 signal transduction. Sci Rep 6: 22450, 2016. doi: 10.1038/srep22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meloche J, Courchesne A, Barrier M, Carter S, Bisserier M, Paulin R, Lauzon-Joset JF, Breuils-Bonnet S, Tremblay É, Biardel S, Racine C, Courture C, Bonnet P, Majka SM, Deshaies Y, Picard F, Provencher S, Bonnet S. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J Am Heart Assoc 2: e005157, 2013. doi: 10.1161/JAHA.112.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natarajan R, Gonzales N, Xu L, Nadler JL. Vascular smooth muscle cells exhibit increased growth in response to elevated glucose. Biochem Biophys Res Commun 187: 552–560, 1992. doi: 10.1016/S0006-291X(05)81529-3. [DOI] [PubMed] [Google Scholar]
- 34.Perrone L, Peluso G, Melone MA. RAGE recycles at the plasma membrane in S100B secretory vesicles and promotes Schwann cells morphological changes. J Cell Physiol 217: 60–71, 2008. doi: 10.1002/jcp.21474. [DOI] [PubMed] [Google Scholar]
- 35.Qin Q, Niu J, Wang Z, Xu W, Qiao Z, Gu Y. Heparanase induced by advanced glycation end products (AGEs) promotes macrophage migration involving RAGE and PI3K/AKT pathway. Cardiovasc Diabetol 12: 37, 2013. doi: 10.1186/1475-2840-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med 203: 1637–1642, 2006. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122: 4306–4313, 2012. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafikov R, Rafikova O, Aggarwal S, Gross C, Sun X, Desai J, Fulton D, Black SM. Asymmetric dimethylarginine induces endothelial nitric-oxide synthase mitochondrial redistribution through the nitration-mediated activation of Akt1. J Biol Chem 288: 6212–6226, 2013. doi: 10.1074/jbc.M112.423269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rafikova O, Meadows ML, Kinchen JM, Mohney RP, Maltepe E, Desai AA, Yuan JX, Garcia JG, Fineman JR, Rafikov R, Black SM. Metabolic Changes Precede the Development of Pulmonary Hypertension in the Monocrotaline Exposed Rat Lung. PLoS One 11: e0150480, 2016. doi: 10.1371/journal.pone.0150480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rafikova O, Rafikov R, Kangath A, Qu N, Aggarwal S, Sharma S, Desai J, Fields T, Ludewig B, Yuan JX, Jonigk D, Black SM. Redox regulation of epidermal growth factor receptor signaling during the development of pulmonary hypertension. Free Radic Biol Med 95: 96–111, 2016. doi: 10.1016/j.freeradbiomed.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rafikova O, Rafikov R, Kumar S, Sharma S, Aggarwal S, Schneider F, Jonigk D, Black SM, Tofovic SP. Bosentan inhibits oxidative and nitrosative stress and rescues occlusive pulmonary hypertension. Free Radic Biol Med 56: 28–43, 2013. doi: 10.1016/j.freeradbiomed.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafikova O, Williams ER, McBride ML, Zemskova M, Srivastava A, Nair V, Desai AA, Langlais PR, Zemskov E, Simon M, Mandarino LJ, Rafikov R. Hemolysis-induced lung vascular leakage contributes to the development of pulmonary hypertension. Am J Respir Cell Mol Biol 59: 334–345, 2018. doi: 10.1165/rcmb.2017-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rai V, Maldonado AY, Burz DS, Reverdatto S, Yan SF, Schmidt AM, Shekhtman A. Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. J Biol Chem 287: 5133–5144, 2012. [Erratum in: J Biol Chem 30: 11283, 2012.] doi: 10.1074/jbc.M111.277731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivero-Gutiérrez B, Anzola A, Martínez-Augustin O, de Medina FS. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal Biochem 467: 1–3, 2014. doi: 10.1016/j.ab.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 45.Rubin LJ. Primary pulmonary hypertension. N Engl J Med 336: 111–117, 1997. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 46.Sacchi R, Gardell AM, Chang N, Kültz D. Osmotic regulation and tissue localization of the myo-inositol biosynthesis pathway in tilapia (Oreochromis mossambicus) larvae. J Exp Zool A Ecol Genet Physiol 321: 457–466, 2014. doi: 10.1002/jez.1878. [DOI] [PubMed] [Google Scholar]
