Abstract
Background:
Behavioral measures of impulsive behavior include the dimensions of behavioral disinhibition, decision-making, and lapses of attention. These behaviors are associated with a range of risky activities during adolescence, including cigarette smoking; however, few studies have evaluated their associations with tobacco treatment outcomes. The current study examined the relationship between impulsive behavior and contingency management treatment outcomes for adolescent smokers.
Methods:
Data from two contingency management smoking cessation trials were combined (N = 189 adolescents). Participants provided breath carbon monoxide (CO) samples with incentives delivered contingent (i.e., active treatment [AT] condition) or non-contingent (i.e., control treatment [CT] condition) on CO level. Dimensions of impulsive behavior were assessed pre-and post-treatment using the Go/Stop Task, a measure of delay discounting, a continuous performance task, while self-reported impulsivity was assessed with the Barratt Impulsiveness Scale-Adolescent. Relationships between impulsive behavior and treatment outcomes (efficacy and adherence) were assessed using linear mixed effects models.
Results:
Participants in the AT condition had significantly lower program CO levels at each treatment phase. Delay discounting in the AT condition predicted CO levels, with those discounting the most lowering their breath CO levels the least. Delay discounting also predicted program adherence across both conditions.
Conclusions:
Delay discounting may be the most relevant dimension of impulsive behavior to predict outcomes for adolescent smokers completing CM programs, both in terms of successful reductions in smoking and program adherence. Suggestions are made to reduce the effects of delay discounting for adolescent smokers using this treatment approach.
Keywords: Impulsive Behavior, Contingency Management, Smoking, Outcomes, Adolescent
1. Introduction
Many individuals have their first experience with tobacco during adolescence, which could establish addictive behaviors that carry into adulthood. In 2013 an estimated 41.1% of adolescents in grades 9-12 had tried cigarette smoking at some point in their lives, and 15.7% had smoked a cigarette in the previous 30 days (Kann et al., 2014). Evidence suggests adolescent tobacco use is likely to continue into adulthood (Riggs et al., 2007). Thus, understanding factors affecting tobacco cessation treatment efficacy among adolescents is critical for decreasing adolescent and adult tobacco use.
Impulsive behavior is associated with a range of risky adolescent behaviors, including tobacco use (for review see Romer, 2010). Different measures have assessed impulsive behavior, which can be categorized as laboratory behavioral assessments or self-report questionnaires. Previous research suggests behavioral and self-report assessments are only modestly correlated (e.g., Mitchell, 1999; Lane et al., 2003; Reynolds et al., 2008a) or not correlated at all (e.g., Reynolds et al., 2006). This lack of correlation may stem from differences in the measurement procedures themselves. For example, behavioral assessments directly measure the behavior of interest, while self-report assessments require the individual to reflect on, and accurately summarize, his or her own behavior across a variety of contexts. Behavioral measures are also narrowly defined (e.g., ability to wait for a reward or ability to inhibit motor responses), while self-report measures assess broadly defined behaviors across a range of situations (e.g., “I am self-controlled” or “I act on impulse”).
Impulsive behavior has been organized into at least three independent dimensions: behavioral inhibition (e.g., stop-task procedures), impulsive decision-making (e.g., discounting tasks), and lapses of attention (e.g., continuous performance tasks) (de Wit, 2009). Factor analyses have confirmed these three independent dimensions of behavior in adolescents (Reynolds et al., 2008a).
Measures of behavioral inhibition assess the ability to inhibit a prepotent motoric response. These tasks typically require the participant to make a response to visual “go-cues” but to inhibit that response following a ‘stop’ or ‘no-go’ signal after the “go-cues.” Individuals who are poor at inhibiting their responses are considered more impulsive. Research has shown that young adult smokers, compared to nonsmokers, have poorer behavioral inhibition (e.g., Luijten et al., 2011), and adult smokers with low behavioral inhibition have higher levels of nicotine dependence (Billieux et al., 2010). Nicotine use can also modulate behavioral inhibition, with improved inhibition following nicotine use in non-smoking young adults with attention deficit disorder (Potter & Newhouse, 2008).
Delay discounting (a measure of impulsive decision-making) represents how much an individual devalues (or ‘discounts’) an outcome based on delay to its occurrence. A person who discounts more impulsively is considered more behaviorally governed by short-term outcomes. Many studies with adolescents and adults have reported that drug users discount more than nonusers (see MacKillop et al., 2011; Reynolds, 2006 for reviews). Specific to adolescents, there is case-control and prospective evidence that delay discounting is associated with the initiation of tobacco use (Audrain-McGovern et al., 2009; Fields et al., 2009; Reynolds & Fields, 2012), greater alcohol use (Field et al., 2007; Fernie et al., 2013), use of marijuana (Richardson & Edalati, 2016), and escalation of drug use broadly (Khurana et al., 2015). Delay discounting also predicts treatment outcomes for adolescent smokers (Krishnan-Sarin et al., 2007) and marijuana users (Stanger et al., 2012).
