Abstract
Background
Host genotype - parasite genotype co-evolutionary dynamics are influenced by local biotic and abiotic environmental conditions. This results in spatially heterogeneous selection among host populations. How such heterogeneous selection influences host resistance, parasite infectivity and virulence remains largely unknown. We hypothesized that different co-evolutionary trajectories of a vertebrate host-parasite association result in specific virulence patterns when assessed on a large geographic scale. We used two reference host populations of three-spined sticklebacks and nine strains of their specific cestode parasite Schistocephalus solidus from across the Northern Hemisphere for controlled infection experiments. Host and parasite effects on infection phenotypes including host immune gene expression were determined.
Results
S. solidus strains grew generally larger in hosts coming from a population with high parasite diversity and low S. solidus prevalence (DE hosts). Hosts from a population with low parasite diversity and high S. solidus prevalence (NO hosts) were better able to control the parasite’s growth, regardless of the origin of the parasite. Host condition and immunological parameters converged upon infection and parasite growth showed the same geographic pattern in both host types.
Conclusion
Our results suggest that NO sticklebacks evolved resistance against a variety of S. solidus strains, whereas DE sticklebacks are less resistant against S. solidus. Our data provide evidence that differences in parasite prevalence can cause immunological heterogeneity and that parasite size, a proxy for virulence and resistance, is, on a geographic scale, determined by main effects of the host and the parasite and less by an interaction of both genotypes.
Electronic supplementary material
The online version of this article (10.1186/s12862-019-1406-3) contains supplementary material, which is available to authorized users.
Keywords: Host-parasite interaction, Immunological heterogeneity, Virulence, Stickleback, Schistocephalus solidus
Background
The interaction of an organism with its environment is a hallmark of life and a pre-requisite for natural selection. Local adaptation is driven by abiotic conditions and biotic interactions within and between species. Among the strongest evolutionary processes is the co-evolution between hosts and parasites [1–5]. Parasites rely on host resources and have the potential to drastically reduce host fitness [6]. To diminish the harm of parasites, effective defence strategies have evolved on the host side [4, 7]. However, heterogeneous environments select for different defence strategies among host populations, which results in immunological heterogeneity [8, 9]. The variation of host defence against parasites can range from mechanisms that decrease the risk of infection to processes that diminish the harm of parasites, such as resistance (i.e. the prevention of infection or the control of parasite growth) and tolerance (i.e. the ability to limit health or fitness effects of a distinct infection intensity) [10, 11]. Likewise, parasite infectivity and virulence (i.e. the detrimental effects on host traits related to fitness) are spatially structured both by environmental parameters and co-evolutionary processes.
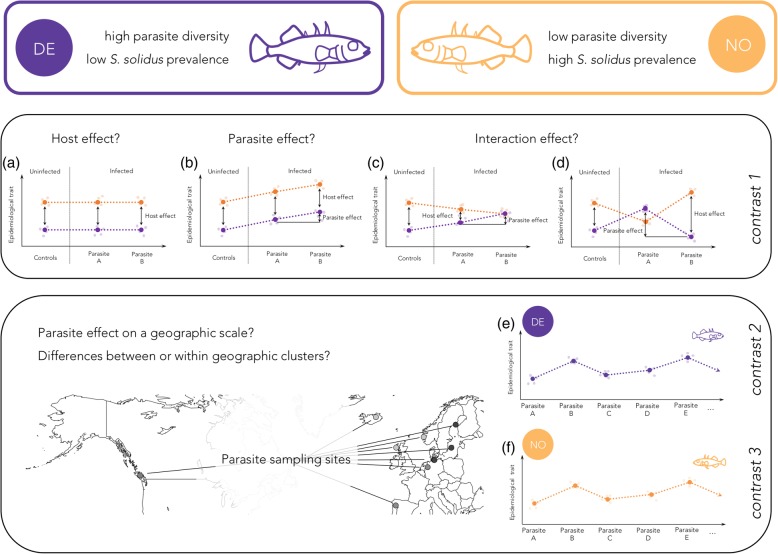
The epidemiological traits are shaped through main effects of the host and the parasite and by interaction effects (Fig. 1). The relative contribution of each of the interaction partners may differ along the infection process and depends on the geographic scale and the degree of environmental heterogeneity. Controlled infection experiments can be used to first identify environmental and evolutionary causes shaping the epidemiological traits and, second, to study the mechanisms and the adaptive significance thereof. Experiments revealed rapid and adaptive co-evolution of host and parasite genotypes in various systems, including phage-bacteria associations [4, 12], malaria systems [13, 14], plant-pathogen interactions [15, 16], and immune gene evolution in three-spined sticklebacks [17, 18]. We chose the association of three-spined sticklebacks and their specific macroparasite Schistocephalus solidus to determine host and parasite effects along the infection process and on different geographic scales.
Fig. 1.
Theoretical framework of the study. Reference hosts came from two contrasting populations, indicated by violet (DE) and orange (NO) dots and lines. For the sake of simplicity, we exemplify possible outcomes with a subset of hypothetical parasites A to E. We asked whether main effects of the host, the parasite, and/or host-parasite interaction effects shaped epidemiological traits (life history traits of the host and/or the parasite). (a, b, c, d) Partitioning of host, parasite, and interaction effects on an epidemiological trait. (a, b) Host genotype and parasite genotype main effects. The host effect (vertical spacing between the two lines) indicates the genetic difference between the two host types. Parallel horizontal lines in (a) indicate absence of a plastic response towards infection. Differences among hosts that are infected with the same parasite (vertical spacing between the dots) indicate a phenotypic plastic response of the parasite. The positive slope in (b) indicates different effects of the two parasite types (parasite effect) and thus a phenotypic plastic response of the host and the parasite. (c) and (d) demonstrate host genotype-parasite genotype interaction effects, because the host effect depends on the parasite type. Crossing reaction norms in (d) clearly show the interaction effect; but note in (c) that the main-effect components can cumulate, causing non-crossing reaction norms. We tested the predictions with data from contrast 1. (e, f) To further understand the parasite effect on a larger geographic scale, each of the two host types was exposed to parasites from different geographic clusters across the Northern Hemisphere. We tested these predictions with data from contrast 2 and contrast 3
Three-spined sticklebacks (Gasterosteus aculeatus; hereafter ‘sticklebacks’) live in numerous freshwater and marine habitats across the Northern Hemisphere. Various studies reported habitat-specific immune responses [19–24]. A lot of attention has been paid on the “supermodel” [25] interaction between sticklebacks and the cestode Schistocephalus solidus, as both can be bred in the laboratory facilitating controlled infection experiments [25]. S. solidus has a three-host life cycle with copepods as first intermediate host and G. aculeatus as specific second intermediate host. S. solidus grows massively in the body cavity of the fish, sometimes even exceeding the host’s weight [26, 27]. Reproduction is confined to the definite host, mostly piscivorous birds. The parasite’s reproductive output is directly related to its size [28]. S. solidus’ detrimental effects on sticklebacks were shown both in nature and in the laboratory and have been linked to the size of the parasite [27–30]. This cestode is assumed to be a driving force of divergent selection in three-spined sticklebacks [31]. Studies using hosts and parasites from different populations from Europe [32], from across continents [33] and in vitro leukocyte responses [34] indicate local adaptation of sticklebacks and S. solidus. It was suggested that S. solidus growth depends on host and/or parasite population-specific traits [35].
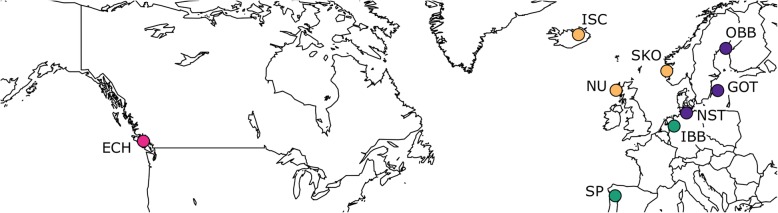
We assumed that sticklebacks evolved environment-specific immunological adaptations to S. solidus and that S. solidus evolved environment-specific virulence. We specifically asked if such divergent evolution could cause different immunological activation in response to a variety of S. solidus strains (i.e. S. solidus parasites from distinct locations). The following was hypothesized: (i) the infection phenotype differs between sticklebacks from heterogeneous environments (indicating a host effect); (ii) the infection phenotype differs between S. solidus strains (indicating a parasite effect); (iii) the infection phenotype differs according to stickleback-S. solidus interactions (indicating an interaction effect) (Fig. 1). These hypotheses were tested with three distinct analyses. First, hosts from two contrasting reference populations of G. aculeatus were experimentally infected with S. solidus from four European locations in order to test if host effects, parasite effects and/or interaction effects influenced S. solidus infection phenotypes in G. aculeatus (the corresponding analyses are referred to as contrast 1; Fig. 1). In order to test the parasite effect in further detail, each of these reference host types was infected with S. solidus strains from across the Northern Hemisphere (the corresponding analyses are referred to as contrast 2 and contrast 3; Fig. 1; Table 1; Additional file 1: Table S1). S. solidus sampling sites covered four geographic areas (clustered localities) corresponding to G. aculeatus phylogeny: the Atlantic region (NU, ISC, SKO), the Baltic region (OBB, NST, GOT), European Inland (SP, IBB), and the Pacific (ECH) (Fig. 2; Table 1).
