Abstract
Background:
Glioblastoma (GBM) is the most malignant brain tumor with a poor prognosis that can be very difficult to cure, and the current treatment options have no optimal outcomes. Hence, it is essential to find new treatment modalities. Histologically, this tumor has high microvascular density that makes it desirable for vascular target agent drugs. Prostate-specific membrane antigen (PSMA) is a novel antigen with unique features that expresses in the vascular endothelium of some malignant tumors.
Materials and Methods:
Formalin-fixed paraffin-embedded tissues from sixty patients who underwent GBM tumor resection from 2012 to 2016 were evaluated for the expression of PSMA by immunohistochemistry. Sections were also assessed for the extent and intensity of endothelial staining in tumor microvessels and for clinicopathologic factor correlation.
Results:
A considerable PSMA expression level was detected in 66% of the cases, and the intensity was strong and moderate in 63%. There was no significant correlation neither between PSMA expression with tumor site, presence of necrosis, and endothelial proliferation nor with age and sex.
Conclusion:
The expression of PSMA in GBM, as observed in the current study, may suggest a new role of PSMA-targeted therapy and indicate more investigations focused on complementary treatment strategies that specifically target tumor vasculature.
Keywords: Glioblastoma, prostate-specific membrane antigen, tumor vasculature
Introduction
Glioblastoma (GBM) is an astrocytic WHO Grade IV tumor, with a median survival time of 14.6 months after diagnosis.[1,2] It is the most common brain and central nervous system malignancy, which accounts for 45.2% of malignant primary brain tumors and diagnosed in the median age of 64 years, 1.6 times higher in men than women.[3]
Surgical resection followed by radiation therapy and chemotherapy is the current standard therapy. However, because of their infiltrating nature, the complete neurosurgical resection of these tumors is impossible,[4] and current GBM treatments have not improved overall patient survival rates. Therefore, new modalities for treatment are required.[5]
GBM in nature is a solid brain tumor with high vascular density, and patients with high tumor microvascular densities (MVDs) have shorter postoperative survival time than the patients with low MVDs, suggesting that the tumor vasculature is an important factor of tumor progression.[6]
This feature makes a favorable field for vascular targeting agents (VTAs) as a complementary therapy. The VTAs all lead to rapid reductions in tumor blood flow and extensive necrosis in tumors.[7]
Prostate-specific membrane antigen (PSMA) is a type II transmembrane glycoprotein with foliate hydrolase 1 and neurocarboxypeptidase activities.[8] Originally, PSMA expression was identified in prostate cancer cells,[9] and it has been shown to be expressed in the endothelial cells of neovasculature from a variety of tumors, including breast and renal cell carcinoma but not normal endothelium.[10,11]
Therefore, it could be beneficial to use PSMA-targeted agents for tumor treatment with PSMA expressing vasculature.
PSMA is qualitatively distinct from other neovascular targets, such as vascular endothelial growth factor, endoglin, or the integrins involved in the general process of angiogenesis, that is not specific to tumor vasculature. PSMA expression has not been reported in normal vasculature and represents the specific currently known neovascular target. This specificity makes PSMA an ideal target for delivery of a cytotoxic agent designed to tumor vasculature destruction.[12] Some studies have revealed the effectiveness of anti-PSMA monoclonal antibody (mAb) J591 in the treatment of tumors expressing PSMA.[12,13,14]
Therefore, in this study, we aimed to show the PSMA expression and its intensity in GBM tumors in patients with GBM referred to Alzahra Hospital from 2012 to 2016.
Materials and Methods
In this study, formalin-fixed paraffin-embedded tissues of sixty patients who underwent tumor resection from 2012 to 2016 for GBM were retrieved from the archive of Alzahra Hospital, Isfahan, Iran. The Medical Ethics Committee of the Isfahan University of Medical Sciences approved the study protocol (research project number: 395820). The inclusion criteria were the adequate material available in the paraffin blocks for immunohistochemistry (IHC) and in the case of improperly fixed specimens, the questionable cases or if the relevant clinical history was not available, the samples were excluded. Immunohistochemical staining was performed on the GBM specimens’ vessels for PSMA expression in tumor endothelium. The tissue samples for CD31 were also stained to verify vessels. From each case, H & E-stained histologic sections were reexamined by a skilled neuropathologist, and also features such as presence of necrosis and endothelial proliferation were recorded.
The sequential sections showing the most viable tumor were selected for IHC.
