Abstract
Background:
Sarcopenia describes the inevitable deterioration in muscle mass and strength that accompanies biological aging. The purpose of this study was to investigate the effects of resistance training (RT) on quadriceps hypertrophy and related biochemistry in sarcopenic and healthy elderly men.
Methods:
A total of 31 elderly men (55–70 years old) were classified as sarcopenic and nonsarcopenic and were divided into two groups. Both groups participated in a progressive RT program for 8 weeks.
Results:
Data indicated that the strength in the sarcopenic group increased more than the healthy group (P < 0.05). Quadriceps cross-sectional area also increased more in the healthy group (P < 0.05). Myostatin concentration decreased in both groups after training (P < 0.05). Follistatin and testosterone increased in the healthy group; in contrast, only testosterone increased in the sarcopenic group after training (P < 0.05).
Conclusions:
The findings from this study suggest that RT improves muscle cross-sectional area and biomarker-related muscle loss in both healthy and sarcopenic elderly men. The findings also demonstrate that growth factor profiles at baseline and changes in testosterone levels play an important role in muscle hypertrophy observed in both groups.
Keywords: Aging, follistatin, hypertrophy, myostatin, resistance training, sarcopenia
Introduction
One of the consequences of the aging process is the progressive loss of muscle mass accompanied by a loss in muscle strength and function, which has implications for the quality of life.[1,2] The gradual but inevitable loss of muscle mass and strength is termed “sarcopenia” and is considered a neuromuscular syndrome.[2] Currently, sarcopenia is a significant health problem affecting the aging population and is associated with an increased risk of fractures and falls.[3]
Sarcopenia is accompanied by several intramuscular and neural events, such as loss of muscle mass, particularly in Type II muscle fibers, a strength decrease, an increase in fat mass, a disruption in coordination,[4,5] increase in protein degradation, particularly contractile protein, and a decrease in the activity of satellite cells.[4] These events are exacerbated by decreasing levels of physical activity and less protein uptake through the diet.[4,5] Even athletes and healthy older adults exhibit a gradual loss of muscle function and mass in advanced age.[6] One of the main mechanisms influencing and aggravating sarcopenia is an age-related change in anabolic hormones and growth factors.[7] This can result in decreases in the level of testosterone and insulin-like growth factor 1 (IGF-1) and an increase in the levels of catabolic factors such as cortisol and myostatin (MSTN).[7,8]
Previously, it has been reported that sarcopenia is not associated with changes in MSTN in different age groups.[8] MSTN is a member of the transforming growth factor beta (TGF-β) group that was initially discovered in 1997 as a regulator of atrophy in a mouse muscle.[9] Transgenic mice with disruption of the MSTN gene developed a 2- to 3-fold increase in muscle mass with no corresponding increase in adipose tissue.[9] Moreover, experimental evidence suggests that follistatin (Fst) plays a role in the regulation of muscle mass, yet the exact mechanism is not completely clear.[10] Fst is an extracellular protein that mainly inhibits the activated and selected members of TGF-β superfamily, including MSTN. Interestingly, the increase in transgenic mice muscle mass is linked with overexpressing of Fst.[10] Ratkevicius et al.[8] indicated that the levels of MSTN and Fst were not different between young men (22 ± 2 years) and mildly and severely sarcopenic elderly (SE) men (69 ± 3 and 76 ± 6 years, respectively); however, free testosterone and IGF-1 were both significantly lower in the elderly groups examined.
