Abstract
Background
Nutrition with ammonium (NH4+) can enhance the drought tolerance of rice seedlings in comparison to nutrition with nitrate (NO3−). However, there are still no detailed studies investigating the response of nitric oxide (NO) to the different nitrogen nutrition and water regimes. To study the intrinsic mechanism underpinning this relationship, the time-dependent production of NO and its protective role in the antioxidant defense system of NH4+- or NO3−-supplied rice seedlings were studied under water stress.
Results
An early NO burst was induced by 3 h of water stress in the roots of seedlings subjected to NH4+ treatment, but this phenomenon was not observed under NO3− treatment. Root oxidative damage induced by water stress was significantly higher for treatment with NO3− than with NH4+ due to reactive oxygen species (ROS) accumulation in the former. Inducing NO production by applying the NO donor 3 h after NO3− treatment alleviated the oxidative damage, while inhibiting the early NO burst by applying the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO) increased root oxidative damage in NH4+ treatment. Application of the nitric oxide synthase (NOS) inhibitor N(G)-nitro-L-arginine methyl ester(L-NAME) completely suppressed NO synthesis in roots 3 h after NH4+ treatment and aggravated water stress-induced oxidative damage. Therefore, the aggravation of oxidative damage by L-NAME might have resulted from changes in the NOS-mediated early NO burst. Water stress also increased the activity of root antioxidant enzymes (catalase, superoxide dismutase, and ascorbate peroxidase). These were further induced by the NO donor but repressed by the NO scavenger and NOS inhibitor in NH4+-treated roots.
Conclusion
These findings demonstrate that the NOS-mediated early NO burst plays an important role in alleviating oxidative damage induced by water stress by enhancing the antioxidant defenses in roots supplemented with NH4+.
Electronic supplementary material
The online version of this article (10.1186/s12870-019-1721-2) contains supplementary material, which is available to authorized users.
Keywords: Ammonium, Nitric oxide, Nitric oxide synthase, Oxidative damage, Antioxidant enzymes, Water stress
Background
As human population and global climate change increase, drought stress is becoming a major abiotic factor limiting crop growth and yield. Plants have evolved several strategies to contend with water stress. These include morphological, physiological, and molecular adaptations [1–3]. As an important signaling molecule in various physiological functions like seed germination, floral transition, stomatal movement, leaf senescence, and yield development, nitric oxide (NO) has gained increasing attention since the 1980s [4–6]. Certain plant responses and adaptations to abiotic stresses also involve NO, and sufficient data indicate that NO mediates plant responses to various stimuli including drought [7], salt [8], and metal toxicity [9] stresses, thereby enhancing plant stress tolerance and survival.
Water deficits simultaneously increase endogenous NO and reactive oxygen species (ROS) production in plants [7, 10]. The accumulation of ROS in water-stressed plants impairs the function of biochemical processes, damages organelles, and ultimately results in cell death [11]. A combination of pharmacological analysis and transgenic technology has indicated that NO induces antioxidant activity and alleviates water stress in plants in several ways: (1) It limits ROS accumulation and ROS-induced cytotoxic activity by inhibiting the ROS-producer nicotinamide adenine dinucleotide phosphate oxidase via S-nitrosylation [12]. (2) It reacts with ROS (e.g. O2.-) to generate transient ONOO−, which is then immediately scavenged by other cellular processes [13]. (3) It induces the expression of genes coding for antioxidant enzymes, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR), and may increase enzyme activity, thereby reducing lipid peroxidation under water stress [14]. (4) It helps maintaining high vacuolar concentrations of osmotically active solutes and amino acids like proline [15]. (5) It acts as a downstream abscisic acid (ABA) signal molecule and participates in “ABA-H2O2-NO-MAPK” signal transduction processes, and thus increases plant antioxidant ability [16]. Therefore, endogenous NO production may enhance plant antioxidant capacity and help plant cells survive under various types of stress.
However, NO has biphasic properties on plants. The duality of its effects depends on stress duration and severity, and on the cell, tissue, and plant species [17]. At low concentration or in the early stage of abiotic stress, NO participates in important functions in higher plants through its involvement in physiological and stress-related processes (as described above). Some authors demonstrated that NO synthesis slightly increased in roots subjected to < 10 h water deficit, but was significantly up-regulated after prolonged drought (≥17 h) [18, 19]. Under severe or protracted stress, NO overproduction in plants can shift the cellular stress status from oxidative stress to severe nitrification stress, finally damaging proteins, nucleic acids, and membranes [13, 20]. Protein tyrosine nitration is considered a good marker to evaluate the process of nitrosative stress under various abiotic stresses [21]. Excess NO can also act synergistically with ROS, resulting in nitro-oxidative stress and eliciting undesirable toxic effects in plant cells [7]. Liao et al. [22] argued that the ability of endogenous or exogenous NO production in plants to alleviate oxidant damage was dose-dependent. Therefore, determining the instantaneous plant NO content under drought stress may not completely reflect the specific role of NO in drought tolerance.
In higher plants, nitrate reductase (NR) and nitric oxide synthase (NOS) are the two key enzymes for NO production [4, 23]. Moreover, NR-dependent NO production occurs in response to pathogen infection [24], aluminum [25], freezing [26], and drought [27]. For a long time, although NOS-like activity had been detected in plants, the gene(s) encoding NOS protein in higher plants remained to be identified [28]. Recently, some authors demonstrated that mammalian NOS inhibitors suppress NO production in response to various stimuli in plants [22, 29], suggesting that an arginine-dependent NOS activity may also occur in plants. Overexpression of rat neuronal NO synthase in plants increased their tolerance to drought stress, also demonstrating the importance of NOS-mediated NO production in tolerance of water deficits [30]. Arasimowicz-Jelonek et al. [18, 19] applied the NO donor sodium nitroprusside (SNP) and S-nitrosoglutathione (GSNO) to water-stressed cucumbers and demonstrated that both NR and NOS participated in drought tolerance. Despite increasing knowledge on NO-mediated plant functions, NO origins and signaling in response to prolonged stress and their regulation in plant drought tolerance remain poorly understood.
Ammonium (NH4+) and nitrate (NO3−) are the two primary N sources for plants. It is known that the negative effects of drought stress on plant development can be more effectively alleviated by NH4+ than NO3− nutrition, as evaluated by plant growth, physiological characteristics, and gene expression levels [2, 31, 32]. NO has a key role in the acclimation of plants to water stress. Nevertheless, information on the dynamic changes in NO production and its role in drought acclimation in plants supplied with NO3− or NH4+ during the early stage of water stress is scarce. In the present study, variations in endogenous NO production were monitored in roots supplied with these two N nutrition supplements during water stress. The specific role and origin of the endogenous NO produced were investigated using pharmacological methods. The present study revealed that an early NO burst is crucial for alleviating the water stress-induced oxidative damage through enhancement of antioxidant defenses in roots of NH4+-supplied plants. Further analyses demonstrated that this early NO burst might be triggered by NOS-like enzyme.
Results
Plant growth and physiological characteristics
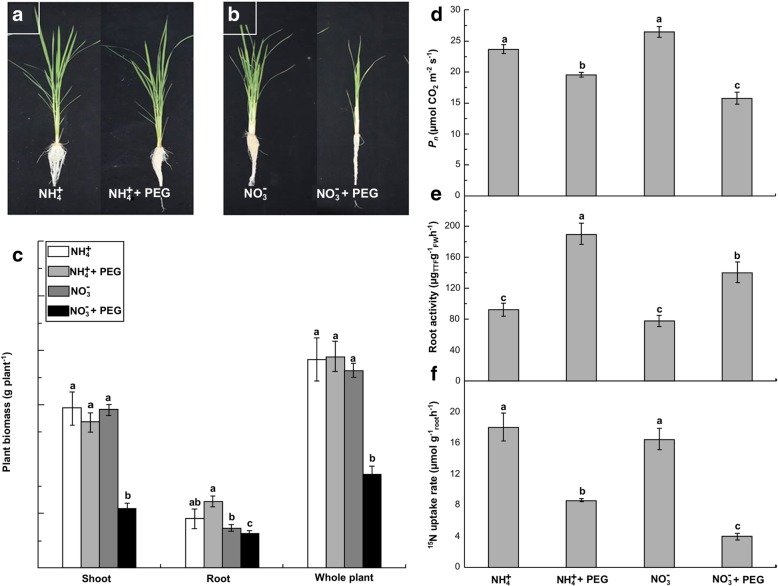
Growth- and physiology-related parameters, such as biomass, net photosynthetic rate (Pn), and root N uptake rate in rice seedlings supplied with different N sources were negatively and differently influenced by water stress (Fig. 1a–f). After 21 days of water stress (as a result of polyethylene glycol [PEG] treatment), root, shoot and total biomass were significantly decreased by 14.1, 62.1 and 52.4% in treatment with NO3− and PEG, compared to its non-water stress treatment (NO3− treatment, as the control treatment) (Fig. 1b, c). However, these values were not significantly affected in NH4+ with PEG treatment. Water stress also reduced leaf (Pn) and root 15N uptake rate in the NO3−-treated plants by 40.4 and 76.1% (P < 0.05) in relation to non-water-stress-treated plants, but that of NH4+-treated plants was reduced by 17.3 and 52.2% (Fig. 1d, f). In contrast, root activity under water stress was increased by 106.2 and 79.6% in the NO3−-treated and NH4+-treated plants, respectively. Thus, it seems that NH4+-supplied rice seedlings can alleviate PEG-induced water stress more effectively than NO3−-supplied rice seedlings.