Lapses of attention refer to ability to maintain attention or vigilance to a goal behavior over an extended period of time. Sustained attention is usually measured with a continuous performance task, which requires sustained attention while avoiding attentional lapses. Adolescent substance users (including tobacco users) have more lapses of attention than non-users (Tarter et al., 1995), and both case-control and prospective studies have shown lapses of attention are associated with daily smoking and initiation of smoking among adolescents (Fields et al., 2009; Harakeh et al., 2012). Lapses of attention have also predicted treatment outcomes for adolescent smokers in a school-based smoking cessation program (Harris et al., 2014). Additional evidence indicates attention improves in adults following cigarette smoking (Pritchard et al., 1992), suggesting some individuals may smoke as a means to improve their attention.
There are limited data on the relationship between impulsive behavior and treatment outcomes for adolescent smokers (for review see Tomko et al., 2016). Several studies have shown that greater self-reported impulsive behavior is associated with poorer treatment outcomes for smokers (Helstrom et al., 2007; Covey et al., 2008; Wegmann et al., 2012). Two smaller studies indicate behavioral measures, including delay discounting (Krishnan-Sarin et al., 2007) and lapses of attention (Harris et al., 2014), may be related to treatment outcomes for adolescent smokers. Lastly, adolescents with greater self-reported impulsivity as measured by the Barratt Impulsivity Scale have shown greater reductions in tobacco smoking when receiving a contingency management (CM) therapy compared to cognitive behavioral therapy (Morean et al., 2015).
Contingency Management therapy is an incentive-based treatment approach that rewards participants for specified changes in behavior (e.g., reductions in smoking). The literature suggests CM therapy is one of the most effective treatment strategies for substance use (Lussier et al., 2006) and tobacco cessation (see Sussman, 2002, for review). Previous research evaluating impulsive behavior and outcomes from incentive-type programs has shown greater delay discounting may be associated with poorer outcomes in both adults and adolescents (Dallery et al., 2007, Krishnan-Sarin et al., 2007).
The purpose of the present analysis was to evaluate prediction of CM treatment response by the three dimensions of impulsive behavior described above. Based on prior research demonstrating greater impulsive behavior is associated with poorer tobacco cessation outcomes, it was hypothesized that measures of impulsive behavior would be associated with poorer treatment outcomes. Additionally, though impulsivity is often considered a personality trait (e.g. Patton et al., 1995), previous evidence has shown at least some of the factors of impulsivity considered here can be state- and trait-like (e.g. impulsive decision-making [Odum and Baumann, 2010; Hamilton et al., 2015] or lapses of attention [Hunter and Eastwood, 2016]). Thus, an exploratory analysis of program effects on impulsive behavior was conducted with the hypothesis that CM treatment would reduce impulsive behavior.
2. Methods
2.1. Participants
Non-treatment seeking adolescents who regularly smoked tobacco cigarettes were recruited from local high schools, recruitment posters, and word-of-mouth referrals into one of two studies. Data from two studies, 62 participants from Reynolds et al. (2015) and 127 participants from a previously unpublished study, were combined for the analysis. Important to note, this CM therapy has shown good overall efficacy for reducing tobacco usage from pre-to post-treatment (Reynolds et al., 2015). For the portions of these data sets that were combined the study procedures and assessments were identical, and participants were not significantly different in age, sex, frequency of cigarette smoking, and baseline CO and cotinine levels. All participants were required to have an afternoon breath CO level ≥ 9 ppm (Bedfont Instruments, Micro III, UK) or a urinary quantitative cotinine value ≥ 100 ng/ml (J2 Laboratories, Tucson, AZ). Participants 18 years of age or older signed Institutional Review Board approved consent forms. Adolescents under 18 signed assent forms and a parent or legal guardian signed the consent form. Two hundred and twenty-seven adolescents were enrolled in both studies and randomized to an active treatment (AT) or control treatment (CT) condition of a CM program. Of these 227 participants, 38 dropped out of the studies (14 prior to randomization, 15 from AT, and 9 from CT) for a total of 189 completers (94 female, 49.7%). The Institutional Review Board of the host institution approved all research protocols.
2.2. CM Program Phases and Contingencies
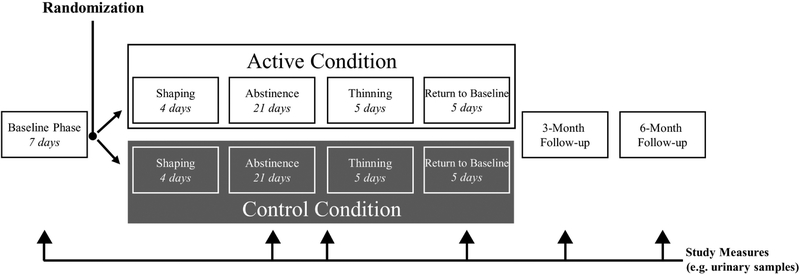
The overall timeline of the study is shown in Figure 2) Participants were assigned to conditions using a simple randomization procedure. A web-based monitoring system (Mōtiv8) was used to verify breath CO measurements and to track vouchers provided as incentives. Breath CO level is an effective measure of recent cigarette smoking (Wald et al., 1981) and was the primary measure for determining smoking throughout the study. Three breath samples were required per day — each separated by at least five hours, but not more than eight hours. The CM treatment program was divided into five separate phases, previously described in detail (Reynolds et al., 2015).
