Abstract
Clinical studies have shown that obesity negatively impacts large arteries’ function. We reported that rats exposed to maternal separation (MatSep), a model of early life stress, display enhanced angiotensin II (ANG II)-induced vasoconstriction in aortic rings cleaned of perivascular adipose tissue (PVAT) under normal diet (ND) conditions. We hypothesized that exposure to MatSep promotes a greater loss of PVAT-mediated protective effects on vascular function and loss of blood pressure (BP) rhythm in rats fed a high-fat diet (HFD) when compared with controls. MatSep was performed in male Wistar-Kyoto rats from days 2 to 14 of life. Normally reared littermates served as controls. On ND, aortic rings from MatSep rats with PVAT removed showed increased ANG II-mediated vasoconstriction versus controls; however, rings from MatSep rats with intact PVAT displayed blunted constriction. This effect was exacerbated by an HFD in both groups; however, the anticontractile effect of PVAT was greater in MatSep rats. Acetylcholine-induced relaxation was similar in MatSep and control rats fed an ND, regardless of the presence of PVAT. HFD impaired aortic relaxation in rings without PVAT from MatSep rats, whereas the presence of PVAT improved relaxation in both groups. On an HFD, immunolocalization of vascular smooth muscle-derived ANG-(1–7) and PVAT-derived adiponectin abundances were increased in MatSep. In rats fed an HFD, 24-h BP and BP rhythms were similar between groups. In summary, MatSep enhanced the ability of PVAT to blunt the heightened ANG II-induced vasoconstriction and endothelial dysfunction in rats fed an HFD. This protective effect may be mediated via the upregulation of vasoprotective factors within the adipovascular axis.
Keywords: early life stress, high-fat diet, maternal separation, PVAT, vascular reactivity
INTRODUCTION
Health problems resulting from being overweight or obese now affect more than 2 billion people worldwide (19a). Obesity leads to vascular dysfunction in humans (2, 43, 54) and is associated with reduced compliance of large arteries independent of conventional risk factors (1). Accordingly, clinical studies have shown that obesity accelerates the development of atherosclerosis and stiffness associated with aging (41, 42).
It is clear that adipose tissue releases endocrine and paracrine factors that influence vascular function and other metabolic processes (43, 66) and is no longer exclusively linked to storage of energy and mechanical protection. This is also true for perivascular adipose tissue (PVAT) that surrounds most systemic vascular structures (10, 57). Normal PVAT, as present in lean subjects, aids in maintaining vascular homeostasis, as its presence diminishes the response to contractile factors, including angiotensin II (ANG II) and the α1-adrenergic agonist phenylephrine (PE) (21, 53). Furthermore, a high-fat diet (HFD) promotes both enhanced vasoconstriction and endothelial dysfunction by leading to a loss of PVAT-mediated anticontractile and vasodilatory function (22, 62). In humans, PVAT mass correlates with hypertension, diabetes, and aortic/coronary calcification (34), whereas the anticontractile effect of PVAT is abolished in patients with obesity and metabolic syndrome (22). Obesity leads to vascular dysfunction in humans (2, 43, 54) similar to the effect of an HFD in rodents (11, 46). In rats fed an HFD, the anticontractile effect of PVAT is also reduced (19, 28) along with impaired vasodilator responses to acetylcholine secondary to the loss of endothelial protection (28, 40).
Humans exposed to adverse childhood experiences, often referred to as early life stress (ELS), displays increased systolic blood pressure, body mass index, and circulating risk factors for metabolic disease, such as C-reactive protein and endothelin-1 (14, 24, 55). We have previously shown that maternal separation (MatSep), a model of ELS in rats, sensitizes the vasoconstrictive responses induced by ANG II in ex vivo aortic ring preparations without PVAT (38). Moreover, rats exposed to MatSep also present with exaggerated ANG II-induced hypertension in vivo (36, 39). Thus, these cardiovascular outcomes in the MatSep model support the concept that psychosocial stress during early life is linked to vascular dysfunction in adulthood. However, little is known about the influence of early life insults on PVAT function in the face of unhealthy dietary conditions, such as an HFD.
Vascular smooth muscle (VSM) of the blood vessel wall is responsible for maintaining normal vascular tone and structural integrity. Notably, the contractile responses of VSM to numerous stimuli have been demonstrated to exhibit time of day variations under normal physiological conditions (7, 61, 64). Under acute or chronic stress conditions, increased glucocorticoids may reset the phase of the circadian clock system that can impact vessel function, including blood pressure control (3). Blood pressure rhythms are disrupted in obese patients and in experimental models of obesity (29, 52, 56). Stressors applied at the beginning of the light phase induced a phase advance, whereas stressing mice at the beginning of the dark phase caused phase delays of clock genes expression (58). It is unknown whether MatSep has any effects on the peripheral and/or central circadian rhythms contributing to the regulation of blood pressure, heart rate (HR), locomotor activity, or autonomic function when exposed to an HFD in induced obesity.
It is well established that an HFD induces the development and progression of vascular derangements (4, 9, 15, 17); however, it is unknown whether the exposure to MatSep further exacerabates these vascular derangements. Therefore, this study was designed to test the hypothesis that exposure to MatSep is associated with a greater loss of PVAT-mediated protective effects on vascular function and loss of blood pressure rhythm in rats fed an HFD when compared with controls. We conducted experiments to evaluate vascular function in isolated aortic rings with intact PVAT, thus preserving the adipovascular axis comprised of the endothelium, VSM, and PVAT and compared these results to experiments examining aortic function with PVAT removed. Furthermore, we utilized telemetry to determine blood pressure rhythms of control and MatSep rats on a normal diet (ND) or HFD. Finally, we determined the abundance of vasoprotective mediators, ANG-(1–7), and adiponectin, in the adipovascular axis as well as circulating metabolic mediators and assessments of autonomic tone to evaluate possible mechanistic influences of MatSep under HFD conditions.
METHODS
Animal model and experimental design.
All animal protocols received prior approval by the institutional animal care and use committee at Augusta University and in accordance with the Animal Research: Reporting of In Vivo Experiments guidelines. The MatSep protocol was performed as previously described (35, 38). Briefly, approximately half of the male pups were separated from their mothers (MatSep) and littermates by transferring the pups to a clean cage in an incubator (30 ± 1°C) for 3 h from day 2 through 14 of life. Normally reared, nonhandled littermates remained with their mothers and served as the control group. At weaning (4 wk of age), rats were placed on an ND or HFD for 17 wk. Experimental groups were comprised by control and MatSep littermates generated from different litters. The HFD consisted of calories from 14% protein, 26% carbohydrates, and 60% fat with 5.49 kcal/g gross energy (F3282 Bioserv, Frenchtown, NJ). Regular chow used as an ND consisted of calories from 24% protein, 58% carbohydrates, and 18% fat with 3.1 kcal/g gross energy (Teklad 8604, Madison, WI). All rats were given tap water ad libitum. After 15 wk of special diets, a set of rats was placed in metabolic cages for urine collection. A week later, 16 h of fasting were allowed before determining insulin and glucose in plasma. At week 17, rats were euthanized for plasma collection and vascular reactivity studies. A separate set of littermates were implanted with telemetry transmitters for blood pressure, HR, locomotor, and frequency domain analysis.
Vascular reactivity.
Each thoracic aorta was sectioned in two segments: one was carefully cleaned of adherent PVAT using microdissection scissors under the microscope to avoid vascular tissue alterations and the other one was left intact, each segment was cut into two concentric rings (~3 mm each), and mounted on pins for wire myography (Danish Myo Technology, Aarhus, Denmark) and tested for viability as previously described (38). One ring derived from each segment was used to test constrictors and the other one to test vasodilators. Briefly, preload tension was fixed to 28 mN in Krebs solution (in mM: 118.4 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25.0 NaHCO3, 11.6 glucose, and 1.9 CaCl2). Cumulative concentration-response (CCR) curves to ANG II (1 × 10−12 to 3 × 10−4 M) or the α1-adrenergic agonist PE (1 × 10−9 to 3 × 10−5 M) were utilized to assess contraction in the presence and absence of PVAT. In each aorta, we studied segments lacking or preserving PVAT. Contraction data are presented as percent increase in force from baseline tension. Endothelial-dependent relaxation was also assessed in additional aortic segments lacking or preserving PVAT following preconstriction with 2 μM PE and generation of CCR curves to acetylcholine (ACh) (1 × 10−9 to 1 × 10−5 M). After recovery, vasorelaxation to the endothelium-independent vasodilator sodium nitroprusside (1 × 10−10 M to 1 × 10−5 M) was assessed in the same aortic segments. Vasorelaxation data are presented as percent relaxation from PE-induced constriction. For all CCR curves, maximum response (Emax) and sensitivity to the vasoactive agonists (−log EC50), as well as area under the curve (AUC), were calculated using Prism Software (GraphPad, San Diego, CA).