Antibodies/reagents
Anti-PSMA mAb clone SP29, peroxidase block, target retrieval solution, peroxidase-labeled polymer conjugated to anti-rabbit immunoglobulin, and DAB+ chromogen were purchased from Biogenex company, CA, USA
The mAb to human CD31 was obtained from Dako Company (DAkO, CA, USA).
Staining procedure
For PSMA staining, paraffin sections were deparaffinized, and rehydrated sections were placed in target retrieval solution (pH 9.0) and heated in a water bath from 95°C to 99°C for 30 min prior to the immunostaining. For CD31 staining, the deparaffinized and rehydrated sections were placed in 0.01 M citrate retrieval solution (pH 6.0) and heated in a pressure cooker for 1 min prior to the immunostaining. The sections were washed in Tris-buffered saline-Tween 20. Peroxidase block was incubated for 5 min. After washing in Tris-buffered saline-Tween 20, mAb SP29 was incubated on the sections for 1 h at room temperature. Antibody binding was detected using peroxidase-labeled polymer conjugated to anti-rabbit immunoglobulins and DAB+ chromogen. The sections were counterstained with 10% hematoxylin.
Prostate adenocarcinoma tissue was considered as a positive control and normal brain tissue as a negative control.
Assessment
Immunostained sections were assessed for the extent and intensity of endothelial staining in tumor microvessels. PSMA extent of vascular endothelial staining (percent staining) was evaluated and scored by an expert neuropathologist as follows:
< 5% staining of vessels (none)
6%–25% staining of vessels (minimal)
26%–50% staining of vessels (moderate)
51%–75% staining of vessels (strong)
76%–100% staining of vessels (very strong).
The staining intensity was scored as follows:
1+: faint, barely perceptible staining
2+: moderately intense staining that was readily apparent at low-power magnification [Figure 1]
3+: maximum intensity staining [Figure 2].
Figure 1.
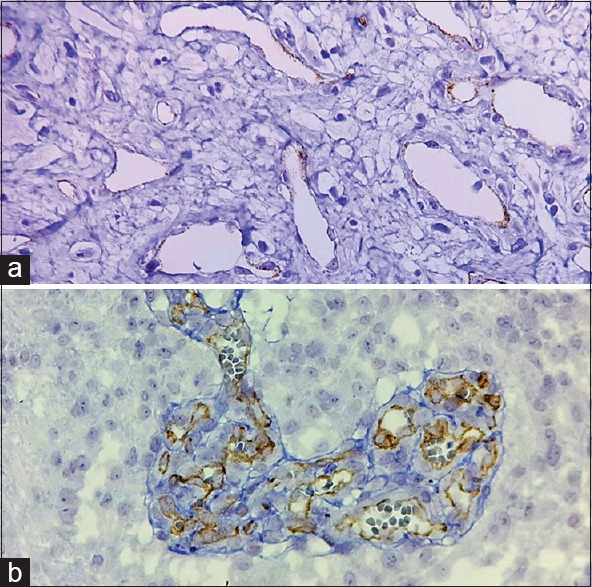
Prostate-specific membrane antigen expression in glioblastoma was scored 1+ (a) and 2+ (b) (×400)
Figure 2.
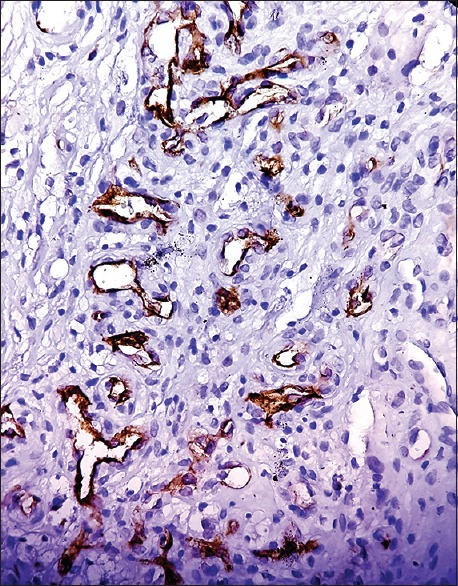
Prostate-specific membrane antigen expression in glioblastoma was scored 3+ (×400)
When there was heterogeneity in the intensity of staining, the score was considered based on the predominant pattern.
Statistical analysis
After data collection, the analysis of quantitative and qualitative data was performed using the Statistical Package for the Social Sciences software version 22 (IBM corporation, New York, USA).
The relationship of variables was investigated using Student's t-test and for categorical variables Pearson's test or Fisher's exact test used. P < 0.05 was considered statistically significant.