Exercise training is a desirable activity that can have positive effects on the health of elderly populations, including increased mobility and a decrease in the risk of falls. It is now well established that aerobic training is greatly beneficial to old people's health, rehabilitation, well-being, and cardiovascular disease reduction. However, aerobic activity does not increase muscle mass and musculoskeletal strength that is lost due to the aging process.[11] In contrast to aerobic training, resistance training (RT) can affect the age-related decline in muscle mass and strength and has been shown to reverse, and in some instances, prevent sarcopenia.[12] Furthermore, resistance exercise has been demonstrated to decrease MSTN[4] and increase muscle protein synthesis and translational efficiency.[13] There have also been increases in markers influencing the activity of satellite cells such as Fst and IGF-1 acutely, and this has resulted in increases in the number of satellite cells chronically.[14] However, evidence exists that histological and body composition changes demonstrating hypertrophic adaptations following structured exercise are limited in healthy versus sarcopenic older populations.[15] Evidence for the effect of RT in the SE is limited, and that growth factor regulation following RT is also inconsistent. We hypothesize that RT provides different effects on elderly people with sarcopenia compared to healthy elderly (HE) individuals without sarcopenia. Therefore, the purpose of this study was to investigate the effects of RT on hypertrophy and biochemical growth factors in both sarcopenic and HE men.
Methods
This study was approved by the Ethics committee of Shahid Chamran University, Ahvaz, Iran. Thirty-one elderly men (aged between 55 and 70 years old) volunteered to participate in the study. The risks and the benefits of the study were explained to all participants, and then, written informed consent was obtained. Ultimately, 31 participants (16 in the SE group and 15 in the HE group) were included this study. All participants were sedentary, and none had been involved in previous RT programs. Participants refrained from participation in any regular physical activity during the study.
The exclusion criteria for the participants were as follows: (1) those engaged in regular moderate-to-heavy training within the past year; (2) vegetarians; (3) those who ingested nutritional supplements or pharmacological substances that would have influenced measurements and responses; (4) people with obesity (body mass index [BMI] >30); and (5) and those with chronic diseases such as neuromuscular and cardiovascular diseases, diabetes, or multiple sclerosis.
Sarcopenia criteria
To determine sarcopenia in the elderly participants, 20 healthy young men (25.80 ± 3.80 years old, BMI: 23.96 ± 3.66 kg/m2) were recruited. The muscle mass values for the participants aged between 20 and 35 years old (33.2 ± 2.7 kg) were employed to develop the classification criteria for muscle loss in older participants. Mild sarcopenia was defined as having a muscle mass between 1 and 2 standard deviation (SD) below the mean value of a sample of 20 healthy young men, while the reduction in muscle mass by 2 SD was used as a cutoff point for severe sarcopenia.[16] Based on these criteria, 22 elderly men were assigned into two groups. Skeletal muscle mass was also calculated, using the anthropometric equation of Lee et al.[17]
Skeletal muscle mass (kg) = Ht − (0.00744 − CAG2 + 0.00088 − C TG2 + 0.00441 − CCG2) + 2.4 − sex − 0.048 − age + race + 7.8, sex = 1 for males, race = −2.0 for Asians.
In this equation, Ht = height (m), CAG = skinfold-corrected upper arm (cm), CTG = skinfold-corrected thigh (cm), CCG = skinfold-corrected calf girths (cm). Using this anthropometric equation and muscle mass measured using magnetic resonance imaging; previously, a correlation of 0.91 was established. The standard error of the estimate for predicting skeletal muscle mass using this technique was 2.2 kg.[17]
One-repetition maximum
One-repetition maximum (1RM) was evaluated before (all exercises) and following (squat and bench press) 8 weeks of training. 1RM started with a warm-up set of 10 submaximal repetitions. Participants had to complete a maximum number (5–15) of repetitions at a resistance that was selected by the investigators. 1RM was estimated using the Brzycki formula.[18] RT exercise sessions were based on 1RM values.
