Abstract
Objective
Mutations in myocilin (MYOC) may cause either juvenile open angle glaucoma (JOAG) or adult-onset primary open angle glaucoma (POAG). MYOC encodes a glycoprotein that is normally secreted from trabecular meshwork cells that regulate intraocular pressure. Prior in vitro, transgenic rodent, and organ culture experiments have suggested that abnormal accumulation of MYOC protein within trabecular meshwork cells is a key step in glaucoma pathophysiology. We investigated the pathogenesis of MYOC glaucoma by examining a donor eye from a patient with JOAG caused by a Tyr437His MYOC mutation.
Design
Case-control, immunohistochemical study of a donor eye from a patient with JOAG caused by a Tyr437His MYOC mutation and age-matched control donor eyes.
Subjects
An eye from a 59-year-old male with JOAG caused by a Tyr437His MYOC mutation and eyes from five donors (ages 51–66) with no known ocular disease were examined.
Methods
Frozen fixed sections of the iridocorneal angle were prepared from the donor eyes of the MYOC glaucoma patient and control eyes. We used antibodies directed against MYOC, collagen IV, and BiP/GRP78 as well as wheat germ agglutinin and concanavalin A lectins to localize MYOC protein in the trabecular meshwork.
Main Outcome Measure
Qualitative comparison of MYOC protein labeling and localization in the trabecular meshwork of donor eyes from a glaucoma patient with a MYOC mutation and from control subjects.
Results
Using immunohistochemistry, we detected more abundant MYOC protein within the trabecular meshwork of the MYOC glaucoma patient’s eye than in control eyes. We further localized MYOC protein within the trabecular meshwork cells of the MYOC glaucoma patient’s eye by co-labeling with the endoplasmic reticulum (ER) marker GRP78 (BiP). Little to no MYOC was identified within the trabecular meshwork cells of control eyes. Minimal extracellular MYOC was detected in both MYOC glaucoma eyes and control eyes.
Conclusions
This is the first histopathological analysis of an eye from a glaucoma patient with a MYOC mutation. Furthermore, this analysis supports our model of MYOC-associated glaucoma, in which MYOC mutations cause abnormal intracellular retention of MYOC within the ER of trabecular meshwork cells as a key step towards development of glaucoma.
Precìs
The first histological analysis of an eye from a patient with glaucoma caused by a myocilin mutation demonstrates abnormal intracellular retention of myocilin protein in the trabecular meshwork.
INTRODUCTION
Glaucoma is the leading cause of irreversible blindness worldwide1,2 and is a disease of the optic nerve and retinal ganglion cells. Optic nerve degeneration from glaucoma has a characteristic appearance on clinical exam, cupping of the optic disc, and is associated with stereotypical patterns of vison loss. Higher intraocular pressure is associated with higher risk for glaucoma, but glaucoma can occur at any intraocular pressure. The most common type of glaucoma is primary open angle glaucoma (POAG), which by definition is diagnosed after 40 years of age. Juvenile-onset open angle glaucoma (JOAG) is diagnosed between 3 and 40 years of age and is notable for markedly high intraocular pressure, autosomal dominant inheritance, and strong family history.3
Primary open angle glaucoma (POAG) has a strong genetic basis. Some cases are caused by the combined action of many genetic risk factors. Over 20 such risk factors for glaucoma have been discovered.4–6 Other cases have autosomal dominant inheritance patterns and are caused primarily by mutations in single genes.4 Three of these glaucoma-causing genes have been identified: myocilin (MYOQ,5 optineurin (OPTN),6 and TANK binding kinase 1 (.TBK1)7 MYOCwas the first glaucoma-causing gene to be identified and mutations in this gene are the most common known genetic cause of glaucoma. MYOC mutations are responsible for 2–4% of POAG cases worldwide5,8–10 and 8–63% of juvenile open angle glaucoma (JOAG).11–14 Over 100 different MYOC mutations have been reported to date http://www.myocilin.com. The vast majority of glaucoma-causing mutations in MYOC are located within exon 3, the olfactomedin-like domain, and are predicted to cause disease through dominant negative mechanisms.15 Some genotype-phenotype relationships have been recognized. Many MYOC mutations cause JOAG (Pro370Leu and Tyr437His) while others are associated with adult-onset POAG (Gln368Stop).9,8
MYOC encodes a secreted glycoprotein of unknown function. Although MYOC mRNA expression is almost ubiquitous throughout the body and eye,16,17 MYOC protein production has been primarily reported within the trabecular meshwork and other ocular tissues.18–20 Studies of cultured trabecular meshwork cells, anterior segment eye culture, transgenic mice, and human aqueous humor from glaucoma patients have shown that wild-type MYOC is secreted into aqueous humor or culture media, while mutant MYOC protein is retained within cells.21–25 Joe and coworkers demonstrated that accumulation of intracellular mutant MYOC protein in the endoplasmic reticulum (ER) causes ER stress,21 which has been replicated in transgenic mice.22 These data have suggested a model of MYOC−associated glaucoma in which disease-causing mutations prevent secretion and cause mutant MYOC to accumulate in trabecular meshwork cells, which stimulates ER stress and ultimately leads to loss of trabecular meshwork cell function and glaucoma.