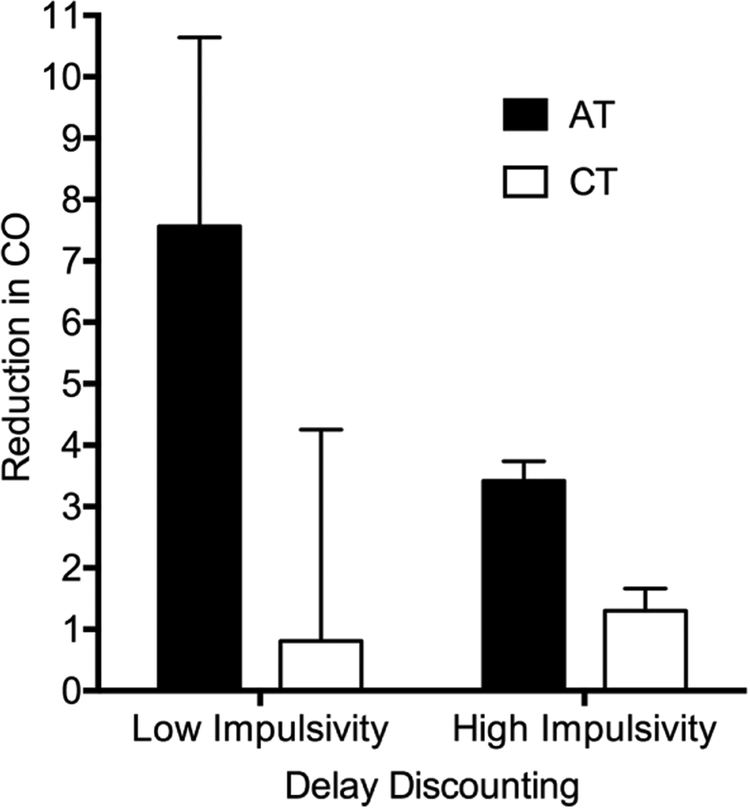
Figure 2. Change in carbon monoxide from Baseline to Return-to-Baseline phases.
Reduction in CO from Baseline to Return-to-Baseline phases. AT = Active Treatment; CT = Control Treatment. Values are mean change in CO (95% CI denoted by error bars) based on parameter estimates derived from a multiple regression model using average values for covariates of age, Fagerstrom Test for Nicotine Dependence, Stage-of-Change Ladder score, and averaged across sexes and studies. Reduction was calculated for −1 SD (High Impulsive behavior; right bars) and +1 SD (Low Impulsive behavior; left bars) pre-treatment delay discounting score.
The program started with a Baseline Phase (seven days). During this phase all participants were required to provide punctual breath samples with no criteria for a specific CO level to earn $6 each day for providing all three samples. Participants were randomized to either the AT or CT condition following the Baseline phase. The AT and CT conditions were alike except incentives in the AT condition were contingent on punctual submission of samples and a specified CO level, whereas in the CT condition incentives were only contingent on punctual submissions. For the Shaping Phase (next four days), AT participants were expected to reduce their CO levels based on calculated “goal-CO” values such that their readings would be ≤ 4 ppm by the end of this phase. Participants received $3 for each sample meeting CO criterion during the Shaping Phase. In the Abstinence Phase (next 21 days) participants in the AT condition were required to provide samples with a CO level ≤ 4 ppm to receive incentives. Four ppm was selected as the threshold based on previous research indicating this value is conservative enough to accurately identify most smoking behavior (see Javors et al., 2005), while not being so low that an excess of false positives would be encountered and potentially disrupt program adherence. For both conditions, incentives during the Abstinence Phase included receiving $3 for the first criterion CO sample, which increased by $0.25 for each consecutive criterion sample. There also was an additional $5 bonus for five consecutive criterion samples. If participants provided a non-criterion sample (e.g., a late sample or a sample with CO ≥4 ppm for the AT condition) they would not receive an incentive for that sample, and the next sample meeting criterion would be awarded $3 regardless of their previous level of incentive. However, following three consecutive criterion samples participants would return to the previous level on the ascending pay schedule prior to submission of the non-criterion sample. During the Thinning Phase (next five days), participants in the AT condition were required to maintain a CO level ≤ 4 ppm to receive incentives. A leaner payment schedule was used to gradually transition participants away from incentives, with $6 awarded for submitting three criterion samples per day. Finally, during the Return-to-Baseline Phase (last five days), there was no specified criterion CO level for participants in the AT condition, and all participants received incentives contingent on providing punctual samples each day. Incentives during this phase were $6 each day for submitting all three CO samples.
2.3. General Procedures
All participants completed a ~2.5 h laboratory session within the two weeks preceding the CM Baseline Phase, which included: a) a demographic questionnaire, b) the Kauffman Brief Intelligence Test – Second Edition (Kaufman and Kaufman, 2004), c) cigarette use questions (e.g., Modified Fagerström Test for Nicotine Dependence [mFTND]), d) measure of readiness to quit smoking (Contemplation Ladder: Stage-of-Change [SCL]), and e) measures of impulsive behavior. These measures were also collected during a post-treatment session within two weeks of finishing the Return-to-Baseline Phase of the CM program.
