Abstract
The total synthesis of hemiketal E2 (HKE2) has been accomplished using a gold(I)-mediated cycloisomerization followed by oxidation of the enol ether product to introduce a unique keto-hemiketal, the core structure of HKE2. Synthetic hemiketal E2 reproduced biosynthetically derived HKE2 in the inhibition of human platelet aggregation.
Graphical Abstract
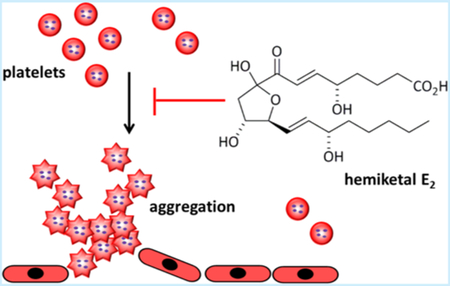
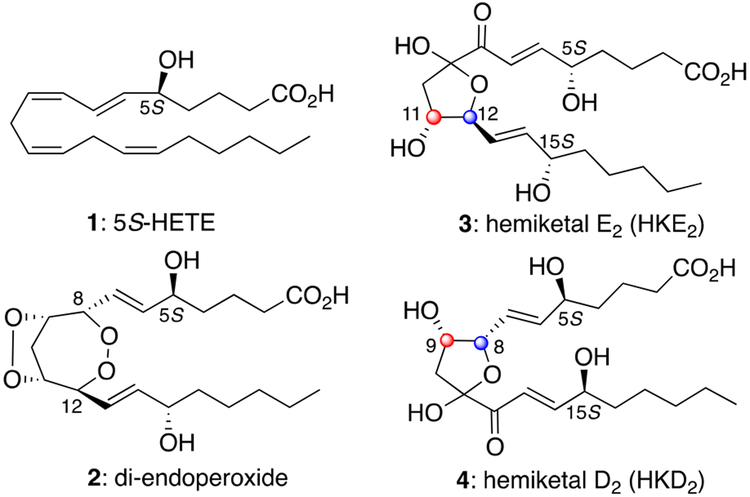
Eicosanoids are produced by enzymatic oxidation of arachidonic acid (AA) by cyclooxygenases (COX-1, COX-2), lipoxygenases (LOX), and cytochromes P450 in response to a variety of cellular stimuli such as hormones, stress, and cytokines.1 These lipid mediators regulate a variety of biological responses and pathological processes such as inflammation, cancer, asthma, and autoimmune diseases.2 Studies on eicosanoid biosynthesis, metabolism, and function have been extensive. The biosynthetic pathways leading to prostaglandins and leukotrienes are generally believed to diverge at the point of the initial enzymatic oxygenation of arachidonic acid by cyclooxygenases and lipoxygenases, respectively.3 However, in 2011, Schneider and co-workers described the biosynthesis of eicosanoids HKD2 (3) and HKE2 (4) as products of the consecutive oxygenation of arachidonic acid by 5-lipoxygenase and cyclooxygenase-2 (AA → 1 → 2 → 3/4, Figure 1).4
Figure 1.
Structures of 5S-HETE (1), diendoperoxide (2), hemiketal E2 (3), and hemiketal D2 (4).\.
The discovery of HKD2 and HKE2 resulted from the hypothesis that 5S-HETE (1), an oxidation product of 5-LOX and arachidonic acid, may serve as a substrate of the COX enzyme. In vitro experiments demonstrated 5S-HETE to be converted by COX-2 (but not COX-1), affording diendoperoxide 2 as the enzymatic product.5 The latter underwent rearrangement to HKD2 and HKE2 in a process reminiscent of the well-known transformation of intermediate endoperoxide PGH2 to PGD2 and PGE2; thus, it is the designated nomenclature for these unusual hemiketals.4 The possible relevance of the crossover pathway to living systems was further demonstrated by their ability to induce tubulogenesis of endothelial cells4 and their biosynthesis following stimulation of 5-LOX and COX-2 in human leukocytes ex vivo using lipopolysaccharide (LPS).6 Further study of HKD2 and HKE2 requires their total synthesis, as currently hemiketals D2/E2 are produced enzymatically in small quantities starting from 5S-HETE and recombinant COX-2.7 Described herein is the first total synthesis of hemiketal E2 (3).