Immunohistochemical analysis of ANG-(1–7) and adiponectin.
Aortas with preserved PVAT were drop-fixed in 4% buffered formalin solution overnight at room temperature, transferred to 70% ethanol for 24 h, and embedded in paraffin. Aortas were cut into 4-μm-thick sections, mounted on Superfrost slides, and stained with primary antibodies specific for rat adiponectin (1:10,000; cat. no. ARG22406; Arigo Biolaboratories, Hsinchu City, Taiwan) and ANG-(1–7) (1:15,000; cat. no. ARG70007; Arigo Biolaboratories). Staining was detected with polymer-conjugated secondary antibody procedures (Biocare Medical, Concord, CA). Localization of protein abundance in each section of aorta section was blindly evaluated within the endothelium, VSM, and perivascular fat regions using a scale from zero (no expression present) to six (highest expression). The numbers are reported as an average of the scores per region in each experimental group.
Telemetry measurements.
Rats fed an HFD were implanted with telemetry transmitters at 8 wk of age (Data Sciences, St. Paul, MN) (36). After recovery from implant surgery (7–10 days), mean arterial pressure (MAP), HR, and locomotor activity were recorded by telemetry transmitters CA-P40 in 10-s bursts every 10 min throughout the experiment. Data were tabulated using ClockLab Analysis 6 (Acitmetrics, Wilmette, IL) for the analysis of time series in chronobiology. Mesor (midline statistic of rhythm, a rhythm-adjusted mean), amplitude (a measure of half the extent of predictable variation within a cycle), and phase (a measure of the time of overall high values recurring in each cycle) were calculated for each animal. Data were expressed in zeitgeber time (ZT), which is a quantification of time defined with reference to environmental regularities or zeitgebers, such as the 12:12 light-dark cycle. For our purposes, ZT0 is defined as the lights-on time, or 7 AM. HR variability parameters were measured using Ponemah 6.3 (Data Sciences International, St. Paul, MN). The maximum derivative of the arterial pulse wave was used for beat-to-beat interval triggering. Active period recordings were taken from ZT14–22 and inactive period from ZT2–10. Frequency domain analysis was completed using 10-s bins using low-frequency (0.2–0.6 Hz) and high-frequency (1.0–3.0 Hz) ranges using the maximum derivative of the blood pressure signal for interbeat interval triggering. Blood pressure frequency domain analysis used the same frequency cutoffs triggered by systolic (maximum) blood pressure from the pulse wave analyzed in 10-min bins (6).
Metabolic profile and plasma measurements.
Body weight and fat pad weight were determined gravimetrically and expressed in grams. The adiposity index was calculated by dividing total weight of retroperitoneal and epididymal adipose tissue by the total body weight then multiplied by 100 and expressed in percent. Blood was obtained from a direct puncture of the abdominal aorta with the rat under anesthesia after 16 h of fasting. Plasma was collected using 7.5% EDTA solution. Plasma levels of leptin, insulin, aldosterone (Cayman, Ann Arbor, MI), and adiponectin (EMD Millipore, Temecula, CA) were performed by enzyme immunoassay. Total cholesterol was determined using an enzymatic kit (Wako Diagnostics, Richmond, VA). Plasma renin activity was assessed using a PRA I125 RIA Kit (DiaSorin, Stillwater, MN). Glucose was measured with an Accu-Check meter (Roche, Indianapolis, IN). Creatinine concentration in plasma was measured by the Jaffé reaction as previously reported (39). Homeostatic model assessment, a method for assessing β-cell function and insulin resistance, was calculated as fasting plasma insulin × fasting plasma glucose (63). After 16 wk on an ND or HFD, rats were placed in metabolic cages to obtain food and water intake and urine output. After 2 days of acclimation, 24-h urine collections were taken. Urinary protein excretion was determined by a colorimetric assay using the Quick Start Bradford Dye Reagent 1X (Bio-Rad, Hercules, CA).
Statistical analysis.
Two-way ANOVA analysis followed by a Bonferroni post hoc test was used to assess the differences in −log EC50 and Emax between MatSep and diet (Tables 1 and 2) or MatSep and PVAT (Figs. 1 and 2) in the vascular reactivity studies using Prism version 5.01 for Windows (GraphPad). For blood pressure and HR variability analysis, the data were analyzed by two-way repeated measures ANOVA with Sidak’s multiple comparisons post hoc test and reported as means ± SE. A value of P < 0.05 was considered statistically significant.
Table 1.
Determination of Emax and −logEC50 in thoracic aorta in absence of PVAT or in the presence of PVAT from control and MatSep males fed an ND or HFD for 17 wk in response to PE and ANG II
| ND |
HFD |
|||||
|---|---|---|---|---|---|---|
| Control | MatSep | Control | MatSep | PDiet | PMatSep | |
| ANG II-induced constriction (% Inc. in Force) in the absence of PVAT | ||||||
| Emax | 19.0 ± 4.0 | 32.1 ± 3.0 | 32 ± 5 | 54 ± 4 | P < 0.05 | P < 0.05 |
| −log EC50 | 7.5 ± 0.1 | 7.1 ± 0.3 | 8.4 ± 0.4# | 8.9 ± 0.2 | P < 0.05 | NS |
| KCl | 43.1 ± 1.3 | 39.2 ± 1.4 | 43.5 ± 1.2 | 42.2 ± 2.6 | NS | NS |
| PE-induced constriction (% Inc. in Force) in the absence of PVAT | ||||||
| Emax | 40.6 ± 6.1 | 36.6 ± 7.2 | 60 ± 7 | 53 ± 4 | P < 0.05 | NS |
| −log EC50 | 6.5 ± 0.2 | 6.2 ± 0.2 | 7.4 ± 0.1 | 6.8 ± 0.2 | P < 0.05 | P < 0.05 |
| KCl | 42.3 ± 1.7 | 43.6 ± 2.1 | 45.1 ± 2.1 | 42.3 ± 2.1 | NS | NS |
| ANG II-induced constriction (% Inc. in Force) in the presence of PVAT | ||||||
| Emax | 13.8 ± 2.1 | 14.3 ± 2.7 | 20 ± 3 | 15 ± 3 | NS | NS |
| −log EC50 | 7.1 ± 0.4 | 6.5 ± 0.3 | 6.4 ± 0.2 | 6.6 ± 0.1 | NS | NS |
| KCl | 37.5 ± 2.7 | 36.4 ± 1.3 | 39.9 ± 0.9 | 38.5 ± 1.1 | NS | NS |
| PE-induced constriction (% Inc. in Force) in the presence of PVAT | ||||||
| Emax | 24.4 ± 2.4 | 28.9 ± 1.8 | 44 ± 4# | 42 ± 8 | NS | NS |
| −log EC50 | 6.3 ± 0.4 | 5.8 ± 0.4 | 6.7 ± 0.1 | 6.4 ± 0.7 | NS | NS |
| KCl | 38.3 ± 1.6 | 41.0 ± 2.7 | 39.9 ± 0.8 | 40.4 ± 2.3 | NS | NS |
Values are means ± SE. Determination of maximum response (Emax) and −logEC50 in thoracic aorta in absence of perivascular adipose tissue (PVAT) or in the presence of PVAT from control and maternal separation (MatSep) males fed a normal diet (ND) or a high-fat diet (HFD) for 17 wk in response to phenylephrine (PE) and angiotensin II (ANG II). The nonreceptor-dependent KCl constrictive response is displayed in each condition. n = 6 per group for ND and 5 per group for HFD; two-way-ANOVA: There was not significant interaction between diet and MatSep.