2.4. Measures of Impulsive Behavior
Behavioral assessments representing each of the three aforementioned dimensions of impulsive behavior were utilized in this study, while a self-report measure of impulsivity was also included.
2.4.1. Go/Stop Task (GST)
The GST is a computerized measure of behavioral inhibition (Dougherty et al., 2003). The outcome measure from this task was the stop-signal reaction time (SSRT), which reflects the average stop signal delay at which a participant was able to successfully stop on 50% of the trials, with larger SSRT values indicating greater disinhibition. Average reaction time to go signals was also an outcome measure.
2.4.2. Question-Based Delay-Discounting Measure (DDQ)
The DDQ is a computerized measure of decision-making (Richards, Zhang, Mitchell, & de Wit, 1999). Indifference points are calculated for each delay interval and represent the smallest amount of immediate money an individual chose instead of a set amount of delayed money ($10). One randomly selected choice from the task was honored to encourage active engagement. The outcome measure was total area under the curve (AUC) of indifference points, with lower AUC values indicating greater impulsive decision-making (Myerson et al. 2001).
2.4.3. Conners’ Continuous Performance Test-II (CPT-II)
The CPT-II is a computerized behavioral measure of inattention (Conners, 2004). The primary outcome measure of this task was number of omission errors (failure to respond to target signals), with greater omissions indicating greater inattention.
2.4.4. Barratt Impulsiveness Scale-Adolescent (BIS-11-A)
The BIS-11-A is a self-report assessment of impulsive behavior, including 30 items rated on a four-point scale adapted from the adult BIS-11 (Fossati et al., 2002; Patton et al., 1995). The outcome measure on this task was total scores and did not include sub-factors based on Fossati and colleagues’ (2002) determination that this “is the most appropriate measure to use in assessing adolescent impulsivity” with this scale.
2.5. Data Analysis
All models included the following covariates: study (i.e. a dichotomous variable for data from Reynolds et al. [2015] or from the previously unpublished study), age, sex, and baseline scores on the mFTND and SCL. Due to group differences on number of peers who smoke (Table 1 this variable was entered as a covariate in all models, but after it did not significantly account for variance in any of the models it was removed. In models examining CO as the dependent variable, treatment adherence (i.e. number of possible CO samples submitted) was included as a covariate to address the possibility participants could have selectively omitted CO samples following recent smoking.
Table 1.
Demographic and Clinical Variables.
| Active | Contro | Dropou | F (p) | |
|---|---|---|---|---|
| N = 94 | N = 95 | N = 38 | ||
| Demographic variables | ||||
| Age, mean (SD) | 16.8 (1.5) | 16.8 (1.51) | 17.3 (1.5) | 1.80 (0.168) |
| Female, N (%) | 46 (48.9) | 48 (50.5) | 13 (34.2) | 3.11† (0.211) |
| Race, N (%) | ||||
| African American | 8 (8.5) | 10 (10.5) | 2 (5.3) | 1.08† (0.898) |
| Caucasian | 78 (83.0) | 76 (80.0) | 32 (84.2) | – |
| Other | 8 (8.5) | 9 (9.5) | 4 (10.5) | – |
| Clinical variables | ||||
| Kauffman Brief Intelligence Test – Second Edition, mean (SD) | 89.0 (10.8) | 88.9 (11.2) | 88.6 (11.3) | 0.02 (0.982) |
| Cigarettes per day,a mean (SD) | 14.0 (10.3) | 11.6 (7.2) | 14.0 (9.4) | 1.98 (0.141) |
| Modified Fagerstrom Test for Nicotine Dependence Score, mean (SD) | 5.2 (1.8) | 5.3 (1.8) | 5.1 (1.8) | 0.18 (0.833) |
| Carbon monoxide (ppm), mean (SD) | 10.1 (6.5) | 10.5 (5.9) | 12.0 (7.1) | 1.22 (0.297) |
| Cotinine (ng/ml), mean (SD) | 1289.2 (951.3) | 1134.2 (943.2) | 1477.4 (702.9) | 2.03 (0.133) |
| Percentage of carbon monoxide samples submitted, mean (SD) | 38.3 (24.8) | 52.7 (27.9) | N/A | 14.05 (<0.001) |
| At last one parent smokes (reporting “yes”), N (%) | 83 (88.3) | 83 (87.4) | 34 (89.5) | 0.12† (0.942) |
| How many peers smoke [categorical],b mean (SD) | 3.9 (0.9) | 3.6 (0.8) | 3.6 (0.9) | 3.36 (0.037) |
| Closest/best friend smokes (reporting “yes”), N (%) | 85 (90.4) | 82 (86.3) | 32 (84.2) | 1.24† (0.537) |
| Contemplation Ladder: Stage-of-Change Score, mean (SD) | 7.0 (1.8) | 6.9 (1.9) | 6.8 (1.9) | 0.17 (0.843) |
| Alcohol use frequency,c mean (SD) | 1.8 (1.0) | 1.7 (1.0) | 1.9 (1.2) | 0.553 (0.576) |
| Marijuana use frequency,c mean (SD) | 2.4 (1.9) | 2.2 (1.8) | 2.6 (2.1) | 0.662 (0.517) |
Cigarettes per week were calculated using a timeline follow back calendar to determine cigarettes smoked each day during the past two weeks;
Friends who smoke was assessed using the following question: “How many of your friends smoke cigarettes/black & milds?”: 1 = none, 2 = some, 3 = half, 4 = most, 5 = all.