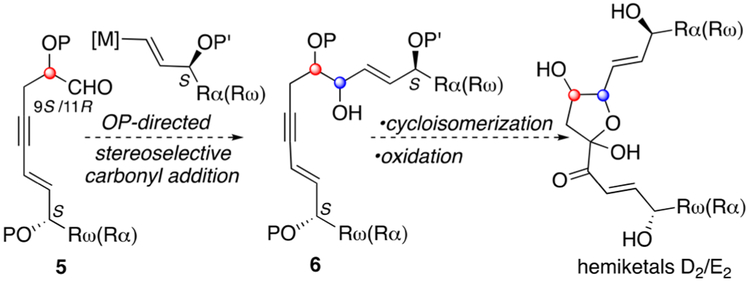
Hemiketals D2 and E2 share common side chains that incorporate secondary alcohols of (S)-configuration of reversed attachment to the heterocyclic core (cf. Figure 2, Rα/Rω). A second difference between the two structures is the configuration of C9 (9S-HKD2) and C11 (11R-HKE2) as reflected in the cis and trans relationships between C8–C9 (HKD2) and C11–C12 (HKE2), respectively (3/4, Figure 1). Based on this structural analysis, we proceeded with a synthetic strategy whereby the absolute stereochemistry of 9S (HKD2) and 11R (HKE2) would be introduced in 2-alkoxy(siloxy)-aldehydes (cf. 5, Figure 2) and relative stereochemistry (C8–C9 and C11–C12) would be established in a vinyl metal addition to 5 directed by the adjacent stereocenter (OP) by way of either a Felkin–Anh (2-siloxyaldehyde) or chelation-controlled (2-alkoxyaldehyde) addition.8 The cyclic hemiketal would be introduced by a two-step process starting with a metal-mediated cycloisomerization followed by oxidation of the intermediate enol ether (Figure 2).9 We chose as our first objective the total synthesis of hemiketal E2 (Scheme 1).
Figure 2.
Synthetic strategy to access hemiketals D2 and E2.
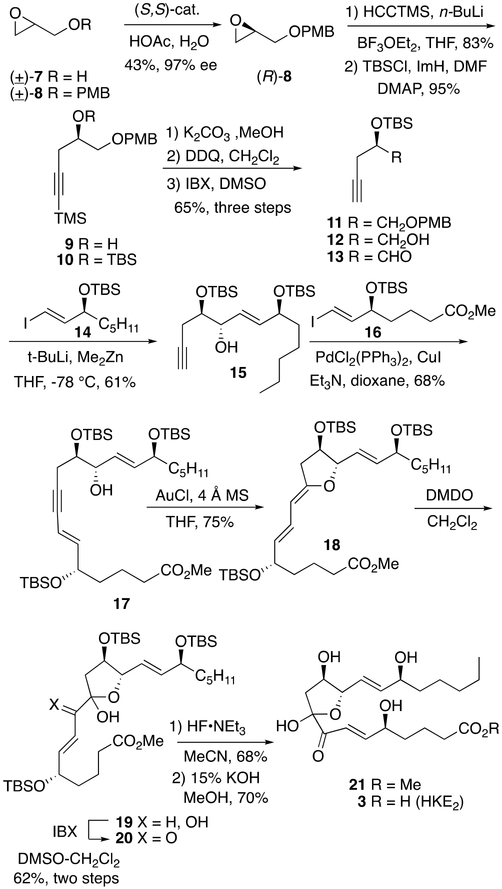
Scheme 1.