Results
In this study, sixty patients in the age range of 19–80 years and a median age of 53 years were enrolled, of which 40 patients were male (66.7%) and 20 were female (33.3%), with a male:female ratio of 2:1.
The frontal and parietal lobes were the most commonly affected sites, representing 30% and 28.3%, respectively.
Moreover, tumor ischemic necrosis was detected in 80% of cases and endothelial proliferation in 86.7% of cases.
PSMA staining was positive in the endothelium of 66.7% of tumor cells, and the intensity was strong and moderate in 63% of cases [Tables 1 and 2]. There was no staining in the vessels of any normal brain specimen.
Table 1.
Extent of vascular staining
| Variable | n (%) |
|---|---|
| Extent of vascular staining | |
| <5% | 20 (33.3) |
| 6%-25% | 12 (20) |
| 26%-50% | 9 (15) |
| 75%-51% | 16 (26.7) |
| 76%-100% | 3 (5) |
Table 2.
Intensity of vascular staining
| Variable | n (%) |
|---|---|
| Intensity of vascular staining | |
| 0 | 15 (25) |
| Mild | 7 (11.7) |
| Moderate | 23 (38.3) |
| Severe | 15 (25) |
No significant correlation was seen between PSMA expression with tumor site, neither with age nor sex [Tables 3 and 4].
Table 3.
Gender distribution in glioblastoma
| Gender | PSMA negative, n (%) | PSMA positive, n (%) | P |
|---|---|---|---|
| Male | 14 (35) | 26 (65) | 0.7 |
| Female | 6 (30) | 14 (70) |
PSMA: Prostate-specific membrane antigen
Table 4.
Age distribution in glioblastoma
| Variable | PSMA negative | PSMA positive | P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 53.6 | 11.6 | 53.2 | 16.5 | 0.93 |
PSMA: Prostate-specific membrane antigen, SD: Standard deviation
Statistical analysis revealed P = 0.55 and 0.56 for the correlation between PSMA status with necrosis and endothelial proliferation, respectively. These values were considered to be statistically insignificant. Hence, there was no correlation between the PSMA status with necrosis [Table 5] and endothelial proliferation.
Table 5.
Presence of necrosis in glioblastoma
| Variable | PSMA negative, n (%) | PSMA positive, n (%) | P |
|---|---|---|---|
| No necrosis | 4 (36.4) | 7 (63.6) | 0.55 |
| Necrosis | 16 (33.3) | 32 (66.7) |
PSMA: Prostate-specific membrane antigen
Discussion
The present study demonstrated remarkable PSMA expression in sixty surgical tissue samples of GBM. The PSMA immunostaining was distinct in tumor vascular endothelium but barely positive in glomeruloid microvascular proliferation. This finding is in concordance with the result of Bychkov et al. which claimed that PSMA expression is not directly related to endothelial cell proliferation in thyroid tumors.[15] Further investigations are needed to evaluate the causes of such findings.
Comparison of the demographic features following observations showed that the number of males exceeded females. Furthermore, frontal and parietal lobes were the most common sites of occurrence. These findings are consistent with the literature.[16,17,18]
In the present study, ischemic necrosis and/or pseudo palisading necrosis was detected in 80% of patients, which is less than the results reported by Homma et al. (87%).[19]
There is a wide variation in the PSMA expression in GBMs, which varies from 6% to 100%.
We detected PSMA expression in 60% of the investigated cases, and the intensity was strong and moderate in 63% of cases.
Wernicke et al. evaluated the expression of PSMA among 32 GBM specimens and revealed that all specimens exhibited staining for PSMA to a variable extent. Of these, 69% had more than 51% vascular staining for PSMA, and the intensity of staining was 2+ (moderate) to 3+ (maximum) in most of the specimens.[20]
In another study, Nomura et al.[21] had evaluated the PSMA expression in blood vessels of gliomas and breast cancer metastases to brain and concluded that highly angiogenic IV graded gliomas show intense PSMA staining.
Mhawech-Fauceglia et al., in a study on GBM samples investigated by IHC using microarray, revealed 49 of 52 (94%) negative results of PSMA. Two samples had weakly and one had moderately positive results with no strong staining.[20]
Recently, Saffar et al. compared PSMA expression in different grades of glioma and reported positive staining in 40.7% of high-grade glial tumor.[22]
Controversial results in different studies might be due to the use of different types of monoclonal antibodies and different IHC methods or the lack of standardization in scoring system.