Marijuana and alcohol use was assessed with the following question: “Thinking about the past six months, how often have you used the following substances?”: 0 = never tried, 1 = tried it, 2 = 1–2 times/month, 3 = once a week, 4 = 2–4 times/week, 5 = 5 or more times a week.
= chi-square statistic.
To examine the relationship between impulsive behavior and treatment efficacy two separate change scores were calculated for each participant based on his or her change in program CO levels: one change score for the Abstinence phase relative to Baseline phase and another for the Return-to-Baseline phase relative to Baseline phase. Analyses of the relationships between impulsive behavior and program CO levels were restricted to the Abstinence and Return-to-Baseline phases because these program stages were non-transitional (as opposed to Shaping and Thinning phases). Treatment adherence was calculated as the percentage of all possible CO samples submitted by a participant during the entire program.
Linear mixed models were used to examine treatment condition, treatment phase, and impulsive behavior when examining CO change values, or treatment condition and impulsive behavior when examining treatment adherence. When significant effects were observed, multiple regression analyses were used for post-hoc analyses.
Between-subjects t-tests were used to compare change in impulsive behavior by treatment condition. All analyses were conducted with the SAS statistical software package, version 9.3 (SAS Institute Inc., Cary, NC). Significance threshold for all analyses was set at p ≤ .05.
3. Results
Demographic and clinical variables are presented in Table 1. Groups were well matched, with the exception that participants in the AT group reported significantly more peers who smoke. Participants in the AT group also submitted significantly fewer CO samples throughout program phases (38.3%) compared to those in the CT group (52.7%).
3.1. Predicting Treatment Outcomes Using Measures of Impulsive Behavior
Results from mixed models examining treatment outcomes as a function of impulsive behavior are presented in Table 2. Consistent with previous data on DDQ area under the curve distributions (e.g. Myerson et al., 2001), these data are positively skewed with skewness of 1.18, and kurtosis of .73. Assumptions for this mixed model, however, only require that the model residuals are normally distributed and do not systematically deviate, which was visually verified. DDQ was predictive of change in CO, indicated by a significant interaction between pre-treatment scores on the DDQ and treatment condition. Results of post-hoc multiple regression analyses for AT and CT conditions indicated a significant relationship between DDQ scores and CO change scores during Return-to-Baseline for individuals in the AT condition only (B=−8.5, p=.049, sr2=.06; Figure 2). Participants in the AT condition who discounted more had higher CO change scores (i.e. smaller reductions in CO) during the Return-to-Baseline phase relative to their less impulsive counterparts. There was also a significant effect of DDQ on treatment adherence (F[1,180]=31.44, p<.001). Estimates generated by the mixed model, using average age, average mFTND and SCL scores, and equal weighting for sexes, conditions, and studies indicated treatment adherence for a −1 SD DDQ score (more impulsive) as 46.0% and a +1 SD DDQ score (less impulsive) as 56.3%.
Table 2.
Predicting change in carbon monoxide levels from Baseline to Abstinence and Return-to-Baseline phases using measures of impulsive behavior.
| Condition | Measure† | Phase | Measure† × Phase | Measure† × Condition | Condition × Phase | Measure† × Condition × Phase | |
|---|---|---|---|---|---|---|---|
| Breath carbon monoxide | |||||||
| Go/Stop task: stop signal delay, F | 2.01 | < 0.01 | < 0.01 | 0.13 | 0.87 | 0.40 | 0.71 |
| Delay Discounting: AUC, F | 3.50 | 0.87 | 0.40 | <0.01 | 4.62* | 1.20 | 1.21 |
| Continuous Performance Task: omissions, F | 2.31 | < 0.01 | 0.13 | < 0.01 | 0.86 | 0.11 | 0.03 |
| Barratt Impulsiveness Scale—Adolescent, F | 3.11 | 0.99 | 1.94 | 1.71 | 0.99 | 4.45* | 4.72* |
| Treatment adherence | |||||||
| Go/Stop task: SSRT, F | 7.80** | 0.56 | — | — | 0.60 | — | — |
| Delay Discounting: AUC, F | 9.82** | 15.61*** | — | — | 0.07 | — | — |
| Continuous Performance Task: omissions, F | 0.38 | 0.13 | — | — | 2.53 | — | — |
| Barratt Impulsiveness Scale—Adolescent, F | 1.01 | 0.19 | — | — | 0.08 | — | — |
= not applicable” for that model; AUC = area under the curve;
= Impulsive behavior measure for that respective row; All values displayed are F values;
p ≤ .05,
p ≤ .01,
p ≤ .001.
A significant interaction between BIS scores, study condition, and phase was indicated for change in CO levels (F[1,124]=4.72, p=.032). Yet, post hoc multiple regression analyses examining change in CO for active and control conditions, during abstinence and return to baseline phases, did not indicated a significant effect of BIS at any phase.