Total Synthesis of HKE2
Our synthesis started with racemic glycidol 7, as derivatives of glycidol are readily resolved into single enantiomeric products using the Jacobsen hydrolytic–kinetic resolution.10 For example, PMB ether 8 was readily resolved into (R)-8 using the (S,S)-Co(salen) catalyst.11 After epoxide opening with trimethylsilyl acetylide, the resulting secondary alcohol (9)12 tected as a TBS ether in anticipation of a Felkin–Anh directed addition to aldehyde 13. Aldehyde 13 was obtained from 10 by way of the three-step deprotection-oxidation sequence as shown in Scheme 1. Next, treatment of known vinyl iodide 14,13 with t-butyllithium afforded an intermediate vinyllithium reagent that was reacted with dimethylzinc leading to a nonbasic vinyl zincate which was added to aldehyde 13 to give allylic alcohol 15 in 61% yield (>95:5 diastereoselectivity). Sonogashira coupling of acetylene 15 with vinyl iodide 1614 gave enyne 17 in 68% yield.
Either a 6-endo or 5-exo-dig mode of cyclization of alkynol 17 was projected to afford the desired hemiketal product following oxidation of either the pyran or furan product, respectively.9 After various conditions were examined, it was determined that gold(I) chloride in tetrahydrofuran proved most efficient to afford the 5-exo product, furan 18, in 75% yield.15 While furan 18 could be purified using buffered silica gel and characterized by NMR analysis, it had a finite lifetime in benzene-d6 of less than 24 h. Typically, 18 was immediately epoxidized using dimethyl dioxirane following isolation. The epoxide product was not isolated, but instead, diol 19, a product of hydrolysis, was obtained and subsequently oxidized using IBX in DMSO to give hemiketal 20 in 62% yield from enol ether 18. Removal of TBS protecting groups was effected using HF-pyridine in acetonitrile to provide methyl ester 21. Saponification of ester 21 provided hemiketal E2 (3), identical by 1H NMR to HKE2 derived enzymatically.7
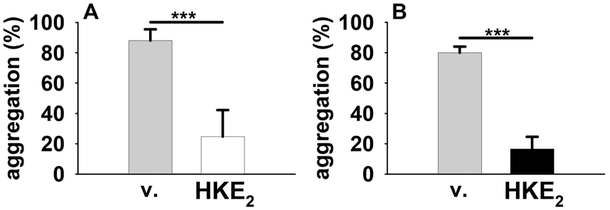
Hemiketal E2 inhibited human platelet aggregation induced by the thromboxane receptor agonist U46,619 in a dose-dependent manner (IC50 < 500 nM). Inhibition was more potent in platelets isolated from individuals in whom PGE2 at 100 nM concentration was unable to inhibit aggregation.16 Chemically synthesized and enzymatically prepared HKE2 (1 μM) were equally effective at inhibiting platelet aggregation (Figure 3).
Figure 3.
HKE2 inhibits platelet aggregation. Human platelet-rich plasma was preincubated with vehicle (v) or HKE2 (1 μM) obtained from chemical synthesis (A) or enzymatic synthesis (B) for 10 min at 37 °C before stimulation of aggregation by the thromboxane receptor agonist U46,619.17
Unfortunately, efforts directed toward the total synthesis of hemiketal D2 starting from 9S-5 (P = Bn) proved unproductive. Problems encountered in effecting the chelation-controlled carbonyl addition included low stereoselectivity and narrow scope of tolerated functionality in the vinyl metal reagent (5 to 6, Figure 2). Access to both hemiketals will provide opportunity for further biological study of these interesting and unusual eicosanoids.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (5R01 GM115722 to G.A.S. and 5R01 GM076592 to C.S.). J.A.G.-B. was supported by a postdoctoral award from the American Heart Association (16POST30690001).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.or-glett.8b01578.