Table 1.
Summary table of sample sizes within contrasts of interest
| Baltic | European Inland | Pacific | Atlantic | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis | Host | OBB | GOT | NST | SP | IBB | ECH | NU | ISC | SKO | control | |||||||||
| contrast 1 | DE (A) | na | na | (a) | 2 | na | (a) | 5 | na | na | (a) | 2 | (d) | 4 | 20 | |||||
| DE (B) | na | na | (b) | 10 | na | (b) | 3 | na | na | (b) | 5 | (a) | 7 | 18 | ||||||
| DE (C) | na | na | (c) | 8 | na | (c) | 10 | na | na | (c) | 5 | (c) | 3 | 20 | ||||||
| NO (A) | na | na | (a) | 2 | na | (a) | 3 | na | na | (a) | 1 | (d) | 4 | 20 | ||||||
| NO (B) | na | na | (b) | 4 | na | (b) | 2 | na | na | (b) | 6 | (a) | 8 | 20 | ||||||
| NO (C) | na | na | (c) | 2 | na | (c) | 2 | na | na | (c) | 5 | (c) | 2 | 20 | ||||||
| contrast 2 | DE (D) | (a) | 4 | (a) | 5 | (a) | 4 | (a) | 1 | na | (a) | 3 | (a) | 1 | na | (a) | 7 | 20 | ||
| DE (E) | (b) | 2 | (b) | 0 | (b) | 3 | (b) | 0 | na | (b) | 2 | (b) | 3 | na | (b) | 0 | 20 | |||
| DE (F) | (c) | 4 | (c) | 3 | (c) | 9 | (c) | 7 | na | (c) | 2 | (c) | 6 | na | (c) | 5 | 20 | |||
| contrast 3 | NO (A) | (a) | 1 | (d) | 1 | (a) | 2 | (a) | 2 | (a) | 3 | (a) | 1 | (b) | 5 | (a) | 1 | (d) | 4 | 20 |
| NO (B) | (b) | 10 | (b) | 4 | (b) | 4 | (d) | 5 | (b) | 2 | (c) | 4 | (d) | 7 | (b) | 6 | (a) | 8 | 20 | |
| NO (C) | (c) | 1 | (a) | 2 | (c) | 2 | (b) | 2 | (c) | 2 | (b) | 0 | (c) | 6 | (c) | 5 | (c) | 2 | 20 | |
Naïve laboratory bred first generation offspring from three-spined sticklebacks Gasterosteus aculeatus from lake Großer Plöner See, Germany (DE), and Lake Skogseidvatnet, Norway (NO), were infected with Schistocephalus solidus parasites from different geographic locations or sham-exposed as controls. The top row indicates S. solidus geographic cluster; abbreviations in the second row refer to S. solidus sampling sites (OBB: Obbola, Sweden; GOT: Gotland, Sweden; NST: Neustädter Binnenwasser, Germany; SP: Xinzo de Limia, Spain; IBB: Ibbenbürener Aa, Germany; ECH: Vancouver Island, Canada; NU: North Uist, Scotland; ISC: Lake Myvatn, Iceland; SKO: Lake Skogseidvatnet, Norway; control: sham-exposed control). Capital letters indicate fish families (offspring of one pair of sticklebacks), lower case letters indicate worm sibships (offspring of one pair of worms). Per treatment, i.e. fish family x worm sibship combination, 100 copepods and subsequently 20 fish were exposed to single infective S. solidus larvae or sham-exposed; combinations with ‘na’ were not included in the respective analysis. Numbers in columns of S. solidus exposed fish indicate the number of infected individuals. We used contrast 1 to test for host, parasite and interaction effects; contrast 2 and contrast 3 were used to test parasite effects on a broader geographic scale. NO data in contrast 1 is a data subset of contrast 3. We accounted for multiple testing
Fig. 2.
Sampling sites. Sticklebacks originated from Lake Großer Plöner See, Germany (DE), ~ 25 km from Neustädter Binnenwasser (NST; one of the sampling sites of S. solidus) and Lake Skogseidvatnet, Norway (NO). S. solidus were sampled from nine different locations across Europe and the Pacific (more information in Additional file 1: Table S1). Colors indicate four geographic clusters (pink: Pacific, orange: Atlantic, violet: Baltic, green: European Inland). The map was drawn with the R package maps [77]; colors were chosen from the ColorBrewer palette [76]
The two host populations differ remarkably in parasite diversity (Shannon diversity index) and abundance (the mean number of parasites per fish) [24, 36]. Parasite diversity is high and S. solidus prevalence (the number of infected individuals) is low (< 1%) in the German habitat (DE), whereas S. solidus prevalence is high and parasite diversity is low in the Norwegian population (NO) (20 to > 50%). Under the assumption that immune defence is costly and co-evolves with parasite virulence [7, 37–40], we hypothesized that sticklebacks from the highly S. solidus exposed (NO) population evolved S. solidus specific resistance, whereas this might not be the case for the rarely S. solidus exposed (DE) population. We suggested that S. solidus specific resistance could be effective against sympatric and potentially even allopatric strains. In order to cover numerous important parameters along the infection process, infection rates and the size of the parasite, as well as host condition and immunological parameters were determined [10]. The size of the parasite is used a measure of host resistance and parasite virulence [11, 32, 41]. The immunological activation was inferred from the size of the major immune organs and by immune gene expression analyses. We asked whether host population and/or parasite strain, cluster or growth caused distinct gene expression profiles. This study investigates evolutionary and proximate (physiological and molecular) causes of immunological heterogeneity, the specificity of resistance and the contribution of host and parasite on infection phenotypes.
Results
Both intermediate hosts (copepods and sticklebacks) were infected with S. solidus from every location (Additional file 1: SI.1; Tables S2 and S3). We obtained 227 plerocercoids from 1342 fish (excluding two infected controls and one double infected fish). The average weight of S. solidus plerocercoids 55 (+/− 2) days post exposure (DPE) was 61.8 mg and varied between 0.6 mg and 151.4 mg. Neither infection rates in copepods nor infection rates in fish influenced S. solidus size in the fish (LMMs for average parasite index (PI) per worm sibship as dependent variable; worm origin, infection rates in copepods and in fish as fixed effects, round as random term).
Contrast 1, the comparison of DE and NO hosts infected with four different European S. solidus strains, included 587 fish: 118 controls (excluding two infected DE controls), 105 infected fish, 364 exposed but uninfected fish; 11 fish died. Contrast 2, testing the parasite effect in DE hosts, included 522 fish: 60 controls, 71 infected fish, 335 exposed but uninfected fish; 14 fish died. Contrast 3, testing the parasite effect in NO hosts, included 60 controls, 92 infected fish, 433 exposed but uninfected fish; 15 fish died.
Constitutive differences between the host populations (contrast 1)
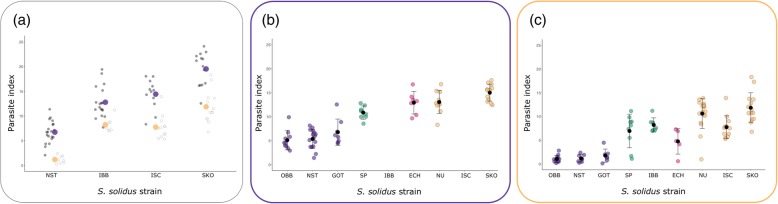
Contrast 1, the combination of the two hosts and four S. solidus strains, was used to test for host effects, parasite effects and host-parasite interaction effects on infection rates and infection phenotypes (Fig. 1; Table 1). S. solidus infection rates were consistent among host populations (host effect: Χ21 = 2.27, p = 0.132; S. solidus effect: Χ23 = 0.882, p = 0.830; host-parasite interaction effect: Χ23 = 6.42, p = 0.093; Additional file 1: Table S4). However, all four S. solidus strains were significantly smaller in NO hosts (parasite index, PI, the relative weight of S. solidus in the host [27]; host effect: F1,95 = 23.48, p < 0.0001). The differences between S. solidus strains were independent of the host population (host-parasite interaction effect on PI: F3,95 = 0.995, p = 0.399) (Fig. 3; Additional file 1: Tables S5-S7).
Fig. 3.
