Abstract
Maternal hypertension during pregnancy is a major risk factor for intrauterine growth restriction (IUGR), which increases susceptibility to cardiovascular and metabolic disease in adulthood through unclear mechanisms. The aim of this study was to characterize the pancreatic β-cell area and function in the fetal rat offspring of a reduced uterine perfusion pressure (RUPP) model of gestational hypertension. At embryonic day 19.5, RUPP dams exhibited lower body weight, elevated mean blood pressure, reduced litter size, and higher blood glucose compared with sham-operated controls. In RUPP placental lysates, a nonsignificant change in mammalian target of rapamycin (mTOR) activity markers, phosphorylated S6 at serine 240, and phosphorylated AKT (at S473) was observed. RUPP offspring showed significantly reduced β-cell-to-pancreas area and increased β-cell death but normal insulin levels in serum. Isolated islets had normal insulin content and secretory function in response to glucose and palmitate. Fetal pancreatic lysates showed a tendency for reduced insulin levels, with a significant reduction in total mTOR protein with RUPP surgery. In addition, its downstream complex 2 targets phosphorylation of AKT at S473, and pAKT at Thr308 tended to be reduced in the fetal RUPP pancreas. Altogether, these data show that RUPP offspring demonstrated increased β-cell death, reduced β-cell area, and altered nutrient-sensor mTOR protein level in the pancreas. This could represent a mechanistic foundation in IUGR offspring’s risk for enhanced susceptibility to type 2 diabetes and other metabolic vulnerabilities seen in adulthood.
Keywords: β-cell, intrauterine growth restriction, islets, mTOR, placenta
INTRODUCTION
The fetal nutrient environment during pregnancy is a major factor that modifies the risk for developing metabolic disease, such as type 2 diabetes (T2D), in adulthood (40). A strong body of epidemiological studies shows robust associations between poor fetal environment and infant growth and the subsequent development of T2D, hypertension, and cardiovascular disease (15, 41). From a public health standpoint, this is a major problem because many pregnancy complications lead to intrauterine growth restriction (IUGR), which effects over 10% of all pregnancies in the United States (4) (39), 4%–8% in 25 European Union countries (45), and up to 5%–75% of pregnancies in developing countries (9). Maternal malnutrition and hypertension during pregnancy are risk factors for IUGR.
Maternal diet manipulation, such as low protein intake during pregnancy, can induce IUGR in the offspring and increase adulthood susceptibility for T2D (40) through long-lasting changes in pancreatic β-cell mass plasticity (2, 5a, 10, 43). Hypertension during pregnancy is another known cause of fetal IUGR (16), but it is not clear whether this form includes similar effects on pancreatic β-cell. Human IUGR infants show reduced circulating insulin levels, impaired β-cell function, and lower β-cell area (5, 22, 46, 47). Since the differentiated population of insulin-producing cells in adulthood is maintained by a very low rate of self-replication in existing β-cells (6), it is generally thought that adult β-cell mass is set by the pool size achieved during the first decade (~4 wk in rats) of postnatal life (11, 14, 31). Therefore, a reduction in postnatal β-cell mass development, resulting from adverse intrauterine exposure, could alter adult metabolism and increase the risk for T2D.
In the present study, we employed the previously characterized and established model of reduced uterine perfusion pressure (RUPP) in rats to closely mimic human hypertension during pregnancy (13) and reduce uterine perfusion pressure by more than 40% (3). This model induces chronic placental ischemia by surgical placement of aortic and uterine artery clips to reduce uteroplacental blood flow starting on embryonic day (e) 14.5, equivalent to the third trimester of human pregnancy (42). The RUPP model has been shown to induce hypertension in the rat dams and IUGR in the offspring (16). In addition to hypertension, chronic reductions of uterine perfusion pressure lead to endothelial dysfunction and limited nutrient availability in the pregnant rat (13). Moreover, metabolic abnormalities, including glucose intolerance, insulin resistance, hyperglycemia, hyperinsulinemia, and hyperleptinemia, had been reported in adult RUPP offspring by 8 wk of age (16, 26). Presently, however, there are very few studies defining the causes of the increased risk for T2D after reduced uteroplacental blood flow-induced IUGR. Specifically, the effects of chronic reduction of uteroplacental blood flow and, possibly, limited nutrient availability on β-cell area and function in the fetal offspring are unknown. We hypothesized that chronic reduction of uteroplacental blood flow and increased pressure during pregnancy in the RUPP rat model would lead to deficits in β-cell number and function in the offspring early in life. A greater understanding of the mechanisms linking maternal hypertension, intrauterine environment, and β-cell area of IUGR offspring could provide potential avenues to reduce the risk of T2D later in life.
MATERIALS AND METHODS
Experimental animals.
The RUPP model was used to induce high blood pressure in pregnancy as previously described (13). Timed-pregnant Sprague-Dawley rats were purchased with breeding weights of 215–225 g (CD IGS strain of rats, Charles River Laboratories, Raleigh, NC). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Minnesota. After 14 days (e14.5) of gestation, initially marked by the appearance of a vaginal plug, each pregnant rat was randomly assigned to a sham or RUPP surgery, as previously described (37). Briefly, a laparotomy was performed through an abdominal incision, and a silver clip (0.203 mm internal diameter) was placed around the aorta above the iliac bifurcation on e14.5. Silver clips (0.1 mm internal diameter) were placed around the left and right uterine arcade at the ovarian artery before the first segmental artery. Control (Ctrl) rats underwent a sham procedure, and clips were not placed on the aorta or the ovarian arteries. This procedure has been shown to reduce uterine perfusion pressure by ~40% (26). Dams were housed in a 12-h light/dark cycle. Fetuses were harvested on e19.5, and sex was determined by post hoc genotyping of tail tissue. The number of animals used is included in the figure legends, and all pups within a litter were included in parameters involving fetal body weight, placental weight, and insulin levels. For β-cell area and Western blotting experiments, samples were randomly tested from at least three different litters. For the insulin secretion study in fetal islets, islets were pooled from all pups from a single litter (RUPP or sham) and considered an n of 1. For Fig. 4, we used two different litters in the control group and four litters in the RUPP group.
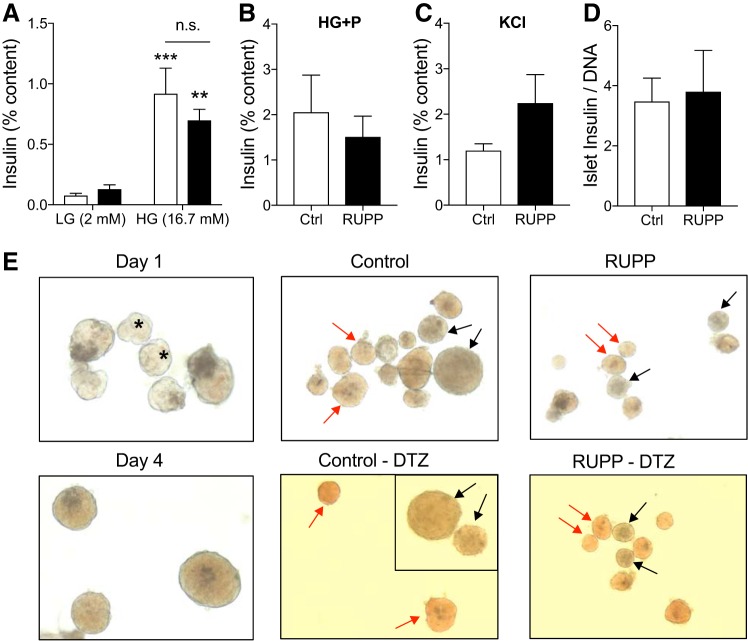
Fig. 4.