General experimental procedures, characterization data, and 1H and 13C NMR spectra of new compounds (PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Funk CD Science 2001, 294, 1871. [DOI] [PubMed] [Google Scholar]
- (2) (a).Harizi H; Corcuff JB; Gualde N Trends Mol. Med 2008, 14, 461. [DOI] [PubMed] [Google Scholar]; (b) Dennis EA; Norris PC Nat. Rev. Immunol 2015, 15, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3) (a).Haeggstrom JZ; Funk CD Chem. Rev 2011, 111, 5866. [DOI] [PubMed] [Google Scholar]; (b) Rouzer CA; Marnett LJ Chem. Rev 2011, 111, 5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Griesser M; Suzuki T; Tejera N; Mont S; Boeglin WE; Pozzi A; Schneider C Proc. Natl. Acad. Sci. U. S. A 2011, 108, 6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Schneider C; Boeglin WE; Yin HY; Stec DF; Voehler MJ Am. Chem. Soc 2006, 128, 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Giménez-Bastida JA; Shibata T; Uchida K; Schneider C FASEB J. 2017, 31, 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gimenez-Bastida JA; Suzuki T; Sprinkel KC; Boeglin WE; Schneider C Prostaglandins Other Lipid Mediators 2017, 132, 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).For a review on the stereoselectivity of alkynyl metals to 2-substituted aldehydes, see: Guillarme S; Ple K; Banchet A; Liard A; Haudrechy A. Chem. Rev 2006, 106, 2355. [DOI] [PubMed] [Google Scholar]
- (9).For an example of a similar cyclization-oxidation sequence, see: Woo SK; Lee E. J. Am. Chem. Soc 2010, 132, 4564. [DOI] [PubMed] [Google Scholar]
- (10).Furrow ME; Schaus SE; Jacobsen EN J. Org. Chem 1998, 63, 6776. [DOI] [PubMed] [Google Scholar]
- (11).Trygstad TM; Pang YC; Forsyth CJ J. Org. Chem 2009, 74, 910. [DOI] [PubMed] [Google Scholar]
- (12).Trost BM; Machacek MR; Faulk BD J. Am. Chem. Soc 2006, 128, 6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Prepared from hexanoyl chloride in six steps:Marron BE; Spanevello RA; Elisseou ME; Serhan CN; Nicolaou KC J. Org. Chem 1989, 54, 5522.Matsumura K; Hashiguchi S; Ikariya T; Noyori RJ Am. Chem. Soc 1997, 119, 8738. Nicolaou KC; Veale CA; Webber SE; Katerinopoulos H. J. Am. Chem. Soc 1985, 107, 7515.Suzuki M; Kiho T; Tomokiyo K; Furuta K; Fukushima S; Takeuchi Y; Nakanishi M; Noyori RJ Med. Chem 1998, 41, 3084.
- (14).Prepared from methyl 4-(chloroformyl)butyrate in six steps:Gotz K; Liermann JC; Thines E; Anke H; Opatz T Org. Biomol. Chem 2010, 8, 2123.Treilhou M; Fauve A; Pougny JR; Prome JC; Veschambre HJ Org. Chem 1992, 57, 3203. Chemin D; Linstrumelle G. Tetrahedron 1992, 48, 1943–1952. Rodriguez AR; Spur BW Tetrahedron Lett. 2012, 53, 86–89.
- (15) (a).Review: Alcaide B; Almendros P; Alonso JM Org. Biomol. Chem 2011, 9, 4405. [DOI] [PubMed] [Google Scholar]; (b) Harkat H; Weibel J-M; Pale P Tetrahedron Lett. 2007, 48, 1439. [Google Scholar]
- (16).Smith JP; Haddad EV; Downey JD; Breyer RM; Boutaud O Arteriosclerosis Thrombosis and Vascular Biology 2010, 30, E219. [Google Scholar]
- (17).Data are presented as mean ± SD from four healthy volunteers. Symbols show differences between the different groups; ***, p < 0.001. [Google Scholar]