While studies in cultured cells and animal models provide powerful and controlled insights into pathophysiology, analyses of human eyes harboring rare disease-causing mutations can offer a unique and robust understanding of how disease affects the production and localization of the mutant protein in the most relevant anatomical and phylogenetic context. In the current article, we investigate the localization of mutant MYOC protein in the first available human donor eye from a patient with glaucoma caused by a Tyr437His MYOC mutation. Immunohistochemical analyses of this human donor eye are consistent with our hypothesis that retention of mutant MYOC protein within trabecular meshwork cells occurs in MYOC-associated glaucoma.
MATERIALS AND METHODS
Human Donor Eyes
A patient with JOAG caused by a Tyr437His MYOC mutation (AR) is a member of a large pedigree that has been described in previous reports.5,9,23 He provided written informed consent for participating in this research project, which was conducted with the approval of the University of Iowa’s institutional review board (IRB) for human subjects research. At 59 years of age, AR’s right eye was painful and blind. His eye was removed (enucleated) and was made available for research. Control eyes were obtained as whole globes from human donors from the Iowa Lions Eye Bank (Iowa City, IA). Full consent for research was obtained from the donor’s next of kin in all cases, and all experiments were performed in accordance with the Declaration of Helsinki.
Following enucleation, AR’s right eye was immediately fixed in 10% neutral-buffered formalin. Another eye from an age-matched donor (age 66) that had no known eye disease was placed in 10% formalin 3.5 hours after death and used as a control. After 96 hours in 10% formalin, the eyes were transferred to 10mM phosphate-buffered saline (PBS). We had access to additional control eyes that had been previously collected. These human donor eyes (ages 51 – 59) with no known ocular disease had been fixed within 6–8 hours after death were used as a second set of controls. One of these control patients (Normal control #3) had been given a 3 day course of oral steroid 2 weeks prior to death and a 3 day intravenous course of steroids 1 week prior to death. The anterior segments of these additional eyes were fixed in 4% paraformaldehyde in 10mM PBS (pH 7.4) for 2 hours.
Anterior segments from all eyes were cryoprotected by passing through a sucrose gradient before being embedded in 20% sucrose in Optimal Cutting Temperature compound (Ted Pella, Redding, CA).24 Sections of 7 μm thickness were collected from each sample using a Microm H505E cryostat (Waldorf, Germany) and mounted on Superfrost plus slides (Ted Pella, Redding, CA).
Immunohistochemistry
Immunofluorescence procedures were performed as previously described.25 Briefly, sections were blocked for 15 minutes using a PBS solution with 1 mg/mL bovine serum albumin. Sections were then incubated in the primary antibody solution for 1 hour, followed by rinsing three times with PBS and incubation in the appropriate Alexa 488-, Alexa 546- and Alexa 633-conjugated secondary antibodies (Invitrogen, Eugene, OR) for 30 minutes.