Reduced uterine perfusion pressure (RUPP) status does not impair fetal islet insulin secretion or content. Insulin secretion as a percentage of total insulin content during a 30-min incubation in low glucose (LG) (2 mM) and stimulatory high glucose (HG) (16.7 mM) (A), high glucose + palmitate (P) (100 μM) (B), or 15 min in potassium chloride (KCl) (30 mM) (C). Insulin content was normalized to DNA from batches of 10 islets (D). Islets were isolated from sham-operated [control (Ctrl), open bars] or RUPP (closed bars) fetal embryonic day 19.5 pancreata (E). The identity of presumed fetal islets was confirmed by culture and dithizone (DTZ) staining. Light microscope image (×10) of fetal islet-like clusters after 1–4 days of culture. Asterisks indicate clusters typical of those selected for islet secretion testing. A mixture of presumed islet clusters (red arrows) and nonislet clusters (black arrows) before and after 5 min of dithizone in fetal islets from sham-operated and RUPP mothers after 2 days of in vitro culture. n = 5 pseudoreplicates per group from 2 independent experiments with pools of 10–12 pancreata per run (from n = 3 litters of RUPP or control dams). Asterisks indicate significance without litter adjustments due to pooling of samples. LG vs. HG. n.s., not significant, refers to Ctrl vs. RUPP. **P ≤ 0.01, ***P ≤ 0.001.
Mean arterial pressure and tissue collection.
On e18.5, a carotid catheter was surgically placed and tunneled to the back of the neck for monitoring blood pressure on e19.5 via a transducer in restrained, unanesthetized dams (26). After blood pressure was monitored, dams were anesthetized with isoflurane, and a midline incision was used to collect blood from the abdominal aorta. Placentas and fetuses were removed and weighed following decapitation and trunk blood sample collection. Tail tissue was frozen for later genotyping. Placentas were flash-frozen. Fetal pancreata were dissected and either snap-frozen or fixed in 10% neutral buffered formalin for 24 h, then transferred to 70% ethanol.
Assessment of blood glucose and insulin.
Maternal blood glucose was assessed using a handheld glucometer (Bayer CONTOUR) during blood collection from the abdominal aorta. For insulin detection, EDTA plasma was collected and frozen. Insulin concentration was determined using the ALPCO Mouse Ultrasensitive Insulin ELISA, following all kit instructions. This ELISA has a cross-reactivity with rat insulin of 120% and 76% (rat insulin 1 and 2, respectively). Some samples were also run on an ALPCO Rat Ultrasensitive Insulin ELISA to confirm parity.
Sex determination by genotyping.
Tail tissue was digested in alkaline lysis buffer (25 mM NaOH, 0.2 mM EDTA, pH 12) and incubated at 95°C for 30 min. The digest was then neutralized (40 mM Tris·HCl, pH 5.2) and amplified by PCR for the sex-determining region Y gene (Forward: TTG TCT AGA GAG CAT GGA GGG CCA TGT CAA, Reverse: CCA CTC CTC TGT GAC ACT TTA GCC CTC CGA). The product was then visualized (Bio-Rad Chemidoc MP) on a 2% agarose gel using ethidium bromide.
Protein isolation and Western blot.
Placenta or pancreatic lysates were prepared by freezing with liquid nitrogen, processing with mortar and pestle, and then resuspending in 1× radioimmunoprecipitation assay lysis (cat. no. 5871, Cell Signaling) buffer with protease inhibitor cocktail (Cell Signaling). Samples were sonicated (Fisher Scientific Sonic Dismembrator model 500; 10 × 3 s, 30% amplitude) and centrifuged to remove the supernatant for total protein quantification via the Pierce BCA Protein Assay Kit (Thermo Scientific), according to the manufacturer’s instructions. For the Western blot procedure, placental and pancreatic proteins were resolved on an 8% or 4%–20% gel, respectively. After transfer to a polyvinylidene fluoride membrane, it was blocked with Tris-buffered saline blocking solution (LI-COR Biosciences) or 5% nonfat dried milk (RPI) in PBS for 30 min and incubated overnight at 4°C with primary antibodies: mammalian target of rapamycin (mTOR) (Cell Signaling), O-GlcNAc transferase (OGT) (Cell Signaling), phospho-S6 (Ser240; Cell Signaling), S6 (Cell Signaling), phospho-Akt (Ser473; Cell Signaling), RL2 (Abcam), β-actin (Cell Signaling), and Vinculin (Cell Signaling). After washing was completed, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG H&L secondary antibodies (GE Healthcare) or 800CW or 680RD infrared fluorescent IgG secondary antibodies (IRDye, LI-COR Biosciences) for 1 h at room temperature. After additional washing, chemiluminescence reactions were carried out using the SuperSignal West Pico PLUS Kit (Thermo Scientific), or membranes were imaged on an LI-COR Odyssey 9120. Densitometry analysis of bands was done with ImageJ or Odyssey software v.3.0.
Immunostaining of pancreatic sections for insulin, TUNEL, S6 S240, or S235.
Formalin-fixed pancreatic tissues were embedded in paraffin for sectioning as previously described (2). After deparaffinization, 5-μm sections underwent antigen retrieval with 0.01 M sodium citrate/citric acid by microwaving for 6 min at 95°C. Sections were permeabilized with 0.01% Triton, then blocked with 5% goat serum before overnight incubation at 4°C in primary antibodies against guinea pig insulin (1:400 dilution, IS002, DAKO) and phosphorylated S6 S240 or S235 at 1:1,000 and 1:500, respectively (Cell Signaling). The following day, sections were washed with PBS-0.01% Tween and incubated in secondary antibodies conjugated to FITC or Cy3 at 1:400 (Jackson Immunoresearch) for 1 h 30 min at 37°C. Additional staining was done with DAPI (cat. no. 62248, Thermo Scientific) based on manufacturer’s instruction. The sections were coverslipped with Fluro-Gel mounting media (Electron Microscopy Sciences). A TUNEL kit from Millipore (ApopTag Red In Situ Apoptosis Detection Kit) was used per manufacturer’s instruction. TUNEL staining was analyzed in insulin-positive cells only. Cell size was assessed by β-cell area/DAPI number using Fiji Software, and ~1,000 embryonic β-cells were counted from each group (n = 3 genotypes). For imaging, a motorized microscope was used (Nikon ECLIPSE NI-E or Zeiss Axio Observer Z.1).
Assessment of β-cell-to-pancreatic area ratio.
Fetal pancreata were sectioned top to the bottom (~400 μm, 5 μm apart). Maternal pancreata were sectioned at 5-μm thickness and separated 200 μm apart every 50 μm of collection. The analysis then entailed assessing total pancreas and insulin-positive cell areas from five insulin-stained sections separated ~100 μm (fetal) or 200 μm (maternal) apart and measured by using Fiji software (National Institutes of Health). Thus, five pancreas sections were analyzed per animal. Automated scanning was done with a Nikon fluorescent microscope (Nikon ECLIPSE NI-E) or Zeiss Axio Observer Z.1. β-Cell mass (average β-cell fraction multiplied by pancreas weight) was not assessed because fetal pancreas weight was not measured. The average ratio of five sections was reported per animal in both male and female embryos.
Embryonic islet isolation.