S. solidus growth differs significantly between host populations and between geographically clustered parasite strains. Naïve laboratory bred F1 offspring from sticklebacks from lake Großer Plöner See, Germany (DE), and Lake Skogseidvatnet, Norway (NO), were experimentally infected with single S. solidus larvae from nine different locations (‘strains’) across the Northern Hemisphere. Fish were dissected 55 (+/− 2) days after exposure to the parasite. The parasite index (PI) was calculated as the proportion of the parasite’s weight from the total weight of infected fish. (a) DE and NO hosts were infected with four different European S. solidus strains (contrast 1). Black and white dots represent individuals; violet: mean parasite indices in DE hosts; orange: mean parasite indices in NO hosts (Additional file 1: Table S6). (b) Parasite indices in DE hosts (contrast 2). Black dots and bars indicate the mean and the standard deviation. Color coding follows Fig. 2. (c) Parasite indices in NO hosts (contrast 3). Black dots and bars indicate the mean and the standard deviation. Color coding follows Fig. 2
We detected constitutive differences in condition and immunological parameters of the two stickleback populations (more information in Additional file 1: SI.3). DE sticklebacks had a significantly higher condition (CF; an estimate of the overall condition [42]) if they were uninfected (Χ21 = 44.252, p < 0.0001) or infected with S. solidus from the Baltic (NST) (Χ21 = 10.48, p = 0.001). Hepatosomatic indices (HSI, an estimate of metabolic reserves [43]) were higher in DE controls compared to NO controls (Χ21 = 26.93, p < 0.0001). Head kidney indices (HKI, the relative weight of the major immune organ in fish) were generally higher in DE fish (Χ24 = 49.47, p < 0.0001) and DE controls showed higher reactive oxygen species (ROS) production of head kidney leukocytes (Χ21 = 24.1, p < 0.0001). Splenosomatic indices (SSI, the relative weight of the major secondary immune organ [44]) were significantly higher in DE controls (Χ21 = 79.38, p < 0.0001) and in DE hosts infected with Baltic (NST) S. solidus (Χ21 = 30.75, p < 0.0001) or European Inland (IBB) S. solidus (Χ21 = 19.02, p < 0.0001). The effects were not directly related to S. solidus size but to S. solidus strain. We detected no significant differences in these condition and immunological parameters between DE and NO sticklebacks if they were infected with S. solidus from two Atlantic populations (SKO, ISC) (Additional file 1: Figure S1).
Total RNA from spleen was used to determine expression levels of 24 key immune genes. We ran non-parametric permutational multivariate analyses of variance (PERMANOVA) including host and parasite main effects and their interaction. The main effects were significant predictors while the interaction did not influence immune gene expression profiles (host effect: PERMANOVAinnate: F1,148 = 10.69, p < 0.0001; PERMANOVAadaptive: F1,148 = 13.58, p < 0.0001; PERMANOVAcomplement: F1,148 = 7.03, p = 0.0001; S. solidus effect: PERMANOVAinnate: F4,148 = 3.74, p = 0.0002; PERMANOVAadaptive: F4,148 = 2.73, p = 0.007; PERMANOVAcomplement: F4,148 = 3.82, p = 0.0002; host-parasite interaction effect: PERMANOVAinnate: F4,148 = 0.93, p = 0.45; PERMANOVAadaptive: F4,148 = 1.01, p = 0.41; PERMANOVAcomplement: F4,148 = 0.40, p = 0.94). Pairwise PERMANOVAs were used a posteriori in order to identify significantly different groups [45].
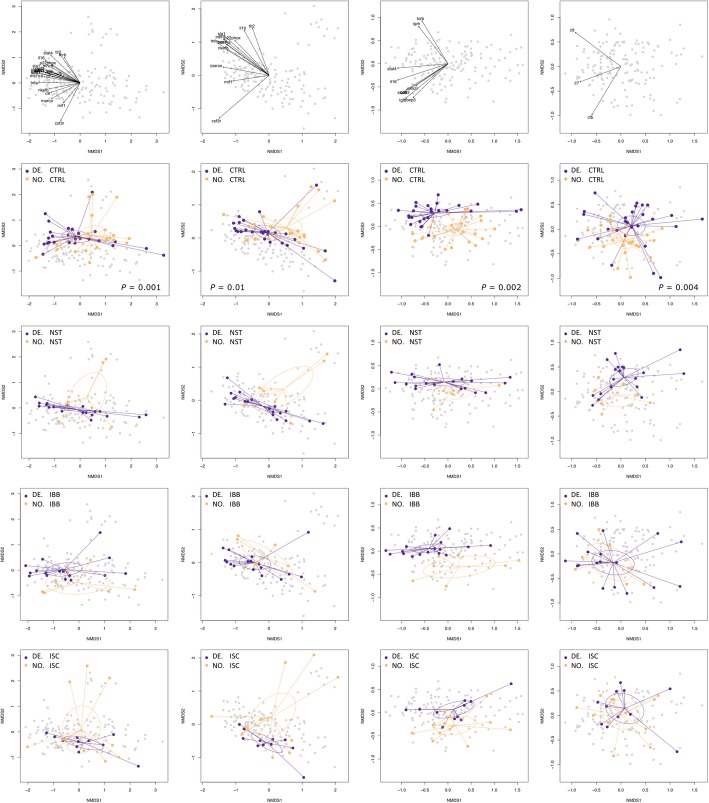
Immune gene expression profiles differed significantly between DE and NO controls (PERMANOVAinnate: F1,48 = 3.32, p < 0.001; PERMANOVAadaptive: F1,48 = 6.76, p = 0.002; PERMANOVAcomplement: F1,48 = 4.78, p = 0.004; Additional file 1: Table S11; Fig. 4). DE sticklebacks had higher expression levels of genes of innate and adaptive immunity, while complement genes were lower expressed than in NO sticklebacks (Additional file 1: Table S8; Figure S6). ISC S. solidus infection caused different innate immune gene expression in DE and NO sticklebacks (PERMANOVAinnate: F1,22 = 3.58, p = 0.004; Additional file 1: Table S9; Fig. 4), which was driven by remarkably low expression of Interleukin-1β (il-1β) in DE sticklebacks (F1,18 = 20.0, p < 0.001) (Additional file 1: Table S9; Figure S6). Expression profiles of NST-, IBB- and SKO-infected fish did not differ significantly between host populations (Fig. 4).
Fig. 4.
Multivariate gene expression patterns differ between DE and NO sticklebacks. Non-metric multidimensional scaling (NMDS) plots on Euclidian distances and two dimensions comparing data from NO and DE sticklebacks (contrast 1). NMDS were based on log10-transformed calibrated normalized relative quantities (CNRQ values) of all 24 immune genes, twelve genes of innate immunity (marco, mst1ra, mif, il-1β, tnfr1, saal1, tlr2, csf3r, p22phox, nkef-b, sla1, cd97), nine genes of adaptive immunity (stat4, stat6, igm, cd83, foxp3, tgf-β, il-16, mhcII, tcr-β), or three genes of the complement system (cfb, c7, c9). Each dot represents one individual; colors refer to the host population. Ellipses represent 95% confidence intervals. P-values are shown if significant after FDR-correction. The contribution of each gene is shown in the first row. The second row shows data from sham-exposed (CTRL) sticklebacks. The third to sixth row show data from infected individuals. Function metaMDS() was used to plot the NMDS; the contribution of each gene was plotted by use of the envfit() function (both functions are implemented in R package vegan [74])
Parasite indices show a geographic pattern in both host types
To further understand the effect of the parasite on infection phenotypes, we exposed DE hosts (contrast 2) and NO hosts (contrast 3) to S. solidus strains from across the Northern Hemisphere (Fig. 1). The infection rates did not differ significantly between parasite strains in DE sticklebacks (contrast 2: Χ26 = 7.15, p = 0.307), but did so in NO sticklebacks (contrast 3: Χ28 = 21.62, p = 0.006) (Additional file 1: Tables S3–S4). Parasite indices differed between parasite strains (contrast 2: F6,62 = 42.39, p < 0.0001; contrast 3: F8,81 = 61.09, p < 0.0001). We found a clear pattern with S. solidus from the Baltic being significantly smaller than worms from the other origins; Atlantic S. solidus were the largest in both host types (Additional file 1: Tables S10-S12; Fig. 3).
Immune gene expression is parasite strain specific
Building on from the idea that S. solidus growth follows a geographic pattern, we asked whether the molecular phenotypes would show the same clustering. We studied the influence of S. solidus strain on stickleback immune gene expression by running pairwise PERMANOVAs within host populations (contrast 2 or contrast 3) and tested (i) if gene expression differed within and/or between geographic clusters (Atlantic, Baltic, European Inland, Pacific) and (ii) if immune gene expression differed between sham-exposed controls and S. solidus infected sticklebacks for each parasite origin. Gene expression neither differed significantly within the clustered localities, nor between Baltic and Atlantic or European parasites, although the parasite indices differed considerably (Figs. 3 and 5). Immune gene expression profiles only differed between clustered localities if sticklebacks were infected with S. solidus from the Pacific (ECH) versus the Baltic or Atlantic region (Fig. 5).