3.2. Change in Impulsive Behavior Measures from Pre- to Post-treatment
An overview of these analyses is presented in Table 3. Results of t-tests examining change in impulsive behavior from pre- to post-treatment indicated the CT group had a significant decrease in BIS-11-A scores, which was significantly different from the AT group. Significant increases were also observed on CPT omissions for individuals in the AT and CT groups, but these changes were not significantly different between groups.
Table 3.
Change in impulsive behavior measures from pre- to post-treatment.
| Active condition | Control condition | Difference between groups | ||
|---|---|---|---|---|
| t | p | |||
| Measure | ||||
| Go/Stop task: SSRT, mean (SEM) | −1.74 (9.66) | 0.60 (9.61) | 0.08 | 0.933 |
| Delay Discounting: AUC, mean (SEM) | −0.01 (0.03) | −0.04 (0.03) | 0.71 | 0.480 |
| Continuous Performance Task: omissions, mean (SEM) | 10.97 (3.82)** | 7.97 (3.80)* | 0.56 | 0.578 |
| Barratt Impulsiveness Scale—Adolescent, mean (SEM) | −1.20 (1.92) | −6.89 (1.91)** | 2.10* | 0.037* |
AUC = area under the curve; Bolded and asterisked mean values represent significant changes from 0.
p ≤ .05.
p ≤ .01.
4. Discussion
This study represents the most comprehensive examination of the relationship between impulsive behavior and CM treatment outcomes for adolescent smokers to date. The primary finding was greater delay discounting was associated with poorer treatment adherence and smaller reductions in CO levels. The relationship between delay discounting and program CO levels is consistent with previous research where delay discounting predicted abstinence from smoking during incentive-based approaches (e.g., Dallery and Raiff, 2007; Krishnan-Sarin et al., 2007). However, to the best of our knowledge, this is the first study in which delay discounting predicted adherence to a smoking cessation program.
Interestingly, delay discounting was not predictive of change in CO levels during the Abstinence phase. This may be because during the Abstinence phase smoking behavior was primarily controlled by the contingent incentives, such that individual differences in delay discounting were not a significant factor when contingent rewards were in place. This explanation is supported by the fact that none of the covariates (i.e. SCL or mFTND scores, gender, or age) were significant predictors during the Abstinence phase for individuals in the AT condition, further suggesting contingent incentives were the primary behavioral determinant. During the Return-to-Baseline phase incentives were no longer contingent upon reductions in smoking and individual differences in delay discounting emerged as a predictor of outcomes. This is similar to results presented by Washio and colleagues (2011) where high and low reward CM conditions were compared for treating cocaine dependency. Their results suggested that individuals who discount greater will have poorer outcomes in low vs high reward CM, while individuals who discount less will do equally well in low and high reward conditions, suggesting that greater CM reward can supersede the influence of individual discounting differences on CM outcomes. Results of the current study also confirm previous research suggesting discounting is predictive of smoking behavior following removal of contingent incentives for tobacco cessation (Dallery & Raiff, 2007). The reader should note that this study used untransformed area under the curve values for discounting (in accordance with Myseron et al., 2001), but results may differ if area under the curve values are transformed (e.g. Borges et al., 2016).
Measures of behavioral inhibition and lapses of attention were not predictive of treatment outcomes, while self-reported impulsive behavior did not have a clear effect according to post-hoc analyses. This finding was unexpected; however, it is important to note the current findings are specifically relevant to adolescent smokers completing a CM program and some of these other types of impulsive behavior may be predictive in different populations (e.g., adult smokers) or different treatment approaches. For example, lapses of attention have been predictive of outcomes in a school-based adolescent smoking cessation program (Harris et al., 2014), and numerous instances where self-reported impulsive behavior has been associated with adolescent smoking treatment outcomes (e.g., Henderson et al., 2008; Wegmann et al., 2012). This suggests adolescents with greater self-reported impulsivity may fare better with a CM treatment for tobacco smoking cessation compared to other treatment modalities. The current study further suggests that for incentive-based approaches with adolescent smokers, delay discounting is likely the most relevant type of impulsive behavior to treatment outcomes.
Overall, there were no conclusive effects of the program on impulsive behavior. Both treatment groups increased their CPT omission rates (indicating increased lapses of attention) from pre- to post-treatment, with no significant difference between groups. This finding is consistent with previous research showing increased omission errors with repeated administrations of the CPT (e.g., Weafer et al., 2013), suggesting an effect of repeated testing rather than a systematic program effect of CM. Also, scores on the BIS-11-A significantly decreased for individuals in the CT, but not the AT, condition. This unexpected finding might be a consequence of differences in program adherence, with greater adherence by participants in the CT than AT condition. Group differences in adherence possibly resulted in greater feelings of responsibility and diminished self-report of impulsive behavior for the CT participants than when they started the research study. This outcome could also reflect a lack of temporal reliability for the BIS-11-A and not represent meaningful change from program effects.