Approximately 10 pooled fetal pancreata were digested with 5 ml of collagenase (1 mg/ml, Roche) by incubating for 10 min at 37°C. Collagenase was deactivated by washing (3×) with cold Hanks’ balanced salt solution with 2% FBS, involving gentle shaking and centrifugation (30 s, 1,000 rev/min) between each round. The reconstituted pellet was filtered through a 70-μm filter and washed off into warm RPMI media (5 mM glucose, 10% vol/vol FBS, 100 IU/ml penicillin, 100 g/ml streptomycin) for handpicking. Islets were allowed to recover overnight before further testing. To ensure that the cell clusters picked from the fetal pancreatic digest were islets, some were cultured for up to 4 days in RPMI, and others were dithizone-stained (0.13 mM in PBS diluted from 10× stock in DMSO) for 5 min at room temperature (23) after 2 days in culture and imaged in situ on a Leica DMi1 inverted microscope with an attached high definition digital camera.
Primary e19.5 islet insulin secretion and content analysis.
After an overnight rest, 30 intact fetal islets (from a pool of 10–12 pancreata per experiment from 3 sham and 4 RUPP dams) were handpicked into Krebs buffer (29) containing 2 mM glucose [low glucose (LG)] for a 2-h preincubation. Islets (n = 10 per well; even size distribution from small to large) were transferred into a cell culture insert with an 8-μm pore screen in a 24-well plate. Islet inserts were successively incubated for 30-min periods in Krebs with LG, high glucose (HG) (16.7 mM), LG, HG + palmitate (HG+P) (100 μM preconjugated 6:1 with 20% BSA), then 15-min periods of LG and potassium chloride (KCl) (30 mM). Incubate solutions were collected for the first LG, HG, HG+P, and KCl. Islets were washed off the insert screens into radioimmunoprecipitation assay buffer containing 100 μM protease inhibitor and sonicated (2 × 15 s, 20% amplitude, Fisher Scientific Sonic Dismembrator model 500;) before testing. Solutions were assayed for insulin content by ELISA kit as described. Islet secretion data were calculated as a percentage of insulin in each incubate solution relative to the insulin in the islets at the end of the experiment. Insulin content was calculated as the sum of insulin released and the final islet insulin, normalized to the DNA content of the islets as assessed by the PicoGreen dsDNA quantitation assay kit (Thermo Fisher).
RNA isolation and quantitative PCR.
After an overnight incubation, fetal islets intended for RNA isolation were stored at −80°C in 20 μl RNAlater solution (Invitrogen) and subsequently isolated using the RNeasy Plus Micro Kit (Qiagen), according to the manufacturer’s protocol. Isolated (eukaryotic) RNA was quantitated using the Agilent RNA 6000 Nano Kit with an Agilent 2100 Bioanalyzer. Total RNA (250 ng) was taken for cDNA synthesis using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Amplification was carried out using Power SYBR Green PCR Master Mix in QuantStudio Flex Real-Time PCR System for 40 cycles (Applied Biosystems). Target gene expression was normalized to the reference gene β-actin and RPLP0. RPLP0 was recently identified as one of the most stable internal reference genes (21, 48).
Statistical analysis.
Data are expressed as means ± SE, and differences were considered significant at P < 0.05. When RUPP and sham treatments for assessing pancreas weight and insulin from the dams were compared, a two-tailed t-test was used. In the case of the other variables from the mother, variances were not equal, so a Satterthwaite’s t-test was employed. When RUPP and sham treatments for e19.5 embryos were compared, a mixed-model ANOVA with random litter effects was used to assess significance since measurements often include more than one pup from the same mother. Means and standard errors in figures and text are reported without litter adjustments.
RESULTS
Phenotype of the RUPP dams.
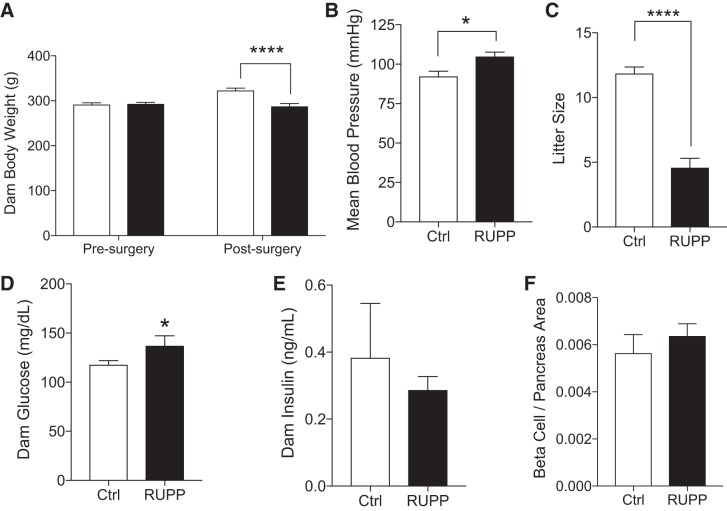
As previously reported, 5-days postsurgery RUPP dams showed significantly lower body weight and higher blood pressure than sham-operated controls (Fig. 1, A and B). Litter size was reduced (Fig. 1C) because of an increase in the percentage of fetuses resorbed (71.66 ± 2.247% vs. 2.774 ± 1.172%, P < 0.0001 RUPP vs. Ctrl, n = 11–12) and a lower survival rate (24.08 ± 4.972% vs. 97.44 ± 1.511%, P < 0.0001 RUPP vs. Ctrl, n = 19–20). No difference in offspring survival was noted between left and right uterine horns; however, the incidence of resorption did vary according to uterine position (data not shown). Maternal blood glucose was significantly elevated (P < 0.05) with RUPP status (Fig. 1D), and circulating insulin was unchanged (Fig. 1E). No differences were observed in the β-cell and pancreas area ratio (Fig. 1F).
Fig. 1.
Reduced uterine perfusion pressure (RUPP) dams show symptoms of maternal hypertension. Body weight pre- and postsurgery in female rats subjected to a sham surgery (Ctrl, open bars) or a restriction of uteroplacental blood flow (closed bars) in the third trimester of pregnancy (n = 21, 27 rats) (A). Postsurgery data is also shown for dam mean blood pressure (n = 11, 7 rats) (B), litter size at birth (n = 21, 31 rats) (C), abdominal aorta blood glucose (n = 7, 17 rats) (D), serum insulin (n = 3 rats) (E), and β-cell-to-pancreas area ratio (n = 4, 5 rats) (F). Asterisks indicate significance RUPP vs. Ctrl by using a mixed-model ANOVA with random litter effects. *P ≤ 0.05, ****P ≤ 0.0001.
mTOR complex 1 activity in RUPP placenta.
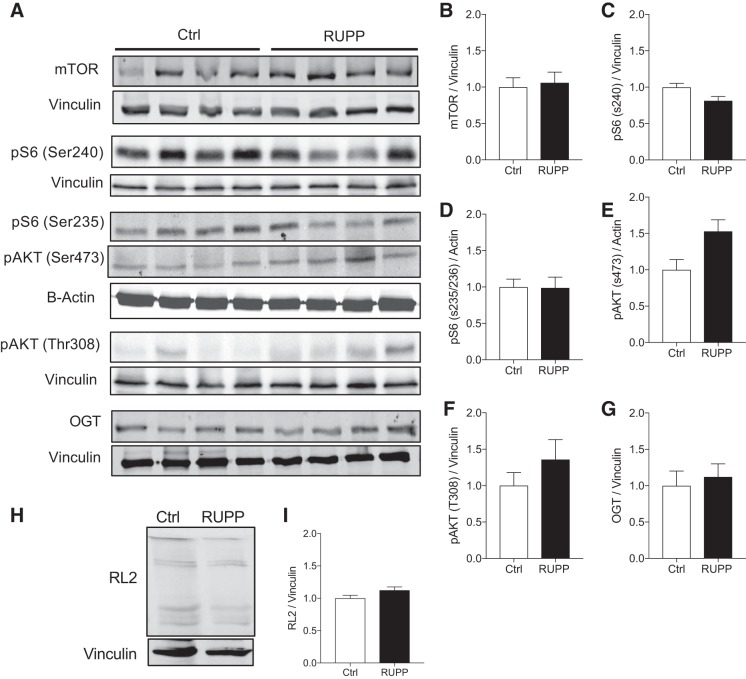
mTOR protein levels are altered in the placenta of IUGR babies (37), although we did not observe this in e19.5 placenta from the RUPP model using Western blot data (Fig. 2, A and B). However, activity downstream of mTOR complex (mTORC)1 tended to be reduced in RUPP placenta at Ser240 phosphorylation of S6 (Fig. 2, A and C) but not at Ser235 S6 (Fig. 2, A and D). Interestingly, only the specific downstream target of mTORC2, phosphorylation of AKT (pAKT) at Ser473, tended to be increased (Fig. 2, A and E) in RUPP versus sham placenta. However, both changes in mTORC1 or mTORC2 activities were not statistically significant after adjusting for litter effect. Insulin-sensitive pAKT (Thr308) protein level was not altered (Fig. 2, A and F) nor was nutrient sensor OGT protein and its activity by assessing global protein O-GlcNAcylation by immunoblotting using RL2 antibody (Fig. 2, A and G–I).