Fig. 1.
(a), (b) and (c) Response of rice agronomic characteristics and biomass in NH4+- and NO3−-supplied rice to water stress stimulated by 10% PEG after 21 days. (d) Effects of water stress on leaf photosynthesis in NH4+- and NO3−-supplied rice after 21 days. (e) Effects of water stress on root activity in NH4+- and NO3−-supplied rice after 21 days. (f) Effects of water stress on root 15N-labeled uptake rate in NH4+- and NO3−-supplied rice after 21 days. Rice leaf photosynthesis, root activity and 15N uptake rate was determined as described in Additional file 4: Method S1. Values represent means ± standard error (SE) (n = 6). Different letters refer to significant differences at P < 0.05. TTF: triphenylformazane; FW: fresh weight
Root endogenous NO production and histochemical analyses of oxidative damage
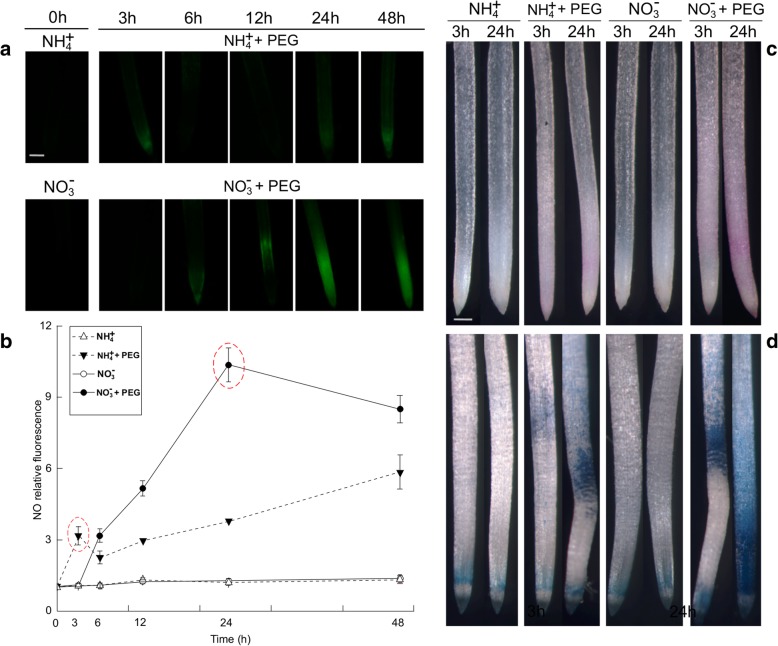
To investigate whether NO participates in water stress acclimation, endogenous NO levels in roots were monitored with the NO-specific fluorescent probe diaminofluorescein-FM diacetate (DAF-FM DA) [25]. Significant differences in endogenous NO production were observed in roots after 48 h of water stress (Fig. 2a). In the NH4+ or NO3−-treated plants, NO production was relatively stable and varied little between the two N nutritions (Fig. 2b). In contrast, water stress significantly induced endogenous NO production 3 h after the roots received NH4+. However, endogenous NO gradually increased only after 6 h in the NO3− treatment. Relative fluorescence indicated a significant early burst of NO at 3 h of water stress in the NH4+ with PEG treatment relative to the NH4+ treatment (Con). The NO level in the seedlings treated with NH4+ was 2.92 times higher than that of NO3−-treated plants. Nevertheless, NO in the NO3−-treated seedlings was 2.72 times higher than in NH4+-treated plants after 24 h of water stress (Fig. 2b).
Fig. 2.
Time-dependent endogenous nitric oxide (NO) production and histochemical detection of oxidative damage in the root apices of NH4+- and NO3−-supplied rice seedlings under water stress. (a) Detection of NO fluorescence using DAF-FM DA staining and a fluorescence microscope. NO generation is indicated by green fluorescence. Bar = 300 μm. (b) NO production is expressed as relative fluorescence. To detect the NO production time course, seedling roots exposed to 10% PEG were collected at 0, 3, 6, 12, 24, and 48 h. (c) and (d) Histochemical detection of the aldehydes derived from lipid peroxidation and Evans blue uptake in root apices of rice seedlings under water stress. Rice seedlings were either untreated or subjected to 3 or 24 h of water stress, respectively. Roots were stained with Schiff’s reagent (c) and Evans blue (d), and then immediately photographed under a Leica S6E stereomicroscope (Leica, Solms, Germany). Red/purple indicates the presence of lipid peroxidation detected with Schiff’s reagent. Bar = 1 mm. Endogenous NO concentrations and histochemical detection of oxidative damage in the root are given. In Fig. 2b, the red dotted oval represents the high endogenous NO production in the NH4+- and NO3−-supplied rice, respectively. Values represent means ± standard error (SE) (n = 6). Different letters refer to significant differences at P < 0.05. Con indicates control treatment for each N nutrition, i.e., plants receiving non-water stress
Histochemical visualization by Schiff’s reagent and Evans blue staining showed that water stress caused severe oxidative damage to the plasma membrane and cell death in the roots of the plants receiving NO3−, whereas the damage was far less pronounced in the seedlings given NH4+ (Fig. 2c, d). The following analysis of the malondialdehyde (MDA) and carbonyl concentrations also confirmed that water stress induced more severe lipid peroxidation in the roots of NO3−-treated than in the roots of NH4+-treated seedlings (Fig. 3c, d).
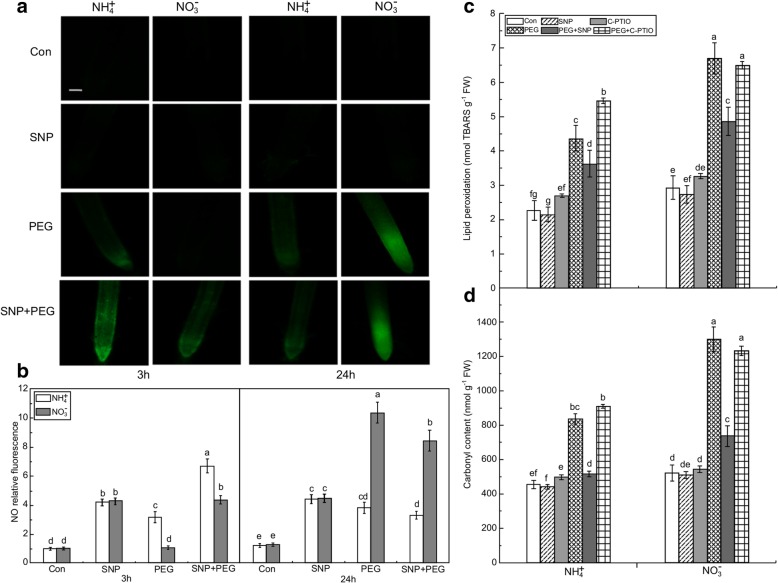
Fig. 3.