Sections were counterstained with 4’−6-diamidino-2-phenylindole (DAPI), washed three times for five minutes in PBS, and coverslipped with Aquamount. All PBS solutions contained 1mM CaCl2 and 0.5mM MgCl2. Antibodies used for immunohistochemistry included anti-MYOC (HPA027364, Sigma-Aldrich, Darmstadt, Germany) used at a concentration of 0.5 ug/mL; anti-collagen IV (M3F7, Development Studies Hybridoma Bank, University of Iowa, Iowa City, IA) used at a concentration of 0.25 ug/mL; anti-CD45 (555480, BD Biosciences, San Jose, CA) used at a concentration of 2.5ug/mL; anti-GRP78 (14–9768-80, Thermo Fisher Scientific, Waltham, MA) used at a concentration of 10ug/mL. Biotinylated wheat germ agglutinin (B- 1025, Vector Laboratories, Burlingame, CA) and biotinylated concanavilin A (B-1005, Vector Laboratories, Burlingame, CA) were used at a concentration of 100ug/mL, followed by incubation in Dylight 488 Streptavidin (SA-5488, Vector Laboratories, Burlingame, CA) for 30 minutes at a concentration of 10ug/mL. Sections were viewed on an Olympus BX41fluorescence microscope with a SPOT RT camera and on a Leica SPS TCE confocal microscope. Photographs were captured using identical exposure, laser intensity, and gain settings for all sections being compared. Hematoxylin and eosin staining was performed under the following conditions: hematoxylin-1 min, rinse in double distilled water, acid alcohol-3 sec, rinse in water, 80% ethanol-1 min, eosin-2 sec, 95% ethanol-30 sec, 100% ethanol-30 sec, and xylenes-twice for 30 sec each. MM 24 mounting media was used to apply coverslips to H&E stained slides.
Optic nerve tissue preparation
A segment of the optic nerve posterior to the eye was fixed in a 1/2K buffer overnight, dehydrated in acetone, embedded in Spurrs resin, and 0.5 μm sections were acquired using a Leica EM UC5 Ultramicrotome with a Histo Diamond Knife. Sections were stained with a 1% paraphenylenediamine (PPD) in a 1:1 isopropanol:methanol solution for one hour, rinsed twice with 1:1 isopropanol:methanol for five minutes, and finally rinsed with xylenes for three minutes. Slides were coverslipped with 1:1 xylenes:Permount. Sections were viewed on an Olympus BX41fluorescence microscope with a SPOT RT camera.
RESULTS
In 1997, we identified a MYOC mutation (Tyr437His) in a very large pedigree that included over 27 family members with juvenile-onset glaucoma.26,5 One of the members of this pedigree, patient AR, donated his right eye for research at 59 years of age in 2017.
Clinical course of glaucoma patient AR with MYOC mutation (Tyr437His)
AR had typical features of juvenile open angle glaucoma, including early age at diagnosis, markedly high intraocular pressure, dominant inheritance of glaucoma, and a strong family history. He was diagnosed with JOAG at age 16 and his highest recorded intraocular
pressures were greater than 60 mm Hg in each eye. AR and his family members were found to have a heterozygous glaucoma-causing MYOC mutation (Tyr437His).
Surgery (trabeculectomy) was required to adequately control AR’s intraocular pressure (left eye at 29 years of age and right eye at 30 years of age). AR had good control of intraocular pressure for over 20 years following surgery with use of topical glaucoma medications. At age 53, however, AR had a series of additional problems in his right eye that began with an ocular infection (endophthalmitis) that was treated with surgery (vitrectomy and lensectomy) and antibiotics. One month later he had a retinal detachment in his right eye that was repaired with another surgery (vitrectomy and scleral buckle). Following these surgeries, AR developed corneal defects and his intraocular pressure become more elevated in his right eye. At 54 years of age AR had another glaucoma surgery on his right eye. A Baerveldt drainage device was placed to improve control of his intraocular pressure. Along with the drainage tube, a corneal transplant and an anterior chamber intraocular lens (AC-IOL) implant were also placed in an attempt to maximize and rehabilitate his vision. Despite these efforts, the vision in his right eye was limited to seeing hand motions. At 59 years of age, AR returned to clinic with bare light perception and pain in his right eye. He was diagnosed with another intraocular infection (endophthalmitis) and was treated with antibiotics and topical steroid. The infection was successfully treated, however, AR had persistent pain with bare light perception vision. Seventeen days after the onset of the infection his right eye was removed for pain control. The eye underwent histopathologic examination and research analysis.