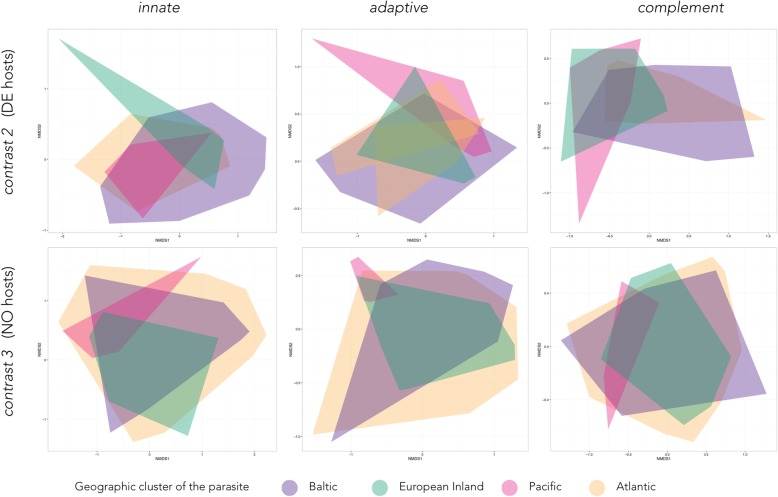
Fig. 5.
Infection with Pacific S. solidus drives significantly different multivariate gene expression patterns. Multivariate patterns in gene expression were visualized by non-metric multidimensional scaling (NMDS) on Euclidian distances and two dimensions using function metaMDS() from vegan [74]. Polygons were plotted using ggplot2 [75]. NMDS were based on log10-transformed calibrated normalized relative quantities (CNRQ values) of twelve genes of innate immunity (marco, mst1ra, mif, il-1β, tnfr1, saal1, tlr2, csf3r, p22phox, nkef-b, sla1, cd97), nine genes of adaptive immunity (stat4, stat6, igm, cd83, foxp3, tgf-β, il-16, mhcII, tcr-β), or three genes of the complement system (cfb, c7, c9). Upper panel: data from DE hosts infected with seven different S. solidus strains from the four clustered localities (contrast 2); lower panel: data from NO hosts infected with nine different S. solidus strains from the four clustered localities (contrast 3). Color coding follows Fig. 1
In DE sticklebacks (contrast 2), Pacific S. solidus infection was associated with higher expression of innate immune genes (PERMANOVAinnate: F1,33 = 3.88, p = 0.018), adaptive immune genes (PERMANOVAadaptive: F1,33 = 4.16, p = 0.013) and complement components (PERMANOVAcomplement: F1,33 = 8.1, p = 0.001) compared to infection with Baltic S. solidus (Table S13). Compared to infection with Atlantic S. solidus, Pacific S. solidus infection was associated with higher expression of adaptive immune genes (PERMANOVAadaptive: F1,26 = 5.84, p < 0.001) and complement components (PERMANOVAcomplement: F1,26 = 3.66, p = 0.016) in DE sticklebacks; only mhcII RNA levels were lower in Pacific S. solidus infections (F1,26 = 15.71, p = 0.0007; Additional file 1: Table S14).
In contrast 3, NO sticklebacks infected with Pacific S. solidus showed differential expression of genes of innate (PERMANOVAinnate: F1,29 = 3.26, p = 0.006) and adaptive immunity (PERMANOVAadaptive: F1,29 = 5.8, p = 0.002) in comparison to infection with Baltic S. solidus. Seven innate immune genes (marco, mif1, tnfr1, p22phox, nkef-b, sla1, cd97) and five adaptive immune genes (stat4, cd83, foxp3, tgf-β, il16) were significantly higher expressed in Pacific S. solidus infections; only RNA levels of mhcII were significantly lower (Additional file 1: Table S15). In comparison to infection with Atlantic S. solidus, Pacific S. solidus infection was linked to higher expression of innate immune genes (PERMANOVAinnate: F1,47 = 2.95, p = 0.014), adaptive immune genes (PERMANOVAadaptive: F1,47 = 5.27, p = 0.004) and complement components (PERMANOVAcomplement: F1,47 = 5.16, p = 0.008) in NO hosts. Seven genes of innate immunity (mst1ra, il-1β, tnfr1, p22phox, nkef-b, sla1, cd97), seven genes of adaptive immunity (stat4, igm, cd83, foxp3, tgf- β, il16, mhcII) and complement c9 were significantly higher expressed in NO sticklebacks infected with Pacific S. solidus in comparison to infection with Atlantic S. solidus (Additional file 1: Table S16).
We next tested if immune gene expression patterns differed between infected and control fish within contrast 2 or contrast 3. Again, gene expression patterns were not related to parasite indices or size but strain-specific.
In DE hosts (contrast 2), expression of genes of all three functional arms of the stickleback’s immune system differed significantly between sham-exposed controls and fish infected with Pacific S. solidus (PERMANOVAinnate: F1,32 = 7.51, p < 0.0001; PERMANOVAadaptive: F1,32 = 6.47, p < 0.001; PERMANOVAcomplement: F1,32 = 5.57, p = 0.007; Additional file 1: Table S17) or Scottish (NU) S. solidus (PERMANOVAinnate: F1,35 = 4.89, p = 0.003; PERMANOVAadaptive: F1,35 = 3.925, p = 0.009; PERMANOVAcomplement: F1,35 = 4.75, p = 0.014; Table S18). Infection with Norwegian (SKO) S. solidus altered expression of adaptive immune genes (PERMANOVAadaptive: F1,35 = 8.76, p < 0.0001) and complement genes (PERMANOVAcomplement: F1,35 = 3.42, p = 0.028; Additional file 1: Table S19).
In NO sticklebacks (contrast 3), innate immune genes and complement components were differentially expressed between controls and hosts infected with Pacific S. solidus (PERMANOVAinnate: F1,26 = 5.43, p = 0.0118; PERMANOVAcomplement: F1,26 = 7.61, p = 0.008; Additional file 1: Table S20). Adaptive immune genes were differentially expressed between controls and Atlantic (NU) S. solidus infections (PERMANOVAadaptive: F1,39 = 5.71, p = 0.002; Additional file 1: Table S20).
Discussion
Parasites are important components of the host’s environment and a crucial agent of natural selection [5, 7, 8, 34, 35, 41]. The co-evolution between hosts and parasites entails complex dynamics, influencing host defense and parasite infectivity and virulence. We used controlled infection experiments of three-spined sticklebacks from two contrasting populations with a variety of Schistocephalus solidus strains in order to characterize specificity and consequences of divergent co-evolution in a vertebrate host-parasite association. We propose that main effects of the host and the parasite determine S. solidus virulence, whereas the interaction might play a minor role.
Immunological differences between host populations
NO sticklebacks come from a population with high S. solidus prevalence and low parasite diversity [24, 36]. Since immune defence is costly and co-evolves with parasite virulence [7, 37–40], we hypothesized that NO sticklebacks evolved specific resistance against S. solidus. Infection rates did not differ significantly between host populations, but S. solidus plerocercoids were consistently smaller in NO hosts. This supports our hypothesis that NO hosts evolved increased resistance against S. solidus as inferred from parasite growth suppression [11, 41]. We found that controls from the DE population had higher immunological activity than NO controls (Fig. 4; SI.3–4). This is in line with the natural situation, as DE hosts are constantly challenged through high parasite diversity and abundance. However, the differences in immunological activation between the two host populations mostly converged upon infection: while immune gene expression profiles and respiratory burst activity of head kidney leukocytes differed significantly between controls, those parameters converged when fish were infected with S. solidus from most origins (Fig. 4; Additional file 1: Figure S1). This resembles the results from among-lake reciprocal transplant experiments [46] and comparisons of wild and laboratory-raised fish [47]. Consistently, these findings emphasize the importance of environmental effects on immune gene expression relative to genetic adaptation. We infer from our data that phenotypic plasticity in response to parasite infection is a stronger contributor to immunological activation than host genotype.
Parasite strain specific immune gene expression
Host immune gene expression did not depend on S. solidus size or geographic cluster, but was parasite strain specific. Immune gene expression profiles differed between NO and DE controls and if fish were infected with Icelandic (ISC) S. solidus (contrast 1). Notably, Icelandic sticklebacks seem to be genetically distinct from other Atlantic populations [48].
Within DE hosts (contrast 1) and within NO hosts (contrast 2), expression profiles of infected fish did not differ between or within clustered parasite localities, but only if sticklebacks were infected with S. solidus from the Pacific (ECH) (Fig. 3). Those parasites originated from the geographically most distant population, indicating the potential of local adaptation at this scale [33]. Infection with Pacific S. solidus was consistently associated with high expression of most immune genes but low expression levels of mhcII. Major histocompatibility complex (MHC) class II molecules are important components of adaptive immunity and activate T-cell mediated humoral immune responses [49]. In our experiments, low expression of mhcII was often associated with low expression of the gene of T-cell receptor subunit TCR-β that is involved in MHC ligand binding (Additional file 1: SI.4). If a speculative active down-regulation of this arm of the immune system in allopatric combinations results from a direct manipulation by S. solidus remains to be answered.
