Abstract
The paraventricular nucleus of the hypothalamus (PVN) contributes to both autonomic and neuroendocrine function. PVN lesion or inhibition blunts cardiorespiratory responses to peripheral chemoreflex activation, suggesting that the PVN is required for full expression of these effects. However, the role of efferent projections to cardiorespiratory nuclei and the neurotransmitters/neuromodulators that are involved is unclear. The PVN sends dense projections to the nucleus tractus solitarii (nTS), a region that displays neuronal activation following hypoxia. We hypothesized that acute hypoxia activates nTS-projecting PVN neurons. Using a combination of retrograde tracing and immunohistochemistry, we determined whether hypoxia activates PVN neurons that project to the nTS and examined the phenotype of these neurons. Conscious rats underwent 2 h normoxia (21% O2, n = 5) or hypoxia (10% O2, n = 6). Hypoxia significantly increased Fos immunoreactivity in nTS-projecting neurons, primarily in the caudal PVN. The majority of activated nTS-projecting neurons contained corticotropin-releasing hormone (CRH). In the nTS, fibers expressing the CRH receptor corticotropin-releasing factor receptor 2 (CRFR2) were colocalized with oxytocin (OT) fibers and were closely associated with hypoxia-activated nTS neurons. A separate group of animals that received a microinjection of adeno-associated virus type 2-hSyn-green fluorescent protein (GFP) into the PVN exhibited GFP-expressing fibers in the nTS; a proportion of these fibers displayed OT immunoreactivity. Thus, nTS CRFR2s appear to be located on the fibers of PVN OT neurons that project to the nTS. Taken together, our findings suggest that PVN CRH projections to the nTS may modulate nTS neuronal activation, possibly via OTergic mechanisms, and thus contribute to chemoreflex cardiorespiratory responses.
Keywords: chemoreflex, corticotropin-releasing factor receptor 2, corticotropin-releasing hormone, oxytocin
INTRODUCTION
Peripheral chemoreflex stimulation by hypoxia leads to compensatory increases in ventilation, arterial pressure, sympathetic nervous system activity, and neuroendocrine secretion (95, 97) to maintain oxygen delivery to tissues. The nucleus tractus solitarii (nTS) is the first central nucleus that receives inputs from sensory afferents, including chemoreceptor afferents. Afferent information undergoes extensive integration in the nTS via a network of interneurons, a wide array of neurotransmitters and neuromodulators, and multiple inputs from other brain regions (2, 36). This integration is critical for eliciting appropriate cardiorespiratory responses to chemoreflex stimulation under different physiological and pathophysiological conditions. Cardiorespiratory chemoreflex responses utilize excitatory projections from the nTS to the rostral ventrolateral medulla (RVLM) (35, 54, 55) and ventral respiratory group (1), and these projections are thought to be essential for the chemoreflex-mediated increase in sympathetic nervous system activity and breathing (37, 82).
The paraventricular nucleus of the hypothalamus (PVN) is a highly integrative nucleus that regulates neuroendocrine and cardiorespiratory function, including responses to peripheral chemoreflex stimulation (48, 71, 77, 78, 86, 95). The PVN receives excitatory inputs from brain-stem cardiorespiratory nuclei (10, 81, 84). These projections are critical for chemoreflex-evoked activation of PVN neurons and full expression of cardiorespiratory responses (9, 52, 89). Hypoxia activates a variety of PVN neurons, including arginine vasopressin (AVP)-, oxytocin (OT)-, corticotropin-releasing hormone (CRH)-, and neuronal nitric oxide synthase (nNOS)-expressing neurons (12, 17, 22, 89). Inhibition or lesion of the PVN blunts autonomic, cardiovascular, and ventilatory effects of peripheral chemoreflex stimulation with KCN (70, 77). Taken together, these findings indicate that the PVN contributes importantly to chemoreflex cardiorespiratory responses, and the activation of neuropeptidergic neurons may play a role.
The efferent projections from the PVN and the neurotransmitters/neuromodulators that shape cardiorespiratory chemoreflex responses have not been fully elucidated. The PVN projects to cardiorespiratory nuclei in the spinal cord and brain stem, including the intermediolateral cell column (IML) (87), phrenic motor nucleus (60, 103), ventrolateral medulla (4, 15, 47, 87), and the nTS (15, 32, 94), and these projections could influence cardiorespiratory responses to hypoxia. Our group recently reported that acute hypoxia does not activate PVN neurons that project to the RVLM or the IML (17), suggesting the PVN likely influences chemoreflex responses via efferent projections to other cardiorespiratory nuclei. Interestingly, the nTS receives the densest projection from the PVN relative to all other brain-stem nuclei (32). The PVN provides inputs to the medial and commissural nTS (32), two subregions that receive chemoreceptor afferent input (54, 63, 65, 66, 82) and that exhibit increased neuronal activation in response to hypoxia (12, 50, 65, 98). The PVN-nTS pathway is highly peptidergic and includes AVP, OT, and CRH neurons (15, 83, 85, 96). Local administration of these neuropeptides or their selective receptor agonists or antagonists into the nTS evokes changes in neuronal excitability and basal or reflex cardiorespiratory function (5, 38, 64, 68, 69, 73). Thus, PVN neuropeptidergic projections to the nTS may contribute to cardiorespiratory chemoreflex responses.
This study examined neuronal activation in the PVN and the nTS in response to peripheral chemoreflex stimulation by acute hypoxia. We hypothesized that hypoxia activates nTS-projecting neuropeptide neurons in the PVN, including CRH, OT, and AVP neurons. Our findings indicate that nTS-projecting PVN neurons are significantly activated by hypoxia, and the majority of these activated neurons contain CRH. Based on these data, we further hypothesized that CRH receptors in the nTS are associated with hypoxia-sensitive nTS neurons. Corticotropin-releasing factor receptor 2 (CRFR2) is the predominant CRH receptor in the nTS (56, 99), and our data show that CRFR2-IR fibers are closely associated with hypoxia-activated nTS neurons. We also determined that CRFR2 colocalizes extensively with OT fibers in the nTS. Taken together, we propose that PVN CRH projections to the nTS contribute to cardiorespiratory chemoreflex responses via activation of nTS CRFR2s, and these effects may be mediated by nTS OT mechanisms.
METHODS
Animals
Tissue from a total of 26 male Sprague-Dawley rats (250–350 g) was used in this study (20 rats with verified injections of tracer in either the nTS, RVLM, or PVN and 6 naïve rats with no central nervous system injections). Rats were housed in a 12-h light/dark cycle at a temperature of 22°C and 40% humidity, with food and water provided ad libitum. All experimental procedures were conducted in accordance with the American Physiological Society’s Guiding Principles for the Care and Use of Vertebrate Animals in Research and Training and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Missouri Animal Care and Use Committee.
Surgical Procedures
Rats were anesthetized (5% for induction and 2%–2.5% for maintenance, isoflurane; Baxter Healthcare, Deerfield, IL), given dexamethasone (2 mg/kg sc), and placed in a stereotaxic apparatus. With the use of aseptic technique, the brain region of interest was exposed. Glass micropipettes (tip outer diameter = 10–20 µm) were used for microinjections into specific brain regions. Injection volumes were verified by monitoring the meniscus level within the pipette using a microscope (×150 magnification, Rolyn Optics, Corvina, CA) with a calibrated eyepiece as described previously (50, 53). All microinjections were performed over the course of at least 1 min, and the pipette remained at the site of injection for at least 5 min to minimize movement of fluid up the injection tract. After removal of the pipette, the incision site was closed. Rats received postoperative treatment with fluids (3 ml, 0.9% saline sc), Baytril antibiotic (5 mg/kg im, Bayer, Shawnee Mission, KS), and Buprenex analgesic (0.05 mg/kg sc, Reckitt Benckiser Pharmaceuticals, Richmond, VA) and were allowed 7–10 days to recover.
nTS-projecting neurons in the PVN were retrogradely labeled in 11 rats. The brain stem was exposed via a limited occipital craniotomy, and micropipettes containing the retrograde tracer cholera toxin subunit B conjugated to AlexaFluor 555 (CTB; 1% in deionized water, List Biological Laboratories, Campbell, CA) were inserted into the nTS at the level of calamus scriptorius (CS): ± 0.4 mm lateral and 0.5 mm ventral from the surface. CTB was injected bilaterally (30 nl each side).
In separate rats, we evaluated PVN-projecting nTS neurons using retrograde labeling from the PVN with CTB (n = 3), or we examined projections from the PVN with fibers in the nTS by expressing green fluorescent protein (GFP) in the PVN under the neuronal promoter synapsin (n = 3). A midline incision was made along the dorsal surface of the skull. Muscle and fascia were bluntly dissected to visualize bregma and lambda, which were positioned in the horizontal plane. A small hole was drilled in the skull and the dura cut. Either CTB or adeno-associated virus (AAV)-hSyn-GFP (Addgene, 50465-AAV2) was microinjected (90 nl) into the PVN bilaterally using the following coordinates: 1.8–2.0 mm caudal to bregma, ± 0.5 mm lateral from midline, and 7.8 mm ventral to the dura (50).
In some animals (n = 3), RVLM-projecting nTS neurons were retrogradely labeled bilaterally with CTB. The dorsal surface of the medulla was exposed and CS visualized. The head was then deflected downward until CS was 2.4 mm posterior to interaural zero, positioning the medulla in a horizontal position. Target stereotaxic coordinates for the RVLM were the following: 0.7–0.8 mm rostral and 1.6–1.8 mm lateral to CS and 3.6–4.2 mm ventral to the dorsal surface of the brain (53).
Hypoxia Exposure
Conscious rats were acclimated to a hypoxia chamber (Biospherix, Redfield, NY) for 3 days before the experiment to reduce the effects of environmental stimuli associated with the chamber. The following day, rats were randomly assigned to groups, placed in the hypoxia chamber, and after an initial 30-min acclimation period in room air, the gas mixture was adjusted to 21% O2 (normoxia) or 10% O2 (hypoxia) for 2 h using a negative feedback control system as reported previously (53). Immediately after normoxic or hypoxic exposure, rats were deeply anesthetized (5% isoflurane) and transcardially perfused with oxygenated, heparinized DMEM (125 ml, pH 7.4, Sigma) followed by 4% paraformaldehyde (500 ml, pH 7.4 Sigma) in 0.01 M phosphate-buffered saline (PBS). Brains were removed and postfixed overnight.
Injection Site Verification
Coronal sections (30 µm) of the forebrain and hindbrain containing the PVN or nTS, respectively, were cut using a vibrating microtome (1000S, Leica, Germany). Sections were viewed using a fluorescence Olympus BX51 microscope. Fluorescent and bright-field images were collected and merged using ImageJ (version 1.48). Microinjection sites were evaluated by examining the intrinsic fluorescence of CTB in the nTS, PVN, RVLM, or GFP in the PVN. For each animal, the center of the injection site was verified histologically using a rat brain atlas (72). The CTB microinjection site was centered in the nTS in all animals used in this study. Injection sites in the PVN and RVLM were verified similarly. Only tissue from animals with verified injections was used for further analysis (nTS CTB, n = 11; RVLM CTB, n = 3; PVN CTB, n = 3; and PVN GFP, n = 3).
Immunohistochemistry
For all immunohistochemical experiments, one in every six consecutive sections of the PVN or nTS was processed for immunoreactivity. All immunohistochemical procedures were carried out using protocols similar to those previously described (50). Briefly, sections were rinsed with 0.01 M phosphate-buffered saline (3 × 10 min PBS), blocked in 10% normal donkey serum (Millipore, S30) in 0.3% Triton-0.01 M PBS (PBS-T), and incubated overnight in 3% normal donkey serum and 0.3% PBS-T containing the primary antibodies. The following day, sections were rinsed in PBS and incubated in appropriate secondary antibodies. Sections were coverslipped with Prolong Diamond (Thermo Fisher, P36970) and sealed with nail polish.
PVN immunohistochemistry.
Immunohistochemistry was performed on PVN sections from 11 rats in which bilateral CTB microinjections into the nTS were verified (21% O2, n = 5; 10% O2, n = 6). All immunohistochemical procedures were carried out simultaneously on tissue from normoxic and hypoxic animals. Fos immunoreactivity (IR) was used as a marker of neuronal activation (23, 27, 15a). All PVN sections were processed for Fos-IR (1:3,000; rabbit anti-Fos, Santa Cruz, sc-52) in combination with one or more of the following antibodies: guinea pig anti-AVP (1:5,000; Peninsula Laboratories T-5048); mouse anti-OT (1:2,000; MAB5296, Millipore); guinea pig anti-CRF (1:1,000; T-5007, Peninsula Laboratories) to label CRH-containing neurons; and goat anti-nNOS (1:2,000; ab1376, Abcam). Immunohistochemistry for CRH was performed in combination with nNOS, whereas immunohistochemistry for OT and AVP were conducted separately. In total, three immunohistochemical experiments were performed on PVN sections from every animal, each in combination with Fos: CRH+nNOS, OT, or AVP.
nTS immunohistochemistry.