Immunohistochemistry of the trabecular meshwork from a MYOC glaucoma patient (AR)
AR’s eye and an age matched normal control eye were identically fixed in formalin, dissected and analyzed for the presence of MYOC protein using various immunocytochemical approaches. Attention was focused on the trabecular meshwork, the primary site for fluid drainage from the eye, which is located at the juncture of the cornea and iris (the iridocorneal angle). We detected intense labeling of MYOC protein in the trabecular meshwork of AR’s eye with the Tyr437His mutation (Figure 1E, 1I, and 1M), while we detected no MYOC in the normal control eye (Figure 1F, 1J and 1N). We confirmed that more MYOC was present in the trabecular meshwork of a MYOC glaucoma patient (AR) than in control eyes by examining more control eyes. Much less MYOC protein was detected in the trabecular meshwork of an additional four eyes from control subjects, (representative images shown in Figure 1G–H, 1K#x2013;L and 1O–P). Specificity of the MYOC antibody was confirmed by preincubation of the antibody with a 10X excess of recombinant MYOC protein (H00004653-P01, Novus biologicals, San Diego, CA) (Supplemental Figure 1).
Figure 1. Immunohistochemical detection of MYOC protein in the trabecular meshwork of an eye with a MYOC Tyr437His mutation.
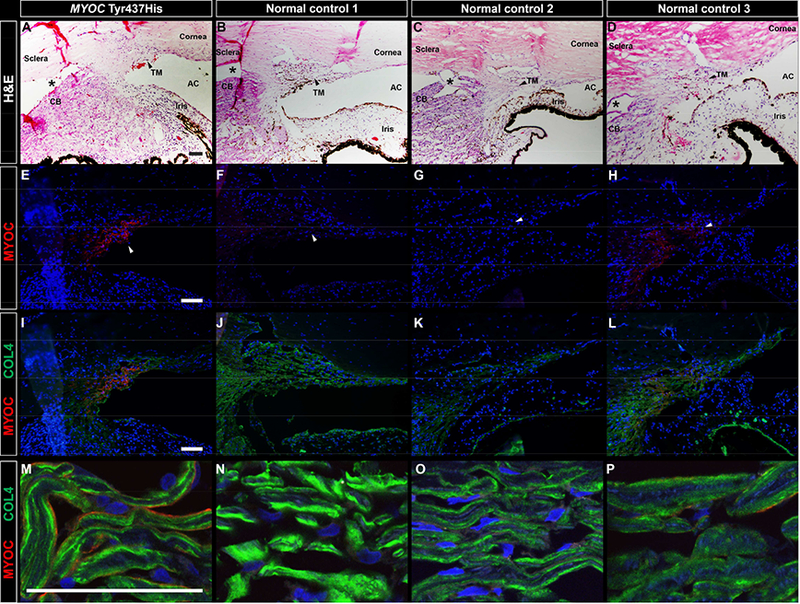
Brightfield microscopic images show H&E staining of the iridocorneal angle in an eye with a MYOC Tyr437His mutation (A) and three normal control eyes (B-D). Intense MYOC immunoreactivity (red) was observed in the trabecular meshwork of the eye with a MYOC Tyr437His mutation (E) No MYOC immunoreactivity was observed in normal control 1 (F), an age-matched donor with no known eye disease whose eye was identically fixed in 10% formalin to match fixation of the MYOC Tyr437His eye. Four additional normal control eyes from donors with no known ocular disease were previously fixed in 4% paraformaldehyde and were available for use as an additional set of controls. No MYOC immunoreactivity was observed in three of the four additional control eyes, as shown with a representative image from normal control 2 (G). One of the four additional control eyes, normal control 3, exhibited minimal positive MYOC labeling (H). The MYOC Tyr437His eye and all of the normal control eyes show collagen IV immunoreactivity (green) of the trabecular meshwork (I-P). At higher magnification, collagen IV is seen deposited along the beams of the trabecular meshwork (M-P), with minimal to no co-localization with MYOC in the MYOC Tyr437His eye (M) or in normal control 3 (P). Artifactitious tissue separation during processing is denoted by an asterisks. Black and white arrows point to the trabecular meshwork. Cell nuclei are labeled with DAPI (blue). TM, trabecular meshwork; AC, anterior chamber; CB, ciliary body. Scale bars = 100μm.
We further localized MYOC within the trabecular meshwork of AR’s eye by co-labeling with an antibody directed against collagen IV (COL4A). Collagen IV has been previously localized in the trabecular meshwork to the basal lamina between the trabecular meshwork cells and the trabecular beams, which are composed largely of collagen I, collagen III, and elastin.27 The anti-COL4A antibody labeled the surface of the trabecular beams in a pattern consistent with the basal lamina (Figure 1I–L, 1M–P). Co-labeling of MYOC and COL4A showed virtually no overlap suggesting that MYOC is localized within the trabecular meshwork cells that line the surface of the trabecular beams.