Fig. 2.
Placental mammalian target of rapamycin (mTOR) signaling in reduced uterine perfusion pressure (RUPP) dams. Representative Western blot images and quantification of placental protein expression from sham-operated (Ctrl, open bars) and RUPP-induced (closed bars) rats (A) (n = 8 rats per group and from at least n = 3 litters). Bar graph data show fold change relative to control for total mTOR protein (B) and downstream activity markers of mTOR complex (mTORC)1 and mTORC2 (C, D, and E) and other nutrient-responsive activity (F–I), including pS6 S240 (C), pS6 S235/236 (D), pAKT (S473) (E), pAKT T308 (F), O-GlcNAc transferase (OGT) (G), and total protein O-GlcNAcylation (RL-2) (H and I). Ctrl, control (sham-operated).
β-Cell loss but normal secretory function in RUPP offspring.
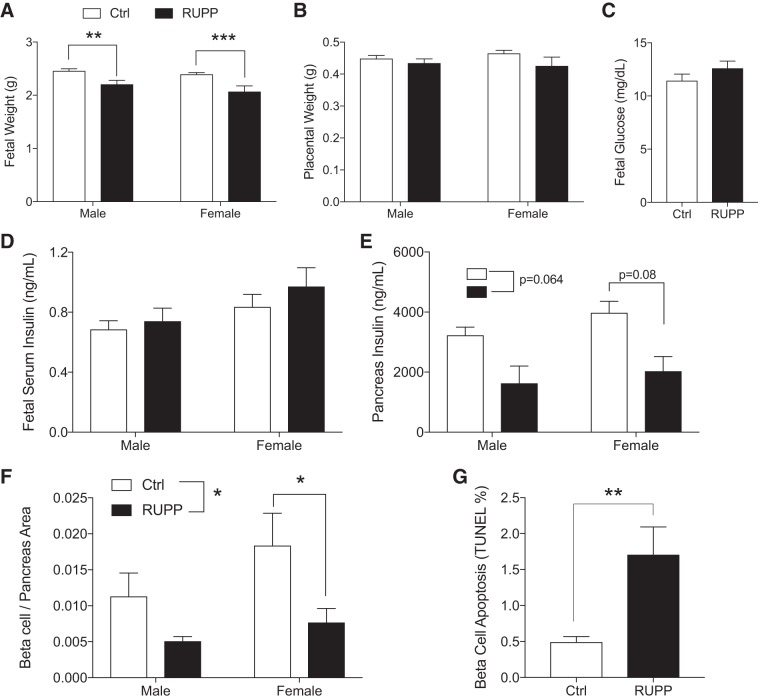
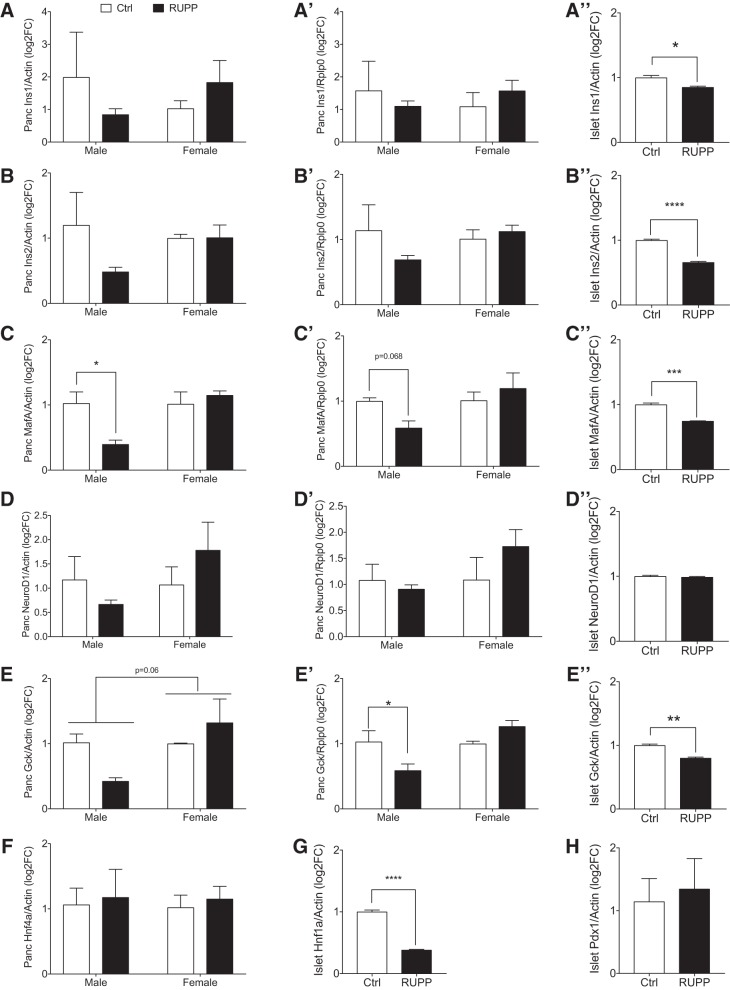
Consistent with IUGR, fetal body weight was lower in both male and female e19.5 RUPP fetuses (Fig. 3A) with no significant changes in placental weight (Fig. 3B). Blood glucose and insulin levels in RUPP fetuses were normal (Fig. 3, C and D), with no significant impact of fetal sex and when adjusted for litter effects. Assessment of total pancreas insulin content showed a reduction in insulin levels both in female and male RUPP compared with Ctrl (Fig. 3E). When litter effects are taken into consideration, however, the overall RUPP effect on total pancreas insulin content resulted in P = 0.064. When both sex and litter effect were included, the RUPP effect in females was P = 0.087 and P = 0.170 for males. These data suggested that changes in β-cell area might be important, and thus we assessed β-cell ratio in five pancreas sections per animal. Indeed, the ratio of β-cell area to pancreas area was significantly reduced in RUPP when including litter effects (Fig. 3F). Only females demonstrated a significant reduction in β-cell area (female P = 0.027, male P = 0.195). The insignificant sex by surgery interaction (P = 0.54) indicated that the β-cell effect is not sex-specific. Comparable average β-cell size was observed (data not shown); however, increased β-cell death by apoptosis was demonstrated in RUPP compared with control pancreata (Fig. 3G). Primary isolated islets pooled from the e19.5 fetal pancreata showed no difference in insulin secretory function in response to glucose and/or fat (palmitate) (Fig. 4, A and B). There was a trend toward increased KCl (maximal) release in RUPP islets (P = 0.14), but total insulin content was unchanged (Fig. 4, C and D). We confirmed that the embryonic cell clusters selected for experiments were islets by observing their maturation in size and golden color after 4 days of culture (Fig. 4E) and through dithizone staining of insulin granules (Fig. 4E). In addition, we assessed changes in transcripts for specific β-cell defining genes (Ins1, Ins2, Pdx1, NeuroD1, MafA, and Gck) in RNA extracted from female and male RUPP pancreas (Fig. 5, A–F and A′–E′) and isolated islets (pooled from multiple embryos, A″–E″, G, and H) using two internal references (β-actin and Rplp0). We observed a consistent reduction in expression of MafA and GcK (Fig. 5, C, E, E′, and E′′). For MafA in pancreatic RNA (Fig. 5, C and C′), this reduction was significant for males when normalized to actin and nearly significant when normalized to Rplp0 (P = 0.06). For Gck normalized to actin (Fig. 5E), no significant reduction was noted when adjusted for litters. Gck normalized to Rplp0 showed a significant reduction in RUPP males and a significant sex effect.