Immunohistochemistry protocols were performed on coronal nTS sections from three sets of animals: 1) naïve rats (without retrograde tracer/AAV microinjections) exposed to 2 h normoxia (21% O2, n = 3) or hypoxia (10% O2, n = 3), 2) rats exposed to 2 h hypoxia that previously received bilateral microinjections of CTB into the RVLM (n = 3) or PVN (n = 3), and 3) rats that received AAV2-hSyn-GFP injections in the PVN (n = 3). In rats that received CTB microinjections into the RVLM or PVN, immunohistochemistry was performed for CRFR2 (1:5,000; rabbit anti-CRFR2, 150510 Abcam) to label CRH type 2 receptors in combination with Fos (goat anti-Fos). In rats that received AAV2-hSyn-GFP injections in the PVN, immunohistochemistry was performed for OT and GFP (1:500; rabbit anti-GFP, ab290, Abcam). In naïve rats, immunohistochemistry was performed for CRFR2 in combination with at least one of the following antibodies: Fos (1:500; goat anti-Fos, sc-52g, Santa Cruz); the neuronal cytosolic marker HUC/D (1:1,000; mouse anti-HUC/D, A-21271, LifeTechnologies); OT (1:1,000; MAB5296, mouse anti-OT,); synaptophysin (1:500; guinea pig anti-synaptophysin, 101004, Synaptic Systems); the catecholaminergic marker tyrosine hydroxylase (TH) (1:1,000, mouse anti-TH, Millipore, MAB318); MAP2 to examine dendrites (1:500; mouse anti-MAP2, M9942, Sigma-Aldrich); the astrocyte marker glial fibrillary acidic protein (GFAP; 1:500; guinea pig anti GFAP, 173004, Synaptic Systems); and the microglia marker IBA1 (1:500; goat anti-IBA1, ab5076, Abcam).
Antibody specificity.
For all immunohistochemical protocols, at least one section served as a control (did not receive primary or secondary antibodies). In addition, all antibodies used in immunohistochemistry protocols were verified in previously published studies or by the vendor using Western blots (3, 16, 24, 26, 39, 80, 101) or preincubation with immunogen (3, 13, 17, 20, 62). Similar to what has been observed previously (20), we found that preincubation with OT immunogen (X50) eliminated OT staining but had no effect on CRFR2 labeling, whereas the blocking peptide for CRFR2 (X50) eliminated nTS CRFR2 staining but had no effect on OT labeling, indicating lack of cross-reactivity. We further verified the CRFR2 antibody using transfection in cell culture followed by Western blot analysis as described below.
Cell culture and transfection.
HEK293 cells (Agilent, 240085) were cultured in DMEM (HyClone, SH30022) containing 10% fetal bovine serum (Serum Source, FB02), 100 U/ml penicillin-0.1 mg/ml streptomycin (HyClone, SV30010), and 1 mM sodium pyruvate (HyClone, SH30239). Cells were seeded onto 10-cm plates and cultured until ~60% confluent. The cells were then transfected with 100 nM of a pool of 4 nontargeting control siRNAs [Dharmacon D-001206–14; 1) 5′-uaaggcuaugaagagauac-3′, 2) 5′-auguauuggccuauuag-3′, 3) 5′-augaacgugaauugcucaa-3′, and 4) 5′-uguuuacaugucgacuaa-3′] or a pool of 4 rat CRFR2-specific siRNAs [CRHR2, Dharmacon M-087983–01; 1) 5′-gcaaguggcucuuccucuu-3′, 2) 5′-gcacacggccaucgucaug-3′, 3) 5′-ggaaccuggugacuuagug-3′, and 4) 5′-cgccugggcaguuggcaaa-3′] using Lipofectamine RNAiMAX (13–778–150,ThermoFisher). After siRNA transfection (24 h), the cells were then transfected with 10 µg of plasmids encoding either mouse cyclophilin-D (positive control) or rat CRHR2, each with a COOH-terminal FLAG tag, using TransIT-293 transfection reagent (MIR2700,Mirus Bio). The cells were then incubated for a further 48 h before being harvested and processed for Western blotting.
Western blotting.
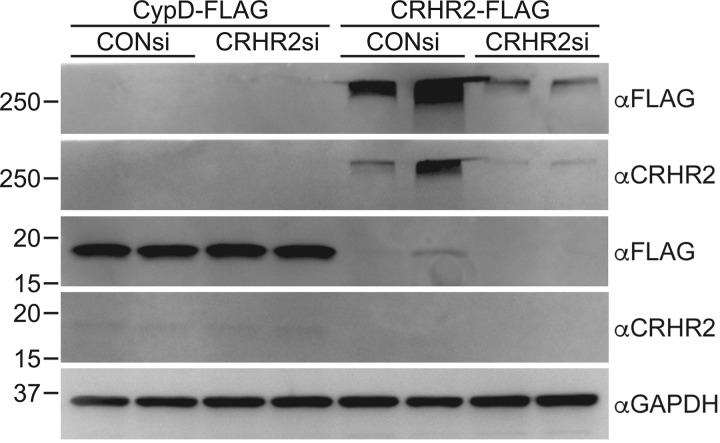
Transfected HEK293 cells were harvested, solubilized, and sonicated in buffer containing 150 mM NaCl, 10 mM Tris pH 7.4, 1% Triton-X100, 1 mM EDTA, and protease/phosphatase inhibitor (PI78442, ThermoFisher) and then centrifuged at 17,000 g for 10 min at 4°C to remove cell debris. Equal amounts of protein, as determined by Bradford assay (5000006, Bio-Rad), in SDS loading buffer were run on 10% SDS/PAGE gels before transfer to PVDF membranes (Bio-Rad, 1620177). Total protein loading was then visualized by staining the membranes with Ponceau-S (P7170, Millipore Sigma). After being blocked in 10% nonfat milk in Tris-buffered saline-Tween 20 (TBS-T), primary antibodies [mouse anti-FLAG (1:1,000; F3165, Millipore Sigma), rabbit anti-CRFR2 (1:1,000; ab150510, Abcam), or mouse anti-GAPDH (1:1,000; Millipore Sigma, MAB374)] were applied to the membranes overnight at 4°C in blocking buffer. After washing in TBS-T, the appropriate anti-mouse (1:1,000; Cell Signaling Technology, 7056) or anti-rabbit (1:1,000; Cell Signaling Technology, 7054) alkaline phosphatase-conjugated antibodies were applied to the membrane for 2 h at room temperature in blocking buffer. Membranes were then washed in TBS-T before chemifluorescent imaging on a Bio-Rad Gel Doc XR using ECF (RPN5785, GE Healthcare Life Sciences). Antibodies for FLAG (αFLAG) recognized both CypD-FLAG and CRHR2-FLAG. In contrast, the anti-CRFR2 antibody (αCRHR2) recognized only the CRHR2-FLAG, indicating its specificity. Note that CRHR2 is observed at a higher than predicted molecular weight, likely because of the fact that CRHRs can form high-molecular-weight oligomers (11, 45, 67). Specificity was further confirmed by a reduced signal in tissue that had been pretreated with siRNAs for CRHR2 (Fig. 1).
Fig. 1.
Validation of the specificity of the anti-corticotropin-releasing factor receptor 2 (anti-CRFR2) antibody. HEK293 cells were transfected with 100 nM of nontargeting control (CONsi) or rat-specific CRHR2 (CRHR2si) siRNAs followed by transfection with 10 μg of plasmids encoding either cyclophilin-D-FLAG (CypD-FLAG) or CRHR2-FLAG 24 h later. After an additional 48 h, cell lysates were immunoblotted for FLAG (αFLAG) and then CRHR2 (αCRHR2). Blotting for GAPDH was used to show equivalent loading between samples.
Microscopy and Image Analysis
Brain sections were viewed using a fluorescence Olympus BX51 microscope with appropriate filter sets. Image stacks were imported into ImageJ (version 1.48v) and merged, and unilateral counts of positively labeled cells were made. Quantification of positively labeled cells from each of the three different immunohistochemical protocols was performed using a custom-made cell counter plugin (53) by individuals blinded to the experimental manipulation.
PVN analysis.
Image stacks of 11 consecutive optical planes (2 µm/plane) were taken from four PVN sections from −1.5 to −2.1 mm relative to bregma (72) and examined for CTB labeling and IR for Fos, AVP, OT, CRH, and/or nNOS. The following criteria were used to identify positively labeled PVN cells: CTB-labeled neurons displayed bright and/or punctate cytosolic labeling; Fos-IR neurons displayed round or ovoid-shaped staining confined to the nucleus; and PVN phenotypes, CRH, AVP, OT, and nNOS, exhibited bright cytosolic labeling with a blank nuclear region. Cells were considered double-, triple-, or quadruple-labeled when the above criteria were met under more than one filter set in the same plane of focus. Data from immunohistochemical experiments were analyzed at four rostral-caudal levels of the PVN and as the total (sum) of counts from the four sections.
Fos-IR and CTB-labeled PVN neurons were quantified in all three immunohistochemical experiments performed for each animal. There were no significant differences in the number of Fos-IR or CTB-labeled cells among immunohistochemical protocols performed in an individual animal. Therefore, the representative overall number of Fos-IR and CTB-labeled cells in each animal was determined by averaging the counts of positively labeled Fos-IR and CTB neurons from the three immunohistochemical protocols. Colocalization of Fos-IR and CTB with the PVN phenotypes, AVP, OT, CRH, or nNOS neurons, was determined using the Fos and CTB counts from the individual respective immunohistochemical experiments.
nTS analysis.
To examine CRH receptors in the nTS, image stacks of 21 consecutive optical planes (1 µm/plane) were taken from 3 coronal brain-stem sections containing the nTS (−180, 0, and 180 µm relative to CS) (72). CRFR2-IR fibers were examined for close associations with nTS neurons that displayed Fos-IR. Close associations were defined as a distance of ≤1 µm between CRFR2-IR fibers and cell bodies within any xy, xz, or yz plane). In addition, in some animals, CRFR2-IR fibers were evaluated for colocalization with OT and the synaptic protein synaptophysin in the nTS. Extent of colocalization of CRFR2- and OT-IR was quantified using the Manders colocalization coefficient (28) (Manders Coefficients plugin; ImageJ).
Statistical Analyses
All statistical analyses were conducted using SigmaPlot (version 12.5; Systat Software, San Jose, CA). Data are presented as means ± SE. Statistical significance was set at P ≤ 0.05. All data sets passed tests for normality (Shapiro-Wilk test) and equal variance (Levene test), either as raw data or following square root transformation. Two-tailed unpaired t-tests were used to compare the number of Fos-IR and the number of PVN phenotypes in normoxic and hypoxic animals. Unpaired t-tests also were used to compare the number of CTB neurons displaying IR for Fos and the number of CTB cells colabeled with AVP, OT, CRH, or nNOS between normoxic and hypoxic animals. One-way repeated measures (RM) ANOVA was used to compare the percentage of CTB-labeled cells that were colabeled with each PVN phenotype examined, and the percentage of Fos-IR CTB-labeled cells that displayed IR for each PVN phenotype was examined. Two-way RM ANOVA was used to compare the percentage of each PVN phenotype that expressed Fos-IR and the percentage of nTS-projecting PVN phenotypes that expressed Fos-IR between normoxic and hypoxic animals. Additionally, two-way RM ANOVA was used to examine the rostral-caudal distributions of the number of positively labeled CTB cells, the number of CTB cells that were colabeled with the phenotypes AVP, OT, CRH, or nNOS, and the number of Fos-IR CTB neurons. When a significant interaction occurred, ANOVAs were followed by post hoc analysis using Fisher’s least significant difference test.
RESULTS
Hypoxia activates PVN neurons.
We examined Fos-IR as an index of neuronal activation in the PVN following exposure to 2 h of normoxia (21% O2) or hypoxia (10% O2) in conscious rats. Fos-IR neurons were observed in both the magnocellular and parvocellular regions of the PVN, similar to previous reports (22, 89). Acute hypoxia significantly increased the overall number of Fos-IR neurons counted in four rostral-caudal sections (−1.5 to −2.1 mm relative to bregma) of the PVN (21% O2, 140 ± 16 cells, n = 5; 10% O2, 747 ± 64 cells, n = 6; t(9) = −10.76, P < 0.001). Two-way RM ANOVA indicated a significant interaction of treatment (normoxia vs. hypoxia) and rostral-caudal PVN level (F[3,27] = 38.18, P < 0.001), and post hoc analyses were performed. Hypoxia increased the number of Fos-IR cells at each PVN level (normoxia vs. hypoxia: bregma −1.5 mm: 27 ± 5 vs. 205 ± 19; bregma −1.7 mm: 20 ± 4 vs. 307 ± 38; bregma −1.9 mm: 45 ± 5 vs.144 ± 17; bregma −2.1 mm: 48 ± 9 vs. 91 ± 10 cells; P < 0.001 for all comparisons). In addition, hypoxia produced a significantly greater number of Fos-IR cells (P < 0.001) in the rostral PVN (−1.5 and −1.7 mm) compared with the caudal PVN (−1.9 and −2.1 mm, relative to bregma).
Characterization of AVP-, OT-, CRH-, and nNOS-IR neurons in the PVN.
We evaluated the number and distribution of neurons expressing AVP, OT, CRH, and nNOS in the PVN. Because we previously observed a high degree of colocalization of CRH- and nNOS-IR (CRH+nNOS) neurons (17), we also examined colabeled CRH and nNOS neurons. Table 1 shows that, as expected, the total number of each neuronal phenotype counted in four levels of the PVN was similar in animals exposed to normoxia or hypoxia. Positively labeled cells of all phenotypes were observed in each PVN level. Consistent with previous studies (17, 89), AVP and OT neurons were primarily located in the posterior magnocellular subregions in the rostral PVN, whereas CRH-IR and nNOS-IR neurons were highly expressed throughout the rostral-caudal extent of the PVN. As with the total number, there were no statistically significant differences in the number of any positively labeled neuronal phenotype between normoxic and hypoxic groups at any level of the PVN examined.
Table 1.