Table 2.
Determination of Emax and −logEC50 in thoracic aorta in absence of PVAT or in the presence of PVAT from control and MatSep males fed an ND or HFD for 17 wk in response to ACh and SNP
| ND |
HFD |
|||||
|---|---|---|---|---|---|---|
| Control | MatSep | Control | MatSep | PDiet | PMatSep | |
| ACh-induced relaxation, (% of PE) in the absence of PVAT | ||||||
| Emax | 83.4 ± 2.0 | 76.8 ± 7.7 | 48 ± 2 | 29 ± 8 | P < 0.05 | P < 0.05 |
| −log EC50 | 7.1 ± 0.2 | 6.8 ± 0.2 | 7.2 ± 0.3 | 6.7 ± 0.3 | NS | NS |
| SNP-induced relaxation, (% of PE) in the absence of PVAT | ||||||
| Emax | 88.8 ± 1.6 | 86.9 ± 3.1 | 97 ± 4 | 98 ± 3 | NS | NS |
| −log EC50 | 6.3 ± 0.3 | 6.2 ± 0.2 | 8.0 ± 0.2 | 7.7 ± 0.5 | NS | NS |
| ACh-induced relaxation, (% of PE) in the presence of PVAT | ||||||
| Emax | 64.5 ± 4.2 | 74.9 ± 3.7 | 118 ± 17 | 98 ± 2 | P < 0.05 | NS |
| −log EC50 | 6.7 ± 0.4 | 7.2 ± 0.3 | 6.9 ± 0.2 | 6.3 ± 0.1 | NS | NS |
| SNP-induced relaxation, (% of PE) in the presence of PVAT | ||||||
| Emax | 84.5 ± 11.2 | 82.1 ± 7.2 | 97 ± 4 | 98 ± 3 | NS | NS |
| −log EC50 | 5.6 ± 0.4 | 5.2 ± 0.2 | 6.8 ± 0.2 | 7.2 ± 0.3 | NS | NS |
Values are means ± SE. Determination of maximum response (Emax) and −logEC50 in thoracic aorta in absence of perivascular adipose tissue (PVAT) or in the presence of PVAT from control and maternal separation (MatSep) males fed a normal diet (ND) or high-fat diet (HFD) for 17 wk in response to acetylcholine (ACh) and sodium nitroprusside (SNP). n = 6 per group for ND and 5 per group for HFD; two-way ANOVA. Pdiet < 0.05 vs. control, PMatSep < 0.05 vs. control. There was not significant interaction between diet and MatSep..
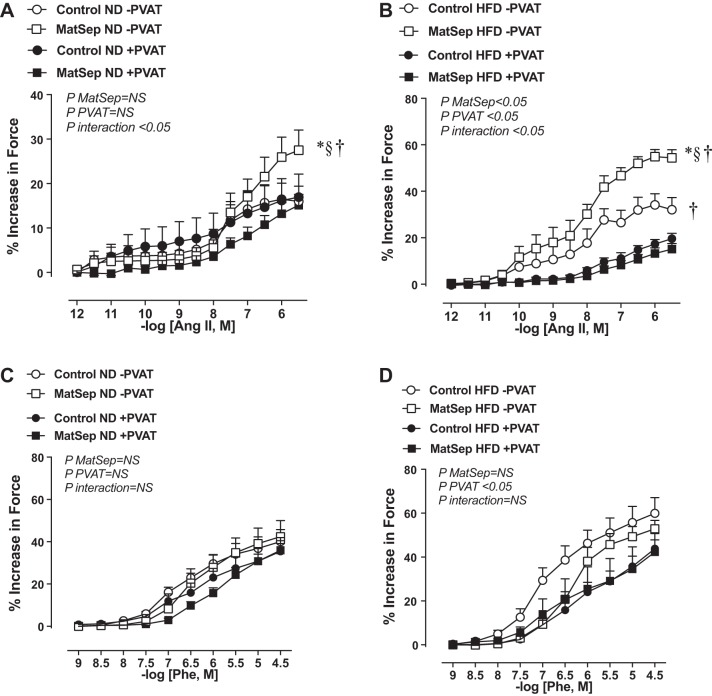
Fig. 1.
Vascular constriction in response to angiotensin II (ANG II) in thoracic aorta from normal diet (ND) (A) and high-fat diet (HFD) (B)-fed rats. Phenylephrine (Phe)-induced constriction in aortic rings from ND (C) and HFD (D)-fed rats. Open symbols, without perivascular adipose tissue (PVAT) ; closed symbols, with PVAT. MatSep, maternal separation. Data were analyzed by two-way ANOVA followed by a Bonferroni multiple comparisons post hoc test and reported as means ± SE. *P < 0.05 vs. control (C); § <0.05 vs. PVAT; †P interaction < 0.05. n = 6 per group for ND and 5 per group for HFD.
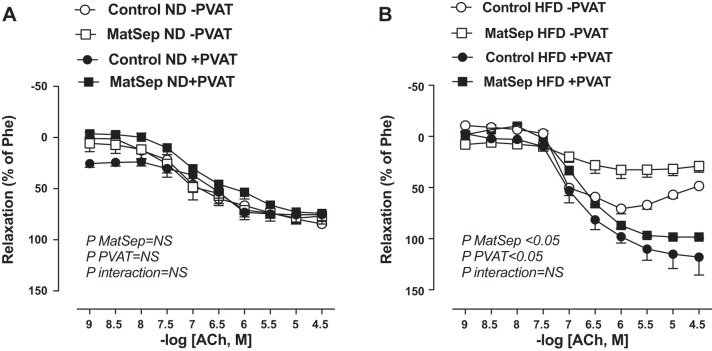
Fig. 2.
Vascular relaxation in response to acetylcholine (ACh) in thoracic aorta from normal diet (ND) (A) and high-fat diet (HFD) (B)-fed rats. Open symbols, without perivascular adipose tissue (PVAT) ; closed symbols, with PVAT. MatSep, maternal separation; Phe, phenylephrine. Data were analyzed by two-way ANOVA followed by a Bonferroni multiple comparisons post hoc test and reported as means ± SE. n = 6 per group for ND and 5 per group for HFD.
RESULTS
Effects of MatSep on PVAT function: ANG II-induced responses.
Consistent with previous reports (38), thoracic aortic rings cleaned of PVAT from MatSep rats on an ND showed heightened maximal ANG II-induced force (Emax) compared with their normally reared control counterparts (Fig. 1A; Table 1). There was no significant effect of PVAT on ANG II-induced contraction from control rats on an ND, whereas PVAT effectively blunted ANG II-induced aortic contraction from MatSep rats on an ND (Fig. 1A; Table 1). Increases in Emax to ANG II were exacerbated in aortic rings lacking PVAT from both groups when fed an HFD (Table 1). However, the increases observed in EC50 in HFD-fed rats were similar in both groups. The PVAT exerted a significant anticontractile effect in vascular tissue from both MatSep and control rats fed an HFD, which resulted in a similar ANG II-induced Emax in both groups (Fig. 1B; Table 1). Therefore, a significantly greater difference in the total AUC was found in MatSep rats fed an HFD compared with control rats (151 ± 23 AUC vs. 36 ± 8.5 AUC, P < 0.05, respectively), regardless of whether PVAT was intact or not. Neither diet nor MatSep influenced the sensitivity of the ANG II-induced responses (EC50) in rings with PVAT. No significant differences were observed with KCl-induced Emax in the absence or presence of PVAT (Table 1).
Effects of MatSep on PVAT function: PE-induced responses.
In response to PE, Emax and EC50 were similar between aortic rings lacking PVAT from control and MatSep groups fed an ND (Fig. 1C; Table 1). These variables were not affected by the presence of PVAT in either group (Fig. 1C; Table 1). An HFD increased PE-induced Emax similarly in aortic rings lacking PVAT from control and MatSep rats compared with aortic rings from rats fed an ND (Fig. 1D; Table 1); however, rings from rats fed an HFD showed a main effect on the attenuated PE response in rings with preserved PVAT without significant changes in EC50 in both groups (Fig. 1D; Table 1).
Effects of MatSep and PVAT on endothelial function.