Responses of endogenous nitric oxide (NO) concentrations and oxidative damage to NO donor (SNP) or NO scavenger (c-PTIO) in root apices under the non-water stress (Con) or water stress conditions. (a) Photographs of NO production after SNP application. Bar = 300 μm. (b) NO production expressed as relative fluorescence. Rice seedlings were either untreated or treated with SNP under water stress. After 3 h and 24 h of treatment, root tips were loaded with 10 μM DAF-FM DA and NO fluorescence was imaged after 20 min using a fluorescence microscope. Endogenous NO concentrations in root are displayed. The lipid peroxidation (c) and carbonyl concentration (d) in rice roots represent the oxidative damage. In the c-PTIO and PEG + c-PTIO treatments, the rice seedlings were pretreated with NO scavenger (c-PTIO) for 3 h followed by non-water stress or water stress. After 3 h, the contents of MDA representing lipid peroxidation and carbonyl group in rice seedling roots were determined. Values represent means ± standard error (SE) (n = 6). Different letters indicate significant differences at P < 0.05. Con indicates control treatment for each N nutrition, i.e., plants receiving non-water stress. FW: fresh weight; TBARS: thiobarbituric acid reactive substances
Effects of the NO donor on root NO production and oxidative damage
To determine the role of NO in water stress tolerance, the NO donor SNP was used to simulate NO production. Pre-experimentation with various SNP concentrations (0–100 μM) was performed to quantify the efficacy of SNP against root oxidative damage. As shown in Additional file 1: Figure S1, root oxidative damage induced by water stress was significantly alleviated by ≤20 μM SNP. However, the remedial effect of SNP on root oxidative damage was reversed at higher application doses (≥ 40 μM), suggesting that high SNP or NO contents are toxic to root growth. Therefore, 20 μM SNP was used in the NO donor experiment conducted in the present study. After 3 h of water stress, SNP application significantly increased root NO fluorescence intensity for both N nutrition. At 3 h, the NO production levels were ~ 2.05 and 3.85 times higher in the SNP + PEG-treated roots of the seedlings receiving NH4+ and NO3−, respectively, than in the PEG-treated roots of Con plants (Fig. 3a, b). However, this phenomenon was not observed after 24 h of water stress.
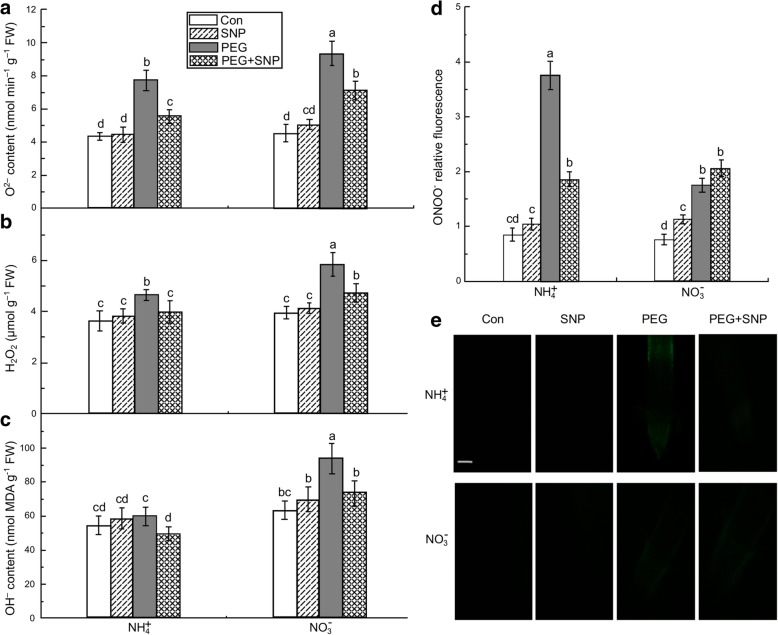
After 3 h of water stress, ROS (O2.-, H2O2, and OH−) levels were increased in the roots of both the NH4+ + PEG- and NO3− + PEG-treated seedlings in relation to their Con seedlings. Under water stress, the O2.-, H2O2, and OH− in the roots given NH4+ and NO3− increased by 78.1 and 107.3%, 28.3 and 47.8%, and 10.6 and 48.4%, respectively (Fig. 4a–c). Root MDA and carbonyl contents were ~ 1.28 and 1.4 times higher in the plants receiving NO3− + PEG than in Con plants, respectively. In turn, MDA and carbonyl levels were significantly higher in NO3− + PEG-treated plants than in plants given NH4+ + PEG (Fig. 3c, d). Water stress induced higher root ONOO− in the NH4+-treated plants than in the NO3−-treated seedlings (Fig. 4d), and exogenous NO application significantly reduced water stress-induced >ROS (O2.- and H2O2) accumulation and oxidative damage (as reflected by MDA and carbonyl) in both N nutritions (Figs. 3, 4).
Fig. 4.
ROS and ONOO− accumulation in root apices of rice seedlings treated with NO donor (SNP) and either receiving non-water stress (Con) or subjected to water stress using PEG. After 3 h, O2.- (a), H2O2 (b), and OH− (c) levels in rice seedlings roots were measured by spectrophotometry. ONOO- production expressed as relative fluorescence (d). The accumulation of ONOO- was detected with 10 μΜ aminophenyl fluorescein (e), Bar =3 00 μm. Fluorescence images and relative fluorescence intensity were analyzed as described in Fig. 2 for NO determination. Values represent means ± standard error (SE) (n = 6). Different letters indicate significant differences at P < 0.05. Con indicates control treatment for each N nutrition, i.e., plants receiving non-water stress. FW: fresh weight
To determine whether the alleviation of water stress-induced oxidative damage by SNP was related to NO production, the NO scavenger c-PTIO was applied to the plants. After pretreatment with 100 μM c-PTIO for 3 h, the alleviation of root oxidative damage induced by SNP application under water stress was reversed (Fig. 3c, d). Depletion of endogenous NO by c-PTIO significantly aggravated root oxidative damage in NH4+-treated plants but had no significant effect on the NO3−-treated plants, in relation to that observed in PEG-treated plants. Therefore, the water stress-induced early NO burst observed in the NH4+-treated plants alleviates root oxidative damage by reducing ROS, such as O2.- and H2O2.
Source of endogenous NO
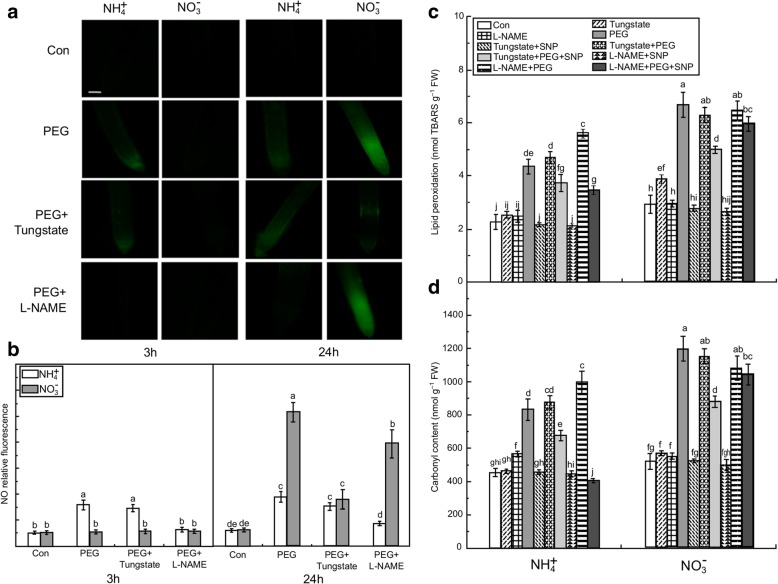
Endogenous plant NO production is mostly driven by NR and NOS activity [4]. Water stress increased NR activity in NO3−-treated roots, and this activity was higher at 24 h than it was at 3 h of water stress (Additional file 2: Figure S2a). The activity of NOS was also significantly elevated at 3 h of water stress, and significantly higher in the NH4+-treated than in the NO3−-treated roots (Additional file 2: Figure S2b). In contrast, water stress suppressed NOS activity in NO3−-treated roots at 24 h. Tungstate and L-NAME, which inhibit NR and NOS activities, respectively, were used to identify the origin of the early NO burst in the NH4+-treated roots [25]. Although L-NAME significantly inhibited endogenous NO production in NH4+-treated roots at 3 h of water stress, it had no significant effect in the NO3−-treated roots. At 24 h, the tungstate and L-NAME applications suppressed NO production in NO3−-treated roots, and tungstate had the stronger inhibitory effect. However, tungstate had no significant effect on NO production in the NH4+-treated roots (Fig. 5a, b).
Fig. 5.
Effects of NR inhibitor (tungstate) and NOS inhibitor (L-NAME) on NO content and oxidative damage in root apices of rice seedlings. Rice seedlings were pretreated with NR inhibitor (100 μM tungstate) or NOS inhibitor (100 μM L-NAME) for 3 h, and then subjected to water treatment. (a) NO fluorescence. Bar = 300 μm. (b) NO production expressed as relative fluorescence. The contents of MDA representing lipid peroxidation (c) and carbonyl group (d) in rice seedling roots were measured after 3 h of water treatment following tungstate or L-NAME pretreatment. Values represent means± standard error (SE) (n = 6). Different letters indicate significant differences at P < 0.05. Con indicates control treatment for each N nutrition, i.e., plants receiving non-water stress. FW: fresh weight; TBARS: thiobarbituric acid reactive substances
The effect of SNP on the alleviation of water stress-induced root oxidative damage was reversed after pretreatment with 100 μM c-PTIO at 3 h. Application of the NOS inhibitor L-NAME significantly aggravated water stress-induced oxidative damage in NH4+-treated roots, and SNP application reversed the effect of the NOS inhibitor but not that of the NR inhibitor (Fig. 5c, d). For the NO3−-treated roots, the application of NR inhibitor or NOS inhibitor had no significant effect on root oxidative damage relative to the water stress treatment.