Anthropometric measures
Body fat percentage (BFP) was assessed by obtaining skinfold thicknesses using a Harpenden skinfold caliper (made in UK) according to methods described previously.[19] In addition, skinfold thickness was recorded at the abdomen, triceps, chest, suprailium, subscapula, midaxilla, and thigh. Three trials were performed, and the mean of the two most similar trials were used in assessing BFP as outlined in methods described previously.[19,20]
Quadriceps and hamstring cross-sectional area
Images of the quadriceps (rectus femoris, vastus lateralis, vastus intermedius, and vastus medialis) and hamstring (semitendinosus, semimembranosus, and biceps femoris) muscle groups were taken from the mid-thigh (the midpoint between trochanterion and tibiale laterale) using a computed tomography scan (Siemens SOMATOM Definition Flash, Forchheim, Germany). Participants rested quietly on the magnet bore in a supine position with extended legs. Quadriceps and hamstring cross-sectional area (QCSA and HCSA) were determined before the protocol and 4 days post the final training session. All measurements were made by an expert radiologist blinded to participant coding in Jundishapur University of Medical Sciences. Intraclass correlation coefficients for inter- and intra-investigator reliability were 0.92 and 0.95, respectively.
Growth factors
Blood samples were collected from the participants before the commencement of the protocol and 3-day post following the final training session between 7:30 am and 9:00 am. It should be mentioned that the participants, who had fasted for 10 h, were seated comfortably while the samples were being taken. Venous blood samples were obtained to determine related biochemistry concentrations. MSTN and Fst concentrations were assessed through enzyme immunoassay using ELISA kits by R and D system (Minneapolis, MN, USA), IGF-1 and testosterone through ELISA kits by Mediagnost (Reutlingen, BW, Germany), and Monobind Inc (Lake Forest, CA, USA), respectively. Intra- and inter-assay coefficients of variance were determined for all the variables and were <10%.
Resistance training
Following a familiarization week, individuals participated in a whole-body progressive RT program 3 days/week for 8 weeks (24 sessions). RT included 10 min of warm-up at the beginning and 10-min cool down in at the end of each session. The RT protocol consisted of 4 sets of 10 repetitions for a total of 8 weeks. Rest periods between exercises and sets were 3 min and 1 min, respectively. The pattern of the repetitions was set by a metronome (each repetition lasting for 3 s, 1.5 s concentric, and 1.5 s eccentric). The RT protocol included bench press, chest press, leg curl, squat, leg extension, cable triceps extension, bicep curl, lat pulldown, and machine shoulder press. The training load in the 1st week was 50% 1RM; with increases in load of 5% 1RM/week (final week: 85% 1RM).
Statistical analysis
Statistical analyses were performed with SPSS Statistics version 21 (IBM SPSS Statistics, Armonk, NY). Descriptive statistics were used to present the index of central tendency and variability with means and SD (mean ± SD). Independent t-tests were used to examine differences between groups at baseline. Paired-t-tetss were employed to compare the effect of RT on measures in each group (pretest to posttest). Analysis of covariance was used to examine between-group differences following the 8-week training program, whereas pretest data was used as a covariate. All significance tests were set at an alpha level of 0.05.
Results
Sarcopenic and nonsarcopenic participants differed significantly in the 1RM-squat and 1RM-bench press from baseline (P < 0.05). After 8 weeks of RT, 1RM of both exercises substantially increased in both groups (P < 0.05). 1RM was augmented more in the SE than the HE group (P < 0.05); however, weight, BMI, and BFP did not change in either group following 8 weeks of RT [Table 1], (P > 0.05).
Table 1.