Endothelial function (ACh-induced vasorelaxation) was similar in aortas from MatSep and control rats fed an ND in the presence or absence of PVAT (Fig. 2A; Table 2). HFD-induced endothelial dysfunction in aortic rings lacking PVAT from control rats was further exaggerated in MatSep rats (Fig. 2B; Table 2). The presence of PVAT restored the endothelial function in both groups (Fig. 2B; Table 2). These changes in Emax were independent of any change of EC50 among control and MatSep groups fed an HFD. In addition, no significant effect of diet or MatSep was observed in the sodium nitroprusside-induced vasorelaxation in Emax or EC50 (Table 2).
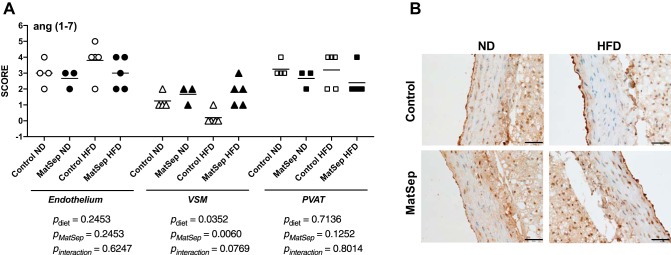
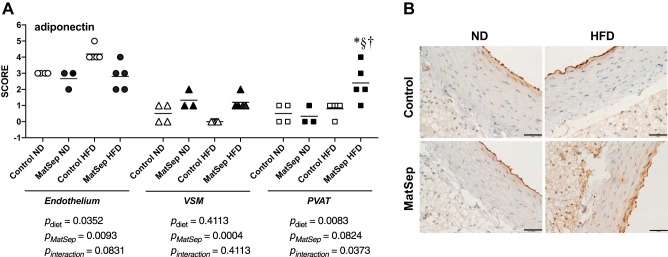
Effects of MatSep on ANG-(1–7) and adiponectin immunolocalization.
Figure 3, A and B, shows the abundance levels and the representative images of ANG-(1–7) protein localization and abundance, respectively. ANG-(1–7) abundance was similar between MatSep and control rats in endothelium and PVAT regardless of diet. However, an HFD significantly increased ANG-(1–7) expression in VSM from MatSep (Fig. 3, A and B). Figure 3, C and D, shows the abundance levels and the representative images of adiponectin protein abundance, respectively. An HFD increased the abundance of adiponectin in endothelium from control rats (Fig. 4, A and B); however, this effect was not observed in endothelium from MatSep rats. Conversely, an HFD significantly increased adiponectin protein abundance in PVAT from MatSep rats compared with controls. Altogether, these data indicate that MatSep promotes the upregulation of vasoprotective factors in VSM and PVAT that may play a role in enhancing the anticontractile function in aortic rings.
Fig. 3.
Angiotensin (Ang) (1–7) expression in endothelium, vascular smooth muscle, and perivascular adipose tissue (PVAT) from control and maternal separation (MatSep) rats fed a normal diet (ND) and a high-fat diet (HFD). A and B: semiquantification and representative pictures of ANG-(1–7) abundance, respectively. Data were analyzed by two-way ANOVA followed by a Bonferroni multiple comparisons post hoc test and reported as means ± SE. n = 4 for Control ND, 3 for MatSep ND, and 5 for Control HFD and MatSep HFD.
Fig. 4.
Adiponectin expression in endothelium, vasculat smooth muscle, and perivascular adipose tissue (PVAT) from control and maternal separation (MatSep) rats fed a normal diet (ND) and a high-fat diet (HFD). A and B: semiquantification and representative pictures of angiotensin (1–7) abundance, respectively. Data were analyzed by two-way ANOVA followed by a Bonferroni multiple comparisons post hoc test and reported as means ± SE. *P < 0.05 vs. control (C); §< 0.05 vs. PVAT; †P interaction < 0.05. n = 4 for Control ND, 3 for MatSep ND, and 5 for Control HFD and MatSep HFD.
Effects of MatSep on blood pressure, HR, locomotor activity, and autonomic function from rats fed an HFD.
MatSep rats did not have a significant difference in 24-h MAP throughout the study compared with control rats (105 ± 2 mmHg vs. 102 ± 3 mmHg, respectively) (Fig. 5; Table 3). Evaluations of the circadian rhythms of MAP and HR revealed that MatSep did not significantly affect any variable (mesor, amplitude, and phase) analyzed (Fig. 5, A and B, Table 3). However, 24-h HR was significantly reduced by ~50 beats/min in both groups after 17 wk on an HFD in MatSep (from 379 ± 7 to 326 ± 6 beats/min) and control rats (from 384 ± 7 to 316 ± 8 beats/min) (Fig. 5; Table 3). After 17 wk on an HFD, MAP, HR, and locomotor activity displayed significantly reduced amplitude and phase in both control and MatSep rats compared with after 8 wk on an HFD (Table 3). A reduction in mesor for HR and activity was found in both groups. Although the systolic blood pressure was similar between groups, the mesor analysis showed an interaction in the control group only, whereas diastolic blood pressure showed an interaction in the amplitude for both MatSep and control rats (Table 3).
Fig. 5.
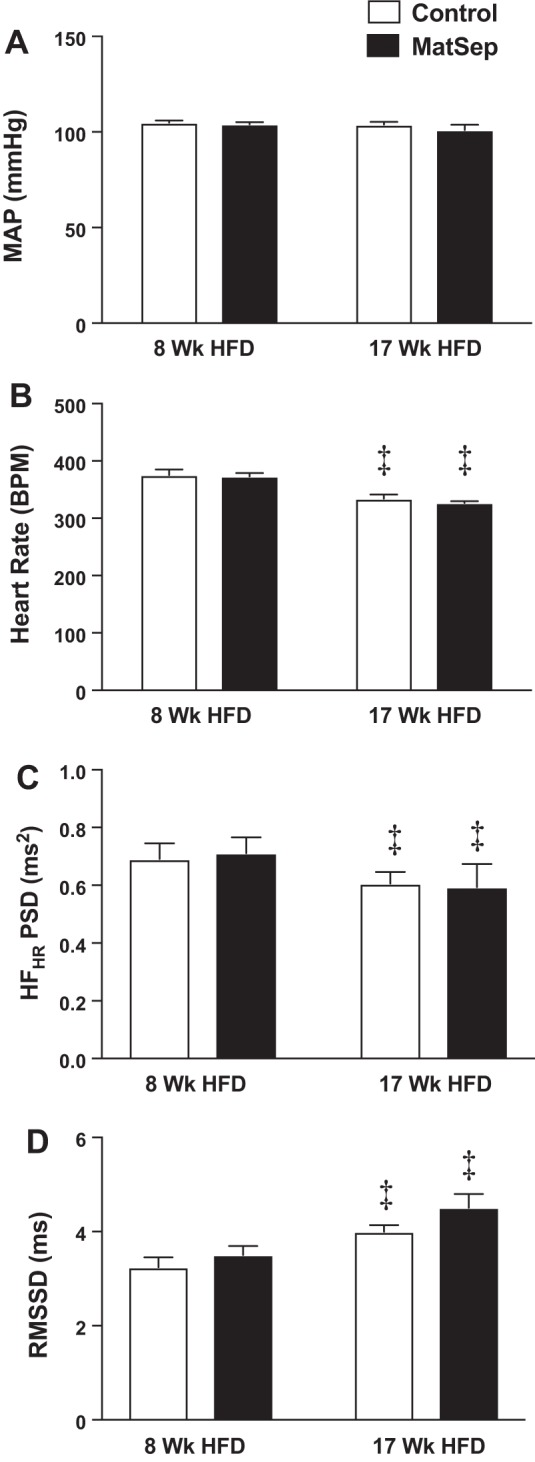
Effect of maternal separation (MatSep) after 8 and 17 wk of high-fat diet (HFD) on mean arterial pressure (MAP) (A), heart rate (HR) (B), high-frequency component of HR variability power spectral density (HFHR PSD) (C), and root mean squared of successive differences (RMSSD) (D). Data were analyzed by two-way repeated measures ANOVA with Sidak’s multiple comparisons post hoc test and reported as means ± SE. ‡P < 0.05 vs. 8 wk HFD. n = 6 control rats and 5 MatSep rats. BPM, beats/min.