Activities of antioxidative enzymes and nitrate/nitrite and arginine/citrulline metabolism
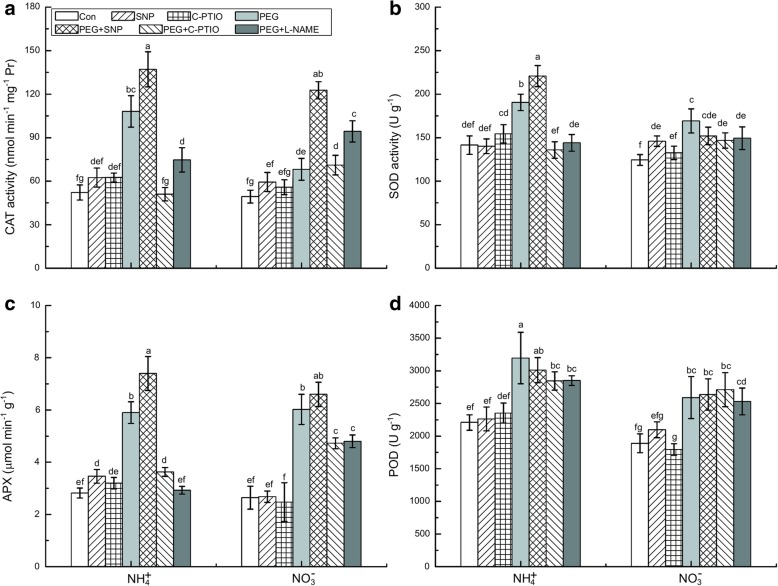
Water stress significantly enhanced the activities of the root antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), and peroxidase (POD) by ~ 107 and 38%, 52 and 36%, 152 and 128%, and 45 and 37% in the NH4+-treated roots and NO3−-treated roots, respectively, compared to their Con roots (Fig. 6). While SNP application further increased CAT, SOD, and APX activities (Fig. 6a–c), these antioxidant enzymes were inhibited by the application of the NO scavenger c-PTIO and by the NOS inhibitor L-NAME in the NH4+-treated roots under water stress.
Fig. 6.
Effects of different treatments on antioxidant enzyme changes in rice seedlings under water stress. Roots were collected to assay CAT (a), SOD (b), APX (c), and POD (d) after 3 h of treatment with non-water stress (Con) or water stress. For the c-PTIO, PEG + c-PTIO, and PEG + L-NAME treatments, the rice seedlings were pretreated with NO scavenger (c-PTIO) or NOS inhibitor (100 μM L-NAME) for 3 h followed by non-water stress or water stress. Values represent means ± standard error (SE) (n = 6). Different letters indicate significant differences at P < 0.05. Con indicates control treatment for each N nutrition, i.e., plants receiving non-water stress
As NR and NOS activities increased in the NO3−-treated roots, water stress lowered the nitrate level in the NR pathway and the arginine level in the NOS pathway (Additional file 3: Figure S3a, b). Similarly, application of NR inhibitor and NOS inhibitor enhanced root nitrate and arginine contents, respectively. In the NH4+-treated roots, water stress significantly decreased the arginine level, indicating that arginine metabolism is relatively high. In this treatment, NR inhibitor had no significant effect on root arginine content. On the other hand, the NOS inhibitor suppressed arginine metabolism, and thus the NH4+-treated roots had higher arginine levels than Con roots (Additional file 3: Figure S3c). These results also indirectly indicate that the NO early production burst in NH4+-treated roots might originate from the NOS pathway.
Discussion
Ample experimental evidence has demonstrated that NO is involved in plant abiotic stress [17]. However, to our knowledge, no detailed study has been conducted to evaluate the role of NO in drought acclimation in plants supplied with NO3− or NH4+. In the present study, plant biomass, root N uptake rate, and leaf photosynthesis were reduced after 21 days of water stress relative to the non-water stress condition (Fig. 1). However, these reductions were less severe for seedlings receiving NH4+, suggesting that NH4+ nutrition can enhance drought tolerance in rice seedlings more effectively than NO3− nutrition [2, 33]. Our study also demonstrated that, in the short term (48 h), endogenous NO production in response to water stress is time-dependent, varying according to water stress duration and N nutrition. This finding is consistent with those reported for other stressors [10, 25]. Early NO bursts were induced at 3 h of water stress in the roots of NH4+-treated seedlings but not in NO3−-treated seedlings. Thus, there might be significant differences between NH4+- and NO3−-supplied plants in terms of NO signal-mediated drought tolerance. In addition, accumulation of ROS, such as O2.-, OH−, and H2O2, and root oxidative damage were significantly lower in the NH4+-treated than in the NO3−-treated roots at 3 h of water stress (Fig. 4). Excessive accumulation of ROS damages cells and plasma membranes under the different abiotic stresses [11]. Whether the early NO burst in response to water stress observed in NH4+-supplied seedlings plays a crucial role in the plant antioxidant defense system needs further investigation, however.
The role of the early NO burst in the water stress tolerance of NH4+−/NO3−-supplied seedlings was confirmed using NO donors and scavengers. Our study demonstrated that NO donor induced NO in the NO3−-treated roots at 3 h but not at 24 h of water stress (Fig. 3a). Plant ROS accumulation and MDA and carbonyl levels under water stress were significantly alleviated after the application of the NO donor in both N nutritions (Fig. 3c, d). Nevertheless, the levels of these substances were higher in the NO3−-treated roots than in the NH4+-treated roots. Therefore, the NO production enhanced at 3 h by the exogenous NO donor can alleviate water stress-induced oxidative damage in the NO3−-treated roots. On the other hand, elimination of the early NO burst by NO scavengers like c-PTIO significantly aggravated water stress-induced oxidative damage (Fig. 3c, d). These results provide direct evidence that the early NO burst plays a crucial role in drought tolerance in NH4+-treated roots. Because the NH4+-supplied roots maintained a higher N uptake rate than NO3−-supplied roots under water stress (Fig. 1f), we hypothesized that the higher NH4+ uptake rate is beneficial for the early NO burst due to the NO production involved in root N metabolism [13, 34]. This NO burst can also be an active adaptation mechanism of plants to abiotic stress as, in addition to drought stress, it has been reported to occur repeatedly in plants challenged by pathogens [35], metal toxicity [9, 25], and cold stress [36].
Our study further demonstrated that an early NO burst improves plant drought tolerance by enhancing the antioxidant defense system of the root. Elevated plant antioxidant enzyme activities and gene expression levels in response to water stress have been widely demonstrated [12, 14, 18]. In the present study, the tips of the NO3−-treated roots presented more serious water stress-induced oxidative damage (due to the excessive production of O2.-, OH−, and H2O2) than those of the NH4+-treated roots (Figs. 2–4). In contrast, NH4+-supplied roots maintained relatively higher antioxidant enzyme (CAT, SOD, and APX) activity levels to catalyze O2.- and H2O2 decomposition (Fig. 6). It has been demonstrated that there is significant crosstalk between NO and ROS in plants. The antioxidant function of NO was explained by its ability to reduce H2O2 and lipid peroxidation, and induce antioxidant gene expression and enzyme activity [1, 14]. Our results showed that enhanced NO level and antioxidant enzyme activities (CAT and SOD) were significantly and simultaneously increased after NO donor application in NO3−-treated roots, thereby reducing ROS concentration and oxidative damage (Figs. 3, 6). The early NO burst observed in NH4+-treated roots can enhance antioxidant enzyme activity and ROS accumulation (O2.-, OH−, and H2O2). These results were confirmed by subsequent experimentation in which the application of NO scavenger significantly suppressed SOD and CAT in NH4+-treated roots. Thus, drought tolerance in the NH4+-treated roots might be associated with the NO-induced up-regulation of antioxidant enzymes and down-regulation of ROS accumulation.
Nitric oxide can also serve as a source of reactive nitrogen species (RNS). Overaccumulation of RNS under abiotic stress can cause tyrosine nitration and inactivate proteins like CAT, manganese-dependent (Mn-)SOD, and GR as well as the peroxidative activity of cytochrome c [37, 38]. Our results showed that NO3−-supplied plants had more severe oxidative damage and accumulated extremely high NO levels after 24 h of water stress (Fig. 3). This latent NO production can be partially alleviated by replenishing the early NO burst at 3 h with SNP. These results indicate that both ROS and RNS metabolism participate in the water stress response. High NO accumulation in the NO3−-treated roots likely caused the nitrosative stress at 24 h, which also damaged root redox balance. A similar phenomenon was described in plants subjected to cold [39], salinity [40], and drought [7] stresses. Because NO competes with oxygen for cytochrome c oxidase binding (Complex IV), it affects both the respiratory chain and oxidative phosphorylation [41, 42]. Thus, under water stress, the higher NO production in the NO3−-treated roots than in the NH4+-treated roots could aggravate respiratory inhibition and induce greater oxidative damage.