The effect of 8 weeks of resistance training on body composition and one-repetition maximum
| Group | Pretest | Posttest | P | |
|---|---|---|---|---|
| Weight (kg) | SE | 78.24±5.94 | 79.09±7.12 | 0.097 |
| HE | 80.24±6.23 | 79.46±5.87 | ||
| BMI (kg/m2) | SE | 25.29±3.18 | 25.68±2.47 | 0.158 |
| HE | 26.84±2.91 | 26.20±2.84 | ||
| BFP (%) | SE | 26.13±1.98 | 25.74±3.09 | 0.134 |
| HE | 23.54±2.18 | 23.69±2.65 | ||
| 1RM-squat (kg) | SE | 34.16±5.14ª | 54.37±4.67* | 0.023# |
| HE | 49.26±8.17 | 64.08±9.03* | ||
| 1RM-bench press (kg) | SE | 36.64±4.43ª | 58.90±6.27* | 0.061 |
| HE | 49.71±6.01 | 61.36±5.91* |
ªRefers to a significant comparison between SE and HE values at the baseline (P<0.05). *P values (P<0.05) refer to the level of significance in the paired t-test. #P values (P<0.05) refer to the level of significance in the analysis of covariance (between-group difference). Pre=Before 8 weeks, Post=After 8 weeks, BMI=Body mass index, BFP=Body fat percentage, 1RM=One-repetition maximum, SE=Sarcopenic elderly group, HE=Healthy elderly group
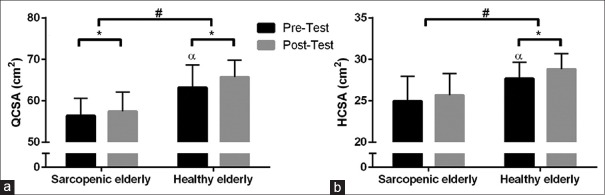
QCSA and HCSA were significantly higher in the HE group at baseline when compared with the SE group (P < 0.05). QCSA notably increased in both groups after 8 weeks of RT [Figure 1], (P < 0.05). In addition, the results indicated that after RT, QCSA, and HSCA were higher in the HE group compared to SE group [Figure 1].
Figure 1.
The effect of resistance training on QCSA (a) and HCSA (b) in the sarcopenic elderly and healthy elderly group. α refers to the significantly higher than other group in the independent t-test (P < 0.05) at the baseline. *P values (P < 0.05) refer to the level of significance in the paired t-test. #P values (P < 0.05) refer to the level of significance in the analysis of covariance (between-group difference). QCSA = Quadriceps cross-sectional area, HCSA = Hamstring cross-sectional area
At baseline and following the training period, no significant difference was observed in both groups regarding the mean levels of serum MSTN (P > 0.05) and Fst (P > 0.05). The mean level of serum IGF-1 was substantially higher in the HE group compared to the SE group at baseline (P < 0.05). At the end of the 8-week RT program, MSTN concentrations significantly decreased, and Fst concentration increased significantly in both groups (P < 0.05). There was no change in IGF-1 concentration in both groups from pre- to posttest; however, IGF-1 remained significantly higher in HE group [Table 2], (P < 0.05). The mean levels of testosterone were significantly higher in the HE in comparison with the SE group at baseline and posttraining (P < 0.05). During the training protocol, testosterone concentrations increased in the HE group (P < 0.05) [Table 2].
Table 2.
The effect of 8 weeks of resistance training on blood biomarker in sarcopenic elderly and healthy elderly group
| Group | Pretest | Posttest | P | |
|---|---|---|---|---|
| MSTN (ng/ml) | SE | 4.31±1.74 | 3.95±1.27* | 0.364 |
| HE | 4.02±2.23 | 3.84±1.94* | ||
| Fst (ng/ml) | SE | 1.45±0.81 | 1.71±0.76* | 0.216 |
| HE | 1.57±0.96 | 1.69±0.92* | ||
| IGF-1 (ng/ml) | SE | 115.56±16.86ª | 113.67±17.06 | 0.026# |
| HE | 130.15±22.23 | 138.11±16.41 | ||
| Testosterone (ng/ml) | SE | 3.32±1.14ª | 3.40±1.12 | 0.001# |
| HE | 4.26±1.12 | 4.92±1.16* |
ªRefers to a significant comparison between SE and HE values at the baseline (P<0.05). *P values (P<0.05) refer to the level of significance in the paired t-test. #P values (P<0.05) refer to the level of significance in the analysis of covariance (between-group difference). MSTN=Myostatin, Fst=Follistatin, IGF-1=Insulin-Like Growth Factor 1, SE=Sarcopenic elderly group, HE=Healthy elderly group
Discussion
The present study is the first study to compare the effect of RT on hypertrophy and growth factors such as MSTN and Fst in sarcopenic and non-SE men. Thirty-one elderly men completed the study. Our results demonstrated that growth factor profiles at baseline and testosterone changes have an important role in quadriceps hypertrophy in elderly men.