- 47.Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest 111: 959–972, 2003. doi: 10.1172/JCI200317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 10: 95, 2009. doi: 10.1186/1465-9921-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saygin D, Highland KB, Farha S, Park M, Sharp J, Roach EC, Tang WHW, Thomas JD, Erzurum SC, Neumann DR, DiFilippo FP. Metabolic and Functional Evaluation of the Heart and Lungs in Pulmonary Hypertension by Gated 2-[18F]-Fluoro-2-deoxy-D-glucose Positron Emission Tomography. Pulm Circ 7: 428–438, 2017. doi: 10.1177/2045893217701917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sobin SS, Tremer HM, Hardy JD, Chiodi HP. Changes in arteriole in acute and chronic hypoxic pulmonary hypertension and recovery in rat. J Appl Physiol 55: 1445–1455, 1983. doi: 10.1152/jappl.1983.55.5.1445. [DOI] [PubMed] [Google Scholar]
- 51.Sorci G, Riuzzi F, Giambanco I, Donato R. RAGE in tissue homeostasis, repair and regeneration. Biochim Biophys Acta 1833: 101–109, 2013. doi: 10.1016/j.bbamcr.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 52.Strauss BH, Rabinovitch M. Adventitial fibroblasts: defining a role in vessel wall remodeling. Am J Respir Cell Mol Biol 22: 1–3, 2000. doi: 10.1165/ajrcmb.22.1.f172. [DOI] [PubMed] [Google Scholar]
- 53.Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol 294: C1407–C1418, 2008. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang H, Chen J, Fraidenburg DR, Song S, Sysol JR, Drennan AR, Offermanns S, Ye RD, Bonini MG, Minshall RD, Garcia JG, Machado RF, Makino A, Yuan JX. Deficiency of Akt1, but not Akt2, attenuates the development of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L208–L220, 2015. doi: 10.1152/ajplung.00242.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson AAR, Lawrie A. Targeting Vascular Remodeling to Treat Pulmonary Arterial Hypertension. Trends Mol Med 23: 31–45, 2017. doi: 10.1016/j.molmed.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, Gera L, Farkas L, Rabinovitch M, Zamanian RT, Inayathullah M, Fridlib M, Rajadas J, Peters-Golden M, Voelkel NF, Nicolls MR. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 5: 200ra117, 2013. doi: 10.1126/scitranslmed.3006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Touré F, Fritz G, Li Q, Rai V, Daffu G, Zou YS, Rosario R, Ramasamy R, Alberts AS, Yan SF, Schmidt AM. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res 110: 1279–1293, 2012. doi: 10.1161/CIRCRESAHA.111.262519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuder RM. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res 367: 643–649, 2017. doi: 10.1007/s00441-016-2539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med 185: 260–266, 2012. doi: 10.1164/rccm.201108-1536PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White K, Dempsie Y, Caruso P, Wallace E, McDonald RA, Stevens H, Hatley ME, Van Rooij E, Morrell NW, MacLean MR, Baker AH. Endothelial apoptosis in pulmonary hypertension is controlled by a microRNA/programmed cell death 4/caspase-3 axis. Hypertension 64: 185–194, 2014. doi: 10.1161/HYPERTENSIONAHA.113.03037. [DOI] [PubMed] [Google Scholar]
- 61.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, Erzurum SC. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA 104: 1342–1347, 2007. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao L, Ashek A, Wang L, Fang W, Dabral S, Dubois O, Cupitt J, Pullamsetti SS, Cotroneo E, Jones H, Tomasi G, Nguyen QD, Aboagye EO, El-Bahrawy MA, Barnes G, Howard LS, Gibbs JS, Gsell W, He JG, Wilkins MR. Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation 128: 1214–1224, 2013. doi: 10.1161/CIRCULATIONAHA.113.004136. [DOI] [PubMed] [Google Scholar]