Our investigation further suggests that the early NO burst in NH4+-treated roots is mainly mediated by NOS at the early stages of water stress. Nitrate reductase-mediated NO generation is known to occur under water deficit [19, 43]. Drought-induced NO generation by NOS-like enzymes in plants has also been demonstrated but this NO production pathway varies significantly with species, tissue type, and plant growth conditions [29, 30]. For the NH4+-treated roots, both NOS activity and NO production increased simultaneously at 3 h of water stress, whereas the application of the NOS inhibitor completely repressed NO synthesis at this time point. The NOS inhibitor also aggravated water stress-induced membrane lipid peroxidation and oxidative protein damage, indicating that some NOS-associated proteins may play an important role in NO-mediated drought-protective responses [8, 23]. In contrast, the NR inhibitor did not significantly affect NO production or membrane lipid peroxidation. The aggravation of lipid peroxidation by L-NAME may have been the result of the alteration of the NOS-mediated early NO burst. In NO3−-treated roots, water stress enhanced NR activity significantly more than NOS activity at 24 h. However, separate NR inhibitor and NOS inhibitor application only partially suppressed NO production. The NO produced by the NR pathway might therefore play an important role in later NO production (24 h), consistent with previous reports [18, 19]. Although several studies support the arginine-dependent NO production model in higher plants, the identification of genes encoding NOS in such plants is still up for debate [28]. For this reason, the nitrate/nitrite and arginine/citrulline levels in the NR and NOS pathways, respectively, were determined. It was found that water stress significantly increased NOS activity and accelerated the conversion of arginine to citrulline in both N nutritions. However, in relation to the Con roots the arginine content was significantly enhanced in the NH4+-treated roots after application of the NOS inhibitor. These results provide additional evidence that the early NO burst in NH4+-treated roots is mainly mediated by NOS (Fig. 7).
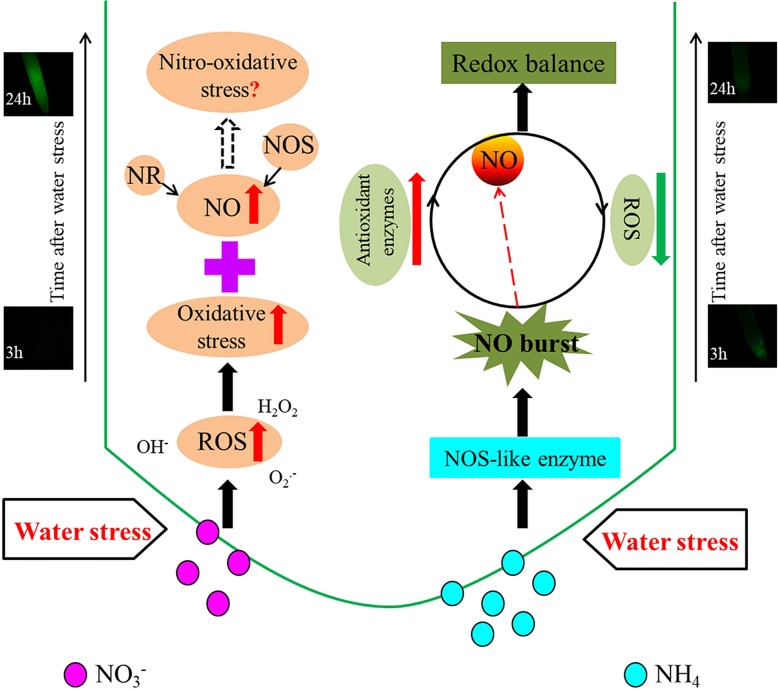
Fig. 7.
Schematic illustration of a proposed model for the different responses of early NO production and its effects on the defense response of rice to water stress. In the roots of NH4+-supplied rice, the NOS-mediated early NO burst (3 h) significantly enhanced plant antioxidant defense by reducing ROS accumulation and enhancing antioxidant enzyme activity; the relative lower NO production after 24 h of water stress in comparison to NO3−-supplied rice also helped maintaining the redox balance in root cells, thus enhancing their drought tolerance. In the roots of NO3−-supplied rice, ROS accumulation and oxidative damage induced by 3 h of water stress were significantly higher than that in NH4+-supplied rice. High NO accumulation in the NO3−-treated roots likely caused the nitrosative stress at 24 h of water stress. A combined effect of oxidative and nitrification stresses might have led to the weak resistance to water stress in NO3−-supplied rice. NR, nitrate reductase. Red arrows represent increase; green arrows represent decrease. Black solid arrows represent defined pathways, dotted arrows represent undefined pathway
Conclusions
Our study demonstrated that the early NO burst in NH4+-treated rice roots significantly enhanced plant antioxidant defense by reducing ROS accumulation and enhancing the activities of antioxidant enzymes, thereby increasing plants’ acclimation to water stress. The early NO burst that occurs in response to water stress may be triggered by NOS-like enzymes in root. Our results provide new insight into how NO-signaling molecules modulate drought tolerance in NH4+-supplied rice plants. However, in future the definite evidencefor this pathway provided by genetic and molecular techniques still need to be developed to achieve the target-specific editing of NO biosynthetic and signaling pathways under water deficits.
Methods
Plant material and growth conditions
Rice (Oryza sativa L. ‘Zhongzheyou No. 1’ hybrid indica) seedlings, obtained from the China National Rice Research Institute, were grown hydroponically in a greenhouse. Seeds were sterilized in 1% (v/v) sodium hypochlorite solution. After germination, seeds were transferred to a 0.5 mM CaCl2 solution (pH 5.5). Three days later, the seedlings were transferred to 1.5-L black plastic pots containing a solution with the following composition: NH4NO3 (0.5 mM), NaH2PO4·2H2O (0.18 mM), KCl (0.18 mM), CaCl2 (0.36 mM), MgSO4·7H2O (0.6 mM), MnCl2·4H2O (9 μM), Na2MoO4·4H2O (0.1 μM), H3BO3 (10 μM), ZnSO4·7H2O (0.7 μM), CuSO4 (0.3 μM), and FeSO4·7H2O–EDTA (ethylenediaminetetraacetic acid) (20 μM). All experiments were performed in a controlled growth room under the following conditions: 14/10 h light/dark photoperiod, 400 μmol·m− 2·s− 1 light intensity, 28/23 °C during the day and night, respectively, and 60% relative humidity. The solution pH was adjusted to 5.5 with 5 mM 2-(N-morpholino)ethanesulfonic acid (MES). The solution was replaced every 3 days.
After 6 days, seedlings of similar size were cultivated under the four following treatments: 1 mM NO3−, 1 mM NO3− + 10% PEG (PEG-6000), 1 mM NH4+ and 1 mM NH4+ + 10% PEG-6000. Water stress was induced by adding 10% PEG-6000. Eight treatments were performed in the NO donor (SNP) experiments: NH4+, NH4+ + SNP, NH4+ + PEG-6000, NH4+ + PEG-6000 + SNP, NO3−, NO3− + SNP, NO3− + PEG-6000, and NO3− + PEG-6000 + SNP. The final SNP concentration was 20 μM. Each treatment had six replicates. For each N nutrition, plants cultivated under non-water stress condition were defined as the control (Con) relative to the water stress (PEG) condition.
To determine the role of NO in the plant antioxidant defense system under water stress, rice seedlings supplied with 1 mM NO3− or 1 mM NH4+ solution were pretreated with 100 μΜ c-PTIO (as NO scavenger) for 3 h, and then subjected to non-water stress (Con treatment) or water stress (PEG) for 24 h under the same condition as those described above. Each treatment had six replicates.
To investigate the origin of the endogenous NO produced under water stress, rice seedlings supplied with 1 mM NO3− or 1 mM NH4+ solution were pretreated with the NR inhibitor (tungstate, 100 μΜ) or NOS inhibitor (L-NAME, 100 μΜ) for 3 h, and then subjected to non-water stress (Con) or water stress for 24 h under the same conditions as described above. There were eight treatments for each N nutrition: tungstate, L-NAME, tungstate + SNP, PEG-6000 + tungstate, PEG-6000 + tungstate + SNP, L-NAME + SNP, PEG-6000 + L-NAME, and PEG-6000 + L-NAME + SNP. Each treatment had six replicates.