In comparison to sham-exposed controls, Pacific S. solidus infection caused high expression of pro-inflammatory and complement genes in hosts of both populations (Additional file 1: Tables S17 and S20). Genes of adaptive immunity were highly expressed (foxp3) or down-regulated (tcr-β and mhcII) in DE hosts. A simultaneous up-regulation of foxp3 is indicative of a T regulatory response [47] that potentially protects the host but may also enable parasite growth through anti-inflammatory activities. Indeed, ECH S. solidus were three times bigger in DE sticklebacks (Fig. 2). Pacific and Atlantic S. solidus reached similar sizes in DE hosts but, except for a potential involvement of tcr-β and/or mhcII, distinct genes were differentially expressed between hosts infected with parasites from different populations (Additional file 1: SI.4.3). We infer that (i) the relative parasite size and immune gene expression profiles are similar in infected fish of the two populations (similar parasite effect) but that (ii) complex ecological and co-evolutionary adaptations at different localities caused distinct levels of virulence and resistance.
Geographic pattern of virulence
Parasite indices were strikingly similar between the two host populations with regard to the geographic origin of the parasite. S. solidus from Atlantic populations grew consistently larger and Baltic parasites were the smallest in both host types (Fig. 3). The geographic pattern of virulence in both host types highlights the parasite main effect. The greatest difference was the suppression of Pacific S. solidus growth through Atlantic (NO) sticklebacks relative to Baltic (DE) sticklebacks. Sticklebacks from the Atlantic region likely originate from the Pacific [50], so we suggest a relatively similar genetic background of Pacific and Atlantic G. aculeatus – S. solidus species pairs. Such a similarity could explain the higher resistance of Atlantic hosts against Pacific parasites. Baltic stickleback populations, in contrast, form a cluster that is distinct from European Inland populations [48]. This, again, is a pattern that we also see in S. solidus growth (Fig. 2). Thus, the geographic pattern of virulence corresponds to the host’s recolonization history after the last glaciation [48]. Based on these data and a previous study [35], we hypothesize that the parasite’s phylogeny resembles the phylogeny of its highly specific host. A genetic basis could explain the same clusters of S. solidus growth in both host types. Latitude or geographical distance between host and parasite source populations did not explain parasite size. This renders the question of what could have selected for different S. solidus types.
We propose that S. solidus evolved different life-history strategies in response to distinct selection by their hosts and habitat-specific trade-offs. Baltic S. solidus from NST, where S. solidus prevalence is extremely low [32], did not reach the proposed minimum weight (50 mg) for sexual reproduction in final hosts [28, 51, 52]. Baltic S. solidus from Swedish populations (OBB, GOT), where S. solidus prevalence is actually high (T. Henrich; pers. comm.), showed the same growth pattern. Hence, parasite prevalence might be one explanation [32, 34, 35, 41], but is certainly not the only cause for different growth strategies, especially in the light of ecological effects on exposure risk [33]. Another possible inference is that S. solidus from the Baltic region reach sexual competence at lower weights than those from other populations, which is supported by the fact that smaller worms can reproduce [26]. Nevertheless, mapping variation on fitness differences in the natural habitat remains to be investigated.
Conclusions
We tested the specificity and immunological activation of three-spined sticklebacks Gasterosteus aculeatus towards various strains of the cestode Schistocephalus solidus at different stages of the infection process. (i) S. solidus infection rates were consistent among the two host populations whereas (ii) the growth of the parasite differed significantly among host populations and among parasite strains from different geographic clusters. Parasite indices were determined by main effects of the host and the parasite with nonsignificant interaction effects. (iii) Immune gene expression profiles were host-parasite combination specific, suggesting stronger interaction effects at this level of the infection process. Our results highlight the differences between mechanisms of distinct stages of the infection process and provide new insights into cestode growth suppression as a form of resistance [41].
We found constitutive immunological population differences but similar responses to infection. Our data provide evidence for (co-)evolutionary and ecological effects on immune functions that favour immunological heterogeneity. We propose that sticklebacks and S. solidus from a population with high S. solidus prevalence (NO) co-evolved high virulence and high resistance. The high resistance of NO hosts against S. solidus (host main effect) was not strain specific on an intermediate geographic scale (across Western Europe). On a larger geographic scale, parasites from the most distant (Pacific) population triggered elevated immunological parameters. The analogous clustering of parasite growth according to geography in the two host populations highlights the strong contribution of the parasite main effect on infection phenotypes. We suggest that patterns of local adaptation are either weak, absent or might be found at large scales [32–35].
Methods
Experimental hosts and parasites
Hosts and parasites were laboratory-raised first generation offspring from wild-caught individuals. Sticklebacks originated from lake Großer Plöner See, Germany (DE), and lake Skogseidvatnet, Norway (NO) and were kept in the institute’s aquaria system at 18 °C and a light:dark rhythm of 16:8 h. All fish were approximately nine months old at the start of the respective experiment. Sticklebacks were experimentally infected in 18 different combinations. We ran two experiments with essentially the same procedures. Each experiment was composed of three rounds using distinct fish families and parasite sibships. ‘Fish family’ refers to offspring from one pair of sticklebacks; ‘parasite sibship’ refers to offspring from one pair of worms. Parasite sibships from one origin are here referred to as ‘strain’. Sham-exposed controls were included in each round. A total of 1345 fish were analysed (Table 1; Table S1). We tested for host, parasite and host-parasite interaction effects using contrast 1. The respective infection experiments were run simultaneously and involved the exact same parasite sibships for both host populations, which should reduce any confounding factors. Parasite effects were further tested within each host type by using S. solidus strains from across the Northern Hemisphere (Table 1; Figs. 1 and 2). Schistocephalus solidus plerocercoids had been sampled from naturally infected sticklebacks from nine different locations (Fig. 2; Additional file 1: Table S1). The sampling sites cover four geographic areas corresponding to G. aculeatus phylogeny: the Atlantic region (NU, ISC, SKO), the Baltic region (OBB, NST, GOT), European Inland (SP, IBB), and the Pacific (ECH). The parasites were bred in vitro in the laboratory in 2012–2014. The eggs were kept at 4 °C in the dark.
Infection experiments
S. solidus eggs developed at 20 °C for three weeks. A 3:8 h light:dark cycle and another light stimulus initiated hatching of the first larval stage (coracidia). Single coracidia were immediately fed to Macrocyclops albidus copepods (first intermediate hosts) from laboratory cultures. Copepods were kept at 16:8 h light:dark cycles at 18 °C and fed with Paramecium three times a week. Infection success was determined by inspection for procercoids (second larval stage) in vivo 7 to 11 DPE. On day 16, sticklebacks were exposed to single infected copepods or uninfected controls. By this time, S. solidus is infective to its second intermediate host and differences in infection success are unlikely to be caused by variation in ontogeny [53, 54]. The fish were starved for two days and isolated in individual tanks. We assigned numbers to each treatment group, i.e. worm sibship and the control, and used a random design for the exposure to avoid any observer bias. The fish were transferred to 16 L aquaria according to their numbers 24 h after exposure. The water was sieved in order to determine the number of ingested copepods per treatment. Sticklebacks were kept in aerated aquaria connected to a flow-through freshwater system at 18 °C and a light:dark rhythm of 16:8 h. The density of 20 individuals per aquarium was maintained by replacing dead fish with spine-clipped sticklebacks from the same fish family.
The fish were fed with frozen Chironomidae larvae three times a week but starved for two to four days before dissection. We dissected the fish in the laboratory 55 (+/− 2) DPE. Fish of every treatment group per experiment were dissected on each day. Sticklebacks were euthanized with MS222 (1 g/L), weighed and measured (standard length, i.e. without tail fin). The head kidneys, spleen, liver, gonads, and, if present, worms were weighted to the nearest 0.1 mg. The carcasses were stored on ice upon dissection. Head kidney cells were immediately prepared for flow cytometric analyses. Spleen, liver and worms were transferred to RNAlater® (Sigma R0901; tenfold volume per weight), kept at 4 °C for one day and stored at − 20 °C until further use.
Phenotypic parameters
Infection rates were calculated with respect to the number of copepods that had not been ingested and include data from double infected hosts and fish that died before the day of dissection. The parasite index (PI) is a proxy for parasite size and host exploitation [32] and is calculated as the proportion of the total weight of an infected fish accounted for by the parasite [27]. The condition factor [42] and the hepatosomatic index (HSI) [43] are estimates of host condition. The splenosomatic index (SSI) [55] and head kidney index (HKI) were used as first proxies of immunological activation. The head kidney is the major immune organ in bony fish [44]. Thus, head kidney leukocytes (HKL) were studied in more detail [56] (Additional file 1: SI.3). Briefly, total cell numbers were determined by a modified protocol [57] of the Standard cell dilution assay [58]. Granulocytes and leukocytes were identified according to their FSC/SSC profiles using a Becton Dickinson FACS Calibur and BD CellQuest™ pro software (Version 6.0). We calculated a granulocyte to lymphocyte ratio (G/L ratio) as a rough activity estimate of the innate versus the adaptive immune system [59], and used a lucigenin-enhanced chemiluminescence assay [59, 60] to measure the phagocytic capacity of HKL by quantifying the respiratory burst reaction in relative luminescence units (RLUs). More details can be found in Additional file 1: SI.3.