Table 3.
Circadian analysis of mean arterial pressure, systolic blood pressure, diastolic blood pressure, heart rate, and activity from control and MatSep rats fed an HFD for 17 wk
| Control |
MatSep |
|||||
|---|---|---|---|---|---|---|
| Mesor | Amplitude | Phase | Mesor | Amplitude | Phase | |
| Mean arterial pressure, mmHg | ||||||
| 8 wk | 105 ± 1 | 5.5 ± 0.9 | 17.1 ± 0.7 | 104 ± 1 | 5.8 ± 1.0 | 16.9 ± 0.3 |
| 17 wk | 104 ± 1 | 3.6 ± 0.4‡ | 16.2 ± 0.9‡* | 101 ± 3 | 2.7 ± 0.2‡ | 16.0 ± 0.2‡* |
| Systolic blood pressure, mmHg† | ||||||
| 8 wk | 125 ± 0.9 | 5.9 ± 0.9 | 17.0 ± 0.4 | 123 ± 1.0 | 6.4 ± 0.9 | 16.9 ± 0.3 |
| 17 wk | 126 ± 1.2 | 4.0 ± 0.6 | 16.1 ± 0.7 | 123 ± 3.2 | 2.6 ± 0.2 | 15.9 ± 0.5 |
| Diastolic blood pressure, mmHg† | ||||||
| 8 wk | 87 ± 1.5 | 5.7 ± 0.6 | 15.7 ± 0.5 | 85 ± 1.2 | 6.3 ± 0.8 | 19.7 ± 0.2 |
| 17 wk | 85 ± 1.2 | 3.9 ± 0.5 | 14.5 ± 0.7 | 82 ± 2.5 | 3.5 ± 0.2 | 18.6 ± 0.2 |
| Heart rate, beats/min† | ||||||
| 8 wk | 375 ± 10 | 58.1 ± 1.3 | 16.1 ± 0.4 | 373 ± 6 | 71.4 ± 4.9 | 16.1 ± 0.3 |
| 17 wk | 334 ± 7* | 60.4 ± 1.6‡* | 15.5 ± 0.3‡* | 326 ± 3* | 59.6 ± 3.1‡* | 15.6 ± 0.3‡* |
| Locomotor activity (arbitrary activity units)† | ||||||
| 8 wk | 3.5 ± 0.3 | 5.3 ± 0.4 | 17.6 ± 0.2 | 3.3 ± 0.3 | 5.3 ± 0.5 | 17.6 ± 0.2 |
| 17 wk | 2.7 ± 0.3‡* | 4.7 ± 0.6‡* | 17.3 ± 0.3 | 2.3 ± 0.2‡* | 3.8 ± 0.5‡* | 17.3 ± 0.1 |
Values are means ± SE. Circadian analysis of mean arterial pressure, systolic blood pressure, diastolic blood pressure, heart rate, and activity from control and maternal separation (MatSep) rats fed a high-fat diet (HFD) for 17 wk. n = 6 for control and 5 for MatSep. Two-way ANOVA:
PTime < 0.05 vs. 8 weeks HFD,
PSubjects < 0.05 vs. 8 weeks HFD,
PInteraction < 0.05 (systolic blood pressure: mesor in control; diastolic blood pressure: amplitude in control and MatSep; heart rate: mesor in control and MatSep; locomotor activity: amplitude in control and MatSep).
Furthermore, there were no differences between MatSep and control rats at 8 or 17 wk of HFD in the high-frequency range of HR variability, although 17 wk of an HFD reduced it in both groups compared with week 8 (Fig. 5C). However, root mean squared of successive differences (Fig. 5D) and standard deviation of normal-to-normal beats showed an increase from weeks 8 to 17 of HFD within both control and MatSep groups (week 8: 2.9 ± 0.3 vs. 3.9 ± 0.2 ms, respectively, PMatSep < 0.05; week 17: 3.6 ± 0.2 vs. 5.3 ± 0.3, respectively, PMatSep < 0.001, P week 8 vs. 17 < 0.05). No day-night differences were observed between groups (data not shown).
Effects of MatSep on cardiometabolic parameters.
The metabolic profile was not different between MatSep and control rats fed an ND (Table 4). An HFD induced increases in body weight and fat pad weight that were similar in MatSep and control rats (Table 4), which estimated adiposity index (~6%) was comparable between groups. An HFD also reduced food intake, diuresis, and proteinuria compared with an ND in both groups (Table 4). Plasma glucose and creatinine showed no significant diet or MatSep effect. While an HFD increased plasma adiponectin levels similarly between groups, MatSep increased leptin and insulin levels (Table 4). Therefore, the homeostatic model assessment-IR index was greater in MatSep rats fed an HFD compared with control (1.39 ± 0.09 vs. 1.02 ± 0.09, P < 0.05). MatSep rats also showed increased levels of plasma aldosterone, but not plasma renin activity, in rats fed an HFD. PVAT-to-aorta tissue weight ratio was similar in MatSep and control rats on an ND and HFD (Table 4).
Table 4.
Metabolic and plasma parameters in MatSep and control males fed an ND or HFD for 17 wk
| Control ND | MatSep ND | Control HFD | MatSep HFD | PDiet | PMatSep | |
|---|---|---|---|---|---|---|
| Body weight and fat distribution | ||||||
| Body weight, g | 327 ± 2 | 333 ± 6 | 375 ± 6 | 382 ± 9 | P < 0.05 | NS |
| Epididymal fat pad, g | 6.1 ± 0.4 | 7.0 ± 0.4 | 12.1 ± 0.8 | 11.5 ± 1.2 | P < 0.05 | NS |
| Peri-renal fat pad, g | 5.6 ± 0.3 | 5.9 ± 1.3 | 11.9 ± 1.4 | 10.4 ± 1.7 | P < 0.05 | NS |
| Adiposity index, % | 3.7 ± 0.3 | 3.5 ± 0.7 | 5.8 ± 0.7 | 6.1 ± 0.9 | NS | NS |
| PVAT-to-aorta ratio | 9.5 ± 0.5 | 9.1 ± 0.8 | 10.3 ± 0.4 | 11.4 ± 0.6 | NS | NS |
| Metabolic profile | ||||||
| Water intake, ml/day | 29.5 ± 1.2 | 27.8 ± 2.3 | 16. ± 1.2 | 16.5 ± 1.7 | P < 0.05 | NS |
| Food intake, g/day | 21.9 ± 1.6 | 24.5 ± 1.2 | 12.7 ± 0.4 | 11.2 ± 1.3 | P < 0.05 | NS |
| Caloric intake, kcal/day | 67.9 ± 6.5 | 75.9 ± 8.2 | 69.7 ± 5.6 | 63.5 ± 7.3 | NS | NS |
| Diuresis, ml/day | 13.7 ± 1.3 | 14.7 ± 1.7 | 9.6 ± 1.1 | 8.8 ± 1.6 | P < 0.05 | NS |
| Proteinuria, mg/day | 10.9 ± 1.5 | 14.9 ± 2.4 | 3.4 v 0.7 | 5.3 ± 0.4 | P < 0.05 | NS |
| Plasma factors | ||||||
| Creatinine, ml | 1.3 ± 0.0 | 1.2 ± 0.1 | 1.7 ± 0.2 | 1.6 ± 0.2 | NS | NS |
| Glucose, mg/dl | 101 ± 1 | 101 ± 2 | 104 ± 1 | 108 ± 4 | NS | NS |
| Insulin, ng/ml | 2.7 ± 0.5 | 3.6 ± 0.2 | 3.5 ± 0.3 | 5.1 ± 0.5 | NS | P < 0.05 |
| Leptin, ng/ml | 5.2 ± 0.4 | 6.1 ± 0.5 | 7.7 ± 1.3 | 12.3 ± 1.4 | P < 0.05 | P < 0.05 |
| Adiponectin, µg/ml | 16.9 ± 1.3 | 15.0 ± 1.2 | 25.9 ± 1.5 | 29.7 ± 1.1 | P < 0.05 | NS |
| Cholesterol, mg/dl | 128.9 ± 2.5 | 134.5 ± 4.3 | 91.6 ± 6 | 98.7 ± 11 | P < 0.05 | NS |
| Aldosterone, ng/ml | 1.5 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.1 | 2.8 ± 0.3† | P < 0.05 | P < 0.05 |
| PRA, ng·ml−1·h−1 | 12.2 ± 0.8 | 12.7 ± 0.7 | 31.1 ± 1.3 | 27 ± 0.3 | P < 0.05 | NS |
Values are means ± SE. Metabolic and plasma parameters in maternal separation (MatSep) and control males fed a normal diet (ND) or high-fat diet (HFD) for 17 wk. n = 6 per group; two-way ANOVA. Pdiet < 0.05 vs. control. PMatSep < 0.05 vs. control. There was not significant interaction between diet and MatSep.