Results indicated that 1RM-squat and -bench press was initially lower in the SE than the HE group; however, strength increased in both groups (more significantly in the SE group) after 8 weeks of training. Similarly, QCSA and HCSA were lower in the SE group at baseline and the end of training; yet, both QSCA and HSCA increased after the training period, an increase that was more recognizable in the HE group [Figure 1a and b]. Recent studies have indicated that muscle loss is associated with age. For example, Ratkevicius et al.[8] demonstrated that muscle volume and CSA were higher in young adults (22 ± 2 years) when compared with older adults (69 ± 3 years). It is well accepted that partial and total muscle volume declines with age, particularly after 50 years.[21] Although loss of muscle mass can lead to loss of strength, neuromuscular and functional changes, such as extension of contraction/relaxation periods can also be affected. Reduced ATPase transport activity, fiber type shift (Type I isoforms consume less ATP than Type II at maximum contraction), and increases in connective tissue or inter- and intra-muscular adipose tissue, can also exacerbate this process.[22]
The higher increase in QCSA in the HE versus the SE group may result from the fact that QCSA was significantly higher in the HE group at baseline. It has been previously reported that muscle size is associated with satellite cell concentration.[23] It has also been suggested that the content of the preexisting satellite cells and baseline muscle size were influential in determining the hypertrophic potential of RT. Dreyer et al.[23] reported that the recruitment of satellite cells was blunted in the elderly in response to exercise training. It has also been observed that the proliferative capacity of satellite cells, as well as the anabolic signaling process, is limited in the elderly.[14,23]
In this study, no significant differences were observed in the level of MSTN and Fst as activation markers of satellite cells at baseline; however, IGF-1 concentration was higher in the HE group.
At baseline, the 1RM results indicated that strength was lower in the SE group. In humans, some studies relate this diminished strength to age-related loss of muscle mass,[24] whereas others report that diminishing strength is greater than the age-related loss of muscle mass.[25] The exact mechanism of these events is unclear, but alterations in the excitation-contraction coupling process,[26] decreases in the firing rates of nerves, and actin-myosin cross-bridge stability[27] can be suggested as potential mechanisms. Interestingly, we found that the SE group gained more strength after the training period. It has already been accepted that the increase in strength throughout the primary weeks of the RT is the result of neural adaptations such as enhanced motor unit recruitment, firing rate of agonists, and reduced contraction of antagonists.[4] There are no studies comparing the impact of RT on the sarcopenic and the HE, but comparative studies investigating the effect of training on different ages have obtained remarkable results. For instance, Mero et al.[4] found that both Type I and II muscle fiber CSA increased more in young men (higher muscle mass), but 1RM of leg concentric extension increased more at baseline to week 10.5 in old (lower muscle mass) versus young men. Bickel et al.[28] and Walker and Häkkinen[29] recorded similar findings, all of which support the results presented here.
Some hormones and growth factors such as MSTN, Fst, IGF-1, and testosterone affect muscle growth and hypertrophy. MSTN has been identified as a candidate to control muscle loss through inhibiting the activation of satellite cells, reducing muscle hypertrophy, or negatively regulating myoblast proliferation.[30] In our data, we observed no significant difference between groups at baseline level for MSTN. Szulc et al.[31] found that MSTN slightly increased with age until 57 years, from which point it slightly decreased in men. Unfortunately, this study included all forms (active C-terminal dimer of MSTN and the N-terminal propeptide) of MSTN, therefore, the MSTN concentration of Szulc et al.[31] were higher when compared with similar studies.