Total number of positively labeled cells counted in four PVN sections
| 21% O2 | 10% O2 | |
|---|---|---|
| AVP | 128 ± 20 | 143 ± 10 |
| OT | 279 ± 14 | 278 ± 14 |
| CRH | 2,040 ± 173 | 1,933 ± 107 |
| nNOS | 589 ± 51 | 527 ± 33 |
| CRH + nNOS | 463 ± 38 | 432 ± 22 |
Values are means ± SE. Arginine vasopressin (AVP), oxytocin (OT), corticotropin-releasing hormone (CRH), neuronal nitric oxide synthase (nNOS), and CRH + NOS colabeled cells in animals exposed to 21% O2 (n = 5) or 10% O2 (n = 6). PVN, paraventricular nucleus of the hypothalamus.
Hypoxia activates AVP-, OT-, CRH-, and nNOS-IR PVN neurons.
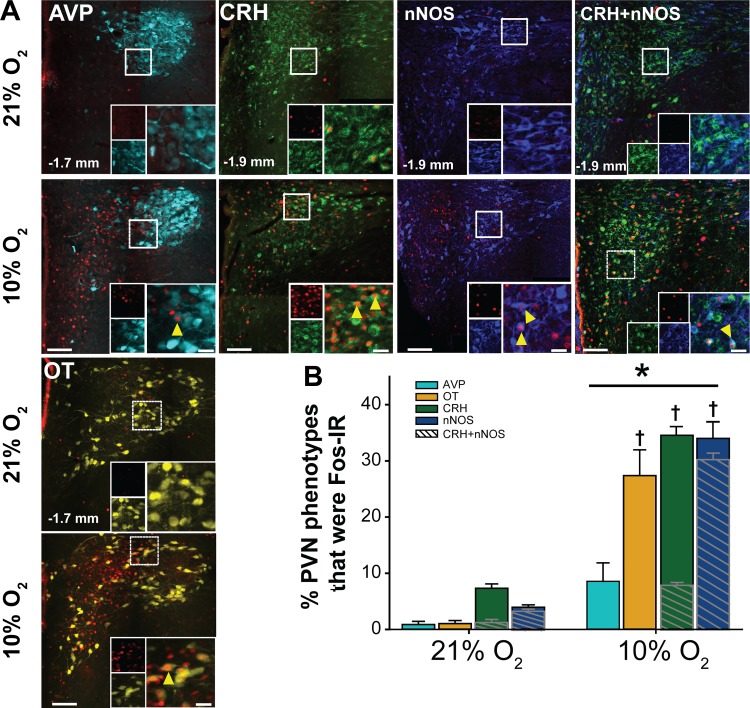
All PVN phenotypes examined were also evaluated for hypoxia-induced neuronal activation via Fos-IR. Figure 2A includes representative photomicrographs from coronal PVN sections showing Fos-IR and positively labeled AVP, OT, CRH, nNOS, and CRH+nNOS cells in PVN tissue from normoxic and hypoxic animals. Analysis of the effects of hypoxia on Fos-IR in PVN neurons revealed a significant interaction of treatment (normoxia vs. hypoxia) and PVN phenotype [F(4,35) = 17.59, P < 0.001]. Hypoxia significantly increased the number of Fos-IR cells in all PVN phenotypes examined (21% O2 vs. 10% O2: AVP, 1 ± 1 vs. 13 ± 5 cells, P = 0.050; OT, 4 ± 2 vs. 72 ± 15 cells, P < 0.001; CRH, 146 ± 12 vs. 645 ± 32 cells, P < 0.001; nNOS, 24 ± 4 vs. 170 ± 20 cells, P < 0.001; and CRH+nNOS, 23 ± 4 vs. 150 ± 13 cells, P < 0.001). AVP and OT neurons displaying Fos-IR were primarily localized to the posterior magnocellular regions of the rostral PVN. In comparison, hypoxia-activated CRH, nNOS, and colabeled CRH+nNOS neurons were observed throughout the PVN. The percentage of each PVN neuronal phenotype expressing Fos-IR is shown in Fig. 2B. There were no significant differences in Fos-IR among phenotypes in animals exposed to normoxia. Hypoxia significantly increased relative activation in all phenotypes examined. In addition, the percentages of OT, CRH, and nNOS cells displaying Fos-IR in response to hypoxia were similar and were greater than the percentage of AVP neurons expressing Fos-IR. We also observed Fos-IR in colabeled CRH+nNOS cells (cross-hatching). Overall, the majority (90 ± 3%) of Fos-IR nNOS cells displayed CRH-IR, and a smaller proportion (23 ± 1%) of Fos-IR CRH cells were nNOS-IR. Taken together, our findings are in agreement with previous studies indicating that acute hypoxia activates a variety of PVN phenotypes, which include AVP, OT, CRH, and nNOS neurons (17, 22, 89, 98).
Fig. 2.
Hypoxia increases neuronal activation in multiple paraventricular nucleus of the hypothalamus (PVN) neuronal phenotypes. A: merged photomicrographs of coronal PVN sections (−1.7 or −1.9 mm relative to bregma) from animals subjected to 2 h normoxia (21% O2) or hypoxia (10% O2) displaying immunoreactivity (IR) for Fos (pseudocolored red) and the following neuronal phenotypes: arginine vasopressin (AVP) (cyan), oxytocin (OT) (yellow), corticotropin-releasing hormone (CRH) (green), neuronal nitric oxide synthase (nNOS) (blue), and CRH+nNOS. Scale bar = 200 µm. Insets: higher magnification of outlined boxes showing colocalization of Fos-IR with PVN phenotypes (arrowheads). Inset scale bars = 25 µm. B: percent of each phenotype that displayed Fos-IR [(Fos + Phenotype)/Phenotype] in normoxic (21% O2, n = 5) and hypoxic (10% O2, n = 6) rats. Cross-hatched overlays represent the percentage of Fos-IR, CRH-IR, or nNOS-IR PVN neurons displaying colabeling. Two-way repeated measures ANOVA indicated a significant interaction between treatment and phenotype (F[4,35] = 17.59, P < 0.001). Subsequent post hoc analysis revealed that hypoxia significantly increased Fos-IR in all phenotypes (AVP, P = 0.05; all other phenotypes, P < 0.001). In addition, hypoxia activated a significantly greater percentage of CRH−, nNOS−, and OT-IR neurons vs. AVP-IR neurons. *10% O2 > 21% O2 for all phenotypes; † >AVP, P < 0.001.
nTS-projecting PVN neurons.
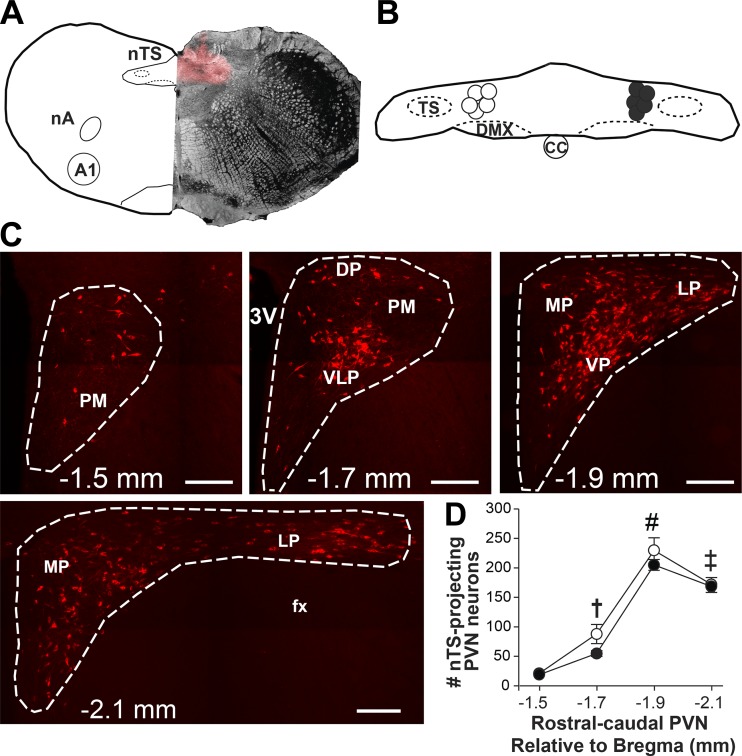
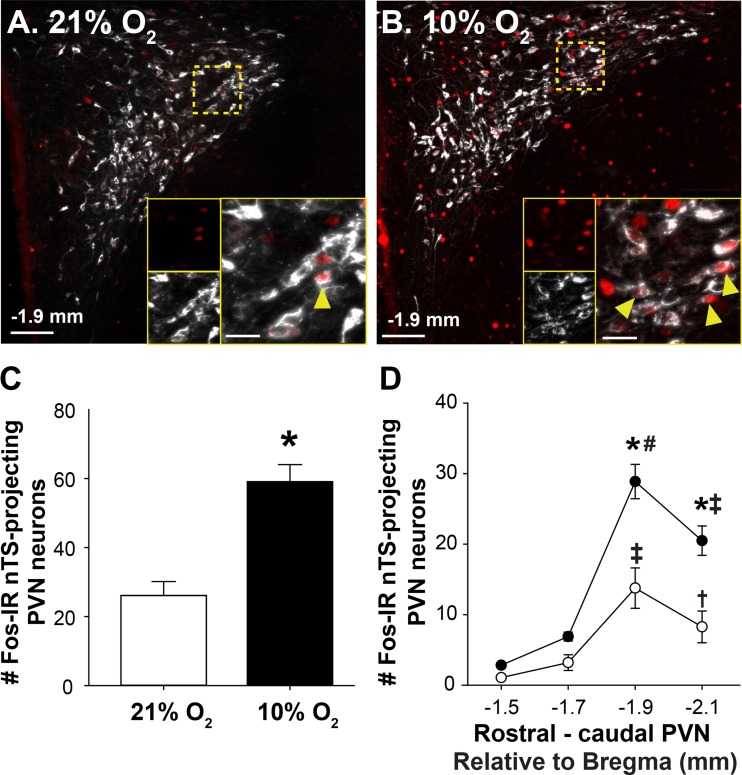
nTS microinjection sites were verified histologically in animals that received bilateral microinjections of the fluorescently tagged retrograde tracer (CTB). Figure 3A includes a diagrammatic representation (left) of a coronal nTS section at the level of CS with a representative photomicrograph showing the CTB microinjection site (right) at the corresponding nTS level (72). Figure 3B shows the center of microinjection sites for normoxic (open circles) and hypoxic (closed circles) animals overlaid on a diagrammatic representation of the nTS at the level of CS. Injection sites were similar between normoxic and hypoxic animals.
Fig. 3.
Nucleus tractus solitarii (nTS) microinjection sites and cholera toxin subunit B (CTB) labeling in the paraventricular nucleus of the hypothalamus (PVN) were verified. A: schematic representation of the nTS at the level of calamus scriptorius (left) and representative photomicrograph showing one side of a CTB microinjection site overlaid on a bright-field image (right). B: schematic representation of the nTS depicting the center of microinjection sites for normoxic (open circles) and hypoxic (filled circles) rats. C: images of four rostral-caudal levels of the PVN (approximately −1.5 to −2.1 mm relative to bregma) depicting CTB labeling (red) from the nTS. D: average number of CTB-labeled cells at each rostral-caudal level of the PVN in normoxic (21% O2, n = 5; open circles) and hypoxic (10% O2, n = 6; closed circles) rats. Two-way repeated measures ANOVA revealed no main effect of treatment (normoxia vs. hypoxia; F[1,27] = 2.07, P = 0.184), indicating the number of CTB-labeled cells in each PVN level was similar between groups. There was a significant main effect of rostral-caudal PVN level (F[3,27] = 198.06, P < 0.001). The caudal PVN sections contained significantly more CTB-labeled cells compared with the rostral sections in both groups. † > −1.5 mm; ‡ > −1.5 and −1.7 mm; # > −1.5, −1.7 and −2.1 mm; P < 0.001 for all comparisons. Scale bars = 200 µm. 3V, third ventricle; A1, noradrenergic cell group; CC, central canal; DP, dorsal parvocellular region; DMX, dorsal motor nucleus of the vagus; fx, fornix; LP, lateral parvocellular region; MP, medial parvocellular region; nA, nucleus ambiguus; PM, posterior magnocellular region; TS, tractus solitarius; VLP, ventrolateral parvocellular region; VP, ventral parvocellular region.
Distribution of nTS-projecting PVN neurons.
PVN neurons with projections to the nTS, identified by cytosolic CTB labeling, were observed throughout the PVN. Figure 3C includes representative photomicrographs showing CTB-labeled neurons in four PVN sections (approximately −1.5 to −2.1 mm, relative to bregma), and Fig. 3D shows the rostral-caudal distribution of positively labeled CTB cells counted from normoxic and hypoxic rats. nTS-projecting neurons were highly abundant in the caudal PVN, in particular the ventrolateral, ventral, and lateral parvocellular regions (Fig. 3C). No nTS-projecting neurons were observed in the posterior magnocellular subregions located in rostral PVN sections. The number of CTB-labeled cells in each PVN section was not different between normoxic and hypoxic animals (Fig. 3D).
Characterization of phenotypes of nTS-projecting PVN neurons.