DISCUSSION
The major finding of this study is that MatSep, a model of ELS in rodents, enhances PVAT function to abolish the ANG II-induced vasoconstriction as well as endothelial dysfunction in response to an HFD. Classically, vascular function is assessed after removal of the PVAT. In conditions with the removal of PVAT, we found that MatSep exaggerated ANG II-induced constriction and promoted endothelial dysfunction in response to an HFD. Conversely, in aortic rings with preserved PVAT, vascular function was improved in both MatSep and control rats, thus abolishing any sign of vascular dysfunction. This improvement of the vascular function was significantly greater in MatSep rats. Furthermore, we found that MatSep increases the capacity to produce anticontractile factors, such as ANG-(1–7) and adiponectin, that may mediate signaling to vasodilator pathways. These findings support the concept that the presence of PVAT-derived factors is necessary to provide protection against exaggerated MatSep-induced vascular dysfunction in rats fed an HFD.
Although ELS is established as an independent risk factor for cardiovascular and metabolic diseases (23, 24, 55), the causative mechanisms for increased vascular dysfunction remain unclear. MatSep is a suitable paradigm to examine the effects of ELS on the cardiovascular system (35, 49, 59, 60). Exposure to MatSep during postnatal life is not associated with increased body weight or blood pressure at baseline; however, this model exerts a long-lasting effect on ANG II-induced vasoconstriction in adult rats fed an ND ex vivo (38) and in vivo (36, 37), yet MatSep does not alter circulating levels of ANG II peptide, plasma renin activity, and aldosterone in ND-fed rats. Contrary to what we observed, Reho et al. (48) has reported that the effects of MatSep on the vascular dysfunction trajectory wanes from postnatal to adult life. However, a large body of literature has shown that the sensitization of the cardiovascular response depends upon strain, stimuli, duration, and timing of the stressor or diet [reviewed in (44)].
Notably, several studies have reported improvements on the endothelial function during early stages of diet-induced obesity. Fat-enriched diets trigger initial compensatory mechanisms in adipose tissue aimed at preventing organ damage and lipotoxicity (18, 26). Ortega-Gil et al. have shown anticontractile effects of PVAT function during obesity. Moreover, their study suggested that a rise in plasma leptin during early diet-induced obesity may facilitate the capacity of PVAT to prevent vascular damage (20). Despite the repeated observations that PVAT elicits vasodilator effects on the vascular tone, no single mechanism for this effect has been identified. A number of factors are proposed to serve as the “PVAT-derived relaxing factor,” including adiponectin (12, 16), H2S (65), nitric oxide (26), ANG-(1–7) (32), and palmitic acid methyl ester (33). Our data indicate that an HFD started at weaning worsens the ACh-induced relaxation in aortic rings from MatSep rats lacking PVAT. Conversely, rings from MatSep rats with preserved PVAT displayed greater improvements on the vascular function compared with controls.
Being that the PVAT-to-aorta tissue weight ratio was similar between MatSep and control rats on an ND and HFD, we reasoned that the vasoprotective impact of the PVAT may be mediated by changes in vasoprotective factors within specific vascular cell types. Thus, we analyzed the expression of two important vasoprotective factors within the adipovascular axis. We found that VSM-derived ANG-(1–7) expression was increased in MatSep rats fed an HFD. Interestingly, it has been shown that ANG-(1–7) stimulation increases adiponectin synthesis in primary adipocyte culture (50). Thus, increases in ANG-(1–7) could exert a beneficial effect on vascular function directly or indirectly by increasing adiponectin levels in PVAT. Accordingly, rings from control rats fed an HFD show a greater adiponectin expression in endothelium compared with rings from control rats on an ND. In mice, adiponectin is highly expressed in aortic intima and costained with the endothelial cell marker, CD31 (30), suggesting a PVAT-independent source of adiponectin. Shen et al. (51) has shown that adiponectin mRNA expression can also be detected in cultured human coronary artery endothelial cells and umbilical vein endothelial cells. In addition, these authors showed that adiponectin staining is localized and associated with endothelial cells lining the lumen of both the artery, vein, vasa vasorum, and capillaries (51). Thus, the data from rodents and the human cell lines suggest that the vasculature and endothelium may be a source of adiponectin production with effects on the modulation of the vascular function. Taken together, these data suggest that endothelial-derived adiponectin may be implicated in the preserved endothelial function displayed by control rats in rings lacking PVAT. However, the presence of PVAT-derived adiponectin could be essential to improve vascular relaxation in rings from MatSep rats. As such, MatSep-induced increases in PVAT-derived adiponectin, potentially mediated via ANG-(1–7) derived from the VSM, may be a critical factor contributing to improve the vascular function.
Typically, studies assessing PVAT control of vascular function use rings lacking PVAT incubated in the presence of excised PVAT. However, in this study, we maintained the aortic ring with intact PVAT during the harvesting and mounting of the ring. We propose that maintaining the original structure of the PVAT and adventitia facilitates the paracrine effects of anticontractile factors in preserving vascular function. The mechanical removal of the adipose tissue may induce damage and increase the local production of, for instance, oxidative stress (28). Other approaches, including incubation of rings lacking PVAT with free PVAT, extracts from homogenized PVAT, or PVAT-conditioned media, may help to clarify whether the contribution of ANG-(1–7) and/or adiponectin to the increased anticontractile function in MatSep rats is dependent of these experimental conditions.
Blood pressure, 24 h and the circadian rhythm, was similar in MatSep rats and control rats fed an HFD. Furthermore, HR and HRV were also similar in MatSep and control rats fed an HFD. Therefore, our data supports the concept that resistance arterial function is similar to what we found with aortic function in response to an HFD or that resistance arterial function is not affected by MatSep. Future studies will focus on this distinction in the different arterial beds. Paradoxically, we found that both control and MatSep rats on an HFD show an upward trend from week 8 to 17 of an HFD in root mean squared of successive differences (Fig. 5D) and standard deviation of normal-to-normal beats (not shown), which has been proven as a sensitive cardiovascular variability index (52). These data suggesting that an HFD may increase the parasympathetic tone of the autonomic nervous system over time could contribute to blood pressure regulation in rats fed an HFD.
We also investigated the effect of MatSep on plasma markers of metabolic dysfunction that are known to influence vascular function. Increases in leptin (25, 31), insulin (5, 46), and/or aldosterone (9) play an important role on the development of endothelial dysfunction in the context of metabolic disease. Recently, it has been reported that ELS correlates with higher circulating levels of leptin and insulin among children with obesity (27). Likewise, male rats exposed to MatSep showed increases in these plasma risk factors compared with controls may be secondary to greater production of adipokines, impairments of the glucose metabolism, or overactivation of the renin angiotensin aldosterone system. To date, only a handful of studies have focused on the metabolic aspects of MatSep in response to an HFD during adult life. However, these studies were performed in females and focused on the adiposity and the endocrine and metabolic function (8, 45, 47). Thus, our study contributes to understanding the effects of MatSep on the vascular function in male HFD-fed rats, with respect to the adipovascular axis.
Perspectives and Significance
Aortic ring preparations from MatSep rats with preserved PVAT showed a greater ability to blunt heightened ANG II-induced vasoconstriction and endothelial dysfunction. Thus, increases in PVAT-mediated anticontractile capacity counterbalances the detrimental effects of combined MatSep and HFD on vascular function. The outcomes of this study in which we explored the effect of ELS on large artery disease support the concept that MatSep enhances the vascular anticontractile function in the adipovascular axis to prevent the development of large artery dysfunction in the face of metabolic insults. If translated to humans, enhanced PVAT anticontractile function may be a means of building resilience against vascular complications of ELS.