It has recently been reported that a single bout of resistance exercise decreases MSTN expression in various ages.[4] In theory, it seems that the decrease in MSTN expression after exercise is positive for muscle growth. Furthermore, through the use of training periods, recent studies have observed that MSTN was downregulated in the quadriceps muscles of young mice, but this result was not found in older mice.[32] Roth et al.[33] demonstrated that MSTN mRNA decreased in the muscles of both young and old men after 9 weeks of RT, whereas Willoughby[34] reported that MSTN mRNA expression did not change after 12 weeks of RT, although strength and muscle mass increased. Interestingly, Jespersen et al.[35] reported that MSTN mRNA may decline after short-term training, but increases after a long-term training period. MSTN mRNA cannot fully represent the active MSTN and MSTN circulation levels in blood because MSTN mRNA undergoes posttranslation modifications after expression.[36] Thus, despite the increased strength and muscle mass in some studies, MSTN mRNA was found to increase. Other reasons for the differences observed could be due to several factors such as the training protocol, exercise duration, gender, and participant characteristics (age, weight, physical activity, etc.). For instance, testosterone concentration is 10-fold more in men than in women. The effect of testosterone on muscle hypertrophy and satellite cells,[37] may contribute to inconsistency in results when comparing different sexes. Our data indicated that testosterone concentration was higher in the HE group which can affect MSTN adaption and contribute to changes in muscle hypertrophy. Furthermore, testosterone increases the number of satellite cells and improves muscle hypertrophy in young and old people. One pathway through which testosterone affects muscle hypertrophy is by upregulating Fst through Wnt signaling.[38]
Several studies have suggested that Fst plays a role in the regulation of muscle mass; also Fst through the actions of binding and neutralizing MSTN prevents the atrophic action of MSTN. The increase in transgenic mice muscle mass was linked with overexpressing of Fst.[10] In our study, Fst results indicated that no marked difference was observed between groups at baseline. Fst regulation response to exercise or during training is unclear; several studies have investigated the effect of exercise/training on the expression of Fst in muscle, but different results have been reported. For instance, Hulmi et al.[39] observed that Fst did not change in the elderly after exercise and 21 weeks of RT. In contrast, Willoughby[34] reported a marked increase in Fst (blood circulation) after 6- and 12-week RT. Previous evidence reported that Fst is secreted from different tissues such as skeletal muscle and liver[40] and that these different secreting tissues may explain the inconsistent results. In our study, at the end of training, Fst concentration significantly increased, however, no significant difference was observed between groups.
At baseline, IGF-1 and testosterone levels were higher in the HE group compared to the SE group. These results may reflect that the lower resting level of IGF-1 and testosterone are a symptom of sarcopenia. Furthermore, recent studies have reported that IGF-1 or insulin is required for Fst-induced muscle hypertrophy. When either IGF-1 or insulin signaling is impaired, Fst still facilitates an anabolic role; but if both factors are impaired, Fst-induced muscle hypertrophy fails.[41] Although our study did not elevate the insulin circulation or metabolic pathway; it confirmed that both the action and the receptor integrity of insulin are impaired by aging. This evidence along with our findings revealed a lower IGF-1 in the SE group at baseline and after training. This demonstrated that despite the lack of difference between the groups in Fst, Fst function, and pathway may be limited in the SE group.
Conclusions
The findings of this study showed that RT improves muscle cross-sectional area and strength in sarcopenic and nonsarcopenic elderly. There was also an associated regulation in anabolic activity related to MSTN and Fst concentrations. However, testosterone only increased in nonsarcopenic participants. Therefore, RT could be an option for improving skeletal muscle quality in both sarcopenic and non-SE men. Findings indicate that the hypertrophy potential in sarcopenia may be reduced. This may be accounted for in part by the lower level of growth factors such as testosterone and IGF-1 in the sarcopenic participants and the failure to increase testosterone and IGF-1 levels through RT.
Financial support and sponsorship
This study was supported by the Shahid Chamran University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Sina Talebvand for his contribution and individuals who participated in the study.