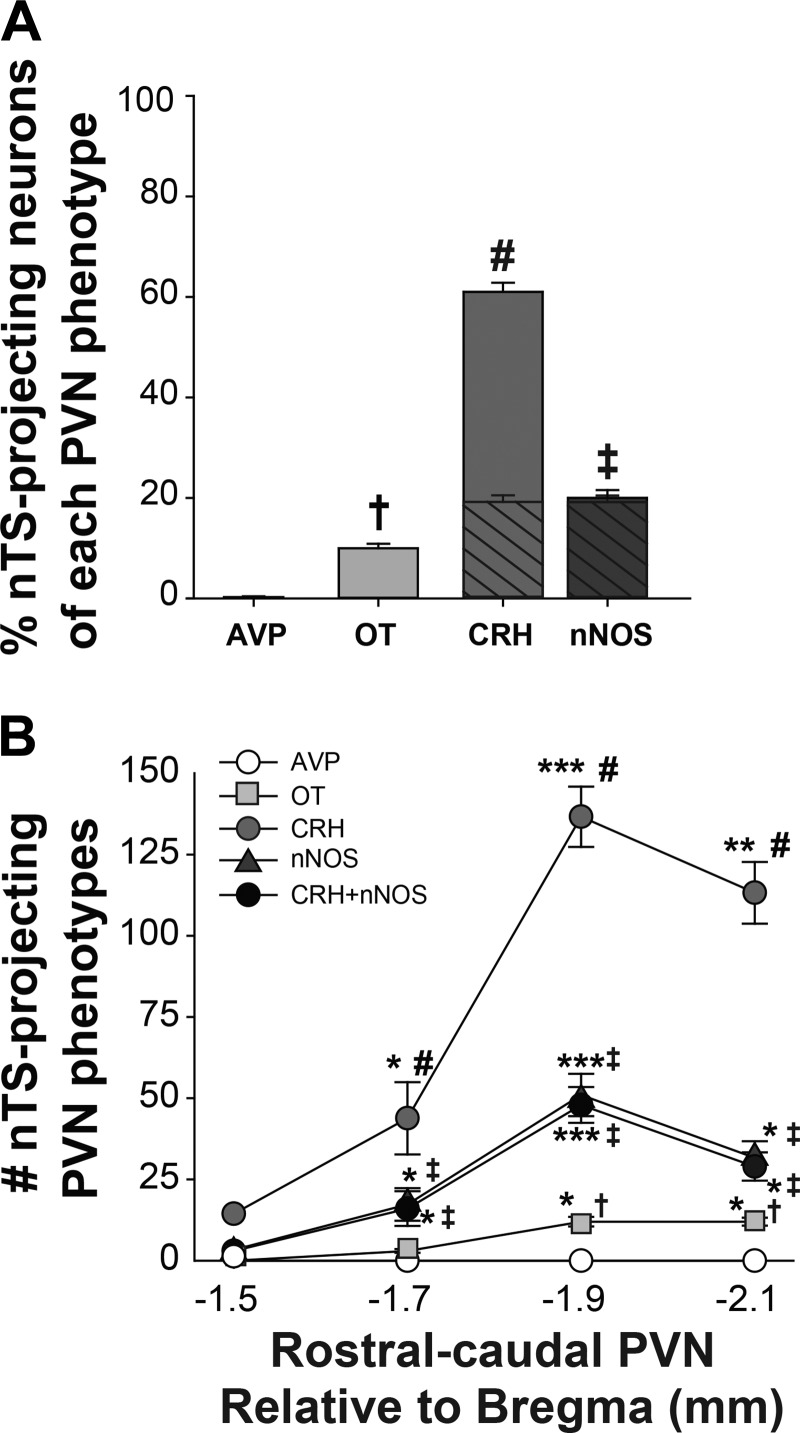
We next determined the phenotypes of PVN neurons with projections to the nTS by evaluating CTB-labeled cells for colocalization with AVP, OT, CRH, and nNOS. The total number of nTS-projecting PVN neurons was similar between normoxic and hypoxic animals (Table 2). Also, there was no significant group difference in either the total number of any specific nTS-projecting neuronal phenotype or the number at any individual PVN level (not shown). Therefore, data from both groups were combined for analysis of colabeled nTS-projecting neurons independent of neuronal activation. Very few (<1%) nTS-projecting neurons displayed AVP-IR, whereas a relatively small percentage of nTS-projecting neurons was OT-IR or nNOS-IR (Fig. 4A). In contrast, CRH-IR cells represented the majority (>60%) of all PVN projections to the nTS. Similar to the overall population (Table 2), a proportion (32 ± 2%) of nTS-projecting CRH neurons displayed nNOS-IR, whereas almost all (93 ± 3%) nTS-projecting nNOS cells were CRH-IR (Fig. 4A, cross-hatching). Overall, colabeling of CRH+nNOS-IR cells was present in ~20% of all PVN projections to the nTS.
Table 2.
Total number of CTB and colabeled nTS-projecting cells in four PVN sections
| 21% O2 | 10% O2 | |
|---|---|---|
| CTB | 510 ± 40 | 448 ± 22 |
| CTB + AVP | 1 ± 0.6 | 1 ± 0.8 |
| CTB + OT | 29 ± 2 | 26 ± 4 |
| CTB + CRH | 349 ± 37 | 320 ± 18 |
| CTB + nNOS | 111 ± 11 | 110 ± 9 |
| CTB + CRH + nNOS | 105 ± 12 | 103 ± 7 |
Values are means ± SE. Total number of cholera toxin subunit B (CTB) and arginine vasopressin (AVP), oxytocin (OT), corticotropin-releasing hormone (CRH), neuronal nitric oxide synthase (nNOS), or CRH + nNOS cells that were colabeled with CTB in animals exposed to 21% O2 (n = 5) or 10% O2 (n = 6). nTS, nucleus tractus solitarii; PVN, paraventricular nucleus of the hypothalamus.
Fig. 4.
Characterization and distribution of nucleus tractus solitarii (nTS)-projecting paraventricular nucleus of the hypothalamus (PVN) neuronal phenotypes. A: overall percentage of nTS-projecting neurons displaying colabeling with specific PVN phenotypes [(CTB + Phenotype)/CTB)] in normoxic and hypoxic animals (n = 11). One-way repeated measures (RM) ANOVA indicated a significant main effect of phenotype (F[4,53] = 444.39, P < 0.001). Overall, there were significantly more corticotropin-releasing hormone (CRH)-immunoreactive (IR) nTS-projecting neurons relative to all other projecting phenotypes examined, and a portion of these also exhibited neuronal nitric oxide synthase (nNOS)-IR (cross-hatching). The majority of nNOS-IR nTS-projecting neurons were also CRH-IR. B: rostral-caudal distribution (−1.5 to −2.1 mm relative to bregma) of colabeled nTS-projecting neurons within the PVN. Two-way RM ANOVA indicated a significant interaction of rostral-caudal PVN level and phenotype (F[12,219] = 221.65, P < 0.001). Post hoc analysis revealed that there were significantly more CTB-labeled neurons displaying colocalization for OT, CRH, and nNOS in the caudal PVN compared with the rostral PVN. Rostral-caudal distribution: * > −1.5 mm; ** > −1.5 and −1.7 mm; *** > −1.5, −1.7 and −2.1 mm. PVN phenotypes: † > AVP; ‡ > AVP and OT; # > AVP, OT and nNOS; for all comparisons, P < 0.001. AVP, arginine vasopressin; CTB, cholera toxin subunit B; OT, oxytocin.
Figure 4B depicts the rostral-caudal distribution of nTS-projecting neuronal phenotypes. Most of the colabeled nTS-projecting neurons were observed in the two caudal PVN sections, consistent with the distribution of CTB-labeled neurons (Fig. 3D). Similar to the overall data, there were significantly more CRH-IR nTS-projecting neurons compared with other phenotypes evaluated in all PVN sections, except the most rostral section examined. We also observed colabeled CRH+nNOS cells throughout the rostral-caudal extent of the PVN. In summary, OT, CRH, and nNOS comprise PVN projections to the nTS, and the predominant phenotype of nTS-projecting neurons was CRH-containing neurons.
Hypoxia activates nTS-projecting PVN neurons.
PVN neurons with nTS projections were examined for activation in response to acute hypoxia. Figure 5 includes representative photomicrographs of caudal PVN sections showing Fos-IR and CTB-labeled neurons following normoxia (Fig. 5A) or hypoxia (Fig. 5B). Higher magnification images (Fig. 5, A and B, insets) show the hypoxia-induced increase in Fos-IR observed in nTS-projecting neurons. Mean data (Fig. 5C) demonstrate that hypoxia significantly increased Fos-IR in nTS-projecting PVN neurons (21% O2: 5 ± 0.5%; 10% O2: 13 ± 0.8%, t(9) = −5.054; P < 0.001). The hypoxia-induced increase in the number of Fos-IR nTS-projecting neurons was greatest in the caudal PVN (Fig. 5D), which contains the majority of nTS-projecting neurons (Fig. 3D). These findings indicate that acute hypoxia activates neurons that comprise a projection from the PVN to the nTS.
Fig. 5.
Hypoxia activates nucleus tractus solitarii (nTS)-projecting paraventricular nucleus of the hypothalamus (PVN) neurons. Merged photomicrographs showing Fos-immunoreactivity (IR) (pseudocolored red) and cholera toxin subunit B (CTB)-labeled (pseudocolored white) neurons in the caudal PVN (−1.9 mm relative to bregma) of rats exposed to normoxia (A) or hypoxia (B). Insets: higher magnification images of the outlined areas showing Fos-IR nTS-projecting neurons (arrowheads). Mean data showing the overall number of nTS-projecting neurons (C) that were Fos-IR following exposure to normoxia (n = 5) or hypoxia (n = 6). Hypoxia increased the number of nTS-projecting neurons displaying Fos-IR [t (9) = −5.054; P < 0.001]. Rostral-caudal distribution of Fos-IR nTS-projecting neurons in the PVN (D). Two-way repeated measures ANOVA revealed a significant interaction between treatment and rostral-caudal PVN level (F[3,27] = 12.96, P < 0.001). Post hoc analysis indicated that hypoxia significantly increased the number of Fos-IR nTS-projecting neurons in the two caudal PVN levels (for both levels, P < 0.001). *10% O2 vs. 21% O2; rostral-caudal level within a group: † > −1.5 (Norm, P = 0.019); ‡ > −1.7 and −1.5 (P < 0.001 for both groups); # > −2.1, −1.7 and −1.5 (hypoxia, P < 0.001). Scale bar = 200 µm. For insets, scale bar = 25 µm.
Relative activation of specific nTS-projecting PVN phenotypes.
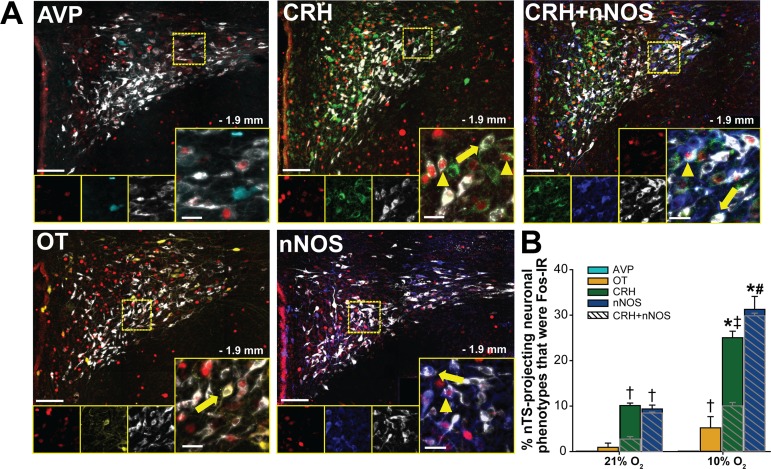
We performed additional immunohistochemical analyses to determine the extent to which individual phenotypes of nTS-projecting PVN neurons were activated by hypoxia. Figure 6A includes representative photomicrographs of the caudal PVN displaying Fos-IR, CTB-labeled cells, and specific PVN neuronal phenotypes following exposure to hypoxia. Hypoxia-induced Fos-IR was observed in OT-, CRH-, nNOS-, and colabeled CRH+nNOS-IR neurons which project to the nTS.
Fig. 6.
Activation of specific phenotypes of nucleus tractus solitarii (nTS)-projecting paraventricular nucleus of the hypothalamus (PVN) neurons. A: merged photomicrographs of the caudal PVN (−1.9 mm relative to bregma) from rats exposed to hypoxia showing Fos- immunoreactivity (IR) (pseudocolored red), cholera toxin subunit B (CTB) labeling (white), and each PVN phenotype examined: arginine vasopressin (AVP) (cyan), oxytocin (OT) (yellow), corticotropin-releasing hormone (CRH) (green), neuronal nitric oxide synthase (nNOS) (blue), and CRH+nNOS neurons. Arrowheads denote nTS-projecting PVN phenotypes displaying Fos-IR; arrows indicate nTS-projecting PVN cells that were negative for Fos-IR. B: percentage of each nTS-projecting neuronal phenotype displaying Fos-IR [(Fos + CTB + Phenotype)/(CTB + Phenotype)] in response to normoxia (n = 5) or hypoxia (n = 6). Two-way repeated measures (RM) ANOVA revealed a significant interaction between treatment and phenotype (F[4,35] = 21.31). Subsequent post hoc analysis indicated that no AVP-IR nTS-projecting neurons expressed Fos-IR. OT-IR nTS-projecting neurons were not significantly activated by hypoxia based on two-way RM ANOVA comparison. However, two-tailed unpaired t-test comparison indicated that hypoxia significantly increased Fos-IR in OT-IR nTS-projecting neurons [t(6) = −2.643, P = 0.038]. Hypoxia significantly increased Fos-IR in CRH- and nNOS-IR nTS-projecting neurons (P < 0.001 for both phenotypes). A portion of activated nTS-projecting CRH-IR cells was nNOS-IR (CRH+nNOS), and the majority of activated nNOS-IR nTS-projecting neurons also contained CRH-IR (cross-hatching). Within a phenotype: *10% O2 vs. 21% O2. Among phenotypes: † > AVP; ‡ > OT and AVP; # > CRH, OT, and AVP; P < 0.001.