We investigated MYOC localization within trabecular meshwork cells using two lectins, wheat germ agglutinin (WGA) and concanavalin (ConA), that have been previously shown to label trabecular meshwork cells.28–30 We observed extensive co-labeling of MYOC with WGA along the surface of the collagen beams in a pattern consistent with localization of MYOC with trabecular meshwork cells (Figure 2A–D). Labeling with ConA produced the same pattern of intracellular localization of MYOC (Figure 2E–H). Given prior reports demonstrating accumulation of mutant MYOC in the ER,22 we further studied MYOC localization in trabecular meshwork cells using antibodies against the ER marker, GRP78 (BiP). We detected co-labeling of GRP78 and MYOC in trabecular meshwork cells of AR’s eye (Figure 2I–L).
Figure 2. Confocal microscopy of MYOC protein within trabecular meshwork cells of an eye with a MYOC Tyr437His mutation.
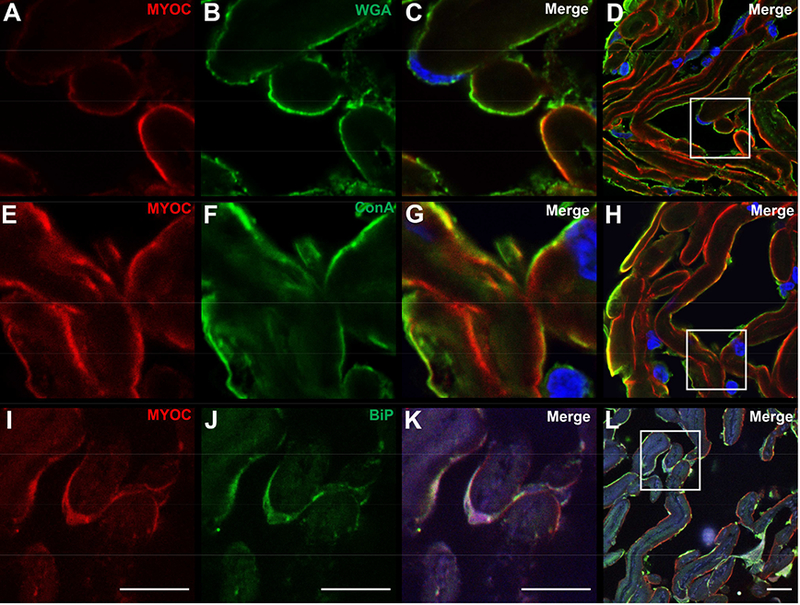
Confocal microscopy shows MYOC immunoreactivity in the trabecular meshwork of the eye with a MYOC Tyr437His mutation that co-localizes with two markers for trabecular meshwork cells, wheat germ agglutinin (WGA) lectin (A-D), and concanavalin (ConA) lectin (E-H). Co-labeling suggests localization of MYOC within trabecular meshwork cells. MYOC immunoreactivity also co-localizes with GRP78/BiP, a marker of ER stress (I-L) Cell nuclei are labeled with DAPI (blue). Scale bars, 10 μm.
Finally, given the history of recent ocular infection, we tested for the presence of leukocytes in the trabecular meshwork by labeling with an anti-CD45 antibody. Although leukocytes were notable in the anterior chamber of this donor eye compared with controls, we observed no co-labeling of MYOC and CD45 (Supplemental Figure 2) further indicating that MYOC is localized within trabecular meshwork cells rather than in infiltrating leukocytes.
Optic nerve histology
We investigated the effects of glaucoma caused by the Tyr437His MYOC mutation on AR’s optic nerve. Sections of the optic nerve were stained with paraphenylenediamine (PPD) to visualize myelin sheaths surrounding axons (Figure 3). We detected a profound loss of axons in both cross-sections and longitudinal sections of AR’s optic nerve, consistent with his severe glaucoma.
Figure 3. Histologic features of the optic nerve of an eye with a MYOC.