Determination of NO and ONOO−contents
The DAF-FM DA probe was used to determine the endogenous root NO level [25]. Root tips (1 cm) were incubated with 10 μM DAF-FM DA in the dark for 30 min, washed three times with 20 mM HEPES–KOH (pH 7.4) to remove excess fluorescence, and then observed and photographed under a Nikon Eclipse 80i fluorescence microscope (Nikon, Tokyo, Japan; excitation filter 460–500 nm, dichroic mirror 505 nm, barrier filter 510–560). The relative fluorescence intensity was measured with Photoshop v. 7.0 (Adobe Systems, Mountain View, CA, USA).
Root endogenous ONOO− was determined using the aminophenylfluorescein (APF) probe method [44]. Root tips were incubated with 10 μM APF dissolved in 10 mM Tris–HCl (pH 7.4) in the dark for 60 min, and then washed three times with 10 mM Tris–HCl. Fluorescence images and relative fluorescence intensities were analyzed as described above for NO.
Histochemical analyses
Lipid peroxidation and root cell death were detected histochemically with Schiff’s reagent and Evans blue [45]. Root tips were incubated in Schiff’s reagent for 20 min and washed by three consecutive immersions in 0.5% (w/v) K2O3S solution. A red/purple endpoint indicated the presence of aldehydes generated by lipid peroxidation. Roots were also washed by performing three serial immersions in distilled water, then incubated in 0.25% (w/v) Evans blue for 15 min, and finally washed three times with distilled water. Roots stained with Schiff’s reagent and Evans blue were immediately photographed under a Leica S6E stereomicroscope (Leica, Solms, Germany).
The oxidative damage level, specifically expressed as membrane lipid peroxidation and protein oxidative damage, was estimated by measuring the concentrations of MDA and carbonyl group with 2,4-dinitrophenylhydrazine (DNPH) [46].
Determination of ROS contents
Root O2.- content was estimated using the method described in Liu et al. [47] with some modifications: about 0.15 g fresh root was powdered with 2 mL of 65 mM phosphate buffer saline (PBS, pH 7.8) in a pre-cooled mortar, and centrifuged at 5000 g and 4 °C for 10 min. Then, 0.9 mL of 65 mM PBS (pH 7.8) and 0.1 mL of 10 mM hydroxylammonium chloride were added to 1 mL of the root extract supernatant, thoroughly mixed, and left to react for 25 min. After this period, 1 mL of 1% (w/v) sulfanilamide and 1 mL of 0.02% (w/v) N-(1-naphthyl)-ethylenediaminedihydrochloride were added to 1 mL of root extract solution and left to react for 30 min. Absorbance was then measured at 540 nm.
Root H2O2 content was determined by the photocolorimetric method [48]: ~ 0.15 g fresh root was powdered with 2 mL acetone in a pre-cooled mortar, and centrifuged at 5000 g and 4 °C for 10 min. Then, 0.1 mL of 5% (w/v) TiSO4 and 0.1 mL pre-cooled ammonium hydroxide were added to 1 mL of the root extract supernatant, which was re-centrifuged at 5000 g for 10 min. The supernatant was discarded and the sediment was re-dissolved in 4 mL of 2 M H2SO4. The absorbance of the root extract solution was measured at 415 nm.
Root OH− content was analyzed by the methods described in a previous study [49]: ~ 0.1 g fresh root was powdered with 3 mL of 50 mM PBS (pH 7.0) in a mortar, and centrifuged at 10,000 g and 4 °C for 10 min. Then, 1.0 mL of 25 mM PBS (pH 7.0) containing 5 mM 2-deoxy-D-ribose and 0.2 mM NADH were added to 1 mL of the root extract supernatant, completely blended, and left to react for 60 min at 35 °C in the dark. Following this incubation, 1 mL of 1% (w/v) thiobarbituric acid and 1 mL glacial acetic acid were added to the filtrate. The mixture was heated to 100 °C for 30 min and then placed on ice for 20 min. The absorbance of the root extract solution was then measured at 532 nm, and the OH− content was inferred from the production of MDA.
Determination of enzyme activities
Fresh rice root samples (0.5 g) were homogenized in 5 mL of 10 mM phosphate buffer (pH 7.0) containing 4% (w/v) polyvinylpyrrolidone and 1 mM ethylenediaminetetraacetic acid. The supernatant was used as crude enzyme solution and collected by centrifugation at 12,000 g and 4 °C for 15 min. The activities of SOD, CAT, APX, and POD were estimated using the photocolorimetric methods described in Jiang and Zhang [11], and Sachadyn-Krol et al. [50].
Root NR and NOS activities were assayed using the methods described in previous studies [25, 26], with some modifications. Briefly, total protein was extracted using a buffer containing 100 mM HEPES–KOH (pH 7.5), 1 mM EDTA, 10% (v/v) glycerol, 5 mM 1,4-dithiothreitol (DTT), 0.5 mM phenylmethylsulfonyl fluoride, 0.1% Triton X-100 (v/v), 1% polyvinylpyrrolidone (PVP), and 20 μM flavin adenine dinucleotide. The supernatant was collected by centrifugation at 12,000 g and 4 °C for 20 min, and then used to determine the NR and NOS activities at 520 nm and 340 nm, respectively.
Specifically, the activity of NR was measured immediately by mixing 250 μL of supernatant with 250 μL pre-warmed (25 °C) assay buffer containing 50 mM HEPES–KOH (pH 7.5), 10 mM MgCl2, 1 mM DTT, 2 mM KNO3 and 200 μM NADH. The reaction was started by adding assay buffer, incubated at 30 °C for 30 min and then stopped by adding 50 μL 0.5 M Zn-acetate. The nitrite produced was measured colorimetrically at 540 nm after adding 1 mlof 1% sulfanilamide in 3 M HCl plus 1 mL of 0.02% N-(1-naphthyl)ethylenediamine in 0.2 M HCl. NOS activity was detected in 1 mL of reaction mixture containing 100 mM phosphate buffer (pH 7.0), 1 mM laevo-arginine(L-Arg), 2 mM MgCl2, 0.3 mM CaCl2, 4 μM BH4, 1 μM FAD, 1 μM flavin mononucleotide (FMN), 0.2 mM DTT, 0.2 mM NADPH, and 200 μL of protein extract. The decrease in absorbance as a result of NADPH consumption was determined at 340 nm for 5 min. NOS activity was calculated using the extinction coefficient of NADPH (ɛ = 6.22 mM− 1·cm− 1).
Determination of arginine and citrulline
Arginine and citrulline contents were estimated using the method described in Salazar et al. [51]. Briefly, 1.0 g root samples were frozen in liquid N2 and extracted with 4 mL 80% (v/v) methanol, and then centrifuged at 10,000 g and 4 °C for 5 min. The supernatant was then used in derivatization and reaction processes. Serial concentrations of amino acid standards were prepared as described above for the derivatizing reagent, and the derivatizing samples were used to determine the arginine and citrulline contents using liquid chromatography/electrospray ionization tandem mass spectroscopy (LC-ESI-MS).
Statistical analyses
All experiments conducted in this study were performed in six replicates, at least. All data, expressed as means ± standard error (SE), were processed in SPSS v. 13.0 (IBM Corp., Armonk, NY, USA). The Least Significant Difference (LSD) test was used to determine statistical significant differences among the treatments (P < 0.05). Figures were drawn in Origin v. 8.0 (OriginLab Corporation, Northampton, MA, USA).
Additional files
Figure S1. Effect of exogenous NO donor (SNP) on root oxidative damage under water stress. Rice roots were exposed to mixed N (NH4+ + NO3−) nutrient solution containing 0 μM, 5 μM, 10 μM, 20 μM, 40 μM, 80 μM, or 100 μM SNP either with or without 10% PEG for 48 h. The contents of MDA representing lipid peroxidation (a) and carbonyl group (b) in rice seedling roots were determined. Values represent means ± SE (n = 6). Different letters indicate significant differences at P < 0.05. Con indicates control treatment for each N nutrition, i.e., plants receiving non-water stress. FW: fresh weight; TBARS: thiobarbituric acid reactive substances. (TIF 71 kb)
Figure S2. Effect of water stress on NR (a) and NOS (b) in roots. Roots were collected for the NR and NOS assays after 3 h and 24 h of water stress, respectively. Values represent means ± SE (n = 6). Different letters indicate significant differences at P < 0.05. Con indicates control treatment for each N nutrition, i.e., plants receiving non-water stress. (TIF 41 kb)
Figure S3. Effect of exogenous NR inhibitor (tungstate) and NOS inhibitor (L-NAME) on the related compounds in NR-mediated and NOS-mediated NO pathways. (a) Levels of nitrate and nitrite in NO3−-treated roots. (b) Levels of arginine and citrulline in NO3−-treated roots. (c) Levels of arginine and citrulline in NH4+-treated roots. For the PEG + tungstate and PEG + L-NAME treatments, the rice seedlings were pretreated with NR inhibitor (100 μM tungstate) or NOS inhibitor (100 μM L-NAME) for 3 h, followed by non-water stress (Con) or water stress treatment. Values represent means ± SE (n = 6). Different letters indicate significant differences at P < 0.05. (TIF 69 kb)
Method S1. Determination of leaf photosynthesis, root N uptake rate, and root nitrate and nitrite contents in rice seedlings after 21 days of non-water stress (Con) or water stress (PEG) treatment. (DOCX 14 kb)
Acknowledgements
We would like to thank Editage (http://www.editage.cn) for English language editing.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (Grant no. LY18C130005); and the National Key Research and Development Program of China (Grant no. 2017YFD0300100, 2016YFD0101801). The supporters did not play any role in the design, analysis, or interpretation of this study and the relevant data.