The relative activation by hypoxia of specific nTS-projecting phenotypes is shown in Fig. 6B. Of the very few positively labeled AVP-IR nTS-projecting neurons (Table 2), none were activated by hypoxia. Fos-IR in nTS-projecting OT neurons was not significantly increased by hypoxia when examined by ANOVA; However, two-tailed unpaired t-test comparison indicated that hypoxia significantly increased the number of Fos- and OT-IR colabeled nTS-projecting neurons. Hypoxia activated a significant percentage of both CRH- and nNOS-IR nTS-projecting PVN neurons. In addition, the percentage of nTS-projecting neurons colabeled with CRH or nNOS that were activated by hypoxia was significantly larger than the percentage of nTS-projecting AVP or OT neurons exhibiting Fos-IR. Of the activated CRH-IR nTS-projecting neurons, 31 ± 1% also displayed nNOS-IR, and nearly all (94 ± 1%) of the activated nNOS-IR nTS-projecting neurons were also CRH-IR (cross-hatching). Taken together, these findings indicate that hypoxia activates OT-, CRH-, and nNOS-IR neuronal projections to the nTS, and activation appears to be most prevalent in CRH-IR and nNOS-IR nTS-projecting neurons.
Hypoxia-activated nTS-projecting PVN neurons are primarily CRH-IR.
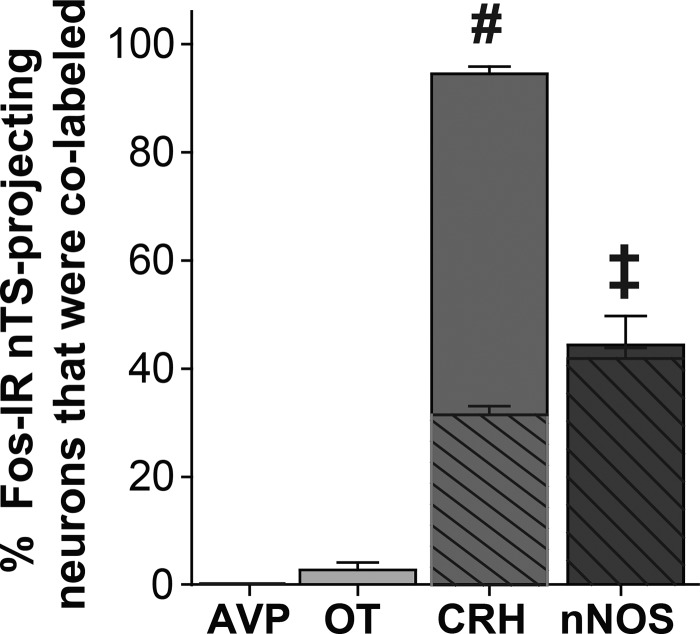
We next determined the phenotypes of hypoxia-activated PVN neurons projecting to the nTS. None of the Fos-IR nTS-projecting neurons displayed AVP-IR, and only a small percentage was colabeled with OT-IR (Fig. 7). In contrast, the majority (>90%) of hypoxia-activated nTS-projecting neurons displayed CRH-IR. As with the CRH-IR PVN-projecting neurons overall (Fig. 6), a portion of these activated neurons were also nNOS-IR. Nearly all (96 ± 3%) of the hypoxia-activated nTS-projecting nNOS-IR neurons also displayed CRH-IR. Thus, acute hypoxia significantly activated PVN neurons that project to the nTS, and the majority of these activated projecting cells contained CRH.
Fig. 7.
Most hypoxia-activated nucleus tractus solitarii (nTS)-projecting neurons display corticotropin-releasing hormone (CRH)-immunoreactivity (IR). Mean data showing the percentage of Fos-IR nTS-projecting neurons that were colabeled with each paraventricular nucleus of the hypothalamus (PVN) phenotype [(Fos + CTB + Phenotype)/(Fos + CTB)] in hypoxic animals (n = 6). Cross-hatched overlays represent colabeled CRH+ neuronal nitric oxide synthase (nNOS) neurons. One-way repeated measures ANOVA revealed a significant difference among phenotypes (F[3,15] = 222.867, P < 0.001). Subsequent post hoc analysis indicated that the percentage of hypoxia-activated nTS-projecting neurons displaying CRH-IR was significantly greater than all other phenotypes. Nearly all hypoxia-activated nTS-projecting nNOS-IR neurons also displayed CRH-IR. ‡ vs. AVP and OT, (P < 0.001); # vs. AVP, OT, and nNOS, (P < 0.001). AVP, arginine vasopressin; CTB, cholera toxin subunit B; OT, oxytocin.
CRFR2-IR is present in the nTS.
The above data demonstrate that most hypoxia-activated nTS-projecting PVN neurons are CRH-IR (Fig. 7), suggesting that these neurons contribute to chemoreflex cardiorespiratory responses. If so, then these effects may be mediated via activation of CRH receptors located in the nTS. Thus, we evaluated the distribution of CRFR2, the predominant CRH receptor in the nTS (99), as well as its relationship to Fos-IR neurons in nTS sections from separate groups of rats exposed to hypoxia.
CRFR2-IR is highly expressed in fibers in the nTS.
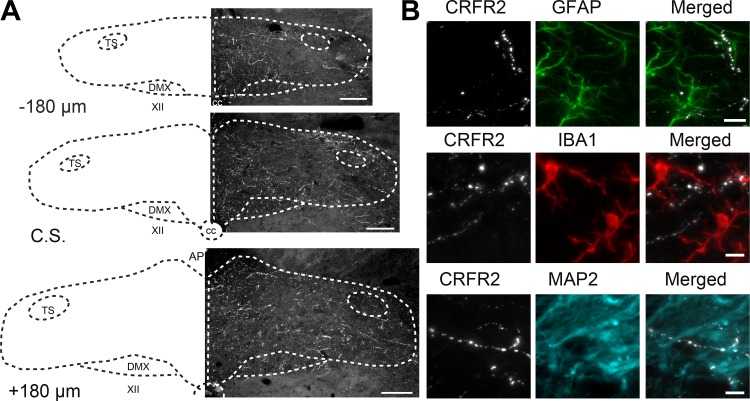
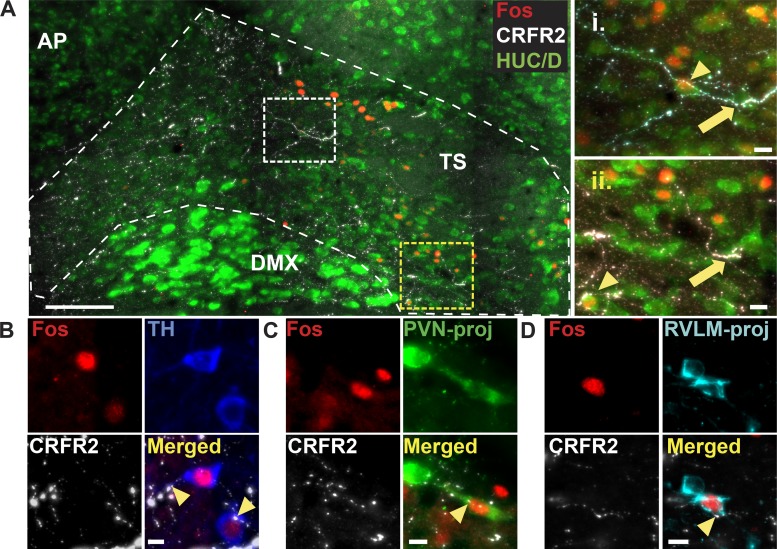
Evaluation of CRFR2-IR in the dorsal brain stem indicated that CRFR2 was present throughout the caudal-rostral extent of the nTS (Fig. 8A). Similar labeling was observed in tissue from all animals in which CRFR2 was examined. Interestingly, CRFR2-IR did not colocalize with GFAP-, IBA1-, MAP2-IR (Fig. 8B), or HUC/D-IR (Fig. 9A), indicating the receptor was not located on nTS astrocytes, microglia, dendrites, or neurons (n = 3 for each cell type). Rather, CRFR2-IR appeared to be located exclusively on fibers. CRFR2-IR fibers were highly localized to the nTS, although the dorsal motor nucleus of the vagus also contained CRFR2-IR fibers. In comparison, CRFR2 was mostly absent in the area postrema, hypoglossal nucleus, or in the solitary tract.
Fig. 8.
Corticotropin-releasing factor receptor 2 (CRFR2)-immunoreactivity (IR) is located in fibers throughout the nucleus tractus solitarii (nTS). A: schematics of 3 coronal nTS sections [relative to calamus scriptorius (CS), −180, 0, and 180 µm, left] and photomicrographs of corresponding nTS sections showing CRFR2-IR (right). CRFR2-IR fibers were highly expressed throughout the nTS in all three sections examined. CRFR2-IR fibers were also observed in the dorsal motor nucleus of the vagus (DMX). In comparison, CRFR2-IR was not seen in the solitary tract (TS), area postrema (AP), or hypoglossal nucleus (XII). Scale bars = 100 µm. All coordinates are relative to CS. B: photomicrographs of coronal nTS sections from naïve rats showing CRFR2-IR fibers (white) and the astrocyte marker glial fibrillary acidic protein (GFAP) (green), the microglial marker IBA1 (red), and the dendritic marker MAP2 (cyan). No colocalization between CRFR2-IR fibers and dendritic or glial markers was observed. Scale bars = 5 µm. CC, central canal.
Fig. 9.
Corticotropin-releasing factor receptor 2 (CRFR2)-immunoreactivity (IR) fibers are closely associated with hypoxia-activated nucleus tractus solitarii (nTS) neurons. A: left: Z-projection photomicrograph of five separate 1-µm thick slices of a coronal nTS section (final image is 5 µm thick) from a rat exposed to hypoxia (2 h of 10% O2). CRFR2-IR (white) fibers and puncta were observed near nTS neurons (HUC/D-IR, green), including hypoxia-activated (Fos-IR, red) neurons. Right: higher magnification images showing close associations (≤1 µm in all three planes) between CRFR2-IR fibers and HUC/D-IR nTS neurons (arrows), including Fos-IR neurons (arrowheads). Scale bar = 100 µm. For insets, scale bars = 10 µm. B–D: photomicrographs of coronal nTS sections from separate animals exposed to 2 h hypoxia displaying similar close associations of CRFR2-IR fibers with Fos-IR catecholaminergic [tyrosine hydroxylase (TH)-IR; B], paraventricular nucleus of the hypothalamus (PVN)-projecting (green, C), and rostral ventrolateral medulla (RVLM)-projecting neurons (cyan, D) nTS neurons (n = 3 each). For B–D, scale bar = 10 µm. AP, area postrema; DMX, dorsal motor nucleus of the vagus; TS, solitary tract.
CRFR2-IR is closely associated with hypoxia-activated nTS neurons.
We examined CRFR2-IR fibers to determine if they were closely associated with hypoxia-activated (Fos-IR) nTS neurons by examining proximity (≤1 µm) in each of the xy, xz, and yz planes. Figure 9A includes merged photomicrographs of a coronal nTS section from an animal exposed to acute hypoxia (2 h, 10% O2). CRFR2-IR was closely associated with, but not localized within, nTS neurons (indicated by the neuronal marker HUC/D) in rats exposed to hypoxia (n = 3), including neurons expressing Fos-IR. Close associations also were made with dendrites in the nTS (Fig. 8B). We then evaluated potential interactions with specific nTS neuronal phenotypes known to express Fos-IR in response to hypoxia. CRFR2-IR fibers exhibited close associations with hypoxia-activated TH-IR catecholaminergic neurons, PVN-projecting, and RVLM-projecting neurons in the nTS (n = 3 each). Examples of close associations with each of these cell types are shown in Fig. 9, B, C, and D. Similar interactions between CRFR2-IR fibers and Fos-IR nTS neurons were observed in all rostral-caudal levels of the nTS examined.
CRFR2 is located presynaptically on OT terminals throughout the nTS.