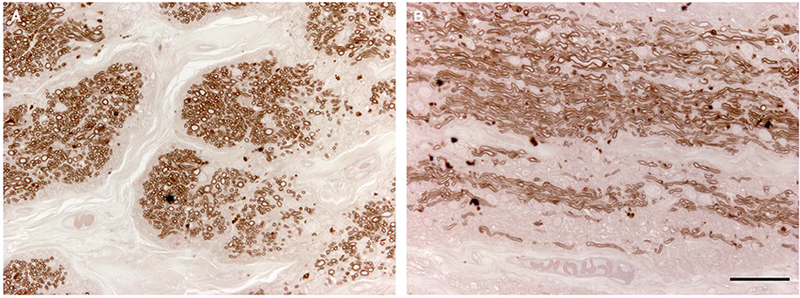
Tyr437His mutation. The myelin sheaths of optic nerve axons were stained with paraphenylenediamine (PPD). Extensive loss of axons caused by severe glaucoma is seen in cross-section (A) and sagittal section (B) of patient AR’s optic nerve. Scale bar = 100μm.
CONCLUSIONS
Human donor eyes from glaucoma patients with disease-causing MYOC mutations are extremely rare. To our knowledge, this is the first report of histological studies of an eye from a glaucoma patient with a MYOC mutation. Prior to histological study, AR’s eye experienced trauma and inflammation from multiple surgeries, intraocular infection, and prior retinal detachment. Each of these events may have caused anatomic or functional changes in the trabecular meshwork of AR’s eye that may have influenced MYOC production and/or localization. Moreover, MYOC production is known to be stimulated by steroids,18 and AR received topical steroid therapy for 17 days prior to removal of his eye. Steroid- induction of MYOC production may have contributed to the increased amount of MYOC detected in AR’s eye. Given AR’s complex clinical history with potential artifactual influences on MYOC protein production and localization, caution must be exercised in interpreting the results. Similarly, one control eye had a small amount of MYOC in its trabecular meshwork cells and came from an individual who received systemic steroids prior to death (Normal control #3), which may have altered MYOC expression in this eye. Despite these limitations, we believe that our studies of the first human donor eye with a glaucoma-causing MYOC mutation have provided valuable insights into the pathogenesis of MYOC−associated glaucoma.
Previous studies of MYOC in human eyes have been limited to donor eyes (not known to carry a MYOC mutation)19 and to surgical samples of aqueous humor and trabeculectomy specimens.31,32 To the best of our knowledge, this report presents the first pathologic analysis of a human donor eye with glaucoma caused by a MYOC mutation. Our study of this eye provides compelling support for our model of MYOC−associated glaucoma that was based primarily on studies of transgenic animals and cell lines. Transfection or viral transduction of cultured human trabecular meshwork cells with MYOC vectors has previously shown that wild-type MYOC protein is secreted, while mutant MYOC protein is retained within cells31 and stimulates ER stress.21 The same pattern of non-secretion / retention of mutant MYOC within the trabecular meshwork cells has also been observed in transgenic Tg-MYCCTyr437His/+ mice22 and in mice whose eyes were transduced with adenovirus carrying a mutant MYOC gene.33 It has been hypothesized that intracellular retention of abnormal MYOC may be toxic to trabecular meshwork cells, stimulate ER stress, and may ultimately lead to trabecular meshwork cell death. Such loss of trabecular meshwork cells and intraocular pressure regulation may be an important early step in glaucoma related to MYOC mutations. In the current report we show that a naturally-occurring MYOC mutation in our patient’s genome causes intracellular retention of MYOC protein in the ER of trabecular meshwork cells of a human eye. Mislocalization and accumulation of MYOC within trabecular meshwork cells is a key step in the pathophysiology of glaucoma in our human patient with glaucoma caused by a MYOC mutation. Moreover, co-localization of MYOC with an ER stress molecule, GRP78 (BiP) provides additional support for the hypothesis that ER stress may be involved in the mechanisms by which mutant MYOC promotes glaucoma.
Supplementary Material
(A) is attenuated by preincubation of the MYOC antibody with 5X (B) and 10X (C) excess recombinant human MYOC protein for 30 min prior to labeling the tissue section. No primary antibody control (D). Cell nuclei are labeled with DAPI (blue). Scale bar = 100um.
Fluorescence microscopy shows rare CD45+ leukocytes in the anterior segment of a normal eye (A) and abundant CD45+ cells in the eye with a MYOC Tyr437His mutation (B). Labeling with the anti-CD45 antibody shows no colocalization of MYOC (B) indicating that MYOC is in trabecular meshwork cells and not in leukocytes. No primary antibody control (C). Cell nuclei are labeled with DAPI (blue). Scale bar = 100µm.