GRANTS
This study was supported by grants from the National Heart, Lung, and Blood Institute to J. S. Pollock and D. M. Pollock (P01-HL-69999 and P01-HL-95499), A. S. Loria (R00-HL-111354, R01-HL-135158), F. T. Spradley (R00-HL-130577), and J. S. Speed (K99-HL-12717); T32 postdoc to C. De Miguel (DK007545) and B. K. Becker (HL007457), T32 predoc to I. E. Obi (HL-007918–19); American Heart Association to J. S. Pollock and D. M. Pollock (5SFRN2390002); and the American Physiological Society Porter Predoctoral Fellowship to I. E. Obi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.L., F.T.S., I.E.O., and C.D.M. performed experiments; A.S.L., F.T.S., I.E.O., B.K.B., C.D.M., J.S.S., D.M.P., and J.S.P. analyzed data; A.S.L., F.T.S., B.K.B., C.D.M., J.S.S., D.M.P., and J.S.P. interpreted results of experiments; A.S.L. prepared figures; A.S.L. and J.S.P. drafted manuscript; A.S.L., F.T.S., I.E.O., B.K.B., C.D.M., D.M.P., and J.S.P. edited and revised manuscript; A.S.L., F.T.S., I.E.O., B.K.B., C.D.M., J.S.S., D.M.P., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the outstanding technical support from Xiaofen Liu, Hiram Ocasio, Amy Dukes, and Joseph Nasworthy.
REFERENCES
- 1.Acree LS, Montgomery PS, Gardner AW. The influence of obesity on arterial compliance in adult men and women. Vasc Med 12: 183–188, 2007. doi: 10.1177/1358863X07079323. [DOI] [PubMed] [Google Scholar]
- 2.Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res 335: 165–189, 2009. doi: 10.1007/s00441-008-0685-6. [DOI] [PubMed] [Google Scholar]
- 3.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347, 2000. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 4.Barton M, Carmona R, Ortmann J, Krieger JE, Traupe T. Obesity-associated activation of angiotensin and endothelin in the cardiovascular system. Int J Biochem Cell Biol 35: 826–837, 2003. doi: 10.1016/S1357-2725(02)00307-2. [DOI] [PubMed] [Google Scholar]
- 5.Belin de Chantemèle EJ, Ali MI, Mintz JD, Rainey WE, Tremblay ML, Fulton DJ, Stepp DW. Increasing peripheral insulin sensitivity by protein tyrosine phosphatase 1B deletion improves control of blood pressure in obesity. Hypertension 60: 1273–1279, 2012. doi: 10.1161/HYPERTENSIONAHA.112.196295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia V, Rarick KR, Stauss HM. Effect of the data sampling rate on accuracy of indices for heart rate and blood pressure variability and baroreflex function in resting rats and mice. Physiol Meas 31: 1185–1201, 2010. doi: 10.1088/0967-3334/31/9/009. [DOI] [PubMed] [Google Scholar]
- 7.Bhatwadekar AD, Beli E, Diao Y, Chen J, Luo Q, Alex A, Caballero S, Dominguez JM II, Salazar TE, Busik JV, Segal MS, Grant MB. Conditional deletion of Bmal1 accentuates microvascular and macrovascular injury. Am J Pathol 187: 1426–1435, 2017. doi: 10.1016/j.ajpath.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobrovskaya L, Maniam J, Ong LK, Dunkley PR, Morris MJ. Early life stress and post-weaning high fat diet alter tyrosine hydroxylase regulation and AT1 receptor expression in the adrenal gland in a sex dependent manner. Neurochem Res 38: 826–833, 2013. doi: 10.1007/s11064-013-0985-4. [DOI] [PubMed] [Google Scholar]
- 9.Briet M, Barhoumi T, Mian MOR, Coelho SC, Ouerd S, Rautureau Y, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-induced vascular remodeling and endothelial dysfunction require functional angiotensin type 1a receptors. Hypertension 67: 897–905, 2016. doi: 10.1161/HYPERTENSIONAHA.115.07074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britton KA, Pedley A, Massaro JM, Corsini EM, Murabito JM, Hoffmann U, Fox CS. Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the Framingham Heart Study. J Am Heart Assoc 1: e004200, 2012. doi: 10.1161/JAHA.112.004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Schölmerich J, Bollheimer LC. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 36: 485–501, 2006. doi: 10.1677/jme.1.01909. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278: 45021–45026, 2003. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 14.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med 163: 1135–1143, 2009. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiéra F, Sharma AM. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol 35: 807–825, 2003. doi: 10.1016/S1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 16.Fésüs G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res 75: 719–727, 2007. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Frigolet ME, Torres N, Tovar AR. The renin-angiotensin system in adipose tissue and its metabolic consequences during obesity. J Nutr Biochem 24: 2003–2015, 2013. doi: 10.1016/j.jnutbio.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Fulop T, Jebelovszki E, Erdei N, Szerafin T, Forster T, Edes I, Koller A, Bagi Z. Adaptation of vasomotor function of human coronary arterioles to the simultaneous presence of obesity and hypertension. Arterioscler Thromb Vasc Biol 27: 2348–2354, 2007. doi: 10.1161/ATVBAHA.107.147991. [DOI] [PubMed] [Google Scholar]
- 19.Gálvez-Prieto B, Dubrovska G, Cano MV, Delgado M, Aranguez I, González MC, Ruiz-Gayo M, Gollasch M, Fernández-Alfonso MS. A reduction in the amount and anti-contractile effect of periadventitial mesenteric adipose tissue precedes hypertension development in spontaneously hypertensive rats. Hypertens Res 31: 1415–1423, 2008. doi: 10.1291/hypres.31.1415. [DOI] [PubMed] [Google Scholar]
- 19a.GBD 2015 Obesity Collaborators Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377: 13–27, 2017. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil-Ortega M, Stucchi P, Guzmán-Ruiz R, Cano V, Arribas S, González MC, Ruiz-Gayo M, Fernández-Alfonso MS, Somoza B. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology 151: 3299–3306, 2010. doi: 10.1210/en.2009-1464. [DOI] [PubMed] [Google Scholar]
- 21.Gollasch M. Vasodilator signals from perivascular adipose tissue. Br J Pharmacol 165: 633–642, 2012. doi: 10.1111/j.1476-5381.2011.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661–1670, 2009. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 23.Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, Su S. Blood pressure trajectories from childhood to young adulthood associated with cardiovascular risk: results from the 23-Year Longitudinal Georgia Stress and Heart Study. Hypertension 69: 435–442, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, Su S. Body mass index trajectories in childhood is predictive of cardiovascular risk: results from the 23-year longitudinal Georgia Stress and Heart study. Int J Obes 42: 923–925, 2018. doi: 10.1038/ijo.2017.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemèle EJ. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 132: 2134–2145, 2015. doi: 10.1161/CIRCULATIONAHA.115.018226. [DOI] [PubMed] [Google Scholar]
- 26.Jebelovszki E, Kiraly C, Erdei N, Feher A, Pasztor ET, Rutkai I, Forster T, Edes I, Koller A, Bagi Z. High-fat diet-induced obesity leads to increased NO sensitivity of rat coronary arterioles: role of soluble guanylate cyclase activation. Am J Physiol Heart Circ Physiol 294: H2558–H2564, 2008. doi: 10.1152/ajpheart.01198.2007. [DOI] [PubMed] [Google Scholar]
- 27.Joung KE, Park KH, Zaichenko L, Sahin-Efe A, Thakkar B, Brinkoetter M, Usher N, Warner D, Davis CR, Crowell JA, Mantzoros CS. Early life adversity is associated with elevated levels of circulating leptin, irisin, and decreased levels of adiponectin in midlife adults. J Clin Endocrinol Metab 99: E1055–E1060, 2014. doi: 10.1210/jc.2013-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J 74: 1479–1487, 2010. doi: 10.1253/circj.CJ-09-0661. [DOI] [PubMed] [Google Scholar]