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Abbreviations
- ABA
Abscisic acid
- APF
Aminophenylfluorescein
- APX
Ascorbate peroxidase
- CAT
Catalase
- c-PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DAF-FM DA
Diaminofluorescein-FM diacetate
- DNPH
2,4-dinitrophenylhydrazine
- DTT
1,4-dithiothreitol
- EDTA
Ethylenediaminetetraacetic acid
- FAD
Flavin adenine dinucleotide
- FMN
Flavin mononucleotide
- GR
Glutathione reductase
- GSNO
S-nitrosoglutathione
- L-Arg
Leavo-arghinine
- LC-ESI-MS
Liquid chromatography/electrospray ionization tandem mass spectroscopy
- L-NAME
N(G)-nitro-L-arginine methyl ester
- MDA
Malondialdehyde
- MES
2-(N-morpholino)ethanesulfonic acid
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- NR
Nitrate reductase
- ONOO−
Peroxynitrite
- PBS
Phosphate buffer saline
- PEG
Polyethylene glycol
- Pn
Net photosynthetic rate
- POD
Peroxidase
- PVP
Polyvinylpyrrolidone
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- SNP
Sodium nitroprusside
- SOD
Superoxide dismutase
Authors’ contributions
XCC, QXM, and QYJ conceived and designed the experiments. XCC, CQZ, and CZ performed the experiments. XCC, LHW, and JHZ analyzed the data. XCC, QXM, and CQZ wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no competing interests exist.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaochuang Cao, Email: caoxiaochuang@126.com.
Chunquan Zhu, Email: 1035305743@qq.com.
Chu Zhong, Email: 526996703@qq.com.
Junhua Zhang, Email: situyajie@126.com.
Lianghuan Wu, Email: 517869546@qq.com.
Qianyu Jin, Email: 11014041@zju.edu.cn.
Qingxu Ma, Email: 592214410@qq.com.
References
- 1.Bogeat-Triboulot MB, Brosché M, Renaut J, Jouve L, Le Thiec D, Fayyaz P, Vinocur B, Witters E, Laukens K, Teichmann T, Altman A, Hausman J, Polle A, Kangasjärvi J, Dreyer E. Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populuseuphratica, a poplar growing in arid regions. Plant Physiol. 2007;143:876–892. doi: 10.1104/pp.106.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo S, Zhou Y, Shen Q, Zhang F. Effect of ammonium and nitrate nutrition on some physiological processes in higher plants - growth, photosynthesis, photorespiration, and water relations. Plant Biol (Stuttg) 2007;9:21–29. doi: 10.1055/s-2006-924541. [DOI] [PubMed] [Google Scholar]
- 3.Slewinski TL. Non-structural carbohydrate partitioning in grass stems: a target to increase yield stability, stress tolerance, and biofuel production. J Exp Bot. 2012;63:4647–4670. doi: 10.1093/jxb/ers124. [DOI] [PubMed] [Google Scholar]
- 4.Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytol. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilson ID, Neill SJ, Hancock JT. Nitric oxide synthesis and signalling in plants. Plant Cell Environ. 2007;31:622–631. doi: 10.1111/j.1365-3040.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 6.Simontacchi M, Galatro A, Ramos-Artuso F, Santa-María GE. Plant survival in a changing environment: the role of nitric oxide in plant responses to abiotic stress. Front Plant Sci. 2015;6:977. doi: 10.3389/fpls.2015.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Signorelli S, Corpas FJ, Omar Borsani O, Barroso JB, Monza J. Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci. 2013;201(202):137–146. doi: 10.1016/j.plantsci.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M, Tian Q, Zhang W. Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol. 2007;144:206–217. doi: 10.1104/pp.107.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González A, Cabrera ML, Henríquez MJ, Contreras RA, Morales B, Moenne A. Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compress a exposed to copper excess. Plant Physiol. 2012;158:1451–1462. doi: 10.1104/pp.111.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Planchet E, Verdu I, Delahaie J, Cukier C, Girard C, Morère-Le Paven M-C, Limami AM. Abscisic acid-induced nitric oxide and proline accumulation in independent pathways under water-deficit stress during seedling establishment in Medicagotruncatula. J Exp Bot. 2014;65:2161–2170. doi: 10.1093/jxb/eru088. [DOI] [PubMed] [Google Scholar]
- 11.Jiang M, Zhang J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot. 2002;53:2401–2410. doi: 10.1093/jxb/erf090. [DOI] [PubMed] [Google Scholar]
- 12.Fan H, Li T, Guan L, Li Z, Guo N, Cai Y, Li Y. Effects of exogenous nitric oxide on antioxidation and DNA methylation of Dendrobium huoshanense grown under drought stress. Plant Cell Tissue Organ Cult. 2012;109:307–314. doi: 10.1007/s11240-011-0096-3. [DOI] [Google Scholar]
- 13.Del Río LA. ROS and RNS in plant physiology: an overview. J Exp Bot. 2015;66:2827–2837. doi: 10.1093/jxb/erv099. [DOI] [PubMed] [Google Scholar]
- 14.Farooq M, Basra SMA, Wahid A, Rehman H. Exogenously applied nitric oxide enhances the drought tolerance in fine grain aromatic rice (Oryza sativa L.) J Agron Crop Sci. 2009;195:254–261. doi: 10.1111/j.1439-037X.2009.00367.x. [DOI] [Google Scholar]
- 15.Verdoy D, Coba De La Peña T, Redondo FJ, Lucas MM, Pueyo JJ. Transgenic Medicagotruncatula plants that accumulate proline display nitrogen-fixing activity with enhanced tolerance to osmotic stress. Plant Cell Environ. 2006;29:1913–1923. doi: 10.1111/j.1365-3040.2006.01567.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, Tang M. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 2007;175:36–50. doi: 10.1111/j.1469-8137.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- 17.Santisree P, Bhatnagar-Mathur P, Sharma KK. NO to drought-multifunctional role of nitric oxide in plant drought: do we have all the answers? Plant Sci. 2015;239:44–55. doi: 10.1016/j.plantsci.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Kubiś J. Involvement of nitric oxide in water stress-induced responses of cucumber roots. Plant Sci. 2009;177:682–690. doi: 10.1016/j.plantsci.2009.09.007. [DOI] [Google Scholar]
- 19.Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Kubiś J. Interaction between polyamine and nitric oxide signaling in adaptive responses to drought in cucumber. J Plant Growth Regul. 2009;28:177–186. doi: 10.1007/s00344-009-9086-7. [DOI] [Google Scholar]
- 20.Groß F, Durner J, Gaupels F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front Plant Sci. 2013;4:419. doi: 10.3389/fpls.2013.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corpas FJ, del Río LA, Barroso JB. Need of biomarkers of nitrosative stress in plants. Trends Plant Sci. 2007;12:436–438. doi: 10.1016/j.tplants.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Liao W, Huang G, Yu J, Zhang M. Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol Biochem. 2012;58:6–15. doi: 10.1016/j.plaphy.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Guo FQ, Okamoto M, Crawford NM. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science. 2003;302:100–103. doi: 10.1126/science.1086770. [DOI] [PubMed] [Google Scholar]
- 24.Shi F, Li Y. Verticilliumdahliae toxins-induced nitric oxide production in Arabidopsis is major dependent on nitrate reductase. BMB Rep. 2008;41:79–85. doi: 10.5483/BMBRep.2008.41.1.079. [DOI] [PubMed] [Google Scholar]
- 25.Sun C, Lu L, Liu L, Liu W, Yu Y, Liu X, Jin C, Lin X. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticumaestivum) New Phytol. 2014;201:1240–1250. doi: 10.1111/nph.12597. [DOI] [PubMed] [Google Scholar]