Gene expression analyses
Differential gene expression of S. solidus infected fish and sham-exposed controls was studied by quantitative real time reverse transcription PCR (RT-qPCR). Total RNA from spleen was extracted with the NucleoSpin®96 Kit (Macherey-Nagel) according to the manufacturer’s manual. Samples were thawed at 4 °C, transferred to new tubes, supplied with ß-mercaptoethanol (1% v/v) containing lysis buffer and homogenized for 2 × 3 min at 30 Hz using Tissue Lyser II (Qiagen). A DNase digestion step was included. RNA was eluted with 40 μL RNase-free H2O. RNA concentration and quality were measured spectrophotometrically (NanoDrop; Thermo Scientific). Samples with concentrations below 6 ng/μL or A260/A280 ratios < 1.9 were excluded. Reverse transcription was performed on 6.4 ng of total RNA using the Omniscript® RT Kit (Qiagen) with oligo dT priming and RNase inhibition (0.2 μL per reaction) at 37 °C for 60 min. 12.8 μL of sample RNA were used if the concentration was below 39 ng/μL. The cDNA was stored at − 20 °C and diluted 1:5 with RNase-free H2O before pre-amplification. Pre-amplification was performed with TaqMan® PreAmp Master Mix (Applied Biosystems) according to the manufacturer’s instructions with 14 cycles. The PCR product was diluted 1:5 with low TE buffer. Differences in transcription levels were tested using 96.96 Dynamic Array IFCs on a Biomark™ HD system (Fluidigm) according to the manufacturer’s protocol. EvaGreen was used as DNA binding dye. Samples were spread across four IFCs. All targets for a given sample were included in the same run and measured in triplicates (technical replicates). Inter-run calibrators, dilution series, and negative controls were included on each IFC. Fluidigm Analysis software was used to assess melting curves of all qPCR assays in order to confirm specific amplification. Samples with suspicious Tm profiles in more than two targets or failed amplifications were excluded. Qbase + 3.0 (Biogazelle) was used for calculation of calibrated normalized relative quantities (CNRQ values). Replicates with variability (difference in quantification cycle, Cq) > 0.5 and wells with Cq > 28 were excluded, resulting in 94% pass rate. The average Cq was calculated as arithmetic mean; targets were scaled to average. We determined target and run specific amplification efficiencies. Expression stability of putative reference targets was inferred from geNorm M and Coefficient of Variation (CV) values [61, 62]. The most stably expressed reference targets rpl13 and ubc (M = 0.133, CV = 0.046) were used for normalization. CNRQs were log10 transformed for analysis. Three missing values from gene csf3r and one missing value from tlr2 were replaced by the mean expression of the respective gene. We analysed gene expression data of a total of 284 individuals from 18 different combinations including controls.
Genes targeted in expression analyses
We used 28 different primer pairs targeting mRNA from immune related genes and putative reference genes (b2m, ef1a, rpl13a, ubc; described in [63]). Targets of interest covered genes of innate immunity (cd97, csf3r, il-1β, marco, mif, mst1ra, nkef-b, tnfr1, saal1, tlr2, p22phox, sla1), adaptive immunity (cd83, foxp3, igm, il-16, stat4, stat6, tgf-β, mhcII, tcr-β) and the complement system (cfb, c7, c9). Primers are described in [46, 47, 64] and in Piecyk, Ritter & Kalbe (in review); the detailed information can be found in Additional file 2.
Statistical analyses
Statistical analyses were performed with R v. 3.2.0; [65]. We used (generalized) mixed effects models (GLMMs) from nlme [66] and lme4 [67] to include random terms and fixed effects according to the experimental design. Infection rates were analysed by using the number of infected individuals as proportional data in GLMMs with binomial error structure and logit link function. The interaction of host and parasite was included in contrast 1 (Table 1). Genotypic variation was generally accounted for by including parasite sibship or ‘round’, i.e. worm sibship x fish family combination, as random term. Models for fish parameters included the sex of the fish as another random effect to account for sex-specific differences. Model selection was based on the Akaike information criterion (AIC) and log likelihood ratio tests. Whenever needed, we incorporated heteroscedasticity in the model fit by definition of the varIdent variance structure for factorial variables. R2 values of mixed effects models [68, 69] were calculated with function sem.model.fits() from piecewiseSEM [70]. Significantly different groups were identified with glht() post hoc tests from multcomp [71] using Tukey’s all-pair comparisons or user defined contrasts according to the respective hypothesis. Multiple testing was accounted for by false discovery rate (FDR) correction [72]. Gene expression data was derived from infected and control fish from each family. Differential immune gene expression was analysed between groups within contrasts by multivariate statistics on data of all 24 immune genes and, if significant, according to functional groups (innate, adaptive, complement). Non-parametric permutational multivariate analyses of variance (PERMANOVA [72]) were calculated on Euclidian distance matrices [73] using function adonis() from vegan [74]. For each test, a random subset of 10,000 permutations was used; permutations were constrained within ‘round’. The weight of the fish was included as covariate to account for size related effects. Post hoc pairwise comparisons were FDR-corrected [72]. If multivariate statistics indicated significant differences, we used linear mixed models (LMMs) to identify which genes were differentially expressed. Again, we accounted for unequal variances and used FDR correction due to multiple testing. In each case, the raw p-values are reported. Data was plotted using ggplot2 [75]; colours for plots and figures were chosen from the ColorBrewer palette [76]. Multivariate patterns in gene expression were visualized by non-metric multidimensional scaling (NMDS) on Euclidian distances and two dimensions (function metaMDS()); the contribution of each gene was plotted by use of the envfit() function (both implemented in vegan). The maps package [77] was used to draw the map of the sampling sites.
Additional files
Supplementary information. Supplementary tables and figures for ‘Specificity of resistance and geographic patterns of virulence in a vertebrate host-parasite system’. (PDF 2743 kb)
Primer information. The primer information is cited from Piecyk, Ritter & Kalbe (in review). (PDF 108 kb)
Acknowledgments
We highly acknowledge and honor Dr. Martin Kalbe, who passed away shortly before submission of this manuscript. We are grateful for his supervision and guidance not only in a scientific context but also in personal relationships. He taught us a lot. G. Augustin, A. Baade, R. Derner, R. Leipnitz, H. Luttmann, D. Martens, I. Moreau, I. Samonte, G. Schmiedeskamp, I. Schultz, M. Schwarz, and N. Wildenhayn helped with experimental procedures, lab work and animal husbandry. Thanks go to N. Erin, M. Hasler, M. Milinski, A. Nolte, M. Ritter, and H. Schulenburg for helpful discussion. Two anonymous reviewers gave useful comments on an earlier version of the manuscript. D. Bolnick, F. Franke, T. Henrich, A. Rahn and J. Scharsack provided S. solidus eggs.
Funding
This study was financially supported by the German Science Foundation DFG (Priority program 1399; received by MK). AP received financial and scientific support from the International Max Planck Research School (IMPRS) for Evolutionary Biology, OR was funded by an ERC Starting Grant (MALEPREG) and grants from the German Research Foundation (349393951, 237263721 & 274695381). These funders had no role in study design, data collection, analysis and interpretation, or preparation of the manuscript.
Availability of data and materials
The data generated and analyzed during this study can be found in EDMOND, the Open Access Data Repository of the Max Planck Society (10.17617/3.21).
Abbreviations
- CF
condition factor
- CNRQ
calibrated normalized relative quantities
- DE, NO
sticklebacks from Lake Großer Plöner See (Germany), Lake Skogseidvatnet (Norway)
- DPE
days post exposure
- ECH, GOT, IBB, ISC, NST, NU, OBB, SKO, SP
parasite strains from Echo Lake (Canada), Gotland (Sweden), Ibbenbürener Aa (Germany), Myvatn (Iceland), Neustädter Binnenwasser (Germany), North Uist (Scotland), Obbola (Sweden), Lake Skogseidvatnet (Norway), Xinzo de Limia (Spain)
- (G)LMM
(generalized) linear mixed model
- FDR
false discovery rate
- G/L
granulocyte to lymphocyte ratio
- HKI
head kidney index
- HKL
head kidney leukocytes
- HSI
hepatosomatic index
- IFC
integrated fluidic circuits
- PERMANOVA
permutational multivariate analysis of variance
- PI
parasite index
- RLU
relative luminescence units
- ROS
reactive oxygen species
- SSI
splenosomatic index
Authors’ contributions
AP carried out the experiments and molecular work, analyzed the data, participated in design of the study and drafted the manuscript. OR helped to interpret the data and revised the manuscript. MK conceived the study and discussed the data. All authors have read and approved the manuscript.
Ethics approval
Animal experiments were approved by the Ministry of Energy Transition, Agriculture, Environment and Rural Areas of Schleswig-Holstein under reference number V 312–7224.123-34.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Martin Kalbe deceased
Contributor Information
Agnes Piecyk, Email: apiecyk@evolbio.mpg.de.