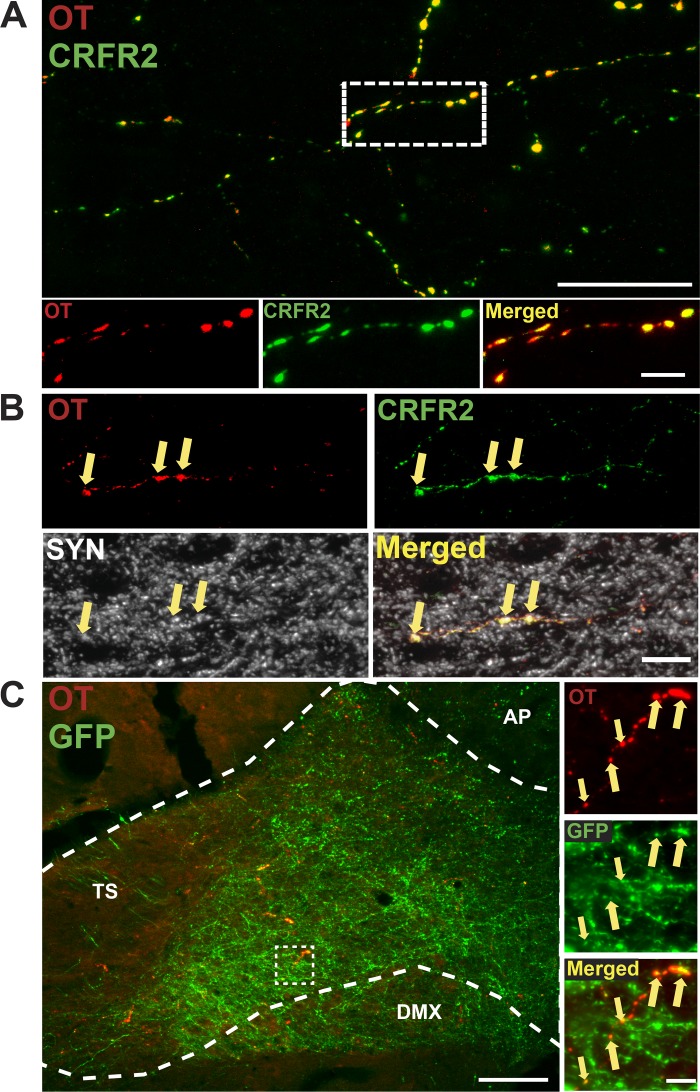
CRFR2 has been reported to colocalize exclusively with OT neurons and fibers in the PVN (20), and in preliminary experiments, we observed similar PVN colocalization (data not shown). In addition, PVN OT neurons send projections to the nTS (79) (Table 2). Therefore, we evaluated CRFR2 fibers for colocalization with OT in the nTS (n = 6). Figure 10A includes a representative photomicrograph of a coronal nTS section at the level of CS. There was extensive colocalization of CRFR2 and OT (0.989 ± 0.0003%, Manders coefficient). Similar colocalization was observed in fibers throughout the rostral-caudal extent of the nTS. CRFR2-IR fibers were characterized by multiple varicosities, suggesting possible release sites. Furthermore, a proportion of CRFR2 + OT-IR puncta was colabeled with the synaptic protein synaptophysin (Fig. 10B). To determine if OT-IR fibers originated from neurons in the PVN, we examined colocalization of OT-IR and GFP-containing fibers in the nTS of animals with prior PVN microinjections of AAV2-hSyn-GFP (n = 3). Figure 10C shows dense GFP-expressing fibers from PVN neurons within the caudal and postremal nTS. As expected, given the relatively small percentage of nTS-projecting PVN neurons that were also OT-IR (Table 2, Fig. 4), many of these GFP fibers did not exhibit OT-IR. However, OT-IR was extensively colocalized with GFP fibers in the nTS. Together, these findings suggest that CRFR2s are located on OT fibers originating from PVN neurons with terminals in the nTS. Close associations of CRFR2-IR fibers with hypoxia-activated neurons (Fig. 9) and colocalization of CRFR2- and OT-IR with synaptophysin (Fig. 10B) raise the possibility that PVN CRH projections to the nTS influence hypoxia-induced nTS neuronal activity via nTS CRFR2 activation, and these effects may involve nTS OTergic mechanisms.
Fig. 10.
Corticotropin-releasing factor receptor 2 (CRFR2)-immunoreactivity (IR) colocalizes with oxytocin (OT)-IR fibers throughout the nucleus tractus solitarii (nTS). A: top: Z-projection photomicrograph of five separate 1-µm thick slices of a coronal nTS section (final image is 5 µm thick) at the level of calamus scriptorius displaying CRFR2-IR (pseudocolored green) and OT-IR (pseudocolored red). Scale bar = 25 µm. Bottom: higher magnification of outlined area revealing extensive colocalization of CRFR2- and OT-IR fibers. Scale bar = 5 µm. B: photomicrographs of a coronal nTS section from a different animal displaying CRFR2-, OT-, and synaptophysin (SYN)-IR (pseudocolored white). Arrows denote colocalization of CRFR2 + OT-IR and SYN-IR puncta in the nTS. Scale bar = 5 µm. C: left: merged photomicrograph of a coronal nTS section from an animal that received paraventricular nucleus of the hypothalamus (PVN) microinjection of AAV2-hSyn-green fluorescent protein (GFP). Dense GFP-IR fibers (green) were observed throughout the nTS, and a proportion of these displayed colocalization with OT-IR (red). Nearly all OT-IR fibers colocalized with GFP fibers. Scale bar = 100 µm. Right: higher magnification image of boxed area. Arrows denote colocalization of GFP- and OT-IR fibers in the nTS. Scale bar = 10 µm. AP, area postrema; DMX, dorsal motor nucleus of the vagus; TS, solitary tract.
DISCUSSION
The PVN is required for full expression of arterial chemoreflex-mediated responses (70, 77), but the neurocircuitry responsible for these effects is not clear. A major finding of this study is that acute hypoxia activates a population of PVN neurons that project to the nTS. Hypoxia primarily activated CRH-IR-projecting neurons, although a small portion of these Fos-IR nTS-projecting neurons were also OT-IR. Based on these findings, we reasoned that a mechanism by which the PVN-nTS pathway may contribute to chemoreflex output is via CRH release and activation of CRH receptors in the nTS. Our data indicate that CRFR2s were highly expressed throughout the nTS, exclusively on OT fibers and terminals from the PVN, suggesting a presynaptic location. Furthermore, CRFR2 fibers were closely associated with hypoxia-activated nTS neurons, including catecholaminergic cells and nTS neurons that project to the PVN or the RVLM. Taken together, these data support the concept that hypoxia activates neuropeptidergic PVN neurons that send projections to the nTS. These projections may in turn influence activation of hypoxia-sensitive nTS neurons via CRH acting at CRFR2s, possibly modulating OTergic signaling within the nTS. This may lead to enhanced chemoreflex output via projections back to the PVN or to the RVLM.
Hypoxia activates a reciprocal nTS-PVN pathway.
Peripheral chemoreflex stimulation activates neurons in the nTS, which integrates this information and transmits it to other cardiorespiratory brain regions. Chemoafferent input is relayed to the PVN via ascending inputs from the brain stem, including catecholaminergic neurons in the nTS and ventrolateral medulla (50, 51). Moreover, depletion of brain-stem catecholaminergic projections to the PVN decreases PVN neuronal activation (89) and blunts ventilatory responses (52) to hypoxia. Thus, catecholaminergic projections to the PVN are critical to arterial chemoreflex adjustments to hypoxia.
Acute hypoxia activates neurons in the magnocellular and parvocellular regions of the PVN (17, 22), likely contributing to increased breathing, sympathetic nerve activity, arterial pressure, and circulating neuropeptides following peripheral chemoreflex stimulation (33, 76, 95, 97). The PVN is required for full expression of chemoreflex function, as PVN inhibition or lesion attenuates sympathoexcitatory and cardiorespiratory responses to potassium cyanide-induced chemoreceptor activation (70, 77). In the present study, we confirm previous reports (17, 22, 89) that hypoxia activates AVP-, OT-, CRH-, and nNOS-IR PVN neurons. Hypoxia-activated AVP cells were almost entirely located in magnocellular subnuclei of the rostral PVN (17, 89), whereas OT-, CRH-, and nNOS-IR neurons were also located in parvocellular PVN regions. Overall, hypoxia activated a greater percentage of OT, CRH, and nNOS neurons compared with AVP neurons, consistent with studies showing less activation in AVP cells compared with other PVN neurons (17, 89) and relatively small increases in AVP secretion (33).
Efferent projections from the PVN to the RVLM and IML contribute to sympathoexcitatory and cardiorespiratory responses to a variety of stimuli (7, 41, 46, 47, 49, 93). However, our previous work found that RVLM- and IML-projecting PVN neurons were not activated by acute hypoxia (17), suggesting that projections from the PVN to other nuclei, such as the nTS, may participate in cardiorespiratory responses during acute hypoxia.
In the present study, cells retrogradely labeled from the nTS were observed in all examined PVN levels, supporting previous work that highlights a dense projection from the PVN to the nTS (32). In contrast to RVLM and IML projections (17), we found that hypoxia significantly activated nTS-projecting PVN neurons. Thus, it appears that hypoxia preferentially activates a population of PVN neurons that project to the nTS. Interestingly, many of these activated nTS-projecting neurons were located in parvocellular subnuclei in the caudal PVN, a site that receives ascending inputs from nTS neurons (19). This raises the possibility that projections from the nTS may influence PVN neurons that project back to the nTS via a monosynaptic or polysynaptic pathway. Taken together, these findings introduce the concept that compensatory responses to hypoxia involve activation of a reciprocal nTS-PVN pathway; nTS neurons provide chemoafferent information to the PVN, which then results in activation of PVN neurons, including a subpopulation of cells that project back to the nTS. It is possible that activation of this pathway amplifies chemoreflex responses.
Our data appear to conflict with a previous report showing that stimulation of peripheral chemoreceptors induces Fos-IR in RVLM-projecting but not in nTS-projecting PVN neurons (18). There are several possibilities that may account for the differences in efferent pathway activation. In that study, peripheral chemoreceptors were activated in conscious rats by repeated injections of KCN. In some cases, KCN-evoked stimulation of peripheral chemoreceptors evokes cardiorespiratory responses comparable to the more natural hypoxic stimulus (8). However, cyanide can evoke aversive, nonspecific responses, and it has been suggested that it constitutes a distinct stimulus (34) which may activate different central pathways. In addition, KCN was injected in an intermittent manner (18), whereas in the present study, conscious rats were exposed to 2 h of continuous hypoxia. Exposure to acute intermittent hypoxia induces long-term facilitation, characterized by increased phrenic and sympathetic nerve activity at baseline and during hypoxia (6, 25), possibly involving activation of different efferent pathways. Thus, differences in both protocol (sustained vs. intermittent activation) and stimulus (hypoxia vs. KCN) may account for discrepancies in activation of descending PVN projections to cardiorespiratory nuclei. Additional studies are required to resolve these differences.
PVN neuropeptidergic projections to the nTS are primarily CRH.
A substantial portion of PVN projections to the nTS are peptidergic neurons, including AVP, OT, and CRH cells (15, 96). Many studies indicate that these neuropeptides influence nTS activity and cardiorespiratory function. For example, AVP presynaptically inhibits glutamate release from sensory afferents (5), and selective AVP receptor blockade in the nTS has been reported to reduce pressor and tachycardic responses to electrical stimulation of the PVN (74). In contrast, nTS OT facilitates glutamatergic transmission (73), and nTS OT receptor antagonism potentiates exercise-induced tachycardia (64), indicating a role for endogenous OT in the nTS. Direct application of CRH into baroreflex regions of the nTS elicits bradycardia and depressor responses, consistent with excitation of neurons involved in baroreflex function (68). Thus, we evaluated PVN neuropeptidergic projections to the nTS.
Overall, AVP was seen in less than 1% of all nTS-projecting neurons. Although some studies suggest that a greater proportion of nTS projections express AVP (43), our data are consistent with other reports indicating a small percentage of AVP inputs to the nTS (15). In comparison, OT comprised a larger proportion of neurons in the PVN-nTS pathway, as shown previously (15, 79). Of the peptides examined in nTS projecting neurons, CRH was the most prevalent and was observed in almost two-thirds of retrogradely labeled cells in the PVN, supporting previous work demonstrating that PVN CRH neurons project to cardiorespiratory brain regions (17, 68, 96). Our data support the concept that a large fraction of nTS-projecting PVN neurons are peptidergic. In fact, we likely are underestimating the proportion of peptidergic neurons, given the fact that we have not evaluated all neuropeptides, and neuropeptides are rapidly transported to axons, potentially reducing the number of immunoreactive neurons observed in the PVN. Taken together, it is possible that PVN neuropeptides that reach the nTS contribute to responses to peripheral chemoreflex stimulation.
Hypoxia activates nTS-projecting PVN neuropeptidergic cells.
Of the very few AVP-IR nTS-projecting cells in the PVN, none expressed Fos following hypoxia, suggesting they likely do not participate in cardiorespiratory chemoreflex responses. In contrast, it appears that hypoxia may produce a small activation of nTS-projecting OT neurons. Thus, activation of PVN OT inputs to the nTS may contribute to chemoreflex responses via actions within the nTS. The concept that OT modulates cardiorespiratory control is supported by previous work demonstrating that OT facilitates neuronal activation and influences cardiorespiratory function via actions in brain-stem nuclei, including the dorsal motor nucleus of the vagus and pre-Botzinger complex (44, 48, 103).
Overall, CRH neurons were the largest population of nTS-projecting neuropeptidergic cells, and we observed robust activation of these neurons following acute hypoxia. Thus, CRH projections to the nTS may be the primary pathway by which the PVN influences nTS neurons to potentially enhance cardiorespiratory chemoreflex function. Interestingly, a portion of these hypoxia-activated cells also displayed nNOS-IR, and nitric oxide (NO) may modulate their activity. In the PVN, NO decreases sympathetic nerve activity and blood pressure (105, 106) associated with potentiated GABAergic signaling to RVLM- and nTS-projecting neurons (58, 59). Furthermore, NO in the PVN tonically restrains cardiorespiratory responses to peripheral chemoreflex activation by KCN (78). We previously observed CRH and nNOS colocalization in PVN neurons that project to the RVLM and IML (17). These neurons were not activated by hypoxia, and it is possible that NO limited hypoxia-induced activation of RVLM- and IML-projecting neurons. However, the presence of nNOS does not appear to prevent activation of nTS-projecting PVN neurons. In addition to actions in the PVN, NO may influence chemoreflex responses via release from PVN terminals in the nTS. For example, microinjection of NO donors or NOS blockade in the nTS influences cardiorespiratory function (42, 57). Taken together, our data suggest that nTS-projecting CRH PVN neurons contribute to cardiorespiratory chemoreflex responses, and these effects may be modulated by NO acting in the PVN or the nTS.
CRFR2s are present in the nTS.