Fig. 3.
Intrauterine growth restriction reduced uterine perfusion pressure (RUPP) offspring show decreased β-cell area. Data show embryonic day 19.5 fetal body weight (nmales = 40, 11 and nfemales = 41,9) (A) and placental weight (nmales = 35,21 and nfemales = 39,12 from at least n = 3 litters) (B) separated by sex. Nonsignificant changes in fetal blood glucose (n = 34,28 from at least n = 3 litters) (C) and circulating insulin (nmales = 44,23 and nfemales = 42,14 from n = 3 litters) (D) taken from trunk blood samples. Whole pancreatic lysates were assayed to determine pancreatic insulin separated by sex (nmales = 4,5 and nfemales = 4,5 from n = 3 litters) (E). The RUPP effect resulted in P = 0.064 and P = 0.08 in the female group. Reduced β-cell-to-pancreas area ratios in RUPP (nmales = 6,4 and nfemales = 5,6 from at least n = 3 litters) (F) and β-cell apoptosis (n = 6,3) (TUNEL; G) were assessed by staining of insulin and DAPI (insulin islet area/DAPI number) and colocalization of insulin and TUNEL-positive cells. Ctrl, control (sham-operated). Asterisks indicate significance RUPP vs. Ctrl by using a mixed-model ANOVA with random litter effects. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Fig. 5.
Gene expression in fetal pancreas and islet. Genes were assessed in total pancreas from male and female reduced uterine perfusion pressure (RUPP) and dams using either β-actin (A–F, n = 3 per sex), Rplp0 (A′–E′, n = 3 per sex) as internal housekeeping gene, or in isolated islets [A′′–E′′, G, H, where n = 3 pseudoreplicates from a pool of 10–12 fetal pancreata (n = 3 litters of Sham or RUPP)]. Ctrl, control (sham-operated). Asterisks indicate significance RUPP vs. Ctrl by using a mixed model ANOVA with random litter effects. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
mTOR protein and activity in pancreas of RUPP offspring.
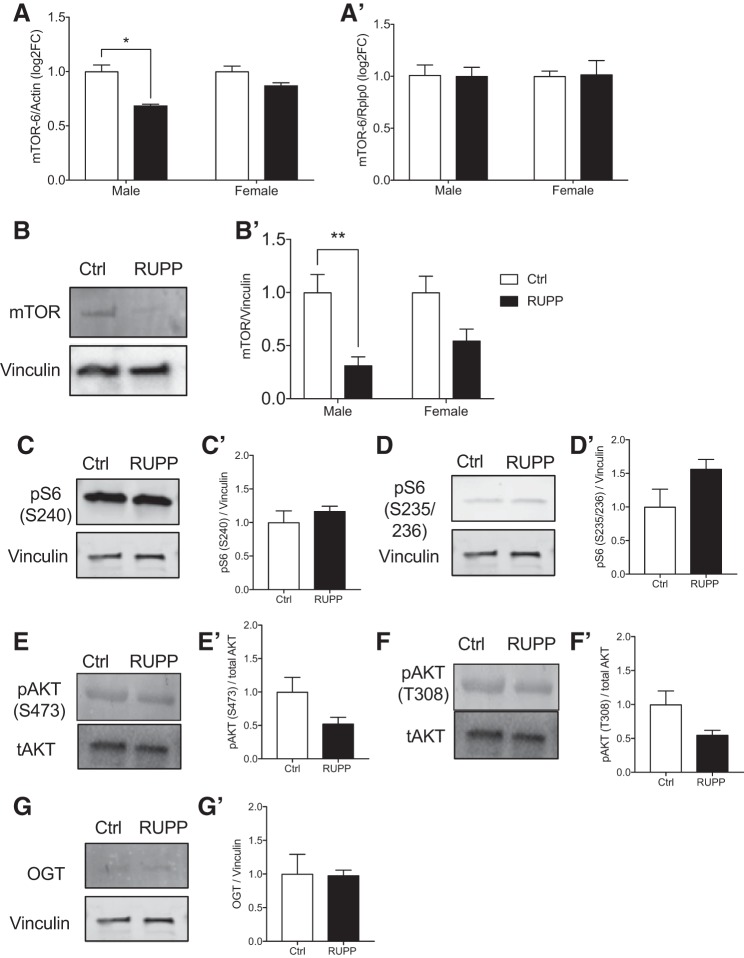
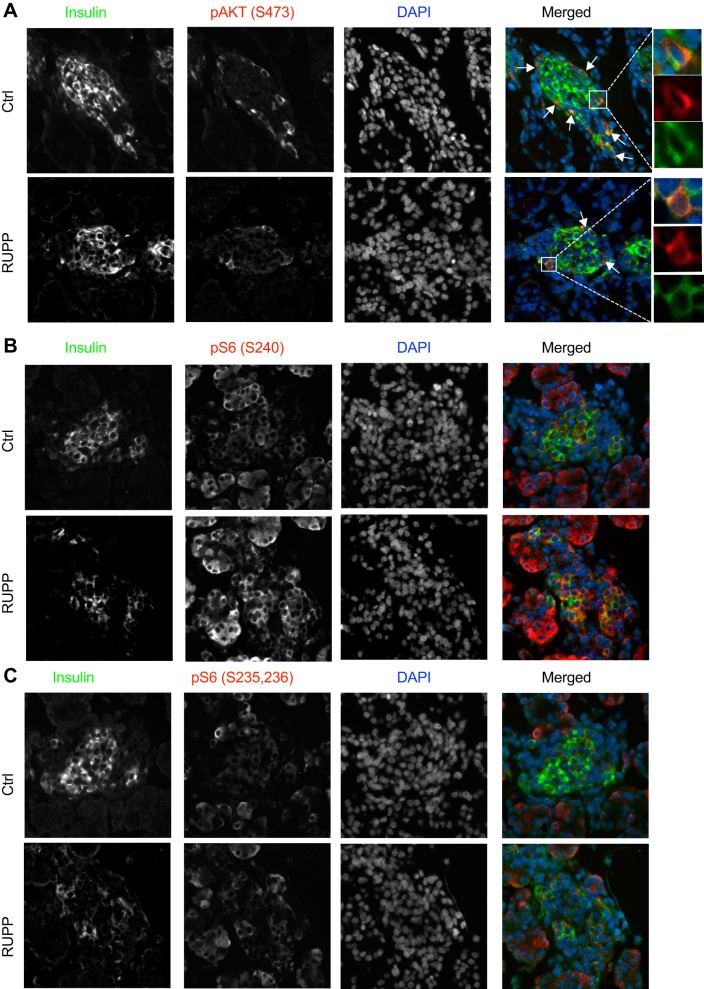
mTOR transcript when normalized with β-actin (Fig. 6A) is affected by both RUPP surgery (P = 0.0045) and sex (P = 0.024). Based on a significant interaction (P = 0.012), the effect in males (P = 0.0005) is more pronounced than in females (P = 0.077). However, this change was not maintained when Rplp0 was used to normalize (Fig. 6A′). Therefore, to validate these changes in transcripts, we assessed RUPP fetal pancreata lysates for mTOR protein and downstream targets. By Western blotting, we showed a 50% loss of total mTOR protein in RUPP compared with controls (P = 0.041, Fig. 6B). There was a significant RUPP effect in males (P = 0.023) but not in females (P = 0.36). Next, we assessed downstream targets of mTOR: pS6 (Ser 240) for mTORC1 and pAKT (Ser473) for mTORC2. No changes were observed in pS6 (Ser240) or pS6 (Ser235) (Fig. 6, C and D). Without litter effect as a dependent variable, we detected a reduction of pAKT (Ser473) as well as T308 (Fig. 6, E, E′, F, and F′), suggesting altered insulin signaling and mTORC2. The nutrient sensor OGT that could impact insulin signaling was not changed (Fig. 6, G and G′). The reduction in the mTORC2 downstream target pAKT (Ser473) was supported by nonquantitative immunofluorescence images showing reduced pAKT (Ser473) staining in insulin-positive regions of the RUPP pancreas (Fig. 7A, note the fewer number of arrows in RUPP images). Consistent with the data on RUPP pancreatic lysates, the immunofluorescence imaging showed comparable staining levels of S6 (Ser240 and Ser235) in insulin-positive cells (Fig. 7, B and C). Together, these data suggest that insulin and mTORC2 signaling may be altered in RUPP samples compared with controls.