These findings indicate adolescents who discount more have more difficulty reducing their smoking during a CM program and more difficulty adhering to the schedule of a CM program. Steps might be taken to reduce program delays and, consequently, reduce the undesirable effects of delay discounting. Perhaps most importantly, reducing delay to incentives could greatly improve program efficacy and adherence. For example, providing each participant with a restricted debit card (e.g., no purchase of tobacco, alcohol, weapons, etc.) that could be immediately updated as financial vouchers are earned would significantly reduce delays to incentives. Also, more frequent contacts between participants and study staff may help offset the effects of program delays for those who discount more. Based on the current findings, these types of delay-related program modifications could lead to improvements in program efficacy and adherence.
Figure 1. Overall Study Timeline.
Highlights.
Greater delay discounting was associated with lower reductions in carbon monoxide
Greater delay discounting predicted poorer treatment adherence
Modifying contingency management for greater discounting could improve efficacy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi SM, Petry NM, Urso J, 2008. Contingency management promotes smoking reductions in residential substance abuse patients. J. Appl. Behav. Anal. 41(4), 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP, 2009. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 103(3), 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billieux J, Gay P, Rochat L, Khazaal Y, Zullino D, Van der Linden M, 2010. Lack of inhibitory control predicts cigarette smoking dependence: Evidence from a non-deprived sample of light to moderate smokers. Drug Alcohol Depend. 112(1-2), 164–167. [DOI] [PubMed] [Google Scholar]
- Borges AM, Kuang J, Milhorn H, Yi R, 2016. An alternative approach to calculating area-under-the-curve (AUC) in delay discounting research. J Exp Anal Behav. 106(2), 145–155. [DOI] [PubMed] [Google Scholar]
- Conners K, 2004. Conners’ rating scales-revised: CRS-R. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Covey LS, Manubay J, Jiang H, Nortick M, Palumbo D, 2008. Smoking cessation and inattention or hyperactivity/impulsivity: A post hoc analysis. Nicotine Tob. Res. 10(12), 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Glenn IM, Raiff BR, 2007. An internet-based abstinence reinforcement treatment for cigarette smoking. Drug Alcohol Depend. 86(2), 230–238. [DOI] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, 2007. Delay discounting predicts cigarette smoking in a laboratory model of abstinence reinforcement. Psychopharmacology. 190(4), 485–496. [DOI] [PubMed] [Google Scholar]
- de Wit H, 2009. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addict. Biol. 14(1), 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, 2003. GoStop impulsivity paradigm San Antonio, TX: Neurobehavioral Research Laboratory and Clinic, University of Texas Health Science Center; Multiple Self-aspects: Structure, Development, Operation, and Implications. Soc. Cogn. 30, 380–395. [Google Scholar]
- Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H, Field M, 2013. Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction. 108(11), 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A, 2007. Delay discounting and the alcohol stroop in heavy drinking adolescents. Addiction. 102(4), 579–586. [DOI] [PubMed] [Google Scholar]
- Fields S, Collins C, Leraas K, Reynolds B, 2009. Dimensions of impulsive behavior in adolescent smokers and nonsmokers. Exp. Clin. Psychopharm. 17(5), 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati A, Barratt ES, Acquarini E, Di Ceglie A, 2002. Psychometric properties of an adolescent version of the Barratt Impulsiveness Scale-11 for a sample of Italian high school students. Percept. Mot. Skills. 95(2), 621–635. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, Lane SD, Lejuez C, Littlefield AK, Luijten M, 2015. Choice impulsivity: Definitions, measurement issues, and clinical implications. Personal. Disord. 6(2): 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakeh Z, de Sonneville L, van den Eijnden RJJM, Huizink AC, Reijneveld SA, Ormel J, Verhulst FC, Monshouwer K, Vollebergh WAM, 2012. The association between neurocognitive functioning and smoking in adolescence: The TRAILS study. Neuropsychology. 26(5), 541. [DOI] [PubMed] [Google Scholar]
- Harris M, Penfold RB, Hawkins A, Maccombs J, Wallace B, Reynolds B, 2014. Dimensions of impulsive behavior and treatment outcomes for adolescent smokers. xp. Clin. Psychopharm. 22(1), 57–64. [DOI] [PubMed] [Google Scholar]
- Helstrom A., Hutchison K., Bryan A., 2007. Motivational enhancement therapy for high-risk adolescent smokers. Addict. Behav. 32(10), 2404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson M, Ecob R, Wight D, Abraham C, 2008. What explains between-school differences in rates of smoking? BMC Public Health. 8(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter A and Eastwood JD, 2016. Does state boredom cause failures of attention? Examining the relations between trait boredom, state boredom, and sustained attention. Exp. Brain Res, 236(9),1–10. [DOI] [PubMed] [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, Harris WA, Lowry R, Olsen EO, McManus T, Chyen D, 2014. Youth Risk Behavior Surveillance — United States, 2013. MMWR Surveill. Summ. 63(Suppl 4), 1–168. [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL, 2004. Kaufman Brief Intelligence Test, second ed. Circle Pines, Minnesota. [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, & Hurt H, 2015. Experimentation versus progression in adolescent drug use: A test of an emerging neurobehavioral imbalance model. Dev. Psychopathol. 27(3), 901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Duhig AM, McKee SA, McMahon TJ, Liss T, McFetridge A, Cavallo DA, 2006. Contingency management for smoking cessation in adolescent smokers. Exp. Clin. Psychopharm. 14(3), 306. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Cavallo DA, Carroll KM, Potenza MN, 2007. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 88(1), 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Rhoades HM, Pietras CJ, Tcheremissine OV, 2003. Relationships among laboratory and psychometric measures of impulsivity: Implications in substance abuse and dependence. Addict. Disord. Their Treat. 2(2), 33–40. [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST, 2006. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 101(2), 192–203. [DOI] [PubMed] [Google Scholar]