This study had some limitations; the criteria for scoring PSMA staining were not well defined in the literature and hence, the results of the study might differ from those of various other studies.
Conclusion
Expression of PSMA in GBM, as observed in the current study, may suggest a new role for PSMA-targeted therapy and indicate more investigations focusing on complementary treatment strategies that specifically target tumor vasculature.
Financial support and sponsorship
This article is derived from proposal no. 395820, Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Purow B, Schiff D. Advances in the genetics of glioblastoma: Are we reaching critical mass? Nat Rev Neurol. 2009;5:419–26. doi: 10.1038/nrneurol.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juratli TA, Schackert G, Krex D. Current status of local therapy in malignant gliomas – A clinical review of three selected approaches. Pharmacol Ther. 2013;139:341–58. doi: 10.1016/j.pharmthera.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6:1359–70. doi: 10.15252/emmm.201302627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim WY, Lee HY. Brain angiogenesis in developmental and pathological processes: Mechanism and therapeutic intervention in brain tumors. FEBS J. 2009;276:4653–64. doi: 10.1111/j.1742-4658.2009.07177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res. 2004;10:415–27. doi: 10.1158/1078-0432.ccr-0642-03. [DOI] [PubMed] [Google Scholar]
- 8.Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci U S A. 1996;93:749–53. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–35. [PubMed] [Google Scholar]
- 10.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB, et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–8. [PubMed] [Google Scholar]
- 11.Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57:3629–34. [PubMed] [Google Scholar]
- 12.Milowsky MI, Nanus DM, Kostakoglu L, Sheehan CE, Vallabhajosula S, Goldsmith SJ, et al. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J Clin Oncol. 2007;25:540–7. doi: 10.1200/JCO.2006.07.8097. [DOI] [PubMed] [Google Scholar]
- 13.Morris MJ, Pandit-Taskar N, Divgi CR, Bender S, O’Donoghue JA, Nacca A, et al. Phase I evaluation of J591 as a vascular targeting agent in progressive solid tumors. Clin Cancer Res. 2007;13:2707–13. doi: 10.1158/1078-0432.CCR-06-2935. [DOI] [PubMed] [Google Scholar]
- 14.Fung EK, Cheal SM, Fareedy SB, Punzalan B, Beylergil V, Amir J, et al. Targeting of radiolabeled J591 antibody to PSMA-expressing tumors: Optimization of imaging and therapy based on non-linear compartmental modeling. EJNMMI Res. 2016;6:7. doi: 10.1186/s13550-016-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bychkov A, Vutrapongwatana U, Tepmongkol S, Keelawat S. PSMA expression by microvasculature of thyroid tumors – Potential implications for PSMA theranostics. Sci Rep. 2017;7:5202. doi: 10.1038/s41598-017-05481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanif F, Muzaffar K, Perveen K, Malhi SM. Simjee S Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18:3–9. doi: 10.22034/APJCP.2017.18.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philips A, Henshaw DL, Lamburn G, Carroll MJ. Brain tumours: Rise in glioblastoma multiforme incidence in England 1995 and –2015 suggests an adverse environmental or lifestyle factor. J Environ Public Health. 2018;2018:10. doi: 10.1155/2018/7910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il’yasova D, et al. Brain tumor epidemiology: Consensus from the brain tumor epidemiology consortium. Cancer. 2008;113:1953–68. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homma T, Fukushima T, Vaccarella S, Yonekawa Y, Di Patre PL, Franceschi S, et al. Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropath Exp Neurol. 2006;65:846–54. doi: 10.1097/01.jnen.0000235118.75182.94. [DOI] [PubMed] [Google Scholar]
- 20.Wernicke AG, Edgar MA, Lavi E, Liu H, Salerno P, Bander NH, et al. Prostate-specific membrane antigen as a potential novel vascular target for treatment of glioblastoma multiforme. Arch Pathol Lab Med. 2011;135:1486–9. doi: 10.5858/arpa.2010-0740-OA. [DOI] [PubMed] [Google Scholar]
- 21.Nomura N, Pastorino S, Jiang P, Lambert G, Crawford JR, Gymnopoulos M, et al. Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. 2014;14:26. doi: 10.1186/1475-2867-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saffar H, Noohi M, Tavangar SM, Saffar H, Azimi S. Expression of prostate-specific membrane antigen (PSMA) in brain glioma and its correlation with tumor grade. Iran J Pathol. 2018;13:45–53. [PMC free article] [PubMed] [Google Scholar]