Fig. 6.
Reduced uterine perfusion pressure (RUPP)-induced intrauterine growth restriction alters pancreatic mammalian target of rapamycin (mTOR) signaling in the offspring. mTOR expression in male and female pancreas using either β-actin (A; n = 3 per sex from 3 different litters) or Rplp0 (A′; n = 3 per sex from 3 different litters) as internal housekeeping gene. Representative images and quantification of pancreatic lysate Western blots showing mTOR protein expression in sham-operated [control (Ctrl), open bars, n = 11] and RUPP-induced (closed bars, n = 14) fetal offspring for total mTOR (B) and when separated by sex (nmales = 4,7 and nfemales = 7,7) (B′). Downstream target of mTOR pS6 S240 (C), pS6 S235/236 (D), pAKT S473 (F), pAKT T308 (G), and O-GlcNAc transferase (OGT) (H), and their quantifications (C′–G′, n = 4,7). ^Significant RUPP vs. Ctrl using two-tailed t-test not adjusted for litter effects. Asterisks indicate significant RUPP vs. Ctrl by using a mixed-model ANOVA with random litter effects.
Fig. 7.
Immunofluorescence images showing mammalian target of rapamycin (mTOR) activity markers in fetal pancreas. Representative images from reduced uterine perfusion pressure (RUPP) and control (Ctrl) embryonic day 19.5 pancreas sections show staining of insulin, nuclear DAPI, and downstream targets of mTOR complex (mTORC)2 activation pAKT S473 (A) and mTORC1 activity pS6 240 (B) and mTORC1-independent and pS6 235/236 (C). Images were taken at ×20 (A–C) magnification. Merged images are shown in pseudo color with green for insulin, blue for DAPI, and red for the specific target.
DISCUSSION
We and others have shown that maternal stress, such as low-protein diet, during pregnancy causes IUGR, a critical factor known to predispose offspring to T2D (40) by causing long-term consequences in total β-ell area and function in the offspring (2, 5a, 10, 43). However, the β-cell area in the IUGR offspring of the RUPP model of gestational hypertension in the rat was unknown. Therefore, the primary goal of the present study was to determine the effect of reduced uteroplacental blood flow and blood pressure from e14.5 to e19.5 on β-cell area and function in the embryos. Here, we reveal that chronic reduction of uteroplacental blood flow and perfusion pressure increased maternal glucose level on day 19.5 of pregnancy and significantly reduced litter size. When litter effects are taken into account, the reduction of placental mTORC1 signaling was not significant, and no changes in the nutrient-sensor OGT protein levels or its activity were observed. Although a reduction in litter size was observed in the RUPP group, a significant reduction in fetal body weight was still evident in the offspring of RUPP compared with sham. A normal circulating level of insulin was observed despite reduced β-cell area and increased β-cell apoptosis in the RUPP offspring, supporting a growing body of evidence that β-cells are exquisitely sensitive to the fetal nutrient environment. These in utero events could significantly impact final adult β-cell mass (44) and risk for T2D later in life (1, 44).
The risk for gestational hypertension during the second half of pregnancy is increased two- to fourfold among women with type 1 or T2D (49). Women without diabetes who have had gestational hypertension are more likely to develop T2D later in life (49). In the RUPP dams, we observed a significant increase in nonfasted blood glucose on gestation day 19.5. These data support previous findings by Gilbert et al. (13), showing increased fasted glucose levels in blood, suggesting that offspring of RUPP dams may experience elevated glucose levels in utero. However, an assessment of glucose levels in e19.5 embryos showed comparable levels between RUPP and sham offspring. Furthermore, we observed comparable β-cell islet area/pancreas area between RUPP and sham dams, suggesting that elevated blood pressure during pregnancy does not cause reduced β-cell islet area. Our data support previous findings by Gilbert et al. (13), demonstrating comparable levels of nonfasted insulin values and glucose tolerance between RUPP and control dams. It would be of interest in the future to assess metabolic syndrome and β-cell function postpregnancy in aged RUPP dams.
Fetal nutrition is primarily determined by the maternal nutrient supply, which is directly dependent on the functionality of the fetal-placental transport interface. mTOR kinase is known to couple signals from nutrient and growth factors, implicating the importance of nutrient transport regulation from the placenta to the growing fetus and its role in pancreas and β-cell development (8, 35). Reduced mTOR signaling is correlated to decreased fetal-placental function in human and rodent models of IUGR (36, 37). Specifically, mTORC1 activity has been shown to play a critical role in the development and function of placental trophoblasts (20) and to regulate amino acid levels (20). In the current study, we identified that the placenta of RUPP fetuses had nonsignificant reductions in mTORC1 activity when taking litter effects into account. Although this is only an associative finding, our data is distinct from other IUGR induction models (low-protein diet, hypoxia, second-hand smoke, and obesity) in human samples that show decreased placental mTOR activity (24, 25, 32). We initially speculated that downregulation of mTOR could lead to compensation by other nutrient sensor proteins, such as the glucose-sensitive OGT. However, OGT level or its activity (i.e., total global protein O-GlcNAcylation), known as placental biomarkers of maternal stress (19), was not altered in the placental RUPP samples compared with control. To directly assess the impact of mTOR, future studies could be directed to the genetic deletion of mTOR kinase, mTORC1/2, specifically in the placenta, to test whether its loss is sufficient to alter metabolic phenotypes in the offspring (e.g., glucose intolerance and loss of β-cell mass).