- 26.Zhao M, Chen L, Zhang L, Zhang W. Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 2009;151:755–767. doi: 10.1104/pp.109.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freschi L, Rodrigues MA, Domingues DS, Purgatto E, Van Sluys MA, Magalhaes JR, Kaiser WM, Mercier H. Nitric oxide mediates the hormonal control of Crassulacean acid metabolism expression in young pineapple plants. Plant Physiol. 2010;152:1971–1985. doi: 10.1104/pp.109.151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zemojtel T, Fröhlich A, Palmieri MC, Kolanczyk M, Mikula I, Wyrwicz LS, Wanker EE, Mundlos S, Vingron M, Martasek P, Durner J. Plant nitric oxide synthase: a never-ending story? Trends Plant Sci. 2006;11:524–525. doi: 10.1016/j.tplants.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Corpas FJ, Palma JM, del Río LA, Barroso JB. Evidence supporting the existence of L-arginine-dependent nitric oxide synthase activity in plants. New Phytol. 2009;184:9–14. doi: 10.1111/j.1469-8137.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- 30.Shi H, Ye T, Zhu JK, Chan Z. Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J Exp Bot. 2014;65:4119–4131. doi: 10.1093/jxb/eru184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, Li Y, Ren B, Ding L, Gao C, Shen Q, Guo S. Drought-induced root aerenchyma formation restricts water uptake in rice seedlings supplied with nitrate. Plant Cell Physiol. 2012;53:495–504. doi: 10.1093/pcp/pcs003. [DOI] [PubMed] [Google Scholar]
- 32.Ding L, Gao C, Li Y, Li Y, Zhu Y, Xu G, Shen Q, Kaldenhoff R, Kai L, Guo S. The enhanced drought tolerance of rice plants under ammonium is related to aquaporin (AQP) Plant Sci. 2015;234:14–21. doi: 10.1016/j.plantsci.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Ren B, Yang X, Xu G, Shen Q, Guo S. Chloroplast downsizing under nitrate nutrition restrained mesophyll conductance and photosynthesis in rice (Oryza sativa L.) under drought conditions. Plant Cell Physiol. 2012;53:892–900. doi: 10.1093/pcp/pcs032. [DOI] [PubMed] [Google Scholar]
- 34.Corpas FJ, Chaki M, Fernández-Ocaña A, Valderrama R, Palma JM, Carreras A, Begara-Morales JC, Airaki M, Del Río LA, Barroso JB. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 2008;49:1711–1722. doi: 10.1093/pcp/pcn144. [DOI] [PubMed] [Google Scholar]
- 35.Floryszak-Wieczorek J, Arasimowicz M, Milczarek G, Jelen H, Jackowiak H. Only an early nitric oxide burst and the following wave of secondary nitric oxide generation enhanced effective defence responses of pelargonium to a necrotrophic pathogen. New Phytol. 2007;175:718–730. doi: 10.1111/j.1469-8137.2007.02142.x. [DOI] [PubMed] [Google Scholar]
- 36.Cantrel C, Vazquez T, Puyaubert J, Rezé N, Lesch M, Kaiser WM, Dutilleul C, Guillas I, Zachowski A, Baudouin E. Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol. 2011;189:415–427. doi: 10.1111/j.1469-8137.2010.03500.x. [DOI] [PubMed] [Google Scholar]
- 37.Clark D, Durner J, Navarre DA, Klessig DF. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant-Microbe Interact. 2000;13:1380–1384. doi: 10.1094/MPMI.2000.13.12.1380. [DOI] [PubMed] [Google Scholar]
- 38.Batthyány C, Souza JM, Durán R, Cassina A, Cerveñansky C, Radi R. Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry. 2005;44:8038–8046. doi: 10.1021/bi0474620. [DOI] [PubMed] [Google Scholar]
- 39.Airaki M, Leterrier M, Mateos RM, Valderrama R, Chaki M, Barroso JB, Del Río LA, Palma JM, Corpas FJ. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low-temperature stress. Plant Cell Environ. 2012;35:281–295. doi: 10.1111/j.1365-3040.2011.02310.x. [DOI] [PubMed] [Google Scholar]
- 40.Tanou G, Filippou P, Belghazi M, Job D, Diamantidis G, Fotopoulos V, Molassiotis A. Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J. 2012;72:585–599. doi: 10.1111/j.1365-313X.2012.05100.x. [DOI] [PubMed] [Google Scholar]
- 41.Millar AH, Day DA. Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS Lett. 1996;398:155–158. doi: 10.1016/S0014-5793(96)01230-6. [DOI] [PubMed] [Google Scholar]
- 42.Yamasaki H, Shimoji H, Ohshiro Y, Sakihama Y. Inhibitory effects of nitric oxide on oxidative phosphorylation in plant mitochondria. Nitric Oxide. 2001;5:261–270. doi: 10.1006/niox.2001.0353. [DOI] [PubMed] [Google Scholar]
- 43.Yu M, Lamattina L, Spoel SH, Loake GJ. Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol. 2014;202:1142–1156. doi: 10.1111/nph.12739. [DOI] [PubMed] [Google Scholar]
- 44.Saito S, Yamamoto-Katou A, Yoshioka H, Doke N, Kawakita K. Peroxynitrite generation and tyrosine nitration in defense responses in tobacco BY-2 cells. Plant Cell Physiol. 2006;47:689–697. doi: 10.1093/pcp/pcj038. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto Y, Kobayashi Y, Matsumoto H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001;125:199–208. doi: 10.1104/pp.125.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- 47.Liu Y, Wu R, Wan Q, Xie G, Bi Y. Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide-involved defense against oxidative stress under salt stress in red kidney bean roots. Plant Cell Physiol. 2007;48:511–522. doi: 10.1093/pcp/pcm020. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Huang J, Bi Y. Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Sci. 2010;179:281–288. doi: 10.1016/j.plantsci.2010.05.014. [DOI] [Google Scholar]
- 49.Liu Y, Jiang H, Zhao Z, An L. Nitric oxide synthase like activity-dependent nitric oxide production protects against chilling-induced oxidative damage in Chorisporabungeana suspension cultured cells. Plant Physiol Biochem. 2010;48:936–944. doi: 10.1016/j.plaphy.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Sachadyn-Król M, Materska M, Chilczuk B, Karás M, Jakubczyk A, Perucka I, Jackowska I. Ozone-induced changes in the content of bioactive compounds and enzyme activity during storage of pepper fruits. Food Chem. 2016;211:59–67. doi: 10.1016/j.foodchem.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 51.Salazar C, Armenta JM, Shulaev V. An UPLC-ESI-MS/MS assay using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate derivatization for targeted amino acid analysis: application to screening of Arabidopsis thaliana mutants. Metabolites. 2012;2:398–328. doi: 10.3390/metabo2030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of exogenous NO donor (SNP) on root oxidative damage under water stress. Rice roots were exposed to mixed N (NH4+ + NO3−) nutrient solution containing 0 μM, 5 μM, 10 μM, 20 μM, 40 μM, 80 μM, or 100 μM SNP either with or without 10% PEG for 48 h. The contents of MDA representing lipid peroxidation (a) and carbonyl group (b) in rice seedling roots were determined. Values represent means ± SE (n = 6). Different letters indicate significant differences at P < 0.05. Con indicates control treatment for each N nutrition, i.e., plants receiving non-water stress. FW: fresh weight; TBARS: thiobarbituric acid reactive substances. (TIF 71 kb)
Figure S2. Effect of water stress on NR (a) and NOS (b) in roots. Roots were collected for the NR and NOS assays after 3 h and 24 h of water stress, respectively. Values represent means ± SE (n = 6). Different letters indicate significant differences at P < 0.05. Con indicates control treatment for each N nutrition, i.e., plants receiving non-water stress. (TIF 41 kb)
Figure S3. Effect of exogenous NR inhibitor (tungstate) and NOS inhibitor (L-NAME) on the related compounds in NR-mediated and NOS-mediated NO pathways. (a) Levels of nitrate and nitrite in NO3−-treated roots. (b) Levels of arginine and citrulline in NO3−-treated roots. (c) Levels of arginine and citrulline in NH4+-treated roots. For the PEG + tungstate and PEG + L-NAME treatments, the rice seedlings were pretreated with NR inhibitor (100 μM tungstate) or NOS inhibitor (100 μM L-NAME) for 3 h, followed by non-water stress (Con) or water stress treatment. Values represent means ± SE (n = 6). Different letters indicate significant differences at P < 0.05. (TIF 69 kb)
Method S1. Determination of leaf photosynthesis, root N uptake rate, and root nitrate and nitrite contents in rice seedlings after 21 days of non-water stress (Con) or water stress (PEG) treatment. (DOCX 14 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).