Strikingly, we found that over 90% of hypoxia-activated nTS-projecting PVN neurons exhibited CRH-IR. Coupled with the fact that the majority of CRH-containing neurons in the PVN are glutamatergic (20), this observation raises the possibility that corelease of glutamate and CRH in the nTS may be important in mediating responses due to activation of the nTS-PVN pathway during hypoxia. Activation of these projections to the nTS thus could enhance neuronal activity via activation of nTS glutamate receptors, and coreleased CRH may contribute to the integrated responses to hypoxia via activation of CRH receptors. CRFR2 is the predominant CRH receptor in the nTS (56, 99). CRH-evoked CRFR2 activation facilitates neuronal excitation via enhanced presynaptic glutamate release in multiple brain regions (21, 88). Microinjection of CRH or selective activation of CRFR2s (69, 102) in baroreflex regions of the nTS produces cardiovascular responses consistent with excitation of nTS neurons involved in baroreflex function (68). In addition, CRFR2s in the nTS appear to play a role in the hypertension associated with exposure to chronic intermittent hypoxia (100). Our data indicate that CRFR2-containing fibers are abundant in the medial and commissural nTS, regions that form the termination site of chemoafferent inputs (29, 30), and exhibit neuronal activation following chemoreflex stimulation (22, 65, 98). Consistent with an influence of CRFR2 activation on hypoxia-induced neuronal activity, CRFR2 fibers exhibited close associations with nTS neurons displaying Fos-IR. Importantly, this includes close associations with hypoxia-activated nTS catecholaminergic cells as well as activated PVN- and RVLM-projecting nTS neurons. Furthermore, neuropeptides, such as CRH and OT, are volume transmitters and therefore may be released from extrasynaptic sites on axons from PVN neurons to influence nTS neuronal activity (31, 40). Given the importance of the nTS-PVN and nTS-RVLM projections in mediating chemoreflex responses (34, 52), this provides an anatomic substrate by which the PVN may augment cardiorespiratory responses to hypoxia. Thus, PVN CRH projections to the nTS may enhance nTS neuronal activation and chemoreflex responses via activation of nTS CRFR2s.
CRH in the nTS may influence OT signaling.
CRFR2s were located exclusively on OT fibers and synaptic terminals in the nTS, similar to colocalization of CRFR2 on OT fibers in bed nucleus of the stria terminalis and nucleus accumbens (20). It is likely that OT fibers within the nTS originate from the PVN. In the PVN, CRFR2 is colocalized with OT neurons (20), and others have reported that the PVN provides the sole source of OT inputs to the nTS (79). Importantly, OT-IR is located on GFP-containing nTS fibers in animals with AAV-mediated expression of GFP in PVN neurons. Our data indicating CRFR2 on OT fibers from the PVN are not consistent with a previous report suggesting that CRFR2s are located on visceral afferent terminals in the nTS (56). The reasons for this discrepancy are unclear. One possibility is that the previous study used autoradiography, whereas we used immunohistochemistry to identify CRFR2 protein. Based on our results, it appears that CRFR2s are presynaptic receptors on OT fibers, and it is plausible that their activation may influence OT release in the nTS.
Previous work supports the concept that functional interactions between CRH and OT occur in central nuclei. CRFR2 activation by CRH regulates OT release in the bed nucleus of the stria terminalis (61). In addition, intracerebroventricular (ICV) injection of CRH increases peripheral OT secretion via activation of CRH receptors in the posterior pituitary gland (14). ICV injection of CRH increases arterial pressure via OT mechanisms, as central blockade of OT receptors completely abolishes the pressor response to ICV CRH (104). Similar interactions may occur in the nTS. nTS-projecting neurons in the PVN are tonically active (90), and our data suggest that hypoxia may activate a population of PVN OT neurons that project to the nTS. In turn, the influence of this OT projection in the nTS may be modulated by activation of CRFR2s by CRH released from terminals of hypoxia-activated nTS-projecting CRH neurons in the PVN. Together, these findings suggest that hypoxia-evoked activation of PVN CRH inputs to the nTS may interact with nTS CRFR2s on OT fibers in the nTS. This interaction may serve to amplify hypoxia-induced nTS neuronal activation and chemoreflex function.
Perspectives and Significance
These studies provide insight regarding the role of the PVN during peripheral chemoreflex stimulation. The PVN sends efferent projections to many essential brain-stem and spinal nuclei, and projections to the RVLM and/or spinal cord have been implicated in responses to diverse stimuli (7, 47, 92). Interestingly, PVN projections to the nTS appear to be preferentially activated by acute hypoxia. The factors that determine activation of RVLM/IML-projecting versus nTS-projecting PVN neurons and the functional implications of their activation remain to be established. In addition, it is possible that other stimuli, as with the effects of exercise (91), may also activate the PVN-NTS pathway, and a role for this projection should be considered when examining the neurocircuitry involved in cardiorespiratory regulation. Regardless of its role in other functional responses, a reciprocal nTS-PVN pathway is engaged during hypoxia and may be essential in mediating cardiorespiratory responses to peripheral chemoreflex stimulation.
CRH neurons comprise a majority of hypoxia-activated nTS-projecting neurons in the PVN. Because these neurons also are glutamatergic (20), they may influence nTS activation directly via activation of nTS glutamate receptors. Furthermore, corelease of CRH from PVN terminals could influence nTS activity by modulating glutamatergic signaling or other peptidergic pathways via activation of CRFR2s. PVN neurons are active at rest (90), hypoxia may activate nTS-projecting PVN OT neurons, and fibers from the PVN to the nTS coexpress OT and CRFR2. These fibers are closely associated with hypoxia-activated nTS neurons, including catecholaminergic, PVN-, and RVLM-projecting neurons known to be important to chemoreflex function. Together, this raises the possibility that hypoxic activation of nTS-projecting PVN CRH neurons may amplify chemoreflex function by multiple mechanisms: direct activation of nTS neurons, modulation of OTergic mechanisms in the nTS, and/or by activating a reciprocal pathway back to the PVN or a projection to the RVLM.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-98602 and Grant RO1-HL-094404.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.C.R., C.M.H., and E.M.H. conceived and designed research; B.C.R., P.J.K., and C.P.B. performed experiments; B.C.R. analyzed data; B.C.R., C.M.H., and E.M.H. interpreted results of experiments; B.C.R., P.J.K., and C.P.B. prepared figures; B.C.R. drafted manuscript; B.C.R., C.M.H., and E.M.H. edited and revised manuscript; B.C.R., C.M.H., and E.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sarah A. Friskey for outstanding technical expertise and Elizabeth Palmieri, Gabrielle Callanan, and Allie Feinberg for assistance with immunohistochemical procedures. We also thank Dr. David Kline for many helpful discussions and critical evaluation of the manuscript.
REFERENCES
- 1.Alheid GF, Jiao W, McCrimmon DR. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neuroscience 190: 207–227, 2011. doi: 10.1016/j.neuroscience.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 3.Austgen JR, Fong AY, Foley CM, Mueller PJ, Kline DD, Heesch CM, Hasser EM. Expression of group I metabotropic glutamate receptors on phenotypically different cells within the nucleus of the solitary tract in the rat. Neuroscience 159: 701–716, 2009. doi: 10.1016/j.neuroscience.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol 28: 95–99, 2001. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- 5.Bailey TW, Jin Y-H, Doyle MW, Smith SM, Andresen MC. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci 26: 6131–6142, 2006. doi: 10.1523/JNEUROSCI.5176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardgett ME, Sharpe AL, Toney GM. Activation of corticotropin-releasing factor receptors in the rostral ventrolateral medulla is required for glucose-induced sympathoexcitation. Am J Physiol Endocrinol Metab 307: E944–E953, 2014. doi: 10.1152/ajpendo.00291.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barros RC, Bonagamba LG, Okamoto-Canesin R, de Oliveira M, Branco LG, Machado BH. Cardiovascular responses to chemoreflex activation with potassium cyanide or hypoxic hypoxia in awake rats. Auton Neurosci 97: 110–115, 2002. doi: 10.1016/S1566-0702(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 9.Bathina CS, Rajulapati A, Franzke M, Yamamoto K, Cunningham JT, Mifflin S. Knockdown of tyrosine hydroxylase in the nucleus of the solitary tract reduces elevated blood pressure during chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 305: R1031–R1039, 2013. doi: 10.1152/ajpregu.00260.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res 15: 254–263, 2005. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- 11.Bender J, Engeholm M, Ederer MS, Breu J, Møller TC, Michalakis S, Rasko T, Wanker EE, Biel M, Martinez KL, Wurst W, Deussing JM. Corticotropin-releasing hormone receptor type 1 (CRHR1) clustering with MAGUKs is mediated via Its C-terminal PDZ binding motif. PLoS One 10: e0136768, 2015. doi: 10.1371/journal.pone.0136768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res 857: 30–40, 2000. doi: 10.1016/S0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- 13.Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, Toga AW, Dong HW. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol 520: 6–33, 2012. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruhn TO, Sutton SW, Plotsky PM, Vale WW. Central administration of corticotropin-releasing factor modulates oxytocin secretion in the rat. Endocrinology 119: 1558–1563, 1986. doi: 10.1210/endo-119-4-1558. [DOI] [PubMed] [Google Scholar]
- 15.Buijs RM, Swaab DF, Dogterom J, van Leeuwen FW. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res 192: 423–435, 1978. doi: 10.1007/BF00224932. [DOI] [PubMed] [Google Scholar]
- 15a.Chan RK, Sawchenko PE. Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J Comp Neurol 348: 433–460, 1994. doi: 10.1002/cne.903480309. [DOI] [PubMed] [Google Scholar]
- 16.Chung DW, Yoo KY, Hwang IK, Kim DW, Chung JY, Lee CH, Choi JH, Choi SY, Youn HY, Lee IS, Won MH. Systemic administration of lipopolysaccharide induces cyclooxygenase-2 immunoreactivity in endothelium and increases microglia in the mouse hippocampus. Cell Mol Neurobiol 30: 531–541, 2010. doi: 10.1007/s10571-009-9477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coldren KM, Li DP, Kline DD, Hasser EM, Heesch CM. Acute hypoxia activates neuroendocrine, but not presympathetic, neurons in the paraventricular nucleus of the hypothalamus: differential role of nitric oxide. Am J Physiol Regul Integr Comp Physiol 312: R982–R995, 2017. doi: 10.1152/ajpregu.00543.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz JC, Bonagamba LG, Machado BH, Biancardi VC, Stern JE. Intermittent activation of peripheral chemoreceptors in awake rats induces Fos expression in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus. Neuroscience 157: 463–472, 2008. doi: 10.1016/j.neuroscience.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham ET Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 274: 60–76, 1988. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 20.Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology 36: 1312–1326, 2011. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG. Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 7: 156, 2013. doi: 10.3389/fnins.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol 71: 359–384, 2003. doi: 10.1016/j.pneurobio.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Dampney RA, Polson JW, Potts PD, Hirooka Y, Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol 23: 597–616, 2003. doi: 10.1023/A:1025080314925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deurveilher S, Lo H, Murphy JA, Burns J, Semba K. Differential c-Fos immunoreactivity in arousal-promoting cell groups following systemic administration of caffeine in rats. J Comp Neurol 498: 667–689, 2006. doi: 10.1002/cne.21084. [DOI] [PubMed] [Google Scholar]
- 25.Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol 92: 87–97, 2007. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- 26.Doméné A, Cavanagh C, Page G, Bodard S, Klein C, Delarasse C, Chalon S, Krantic S. Expression of phenotypic astrocyte marker is increased in a transgenic mouse model of Alzheimer’s disease versus age-matched controls: a presymptomatic stage study. Int J Alzheimers Dis 2016: 5696241, 2016. doi: 10.1155/2016/5696241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29: 261–265, 1989. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 28.Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol 300: C723–C742, 2011. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felder RB, Mifflin SW. Baroreceptor and chemoreceptor afferent processing in the solitary tract nucleus. In: Nucleus of the Solitary Tract, edited by Barraco IRA. Boca Raton, FL: CRC, 1994, p. 169–186. [Google Scholar]
- 30.Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res 572: 108–116, 1992. doi: 10.1016/0006-8993(92)90458-L. [DOI] [PubMed] [Google Scholar]
- 31.Garcia I, Quast KB, Huang L, Herman AM, Selever J, Deussing JM, Justice NJ, Arenkiel BR. Local CRH signaling promotes synaptogenesis and circuit integration of adult-born neurons. Dev Cell 30: 645–659, 2014. doi: 10.1016/j.devcel.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol 518: 1460–1499, 2010. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffen SC, Raff H. Vasopressin responses to hypoxia in conscious rats: interaction with water restriction. J Endocrinol 125: 61–66, 1990. doi: 10.1677/joe.0.1250061. [DOI] [PubMed] [Google Scholar]
- 34.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 36.Guyenet PG, Koshiya N. Working model of the sympathetic chemoreflex in rats. Clin Exp Hypertens 17: 167–179, 1995. doi: 10.3109/10641969509087063. [DOI] [PubMed] [Google Scholar]
- 37.Guyenet PG, Koshiya N, Huangfu D, Baraban SC, Stornetta RL, Li Y-W. Role of medulla oblongata in generation of sympathetic and vagal outflows. In: Progress in Brain Research, edited by Holstege G, Bandler R, Saper CB Amsterdam, The Netherlands: Elsevier Science B. V. 1996, p. 127–144. doi: 10.1016/S0079-6123(08)61862-2. [DOI] [PubMed] [Google Scholar]