Olivia Roth, Email: oroth@geomar.de.
References
- 1.Ebert D, Hamilton WD. Sex against virulence: the coevolution of parasitic diseases. Trends Ecol Evol. 1996;11(2):79–82. doi: 10.1016/0169-5347(96)81047-0. [DOI] [PubMed] [Google Scholar]
- 2.Bull JJ. Perspective: virulence. Evolution. 1994;48(5):1423. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JN. Rapid evolution as an ecological process. Trends Ecol Evol. 1998;13(8):329–332. doi: 10.1016/s0169-5347(98)01378-0. [DOI] [PubMed] [Google Scholar]
- 4.Buckling A, Rainey PB. Antagonistic coevolution between a bacterium and a bacteriophage. Proc Biol Sci. 2002;269(1494):931–936. doi: 10.1098/rspb.2001.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulenburg H, Kurtz J, Moret Y, Siva-Jothy MT. Introduction. Ecological immunology. Philos Trans R Soc B Biol Sci. 2009;364(1513):3–14. doi: 10.1098/rstb.2008.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid-Hempel P. Evolutionary parasitology the integrated study of infections, immunology, ecology, and genetics. New York, NY, USA: Oxford University Press, Inc.; 2011. [Google Scholar]
- 7.Jokela J, Schmid-Hempel P, Rigby MC. Dr. Pangloss restrained by the red queen – steps towards a unified defence theory. Oikos. 2000;89(2):267–274. [Google Scholar]
- 8.Lazzaro BP, Little TJ. Immunity in a variable world. Philos Trans R Soc B Biol Sci. 2009;364(1513):15–26. doi: 10.1098/rstb.2008.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham AL. Optimal immunity meets natural variation: the evolutionary biology of host defence. Parasite Immunol. 2013;35(11):315–317. doi: 10.1111/pim.12073. [DOI] [PubMed] [Google Scholar]
- 10.Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proc R Soc Lond B Biol Sci. 2003;270(1513):357–366. doi: 10.1098/rspb.2002.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Råberg L. How to live with the enemy: understanding tolerance to parasites. PLoS Biol. 2014;12(11):e1001989. doi: 10.1371/journal.pbio.1001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson S, Vogwill T, Buckling A, Benmayor R, Spiers AJ, Thomson NR, et al. Antagonistic coevolution accelerates molecular evolution. Nature. 2010;464(7286):275–278. doi: 10.1038/nature08798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambrechts L, Halbert J, Durand P, Gouagna LC, Koella JC. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to plasmodium falciparum. Malar J. 2005;4(1):3. doi: 10.1186/1475-2875-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grech K, Watt K, Read AF. Host–parasite interactions for virulence and resistance in a malaria model system. J Evol Biol. 2006;19(5):1620–1630. doi: 10.1111/j.1420-9101.2006.01116.x. [DOI] [PubMed] [Google Scholar]
- 15.Burdon JJ, Jarosz AM. Host-pathogen interactions in natural populations of Linum marginale and Melampsora lini: I. Patterns of resistance and racial variation in a large host population. Evolution. 1991;45(1):205–217. doi: 10.1111/j.1558-5646.1991.tb05278.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaltz O, Shykoff JA. Within- and among-population variation in infectivity, latency and spore production in a host–pathogen system. J Evol Biol. 2002;15(5):850–860. [Google Scholar]
- 17.Eizaguirre C, Lenz TL, Kalbe M, Milinski M. Rapid and adaptive evolution of MHC genes under parasite selection in experimental vertebrate populations. Nat Commun. 2012;3:621. doi: 10.1038/ncomms1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eizaguirre C, Lenz TL, Kalbe M, Milinski M. Divergent selection on locally adapted major histocompatibility complex immune genes experimentally proven in the field. Ecol Lett. 2012;15(7):723–731. doi: 10.1111/j.1461-0248.2012.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wegner KM, Reusch TBH, Kalbe M. Multiple parasites are driving major histocompatibility complex polymorphism in the wild. J Evol Biol. 2003;16(2):224–232. doi: 10.1046/j.1420-9101.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- 20.Kalbe M, Kurtz J. Local differences in immunocompetence reflect resistance of sticklebacks against the eye fluke Diplostomum pseudospathaceum. Parasitology. 2006;132(01):105–116. doi: 10.1017/S0031182005008681. [DOI] [PubMed] [Google Scholar]
- 21.Scharsack JP, Kalbe M, Harrod C, Rauch G. Habitat-specific adaptation of immune responses of stickleback (Gasterosteus aculeatus) lake and river ecotypes. Proc R Soc B Biol Sci. 2007;274(1617):1523–1532. doi: 10.1098/rspb.2007.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eizaguirre C, Lenz TL, Sommerfeld RD, Harrod C, Kalbe M, Milinski M. Parasite diversity, patterns of MHC II variation and olfactory based mate choice in diverging three-spined stickleback ecotypes. Evol Ecol. 2011;25(3):605–622. [Google Scholar]
- 23.Lenz TL, Eizaguirre C, Rotter B, Kalbe M, Milinski M. Exploring local immunological adaptation of two stickleback ecotypes by experimental infection and transcriptome-wide digital gene expression analysis. Mol Ecol. 2013;22(3):774–786. doi: 10.1111/j.1365-294X.2012.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Chain FJJ, Panchal M, Eizaguirre C, Kalbe M, Lenz TL, et al. Transcriptome profiling of immune tissues reveals habitat-specific gene expression between lake and river sticklebacks. Mol Ecol. 2016 Jan 1;n/a-n/a. [DOI] [PMC free article] [PubMed]
- 25.Barber I. Sticklebacks as model hosts in ecological and evolutionary parasitology. Trends Parasitol. 2013;29(11):556–566. doi: 10.1016/j.pt.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Clarke AS. Studies on the life cycle of the pseudophyllidean cestode Schistocephalus solidus. Proc Zool Soc Lond. 1954;124(2):257–302. [Google Scholar]
- 27.Arme C, Owen RW. Infections of the three-spined stickleback, Gasterosteus aculeatus L., with the plerocercoid larvae of Schistocephalus solidus (Müller, 1776), with special reference to pathological effects. Parasitology. 1967;57(02):301–314. doi: 10.1017/s0031182000072103. [DOI] [PubMed] [Google Scholar]
- 28.Tierney JF, Crompton DWT. Infectivity of Plerocercoids of Schistocephalus solidus (Cestoda: Ligulidae) and fecundity of the adults in an experimental definitive host, Gallus gallus. J Parasitol. 1992;78(6):1049–1054. [PubMed] [Google Scholar]
- 29.Heins DC, Singer SS, Baker JA. Virulence of the cestode Schistocephalus solidus and reproduction in infected threespine stickleback, Gasterosteus aculeatus. Can J Zool. 1999;77(12):1967–1974. [Google Scholar]
- 30.Heins DC, Barry KA, Petrauskas LA. Consistency of host responses to parasitic infection in the three-spined stickleback fish infected by the diphyllobothriidean cestode Schistocephalus solidus. Biol J Linn Soc. 2014;113(4):958–968. [Google Scholar]
- 31.MacColl ADC. Parasite burdens differ between sympatric three-spined stickleback species. Ecography. 2009;32(1):153–160. [Google Scholar]
- 32.Kalbe M, Eizaguirre C, Scharsack JP, Jakobsen PJ. Reciprocal cross infection of sticklebacks with the diphyllobothriidean cestode Schistocephalus solidus reveals consistent population differences in parasite growth and host resistance. Parasit Vectors. 2016;9:130. doi: 10.1186/s13071-016-1419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber JN, Kalbe M, Shim KC, Erin NI, Steinel NC, Ma L, et al. Resist globally, infect locally: a transcontinental test of adaptation by stickleback and their tapeworm parasite. Am Nat 2016 Nov 18;E000–E000. [DOI] [PubMed]
- 34.Franke F, Rahn AK, Dittmar J, Erin N, Rieger JK, Haase D, et al. In vitro leukocyte response of three-spined sticklebacks (Gasterosteus aculeatus) to helminth parasite antigens. Fish Shellfish Immunol. 2014;36(1):130–140. doi: 10.1016/j.fsi.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Scharsack JP, Franke F, Erin NI, Kuske A, Büscher J, Stolz H, et al. Effects of environmental variation on host–parasite interaction in three-spined sticklebacks (Gasterosteus aculeatus) Zoology. 2016;119(4):375–383. doi: 10.1016/j.zool.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Feulner PGD, Chain FJJ, Panchal M, Huang Y, Eizaguirre C, Kalbe M, et al. Genomics of divergence along a continuum of Parapatric population differentiation. PLoS Genet. 2015;11(2):e1004966. doi: 10.1371/journal.pgen.1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11(8):317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- 38.Duffy MA, Ochs JH, Penczykowski RM, Civitello DJ, Klausmeier CA, Hall SR. Ecological context influences epidemic size and parasite-driven evolution. Science. 2012;335(6076):1636–1638. doi: 10.1126/science.1215429. [DOI] [PubMed] [Google Scholar]