ACKNOWLEDGEMENTS
This research was supported in part by NIH R01EY023512 (JHF), NIH P30 EY025580 (RFM), and by funds from the Marlene and Leonard Hadley and Martin Carver Chair in Glaucoma (JHF). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Quigley HA, Broman AT: The number of people with glaucoma worldwide in 2010 and 2020. The British journal of ophthalmology 2006, 90:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hons Y-CTB, BSc XL, PhD TYWF, MD HAQ, PhD TAFE, PhD C-YCM: Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology 2014, 121:1–1024388392 [Google Scholar]
- 3.Kwon YH, Fingert JH, Kuehn MH, Alward WLM: Primary Open-Angle Glaucoma. The New England journal of medicine 2009, 360:1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fingert JH: Primary open-angle glaucoma genes. Eye 2011, 25:587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone EM, Fingert JH, Alward WLM, Nguyen TD, Polansky JR, Sunden SLF, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC: Identification of a Gene That Causes Primary Open Angle Glaucoma. Science (New York, NY) 275:668–670 [DOI] [PubMed] [Google Scholar]
- 6.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Héon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M: Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science (New York, NY) 2002, 295:1077–1079 [DOI] [PubMed] [Google Scholar]
- 7.Fingert JH, Robin AL, Ben R Roos, Davis LK, Scheetz TE, Wassink TH, Kwon YH, Alward WLM, Mullins RF, Sheffield VC, Stone EM: Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Human Molecular Genetics 2011, 20:2482–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam MF, Belmouden A, Binisti P, Brézin AP, Valtot F, Béchetoille A, Dascotte JC, Copin B, Gomez L, Chaventré A, Bach JF, Garchon H-J: Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Human Molecular Genetics 1997, 6:2091–2097 [DOI] [PubMed] [Google Scholar]
- 9.Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM: Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). The New England journal of medicine 338:1022–1027 [DOI] [PubMed] [Google Scholar]
- 10.Fingert JH, Héon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM: Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Human Molecular Genetics 1999, 8:899–905 [DOI] [PubMed] [Google Scholar]
- 11.Wiggs JL, Allingham RR, Vollrath D, Jones KH, La Paz De M, Kern J, Patterson K, Babb VL, Del Bono EA, Broomer BW, Pericak-Vance MA, Haines JL: Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. American journal of human genetics 1998, 63:1549–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen Y-C, Yang J-J, Chou M-C, Li S-Y: Identification of mutations in the myocilin (MYOC) gene in Taiwanese patients with juvenile-onset open-angle glaucoma. Molecular vision 2007, 13:1627−1634 [PubMed] [Google Scholar]
- 13.Bruttini M, Longo I, Frezzotti P, Ciappetta R, Randazzo A, Orzalesi N, Fumagalli E, Caporossi A, Frezzotti R, Renieri A: Mutations in the myocilin gene in families with primary open-angle glaucoma and juvenile open-angle glaucoma. Archives of ophthalmology 2003, 121:1034–1038 [DOI] [PubMed] [Google Scholar]
- 14.Stoilova D, Child A, Brice G, Desai T, Barsoum-Homsy M, Ozdemir N, Chevrette L, Adam MF, Garchon H-J, Pitts Crick R, Sarfarazi M: Novel TIGR/MYOC mutations in families with juvenile onset primary open angle glaucoma. Journal of Medical Genetics 1998, 35:989–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon YH, Fingert JH, Kuehn MH, Alward WLM: Primary Open-Angle Glaucoma. The New England journal of medicine 2009, 360:1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fingert JH, Ying L, Swiderski RE, Nystuen AM, Arbour NC, Alward WL, Sheffield VC, Stone EM: Characterization and comparison of the human and mouse GLC1A glaucoma genes. Genome Research 1998, 8:377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swiderski RE, Ross JL, Fingert JH, Clark AF, Alward WL, Stone EM, Sheffield VC: Localization of MYOC transcripts in human eye and optic nerve by in situ hybridization. Investigative Ophthalmology & Visual Science 2000, 41:3420–3428 [PubMed] [Google Scholar]
- 18.Polansky JR, Fauss DJ, Chen P, Chen H, Lütjen-Drecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD: Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica Journal international d’ophtalmologie International journal of ophthalmology Zeitschrift für Augenheilkunde 1997, 211:126–139 [DOI] [PubMed] [Google Scholar]