We speculate that loss of pancreatic β-cell number could partly explain the glucose intolerance of adult RUPP offspring (16). The growing β-cell is sensitive to nutrient levels in utero (8, 17), particularly the availability of amino acids, known to be regulated by mTORC1 activity in the placenta (38). Our finding that β-cell area in the e19.5 RUPP fetus is significantly decreased compared with controls is in accordance with other IUGR models, such as maternal low-protein diet throughout pregnancy (LP0.5) in rodents (1, 7) and in sheep (27, 28). In particular, LP0.5 offspring in mice also show reduced β-cell area at birth, which subsequently normalizes in adulthood (1). Furthermore, a different artery ligation-IUGR model, induced at e19.5 of a 21.5-day gestation period, produced offspring with normal β-cell mass at birth but a significant reduction by 10 and 15 wk of age (41). Thus, we hypothesize that a longer duration of uteroplacental blood flow reduction (e14.5–19.5) resulted in loss of β-cell area at e19.5. The induction period of uteroplacental blood flow reduction may affect pancreas development. At e14.5, the developing pancreas is sensitive to nutrient levels (8), as progenitors differentiate to β-cell and growth expansion of insulin-expressing cells occurs (44). Thus, the induction of uteroplacental blood flow and pressure reduction leading to maternal hypertension and, possibly, limited nutrient supply to the fetus could affect β-cell area by potentially impacting the secondary transition or the specification and differentiation period of endocrine cells between e12.5 and e15.5 (30). Like our RUPP model (16), the offspring of RUPP dams from e19.5 to e21.5 are glucose intolerant (41). It is important to point out that glucose intolerance and insulin resistance in this specific model of acute uteroplacental blood flow reduction were associated with a loss of β-cell mass in adulthood (41). Because of the longer duration of uteroplacental blood flow reduction (e14.5 to e21.5), it is likely that adult RUPP offspring would show reduced β-cell mass, but this remains to be studied. The results of these studies and ours continue to emphasize the important connections between in utero environment, perinatal β-cell development, and adult β-cell mass.
We discovered reduced β-cell area in RUPP offspring, in part explained by increased cell death. The rate of self-duplication could have also been altered, as other IUGR models in rats have demonstrated normal (12) or reduced β-cell proliferation (34, 43). Assessment of circulating insulin in serum was not altered in either male or female e19.5 RUPP compared with control. Interestingly, young adult RUPP offspring (9 wk old) display hyperinsulinemia, a possible response to insulin resistance in RUPP compared with control offspring (16). In another model where uteroplacental blood flow was reduced in e19.5–21.5 (vs. RUPP, e14.5–19.5), β-cell mass was normal on day 7. Fasting insulin levels in these IUGR rats are comparable to control on day 7 (41). However, by 7–10 wk of age, they developed hyperinsulinemia because of insulin resistance (41). IUGR children have also been shown to have specific impairment in insulin sensitivity (47). Functional insulin secretion assays in vitro, independent of hormonal and neural stimuli, showed comparable glucose or glucose and palmitate insulin secretion from isolated islets of e19.5 offspring of RUPP and control dams. Although, in a different study [in vivo glucose-stimulated insulin secretion versus in vitro (controlled number of islets); experiments were done in different in time of development], impaired insulin secretion was observed in response to glucose in 1, 15, and 26-wk-old IUGR rats (41). Glucose-stimulated insulin secretion and glucose removal are impaired in the severely IUGR human fetus (33). The current data suggest that the reduction in β-cell area in e19.5 RUPP is not sufficient to alter insulin levels at this stage of development. However, future studies could look at the insulin secretion in adult RUPP rats.
Perspectives and Significance
The data presented here support the hypothesis that an abnormal intrauterine milieu, induced by reduced placental blood flow and increased maternal pressure from e14.5–19.5 gestation, can induce changes in β-cell area early in life, which could contribute to glucose intolerance and susceptibility to metabolic disease such as T2D in adulthood.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants (K01-DK-103823, R21-DK-112144, R03-DK-114465, and R01-DK-115720) to E. U. Alejandro, R03-DK-11446501A1 to B. Akhaphong, and National Heart, Lung, and Blood Institute Grant 2-R15-HL-109843–03 to J. F. Regal.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.U.A. conceived the project; B.A., A.L., J.A.W., C.R.W., J.F.R., and E.U.A. designed research; B.A., A.L., S.J., R.M., J.A.W., C.R.W., J.F.R., and E.U.A. performed experiments; B.A., A.L., S.J., R.M., J.A.W., C.R.W., J.F.R., and E.U.A. analyzed data; B.A., A.L., S.J., R.M., J.F.R., and E.U.A. interpreted results of experiments; B.A., A.L., S.J., R.M., and E.U.A. prepared figures; B.A., A.L., and E.U.A. drafted manuscript; B.A., A.L., S.J., R.M., J.F.R., and E.U.A. edited and revised manuscript; A.L., S.J., R.M., J.A.W., C.R.W., J.F.R., and E.U.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Daniel Baumann, Kate Root, Connor Laule, Luke McCutcheon, Ingrid Bender, Michelle Sia, and Alicia Wong for technical support. We thank Dr. Thomas Pengo for assistance in Fiji and the University of Minnesota Imaging Center. The neonatal tissue processing and embedding was performed at the laboratory of Dr. Jop van Berlo, Lillehei Heart Institute, University of Minnesota. The authors acknowledge Dr. Ronald Regal, Department of Mathematics and Statistics, University of Minnesota Duluth, for assistance with the statistical analysis.
REFERENCES
- 1.Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Méneur C, Bernal-Mizrachi E. Natural history of β-cell adaptation and failure in type 2 diabetes. Mol Aspects Med 42: 19–41, 2015. doi: 10.1016/j.mam.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alejandro EU, Gregg B, Wallen T, Kumusoglu D, Meister D, Chen A, Merrins MJ, Satin LS, Liu M, Arvan P, Bernal-Mizrachi E. Maternal diet-induced microRNAs and mTOR underlie β cell dysfunction in offspring. J Clin Invest 124: 4395–4410, 2014. doi: 10.1172/JCI74237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 4.Ananth CV, Vintzileos AM. Distinguishing pathological from constitutional small for gestational age births in population-based studies. Early Hum Dev 85: 653–658, 2009. doi: 10.1016/j.earlhumdev.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Béringue F, Blondeau B, Castellotti MC, Bréant B, Czernichow P, Polak M. Endocrine pancreas development in growth-retarded human fetuses. Diabetes 51: 385–391, 2002. doi: 10.2337/diabetes.51.2.385. [DOI] [PubMed] [Google Scholar]
- 5a.Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hote JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes 40, Suppl 2: 115–120, 1991. doi: 10.2337/diab.40.2.S115. [DOI] [PubMed] [Google Scholar]
- 6.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 7.Dumortier O, Hinault C, Gautier N, Patouraux S, Casamento V, Van Obberghen E. Maternal protein restriction leads to pancreatic failure in offspring: role of misexpressed microRNA-375. Diabetes 63: 3416–3427, 2014. doi: 10.2337/db13-1431. [DOI] [PubMed] [Google Scholar]
- 8.Elghazi L, Blandino-Rosano M, Alejandro E, Cras-Méneur C, Bernal-Mizrachi E. Role of nutrients and mTOR signaling in the regulation of pancreatic progenitors development. Mol Metab 6: 560–573, 2017. doi: 10.1016/j.molmet.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fall CH, Yajnik CS, Rao S, Davies AA, Brown N, Farrant HJ. Micronutrients and fetal growth. J Nutr 133, Suppl 2: 1747S–1756S, 2003. doi: 10.1093/jn/133.5.1747S. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Twinn DS, Ozanne SE. Early life nutrition and metabolic programming. Ann NY Acad Sci 1212: 78–96, 2010. doi: 10.1111/j.1749-6632.2010.05798.x. [DOI] [PubMed] [Google Scholar]