- 39.Hasu T, Benesh DP, Valtonen ET. Differences in parasite susceptibility and costs of resistance between naturally exposed and unexposed host populations. J Evol Biol. 2009;22(4):699–707. doi: 10.1111/j.1420-9101.2009.01704.x. [DOI] [PubMed] [Google Scholar]
- 40.Duncan AB, Fellous S, Kaltz O. Reverse evolution: selection against costly resistance in disease-free microcosm populations of Paramecium Caudatum. Evolution. 2011;65(12):3462–3474. doi: 10.1111/j.1558-5646.2011.01388.x. [DOI] [PubMed] [Google Scholar]
- 41.Weber JN, Steinel NC, Shim KC, Bolnick DI. Recent evolution of extreme cestode growth suppression by a vertebrate host. Proc Natl Acad Sci U S A. 2017;114(25):6575–6580. doi: 10.1073/pnas.1620095114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frischknecht M. The breeding colouration of male three-spined sticklebacks (Gasterosteus aculeatus) as an indicator of energy investment in vigour. Evol Ecol. 1993;7(5):439–450. [Google Scholar]
- 43.Chellappa S, Huntingford FA, Strang RHC, Thomson RY. Condition factor and hepatosomatic index as estimates of energy status in male three-spined stickleback. J Fish Biol. 1995;47(5):775–787. [Google Scholar]
- 44.Rauta PR, Nayak B, Das S. Immune system and immune responses in fish and their role in comparative immunity study: a model for higher organisms. Immunol Lett. 2012;148(1):23–33. doi: 10.1016/j.imlet.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26(1):32–46. [Google Scholar]
- 46.Stutz WE, Schmerer M, Coates JL, Bolnick DI. Among-lake reciprocal transplants induce convergent expression of immune genes in threespine stickleback. Mol Ecol. 2015;24(18):4629–4646. doi: 10.1111/mec.13295. [DOI] [PubMed] [Google Scholar]
- 47.Robertson S, Bradley JE, MacColl ADC. Measuring the immune system of the three-spined stickleback – investigating natural variation by quantifying immune expression in the laboratory and the wild. Mol Ecol Resour. 2015 Dec 1;n/a-n/a. [DOI] [PMC free article] [PubMed]
- 48.Fang B, Merilä J, Ribeiro F, Alexandre CM, Momigliano P. Worldwide phylogeny of three-spined sticklebacks. Mol Phylogenet Evol [Internet]. 2018 Jun 12 [cited 2018 Jul 17]; Available from: http://www.sciencedirect.com/science/article/pii/S1055790317308527 [DOI] [PubMed]
- 49.Zhu L, Nie L, Zhu G, Xiang L, Shao J. Advances in research of fish immune-relevant genes: a comparative overview of innate and adaptive immunity in teleosts. Dev Comp Immunol. 2013;39(1–2):39–62. doi: 10.1016/j.dci.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Ortí G, Bell MA, Reimchen TE, Meyer A. Global survey of mitochondrial Dna sequences in the Threespine stickleback: evidence for recent migrations. Evolution. 1994;48(3):608–622. doi: 10.1111/j.1558-5646.1994.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 51.Scharsack JP, Koch K, Hammerschmidt K. Who is in control of the stickleback immune system: interactions between Schistocephalus solidus and its specific vertebrate host. Proc R Soc B Biol Sci. 2007;274(1629):3151–3158. doi: 10.1098/rspb.2007.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammerschmidt K, Kurtz J. Chapter 5 Ecological Immunology of a Tapeworms’ Interaction with its Two Consecutive Hosts. In: Joanne P. Webster, editor. Advances in Parasitology [Internet]. Academic Press; 2009 [cited 2013 Dec 8]. p. 111–37. (Natural History of Host-Parasite Interactions; vol. Volume 68). Available from: http://www.sciencedirect.com/science/article/pii/S0065308X08006052 [DOI] [PubMed]
- 53.Hammerschmidt K, Koch K, Milinski M, Chubb JC, Parker GA. When to go: optimization of host switching in parasites with complex life cycles. Evolution. 2009;63(8):1976–1986. doi: 10.1111/j.1558-5646.2009.00687.x. [DOI] [PubMed] [Google Scholar]
- 54.Benesh DP, Hafer N. Growth and ontogeny of the tapeworm Schistocephalus solidus in its copepod first host affects performance in its stickleback second intermediate host. Parasit Vectors. 2012;5:90. doi: 10.1186/1756-3305-5-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seppänen E, Kuukka H, Voutilainen A, Huuskonen H, Peuhkuri N. Metabolic depression and spleen and liver enlargement in juvenile Arctic charr Salvelinus alpinus exposed to chronic parasite infection. J Fish Biol. 2009;74(3):553–561. doi: 10.1111/j.1095-8649.2008.02144.x. [DOI] [PubMed] [Google Scholar]
- 56.Scharsack JP, Kalbe M. Differences in susceptibility and immune responses of three-spined sticklebacks (Gasterosteus aculeatus) from lake and river ecotypes to sequential infections with the eye fluke Diplostomum pseudospathaceum. Parasit Vectors. 2014;7(1):109. doi: 10.1186/1756-3305-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scharsack JP, Kalbe M, Derner R, Kurtz J, Milinski M. Modulation of granulocyte responses in three-spined sticklebacks Gasterosteus aculeatus infected with the tapeworm Schistocephalus solidus. Dis Aquat Org. 2004;59(2):141–150. doi: 10.3354/dao059141. [DOI] [PubMed] [Google Scholar]
- 58.Pechhold K, Pohl T, Kabelitz D. Rapid quantification of lymphocyte subsets in heterogeneous cell populations by flow cytometry. Cytometry. 1994;16(2):152–159. doi: 10.1002/cyto.990160209. [DOI] [PubMed] [Google Scholar]
- 59.Kurtz J, Kalbe M, Aeschlimann PB, Häberli MA, Wegner KM, Reusch TBH, et al. Major histocompatibility complex diversity influences parasite resistance and innate immunity in sticklebacks. Proc R Soc Lond B Biol Sci. 2004;271(1535):197–204. doi: 10.1098/rspb.2003.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scott AL, Klesius PH. Chemiluminescence: a novel analysis of phagocytosis in fish. Dev Biol Stand. 1981;49:243–254. [Google Scholar]
- 61.Vandesompele J, Preter KD, Pattyn F, Poppe B, Roy NV, Paepe AD, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hibbeler S, Scharsack JP, Becker S. Housekeeping genes for quantitative expression studies in the three-spined stickleback Gasterosteus aculeatus. BMC Mol Biol. 2008;9(1):18. doi: 10.1186/1471-2199-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunner FS, Anaya-Rojas JM, Matthews B, Eizaguirre C. Experimental evidence that parasites drive eco-evolutionary feedbacks. Proc Natl Acad Sci. 2017;16:201619147. doi: 10.1073/pnas.1619147114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: h ttp. Www R-Proj Org. 2015;
- 66.Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core Team (2014) nlme: linear and nonlinear mixed effects models. R package version 3.1–117. Available H TtpCRAN R-Proj Orgpackage Nlme. 2014;
- 67.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models using lme4. ArXiv14065823 Stat [Internet]. 2014 Jun 23 [cited 2015 Jun 23]; Available from: https://arxiv.org/abs/1406.5823.
- 68.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133–142. [Google Scholar]
- 69.Johnson PCD. Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods Ecol Evol. 2014;5(9):944–946. doi: 10.1111/2041-210X.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lefcheck JS. piecewiseSEM: piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol Evol. 2016;7(5):573–579. [Google Scholar]
- 71.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 72.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 73.D’haeseleer P. How does gene expression clustering work? Nat Biotechnol. 2005;23(12):1499–1502. doi: 10.1038/nbt1205-1499. [DOI] [PubMed] [Google Scholar]
- 74.Oksanen JF, Blanchet G, Kindt R, Legendre P, Minchin PR, O’Hara RB, et al. vegan: community ecology package. 2.0–0. R package version 2.3–0. 2015.
- 75.Wickham H. Elegant graphics for data analysis (ggplot2) New York, NY: Springer-Verlag; 2009. [Google Scholar]
- 76.Neuwirth E. ColorBrewer Palettes. R package version 1.1–2. 2014. 2015.
- 77.Brownrigg R. Maps: Draw Geographical Maps. R package version 2.3–9. Enhancements by Thomas P Minka. Original S code by Richard A. Becker and Allan R. Wilks. 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information. Supplementary tables and figures for ‘Specificity of resistance and geographic patterns of virulence in a vertebrate host-parasite system’. (PDF 2743 kb)
Primer information. The primer information is cited from Piecyk, Ritter & Kalbe (in review). (PDF 108 kb)
Data Availability Statement
The data generated and analyzed during this study can be found in EDMOND, the Open Access Data Repository of the Max Planck Society (10.17617/3.21).