- 29.Kolbe I, Carrasco-Benso MP, López-Mínguez J, Luján J, Scheer FAJL, Oster H, Garaulet M. Circadian period of luciferase expression shortens with age in human mature adipocytes from obese patients. FASEB J. 33: 2018. doi: 10.1096/fj.201800441R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komura N, Maeda N, Mori T, Kihara S, Nakatsuji H, Hirata A, Tochino Y, Funahashi T, Shimomura I. Adiponectin protein exists in aortic endothelial cells. PLoS One 8: e71271, 2013. doi: 10.1371/journal.pone.0071271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol 295: H1514–H1521, 2008. doi: 10.1152/ajpheart.00479.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens 27: 782–790, 2009. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- 33.Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, Chen PY, Kuo JS, Lee TJ. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation 124: 1160–1171, 2011. doi: 10.1161/CIRCULATIONAHA.111.027375. [DOI] [PubMed] [Google Scholar]
- 34.Lehman SJ, Massaro JM, Schlett CL, O’Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis 210: 656–661, 2010. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loria AS, Osborn JL. Maternal separation diminishes α-adrenergic receptor density and function in renal vasculature from male Wistar-Kyoto rats. Am J Physiol Renal Physiol 313: F47–F54, 2017. doi: 10.1152/ajprenal.00591.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension 55: 494–499, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loria AS, Pollock DM, Pollock JS. Angiotensin II is required to induce exaggerated salt sensitivity in Dahl rats exposed to maternal separation. Physiol Rep 3: e12408, 2015. doi: 10.14814/phy2.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension 58: 619–626, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol 304: R121–R129, 2013. doi: 10.1152/ajpregu.00364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma L, Ma S, He H, Yang D, Chen X, Luo Z, Liu D, Zhu Z. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res 33: 446–453, 2010. doi: 10.1038/hr.2010.11. [DOI] [PubMed] [Google Scholar]
- 41.Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci (Lond) 103: 371–377, 2002. doi: 10.1042/cs1030371. [DOI] [PubMed] [Google Scholar]
- 42.Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. The vascular impact of aging and vasoactive drugs: comparison of two digital volume pulse measurements. Am J Hypertens 16: 467–472, 2003. doi: 10.1016/S0895-7061(03)00569-7. [DOI] [PubMed] [Google Scholar]
- 43.Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord 28, Suppl 4: S58–S65, 2004. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- 44.Murphy MO, Cohn DM, Loria AS. Developmental origins of cardiovascular disease: Impact of early life stress in humans and rodents. Neurosci Biobehav Rev 74: 453–465, 2017. doi: 10.1016/j.neubiorev.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy MO, Herald JB, Wills CT, Unfried SG, Cohn DM, Loria AS. Postnatal treatment with metyrapone attenuates the effects of diet-induced obesity in female rats exposed to early-life stress. Am J Physiol Endocrinol Metab 312: E98–E108, 2017. doi: 10.1152/ajpendo.00308.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naderali EK, Williams G. Prolonged endothelial-dependent and -independent arterial dysfunction induced in the rat by short-term feeding with a high-fat, high-sucrose diet. Atherosclerosis 166: 253–259, 2003. doi: 10.1016/S0021-9150(02)00367-2. [DOI] [PubMed] [Google Scholar]
- 47.Paternain L, Martisova E, Milagro FI, Ramírez MJ, Martínez JA, Campión J. Postnatal maternal separation modifies the response to an obesogenic diet in adulthood in rats. Dis Model Mech 5: 691–697, 2012. doi: 10.1242/dmm.009043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reho JJ, Fisher SA. The stress of maternal separation causes misprogramming in the postnatal maturation of rat resistance arteries. Am J Physiol Heart Circ Physiol 309: H1468–H1478, 2015. doi: 10.1152/ajpheart.00567.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders BJ, Anticevic A. Maternal separation enhances neuronal activation and cardiovascular responses to acute stress in borderline hypertensive rats. Behav Brain Res 183: 25–30, 2007. doi: 10.1016/j.bbr.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santos SH, Braga JF, Mario EG, Pôrto LC, Rodrigues-Machado MG, Murari A, Botion LM, Alenina N, Bader M, Santos RA. Improved lipid and glucose metabolism in transgenic rats with increased circulating angiotensin-(1-7). Arterioscler Thromb Vasc Biol 30: 953–961, 2010. doi: 10.1161/ATVBAHA.109.200493. [DOI] [PubMed] [Google Scholar]
- 51.Shen L, Evans IM, Souza D, Dreifaldt M, Dashwood MR, Vidya MA. Adiponectin: an endothelium-derived vasoprotective factor? Curr Vasc Pharmacol 14: 168–174, 2016. doi: 10.2174/1570161114666151202210128. [DOI] [PubMed] [Google Scholar]
- 52.Skene DJ, Skornyakov E, Chowdhury NR, Gajula RP, Middleton B, Satterfield BC, Porter KI, Van Dongen HPA, Gaddameedhi S. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc Natl Acad Sci USA 115: 7825–7830, 2018. doi: 10.1073/pnas.1801183115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A 13: 277–296, 1991. [DOI] [PubMed] [Google Scholar]
- 54.Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension 40: 441–447, 2002. doi: 10.1161/01.HYP.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- 55.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation 131: 1674–1681, 2015. doi: 10.1161/CIRCULATIONAHA.114.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su W, Xie Z, Guo Z, Duncan MJ, Lutshumba J, Gong MC. Altered clock gene expression and vascular smooth muscle diurnal contractile variations in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol 302: H621–H633, 2012. doi: 10.1152/ajpheart.00825.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag 9: 105–116, 2013. doi: 10.2147/VHRM.S33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tahara Y, Shiraishi T, Kikuchi Y, Haraguchi A, Kuriki D, Sasaki H, Motohashi H, Sakai T, Shibata S. Entrainment of the mouse circadian clock by sub-acute physical and psychological stress. Sci Rep 5: 11417, 2015. doi: 10.1038/srep11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trombini M, Hulshof HJ, Graiani G, Carnevali L, Meerlo P, Quaini F, Sgoifo A. Early maternal separation has mild effects on cardiac autonomic balance and heart structure in adult male rats. Stress 15: 457–470, 2012. doi: 10.3109/10253890.2011.639414. [DOI] [PubMed] [Google Scholar]
- 60.Tucker DC, Johnson AK. Influence of neonatal handling on blood pressure, locomotor activity, and preweanling heart rate in spontaneously hypertensive and Wistar Kyoto rats. Dev Psychobiol 17: 587–600, 1984. doi: 10.1002/dev.420170603. [DOI] [PubMed] [Google Scholar]
- 61.Uluoglu C, Durakoglugil DB, Karasu C, Ozbey G, Gunes A, Zengil H. The effect of experimental diabetes on the twenty-four-hour pattern of the vasodilator responses to acetylcholine and isoprenaline in the rat aorta. Chronobiol Int 24: 1081–1094, 2007. doi: 10.1080/07420520701795332. [DOI] [PubMed] [Google Scholar]
- 62.Van de Voorde J, Boydens C, Pauwels B, Decaluwé K. Perivascular adipose tissue, inflammation and vascular dysfunction in obesity. Curr Vasc Pharmacol 12: 403–411, 2014. doi: 10.2174/1570161112666140423220628. [DOI] [PubMed] [Google Scholar]
- 63.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 64.Witte K, Hasenberg T, Rueff T, Hauptfleisch S, Schilling L, Lemmer B. Day-night variation in the in vitro contractility of aorta and mesenteric and renal arteries in transgenic hypertensive rats. Chronobiol Int 18: 665–681, 2001. doi: 10.1081/CBI-100106080. [DOI] [PubMed] [Google Scholar]
- 65.Wójcicka G, Jamroz-Wiśniewska A, Atanasova P, Chaldakov GN, Chylińska-Kula B, Bełtowski J. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol Res 63: 68–76, 2011. doi: 10.1016/j.phrs.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 66.Xu A, Wang Y, Lam KS, Vanhoutte PM. Vascular actions of adipokines molecular mechanisms and therapeutic implications. Adv Pharmacol 60: 229–255, 2010. doi: 10.1016/B978-0-12-385061-4.00008-8. [DOI] [PubMed] [Google Scholar]