- 11.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44: 249–256, 1995. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- 12.Garofano A, Czernichow P, Bréant B. In utero undernutrition impairs rat beta-cell development. Diabetologia 40: 1231–1234, 1997. doi: 10.1007/s001250050812. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert J, Dukes M, LaMarca B, Cockrell K, Babcock S, Granger J. Effects of reduced uterine perfusion pressure on blood pressure and metabolic factors in pregnant rats. Am J Hypertens 20: 686–691, 2007. doi: 10.1016/j.amjhyper.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 97: 3197–3206, 2012. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35: 595–601, 1992. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 16.Heltemes A, Gingery A, Soldner EL, Bozadjieva N, Jahr KN, Johnson BK, Gilbert JS. Chronic placental ischemia alters amniotic fluid milieu and results in impaired glucose tolerance, insulin resistance and hyperleptinemia in young rats. Exp Biol Med (Maywood) 235: 892–899, 2010. doi: 10.1258/ebm.2010.009357. [DOI] [PubMed] [Google Scholar]
- 17.Hill DJ. Nutritional programming of pancreatic β-cell plasticity. World J Diabetes 2: 119–126, 2011. doi: 10.4239/wjd.v2.i8.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howerton CL, Bale TL. Targeted placental deletion of OGT recapitulates the prenatal stress phenotype including hypothalamic mitochondrial dysfunction. Proc Natl Acad Sci USA 111: 9639–9644, 2014. doi: 10.1073/pnas.1401203111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta 33, Suppl 2: e23–e29, 2012. doi: 10.1016/j.placenta.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarczak J, Kaba J, Bagnicka E. The validation of housekeeping genes as a reference in quantitative Real Time PCR analysis: application in the milk somatic cells and frozen whole blood of goats infected with caprine arthritis encephalitis virus. Gene 549: 280–285, 2014. doi: 10.1016/j.gene.2014.07.063. [DOI] [PubMed] [Google Scholar]
- 22.Jensen CB, Storgaard H, Dela F, Holst JJ, Madsbad S, Vaag AA. Early differential defects of insulin secretion and action in 19-year-old caucasian men who had low birth weight. Diabetes 51: 1271–1280, 2002. doi: 10.2337/diabetes.51.4.1271. [DOI] [PubMed] [Google Scholar]
- 23.Kebede MA, Oler AT, Gregg T, Balloon AJ, Johnson A, Mitok K, Rabaglia M, Schueler K, Stapleton D, Thorstenson C, Wrighton L, Floyd BJ, Richards O, Raines S, Eliceiri K, Seidah NG, Rhodes C, Keller MP, Coon JL, Audhya A, Attie AD. SORCS1 is necessary for normal insulin secretory granule biogenesis in metabolically stressed β cells. J Clin Invest 124: 4240–4256, 2014. doi: 10.1172/JCI74072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimball R, Wayment M, Merrill D, Wahlquist T, Reynolds PR, Arroyo JA. Hypoxia reduces placental mTOR activation in a hypoxia-induced model of intrauterine growth restriction (IUGR). Physiol Rep 3: e12651, 2015. doi: 10.14814/phy2.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lager S, Samulesson AM, Taylor PD, Poston L, Powell TL, Jansson T. Diet-induced obesity in mice reduces placental efficiency and inhibits placental mTOR signaling. Physiol Rep 2: e00242, 2014. doi: 10.1002/phy2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lillegard KE, Johnson AC, Lojovich SJ, Bauer AJ, Marsh HC, Gilbert JS, Regal JF. Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol 56: 91–97, 2013. doi: 10.1016/j.molimm.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limesand SW, Jensen J, Hutton JC, Hay WW Jr. Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 288: R1297–R1305, 2005. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- 28.Limesand SW, Rozance PJ, Macko AR, Anderson MJ, Kelly AC, Hay WW Jr. Reductions in insulin concentrations and β-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am J Physiol Endocrinol Metab 304: E516–E523, 2013. doi: 10.1152/ajpendo.00435.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lockridge AD, Baumann DC, Akhaphong B, Abrenica A, Miller RF, Alejandro EU. Serine racemase is expressed in islets and contributes to the regulation of glucose homeostasis. Islets 8: 195–206, 2016. doi: 10.1080/19382014.2016.1260797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastracci TL, Sussel L. The endocrine pancreas: insights into development, differentiation, and diabetes. Wiley Interdiscip Rev Dev Biol 1: 609–628, 2012. doi: 10.1002/wdev.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57: 1584–1594, 2008. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mejia C, Lewis J, Jordan C, Mejia J, Ogden C, Monson T, Winden D, Watson M, Reynolds PR, Arroyo JA. Decreased activation of placental mTOR family members is associated with the induction of intrauterine growth restriction by secondhand smoke in the mouse. Cell Tissue Res 367: 387–395, 2017. doi: 10.1007/s00441-016-2496-5. [DOI] [PubMed] [Google Scholar]
- 33.Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res 22: 426–430, 1990. doi: 10.1055/s-2007-1004939. [DOI] [PubMed] [Google Scholar]
- 34.Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, Hill DJ. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology 140: 4861–4873, 1999. doi: 10.1210/endo.140.10.7042. [DOI] [PubMed] [Google Scholar]
- 35.Rachdi L, Aïello V, Duvillié B, Scharfmann R. L-leucine alters pancreatic β-cell differentiation and function via the mTor signaling pathway. Diabetes 61: 409–417, 2012. doi: 10.2337/db11-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roos S, Jansson N, Palmberg I, Säljö K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 582: 449–459, 2007. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol 296: C142–C150, 2009. doi: 10.1152/ajpcell.00330.2008. [DOI] [PubMed] [Google Scholar]
- 38.Rosario FJ, Dimasuay KG, Kanai Y, Powell TL, Jansson T. Regulation of amino acid transporter trafficking by mTORC1 in primary human trophoblast cells is mediated by the ubiquitin ligase Nedd4-2. Clin Sci (Lond) 130: 499–512, 2016. doi: 10.1042/CS20150554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saleem T, Sajjad N, Fatima S, Habib N, Ali SR, Qadir M. Intrauterine growth retardation–small events, big consequences. Ital J Pediatr 37: 41, 2011. doi: 10.1186/1824-7288-37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandovici I, Hammerle CM, Ozanne SE, Constância M. Developmental and environmental epigenetic programming of the endocrine pancreas: consequences for type 2 diabetes. Cell Mol Life Sci 70: 1575–1595, 2013. doi: 10.1007/s00018-013-1297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50: 2279–2286, 2001. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 42.Smith GC. First trimester origins of fetal growth impairment. Semin Perinatol 28: 41–50, 2004. doi: 10.1053/j.semperi.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate 57: 107–118, 1990. doi: 10.1159/000243170. [DOI] [PubMed] [Google Scholar]
- 44.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 445: 886–891, 2007. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 45.Thümmler K, Britton A, Kirch W. Data and Information on Women’s Health in the European Union. Luxembourg: Office for Official Publications of the European Communities, 2009. doi: 10.2785/27266. [DOI] [Google Scholar]
- 46.Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol 84: 751–753, 1977. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- 47.Veening MA, van Weissenbruch MM, Heine RJ, Delemarre-van de Waal HA. Beta-cell capacity and insulin sensitivity in prepubertal children born small for gestational age: influence of body size during childhood. Diabetes 52: 1756–1760, 2003. doi: 10.2337/diabetes.52.7.1756. [DOI] [PubMed] [Google Scholar]
- 47a.Veening MA, Van Weissenbruch MM, Delemarre-Van De Waal HA. Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. J Clin Endocrinol Metab 87: 4657–4661, 2002. doi: 10.1210/jc.2001-011940. [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Liang ZA, Sandford AJ, Xiong XY, Yang YY, Ji YL, He JQ. Selection of suitable housekeeping genes for real-time quantitative PCR in CD4(+) lymphocytes from asthmatics with or without depression. PLoS One 7: e48367, 2012. doi: 10.1371/journal.pone.0048367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep 15: 9, 2015. doi: 10.1007/s11892-015-0579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]