- Luijten M, Littel M, Franken IH, 2011. Deficits in inhibitory control in smokers during a go/nogo task: An investigation using event-related brain potentials. PloS One, 6(4), e18898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR, 2011. Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology. 216(3), 305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH, 1999. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 146(4), 455–464. [DOI] [PubMed] [Google Scholar]
- Morean ME, Kong G, Camenga DR, Cavallo DA, Carroll KM, Pittman B, & Krishnan-Sarin S, 2015. Contingency management improves smoking cessation treatment outcomes among highly impulsive adolescent smokers relative to cognitive behavioral therapy. Addict. Behav. 42, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M, 2001. Area under the curve as a measure of discounting. J. Exp. Anal. Behav. 76(2), 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Baumann AA, 2010. Delay discounting: State and trait variable In Madden GJ & Bickel WK (Eds.), Impulsivity: The behavioral and neurological science of discounting (pp. 39–65). Washington, DC, US: American Psychological Association. [Google Scholar]
- Patton JH, Stanford MS, 1995. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 51(6), 768–774. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA, 2008. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol. Biochem. Behav. 88(4), 407–417. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Robinson JH, Guy TD, 1992. Enhancement of continuous performance task reaction time by smoking in non-deprived smokers. Psychopharmacology. 108(4), 437–442. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Dallery J, Shroff P, Patak M, Leraas K, 2008. A web-based contingency management program with adolescent smokers. J. Appl. Behav. Anal. 41(4), 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Fields S, 2012. Delay discounting by adolescents experimenting with cigarette smoking. Addiction (Abingdon, England). 107(2), 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Harris M, Slone SA, Shelton BJ, Dallery J, Stoops W, Lewis R, 2015. A feasibility study of home-based contingency management with adolescent smokers of rural Appalachia. Exp. Clin. Psychopharmacol. 23(6), 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H, 2006. Dimensions of impulsive behavior: Personality and behavioral measures. Pers. Individ. Dif. 40(2), 305–315. [Google Scholar]
- Reynolds B, Penfold RB, Patak M, 2008a. Dimensions of impulsive behavior in adolescents: Laboratory behavioral assessments. Exp. Clin. Psychopharmacol. 16(2), 124. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H, 1999. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. J. Exp. Anal. Behav. 71(2), 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson CG, Edalati H, 2016. Application of a brief measure of delay discounting to examine the relationship between delay discounting and the initiation of substance use among adolescents. Subst. Use Misuse. 51(4), 540–544. [DOI] [PubMed] [Google Scholar]
- Riggs NR, Chou CP, Li C, Pentz MA, 2007. Adolescent to emerging adulthood smoking trajectories: When do smoking trajectories diverge, and do they predict early adulthood nicotine dependence? Nicotine Tob. Res. 9(11), 1147–54. [DOI] [PubMed] [Google Scholar]
- Roll JM, 2005. Assessing the feasibility of using contingency management to modify cigarette smoking by adolescents. J. Appl. Behav. Anal. 38(4), 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, 2010. Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Dev. Psychobiol. 52(3), 263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Jarvik ME, Ling W, Rawson RA, 1996. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addict. Behav. 21(3), 409–412. [DOI] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, Budney AJ, 2012. Delay discounting predicts adolescent substance abuse treatment outcome. Exp. Clin. Psychopharmacol. 20(3), 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, 2002. Effects of sixty six adolescent tobacco use cessation trials and seventeen prospective studies of self-initiated quitting. Tob. Induc. Dis. 1(1), 1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh YC, Parks SM, 1995. Cognitive capacity in female adolescent substance abusers. Drug Alcohol Depend. 39(1), 15–21. [DOI] [PubMed] [Google Scholar]
- Tomko RL, Bountress KE, & Gray KM, 2016. Personalizing substance use treatment based on pre-treatment impulsivity and sensation seeking: A review. Drug Alcohol Depend. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald NJ, Idle M, Boreham J, Bailey A, 1981. Carbon monoxide in breath in relation to smoking and carboxyhaemoglobin levels. Thorax. 36(5): 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washio Y, Higgins ST, Heil SH, McKerchar TL, Badger GJ, Skelly JM, Dantona RL, 2011. Delay discounting is associated with treatment response among cocaine-dependent outpatients. Exp. Clin. Psychopharm. 19(3), 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Baggott MJ, de Wit H, 2013. Test-retest reliability of behavioral measures of impulsive choice, impulsive action, and inattention. Exp. Clin. Psychopharmacol. 21(6), 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann L, Bühler A, Strunk M, Lang P, Nowak D, 2012. Smoking cessation with teenagers: The relationship between impulsivity, emotional problems, program retention and effectiveness. Addict. Behav. 37(4), 463–468. [DOI] [PubMed] [Google Scholar]