- 38.Higa-Taniguchi KT, Felix JV, Michelini LC. Brainstem oxytocinergic modulation of heart rate control in rats: effects of hypertension and exercise training. Exp Physiol 94: 1103–1113, 2009. doi: 10.1113/expphysiol.2009.049262. [DOI] [PubMed] [Google Scholar]
- 39.Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC, Katibah GE, Amora R, Boydston EA, Zeitler B, Meng X, Miller JC, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jaenisch R. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol 27: 851–857, 2009. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hökfelt T, Broberger C, Xu ZQ, Sergeyev V, Ubink R, Diez M. Neuropeptides–an overview. Neuropharmacology 39: 1337–1356, 2000. doi: 10.1016/S0028-3908(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 41.Horiuchi J, Potts PD, Polson JW, Dampney RA. Distribution of neurons projecting to the rostral ventrolateral medullary pressor region that are activated by sustained hypotension. Neuroscience 89: 1319–1329, 1999. doi: 10.1016/S0306-4522(98)00399-6. [DOI] [PubMed] [Google Scholar]
- 42.Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, Ferguson AV. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol Regul Integr Comp Physiol 266: R306–R313, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Jackson K, Silva HM, Zhang W, Michelini LC, Stern JE. Exercise training differentially affects intrinsic excitability of autonomic and neuroendocrine neurons in the hypothalamic paraventricular nucleus. J Neurophysiol 94: 3211–3220, 2005. doi: 10.1152/jn.00277.2005. [DOI] [PubMed] [Google Scholar]
- 44.Jameson H, Bateman R, Byrne P, Dyavanapalli J, Wang X, Jain V, Mendelowitz D. Oxytocin neuron activation prevents hypertension that occurs with chronic intermittent hypoxia/hypercapnia in rats. Am J Physiol Heart Circ Physiol 310: H1549–H1557, 2016. doi: 10.1152/ajpheart.00808.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jappelli R, Perrin MH, Lewis KA, Vaughan JM, Tzitzilonis C, Rivier JE, Vale WW, Riek R. Expression and functional characterization of membrane-integrated mammalian corticotropin releasing factor receptors 1 and 2 in Escherichia coli. PLoS One 9: e84013, 2014. doi: 10.1371/journal.pone.0084013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kantzides A, Badoer E. nNOS-containing neurons in the hypothalamus and medulla project to the RVLM. Brain Res 1037: 25–34, 2005. doi: 10.1016/j.brainres.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 47.Kc P, Balan KV, Tjoe SS, Martin RJ, Lamanna JC, Haxhiu MA, Dick TE. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J Physiol 588: 725–740, 2010. doi: 10.1113/jphysiol.2009.184580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kc P, Dick TE. Modulation of cardiorespiratory function mediated by the paraventricular nucleus. Respir Physiol Neurobiol 174: 55–64, 2010. doi: 10.1016/j.resp.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kc P, Haxhiu MA, Tolentino-Silva FP, Wu M, Trouth CO, Mack SO. Paraventricular vasopressin-containing neurons project to brain stem and spinal cord respiratory-related sites. Respir Physiol Neurobiol 133: 75–88, 2002. doi: 10.1016/S1569-9048(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 50.King TL, Heesch CM, Clark CG, Kline DD, Hasser EM. Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol 302: R1219–R1232, 2012. doi: 10.1152/ajpregu.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King TL, Kline DD, Ruyle BC, Heesch CM, Hasser EM. Acute systemic hypoxia activates hypothalamic paraventricular nucleus-projecting catecholaminergic neurons in the caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 305: R1112–R1123, 2013. doi: 10.1152/ajpregu.00280.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.King TL, Ruyle BC, Kline DD, Heesch CM, Hasser EM. Catecholaminergic neurons projecting to the paraventricular nucleus of the hypothalamus are essential for cardiorespiratory adjustments to hypoxia. Am J Physiol Regul Integr Comp Physiol 309: R721–R731, 2015. doi: 10.1152/ajpregu.00540.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience 167: 510–527, 2010. doi: 10.1016/j.neuroscience.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol 270: R1273–R1278, 1996. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- 55.Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Res 609: 174–184, 1993. doi: 10.1016/0006-8993(93)90871-J. [DOI] [PubMed] [Google Scholar]
- 56.Lawrence AJ, Krstew EV, Dautzenberg FM, Rühmann A. The highly selective CRF(2) receptor antagonist K41498 binds to presynaptic CRF(2) receptors in rat brain. Br J Pharmacol 136: 896–904, 2002. doi: 10.1038/sj.bjp.0704783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewis SJ, Ohta H, Machado B, Bates JN, Talman WT. Microinjection of S-nitrosocysteine into the nucleus tractus solitarii decreases arterial pressure and heart rate via activation of soluble guanylate cyclase. Eur J Pharmacol 202: 135–136, 1991. doi: 10.1016/0014-2999(91)90269-V. [DOI] [PubMed] [Google Scholar]
- 58.Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol 88: 2664–2674, 2002. doi: 10.1152/jn.00540.2002. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience 118: 585–601, 2003. doi: 10.1016/S0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 60.Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J Appl Physiol (1985) 92: 826–834, 2002. doi: 10.1152/japplphysiol.00839.2001. [DOI] [PubMed] [Google Scholar]
- 61.Martinon D, Dabrowska J. Corticotropin-releasing factor receptors modulate oxytocin release in the dorsolateral bed nucleus of the stria terminalis (BNST) in male rats. Front Neurosci 12: 183, 2018. doi: 10.3389/fnins.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masliukov PM, Moiseev KY, Korzina MB, Porseva VV. Development of nNOS-positive neurons in the rat sensory ganglia after capsaicin treatment. Brain Res 1618: 212–221, 2015. doi: 10.1016/j.brainres.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 63.Massari VJ, Shirahata M, Johnson TA, Gatti PJ. Carotid sinus nerve terminals which are tyrosine hydroxylase immunoreactive are found in the commissural nucleus of the tractus solitarius. J Neurocytol 25: 197–208, 1996. doi: 10.1007/BF02284796. [DOI] [PubMed] [Google Scholar]
- 64.Michelini LC. Oxytocin in the NTS. A new modulator of cardiovascular control during exercise. Ann NY Acad Sci 940: 206–220, 2001. doi: 10.1111/j.1749-6632.2001.tb03678.x. [DOI] [PubMed] [Google Scholar]
- 65.Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol 263: R368–R375, 1992. doi: 10.1152/ajpregu.1992.263.2.R368. [DOI] [PubMed] [Google Scholar]
- 66.Mifflin SW. Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. Am J Physiol Regul Integr Comp Physiol 271: R870–R880, 1996. doi: 10.1152/ajpregu.1996.271.4.R870. [DOI] [PubMed] [Google Scholar]
- 67.Milan-Lobo L, Gsandtner I, Gaubitzer E, Rünzler D, Buchmayer F, Köhler G, Bonci A, Freissmuth M, Sitte HH. Subtype-specific differences in corticotropin-releasing factor receptor complexes detected by fluorescence spectroscopy. Mol Pharmacol 76: 1196–1210, 2009. doi: 10.1124/mol.109.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milner TA, Reis DJ, Pickel VM, Aicher SA, Giuliano R. Ultrastructural localization and afferent sources of corticotropin-releasing factor in the rat rostral ventrolateral medulla: implications for central cardiovascular regulation. J Comp Neurol 333: 151–167, 1993. doi: 10.1002/cne.903330203. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura T, Kawabe K, Sapru HN. Cardiovascular responses to microinjections of urocortin 3 into the nucleus tractus solitarius of the rat. Am J Physiol Heart Circ Physiol 296: H325–H332, 2009. doi: 10.1152/ajpheart.01044.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olivan MV, Bonagamba LG, Machado BH. Involvement of the paraventricular nucleus of the hypothalamus in the pressor response to chemoreflex activation in awake rats. Brain Res 895: 167–172, 2001. doi: 10.1016/S0006-8993(01)02067-4. [DOI] [PubMed] [Google Scholar]
- 71.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev 5: 73–86, 2000. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 72.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Cambridge, MA: Academic, 2007. [Google Scholar]
- 73.Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci 28: 11731–11740, 2008. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pittman QJ, Franklin LG. Vasopressin antagonist in nucleus tractus solitarius/vagal area reduces pressor and tachycardia responses to paraventricular nucleus stimulation in rats. Neurosci Lett 56: 155–160, 1985. doi: 10.1016/0304-3940(85)90122-3. [DOI] [PubMed] [Google Scholar]
- 76.Raff H, Fagin KD. Measurement of hormones and blood gases during hypoxia in conscious cannulated rats. J Appl Physiol Respir Environ Exerc Physiol 56: 1426–1430, 1984. doi: 10.1152/jappl.1984.56.5.1426. [DOI] [PubMed] [Google Scholar]
- 77.Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol 289: R789–R797, 2005. doi: 10.1152/ajpregu.00222.2005. [DOI] [PubMed] [Google Scholar]
- 78.Reddy MK, Schultz HD, Zheng H, Patel KP. Altered nitric oxide mechanism within the paraventricular nucleus contributes to the augmented carotid body chemoreflex in heart failure. Am J Physiol Heart Circ Physiol 292: H149–H157, 2007. doi: 10.1152/ajpheart.00117.2006. [DOI] [PubMed] [Google Scholar]
- 79.Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol 399: 101–109, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 80.Romero-Alemán MM, Monzón-Mayor M, Santos E, Yanes C. Expression of neuronal markers, synaptic proteins, and glutamine synthetase in the control and regenerating lizard visual system. J Comp Neurol 518: 4067–4087, 2010. doi: 10.1002/cne.22444. [DOI] [PubMed] [Google Scholar]
- 81.Saphier D. Electrophysiology and neuropharmacology of noradrenergic projections to rat PVN magnocellular neurons. Am J Physiol Regul Integr Comp Physiol 264: R891–R902, 1993. doi: 10.1152/ajpregu.1993.264.5.R891. [DOI] [PubMed] [Google Scholar]
- 82.Sapru HN. Carotid chemoreflex. Neural pathways and transmitters. Adv Exp Med Biol 410: 357–364, 1996. doi: 10.1007/978-1-4615-5891-0_55. [DOI] [PubMed] [Google Scholar]
- 83.Sawchenko PE. Evidence for differential regulation of corticotropin-releasing factor and vasopressin immunoreactivities in parvocellular neurosecretory and autonomic-related projections of the paraventricular nucleus. Brain Res 437: 253–263, 1987. doi: 10.1016/0006-8993(87)91641-6. [DOI] [PubMed] [Google Scholar]
- 84.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 257: 275–325, 1982. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 85.Sawchenko PE, Swanson LW, Vale WW. Corticotropin-releasing factor: co-expression within distinct subsets of oxytocin-, vasopressin-, and neurotensin-immunoreactive neurons in the hypothalamus of the male rat. J Neurosci 4: 1118–1129, 1984. doi: 10.1523/JNEUROSCI.04-04-01118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schlenker EH. Integration in the PVN: another piece of the puzzle. Am J Physiol Regul Integr Comp Physiol 289: R653–R655, 2005. doi: 10.1152/ajpregu.00386.2005. [DOI] [PubMed] [Google Scholar]
- 87.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998. doi: 10.1016/S0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 88.Silberman Y, Winder DG. Corticotropin releasing factor and catecholamines enhance glutamatergic neurotransmission in the lateral subdivision of the central amygdala. Neuropharmacology 70: 316–323, 2013. doi: 10.1016/j.neuropharm.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith DW, Buller KM, Day TA. Role of ventrolateral medulla catecholamine cells in hypothalamic neuroendocrine cell responses to systemic hypoxia. J Neurosci 15: 7979–7988, 1995. doi: 10.1523/JNEUROSCI.15-12-07979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537: 161–177, 2001. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stern JE, Sonner PM, Son SJ, Silva FCP, Jackson K, Michelini LC. Exercise training normalizes an increased neuronal excitability of NTS-projecting neurons of the hypothalamic paraventricular nucleus in hypertensive rats. J Neurophysiol 107: 2912–2921, 2012. doi: 10.1152/jn.00884.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 287: R1172–R1183, 2004. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- 93.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 95.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410–417, 1980. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 96.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36: 165–186, 1983. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 97.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90: 675–754, 2010. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- 98.Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol 388: 169–190, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 99.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 100.Wang LA, Nguyen DH, Mifflin SW. CRHR2 (corticotropin-releasing hormone receptor 2) in the nucleus of the solitary tract contributes to intermittent hypoxia-induced hypertension. Hypertension 72: 994–1001, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu M, Yang L, Rong JG, Ni Y, Gu WW, Luo Y, Ishidoh K, Katunuma N, Li ZS, Zhang HL. Inhibition of cysteine cathepsin B and L activation in astrocytes contributes to neuroprotection against cerebral ischemia via blocking the tBid-mitochondrial apoptotic signaling pathway. Glia 62: 855–880, 2014. doi: 10.1002/glia.22645. [DOI] [PubMed] [Google Scholar]
- 102.Yamazaki T, Waki H, Kohsaka A, Nakamura T, Cui H, Yukawa K, Maeda M. Microinjection of urocortin into the rat nucleus tractus solitarii decreases arterial blood pressure. Auton Neurosci 142: 51–54, 2008. doi: 10.1016/j.autneu.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 103.Yeh ER, Erokwu B, LaManna JC, Haxhiu MA. The paraventricular nucleus of the hypothalamus influences respiratory timing and activity in the rat. Neurosci Lett 232: 63–66, 1997. doi: 10.1016/S0304-3940(97)00579-X. [DOI] [PubMed] [Google Scholar]
- 104.Yosten GL, Samson WK. Neural circuitry underlying the central hypertensive action of nesfatin-1: melanocortins, corticotropin-releasing hormone, and oxytocin. Am J Physiol Regul Integr Comp Physiol 306: R722–R727, 2014. doi: 10.1152/ajpregu.00396.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol 281: H995–H1004, 2001. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- 106.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol 275: R728–R734, 1998. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]