- 19.Lütjen-Drecoll E, May CA, Polansky JR, Johnson DH, Bloemendal H, Nguyen TD: Localization of the stress proteins alpha B-crystallin and trabecular meshwork inducible glucocorticoid response protein in normal and glaucomatous trabecular meshwork. Investigative Ophthalmology & Visual Science 1998, 39:517–525 [PubMed] [Google Scholar]
- 20.Clark AF, Steely HT, Dickerson JE, English-Wright S, Stropki K, McCartney MD, Jacobson N, Shepard AR, Clark JI, Matsushima H, Peskind ER, Leverenz JB, Wilkinson CW, Swiderski RE, Fingert JH, Sheffield VC, Stone EM: Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Investigative Ophthalmology & Visual Science 2001, 42:1769. –1780 [PubMed] [Google Scholar]
- 21.Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C: Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochemical and Biophysical Research Communications 2003, 312:592–600 [DOI] [PubMed] [Google Scholar]
- 22.Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC: Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. The Journal of clinical investigation 2011, 121:3542–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fingert JH, Héon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM: Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Human Molecular Genetics 1999, 8:899–905 [DOI] [PubMed] [Google Scholar]
- 24.Barthel LK, Raymond PA: Improved method for obtaining 3-microns cryosections for immunocytochemistry. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 1990, 38:1383–1388 [DOI] [PubMed] [Google Scholar]
- 25.Chirco KR, Hazlewood RJ, Miller K, Workalemahu G, Jampol LM, Lesser GR, Mullins RF, Kuehn MH, Fingert JH: MMP19 expression in the human optic nerve. Molecular vision 2016, 22:1429–1436 [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson AT, Drack AV, Kwitek AE, Cannon RL, Stone EM, Alward WL: Clinical features and linkage analysis of a family with autosomal dominant juvenile glaucoma. Ophthalmology 1993, 100:524–529 [DOI] [PubMed] [Google Scholar]
- 27.Tamm ER: Functional Morphology of the Trabecular Meshwork Outflow Pathways. Glaucoma 2015, 40–46 [Google Scholar]
- 28.Kurosawa A, Elner VM, Yue BY, Elvart JL, Tso MO: Cultured trabecular-meshwork cells: immunohistochemical and lectin-binding characteristics. Experimental Eye Research 1987, 45:239–251 [DOI] [PubMed] [Google Scholar]
- 29.Tripathi BJ, Marcus CH, Tripathi RC, Millard CB, Gulcher J, Stefansson K: Monoclonal antibodies and lectins as probes for investigation of the cell biology of human trabecular meshwork: a preliminary report. Ophthalmic research 1989, 21:27–32 [DOI] [PubMed] [Google Scholar]
- 30.Tuori A, Virtanen I, Uusitalo H: Lectin binding in the anterior segment of the bovine eye. The Histochemical journal 1994, 26:787–798 [PubMed] [Google Scholar]
- 31.Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC: Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Human Molecular Genetics 2001, 10:117–125 [DOI] [PubMed] [Google Scholar]
- 32.Hamanaka T, Kimura M, Sakurai T, Ishida N, Yasuda J, Nagasaki M, Nariai N, Endo A, Homma K, Katsuoka F, Matsubara Y, Yamamoto M, Fuse N: A Histologic Categorization of Aqueous Outflow Routes in Familial Open-Angle Glaucoma and Associations With Mutations in the MYOC Gene in Japanese Patients. Investigative Ophthalmology & Visual Science 2017, 58:2818–2831 [DOI] [PubMed] [Google Scholar]
- 33.McDowell CM, Luan T, Zhang Z, Putliwala T, Wordinger RJ, Millar JC, John SWM, Pang I-H, Clark AF: Mutant human myocilin induces strain specific differences in ocular hypertension and optic nerve damage in mice. Experimental Eye Research 2012, 100:65–72] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) is attenuated by preincubation of the MYOC antibody with 5X (B) and 10X (C) excess recombinant human MYOC protein for 30 min prior to labeling the tissue section. No primary antibody control (D). Cell nuclei are labeled with DAPI (blue). Scale bar = 100um.
Fluorescence microscopy shows rare CD45+ leukocytes in the anterior segment of a normal eye (A) and abundant CD45+ cells in the eye with a MYOC Tyr437His mutation (B). Labeling with the anti-CD45 antibody shows no colocalization of MYOC (B) indicating that MYOC is in trabecular meshwork cells and not in leukocytes. No primary antibody control (C). Cell nuclei are labeled with DAPI (blue). Scale bar = 100µm.