References
- 1.Lauretani F, Bautmans I, De Vita F, Nardelli A, Ceda GP, Maggio M, et al. Identification and treatment of older persons with sarcopenia. Aging Male. 2014;17:199–204. doi: 10.3109/13685538.2014.958457. [DOI] [PubMed] [Google Scholar]
- 2.Judge LW, Bellar DM, Hoover DL, Biggs D, Leitzelar BN, Craig BW, et al. Effects of acute androstenedione supplementation on testosterone levels in older men. Aging Male. 2016;19:161–7. doi: 10.3109/13685538.2016.1167180. [DOI] [PubMed] [Google Scholar]
- 3.Thompson DD. Aging and sarcopenia. J Musculoskelet Neuronal Interact. 2007;7:344–5. [PubMed] [Google Scholar]
- 4.Mero AA, Hulmi JJ, Salmijärvi H, Katajavuori M, Haverinen M, Holviala J, et al. Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol. 2013;113:641–50. doi: 10.1007/s00421-012-2466-x. [DOI] [PubMed] [Google Scholar]
- 5.Delbono O. Sarcopenia – Age-Related Muscle Wasting and Weakness. Netherlands: Springer; 2011. Excitation-contraction coupling regulation in aging skeletal muscle; pp. 113–4. [Google Scholar]
- 6.Yamauchi J, Mishima C, Nakayama S, Ishii N. Force-velocity, force-power relationships of bilateral and unilateral leg multi-joint movements in young and elderly women. J Biomech. 2009;42:2151–7. doi: 10.1016/j.jbiomech.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F, et al. The GH/IGF1 axis and signaling pathways in the muscle and bone: Mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–10. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 8.Ratkevicius A, Joyson A, Selmer I, Dhanani T, Grierson C, Tommasi AM, et al. Serum concentrations of myostatin and myostatin-interacting proteins do not differ between young and sarcopenic elderly men. J Gerontol A Biol Sci Med Sci. 2011;66:620–6. doi: 10.1093/gerona/glr025. [DOI] [PubMed] [Google Scholar]
- 9.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 10.Lee YS, Lehar A, Sebald S, Liu M, Swaggart KA, Talbot CC, Jr, et al. Muscle hypertrophy induced by myostatin inhibition accelerates degeneration in dysferlinopathy. Hum Mol Genet. 2015;24:5711–9. doi: 10.1093/hmg/ddv288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbari Kamrani AA, Zamani Sani SH, Fathire-Zaie Z, Bashiri M, Ahmadi E. The psychometric characteristics of the exercise benefits/barriers scale among Iranian elderly. Iran J Public Health. 2014;43:362–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Maltais ML, Ladouceur JP, Dionne IJ. The effect of resistance training and different sources of postexercise protein supplementation on muscle mass and physical capacity in sarcopenic elderly men. J Strength Cond Res. 2016;30:1680–7. doi: 10.1519/JSC.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–7. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64:332–9. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291:E937–46. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee RC, Wang Z, Heo M, Ross R, Janssen I, Heymsfield SB, et al. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000;72:796–803. doi: 10.1093/ajcn/72.3.796. [DOI] [PubMed] [Google Scholar]
- 18.Brzycki M. A practical approach to strength training. Contemporary Books. 1995 [Google Scholar]
- 19.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 20.Siri WE. Body composition from fluid spaces and density: Analysis of methods. Tech Meas Body Compos. 1961;61:223–44. [PubMed] [Google Scholar]
- 21.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 2006;101:531–44. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 22.Perkisas S, De Cock A, Verhoeven V, Vandewoude M. Physiological and architectural changes in the ageing muscle and their relation to strength and function in sarcopenia. Eur Geriatr Med. 2016;7:201–6. [Google Scholar]
- 23.Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33:242–53. doi: 10.1002/mus.20461. [DOI] [PubMed] [Google Scholar]
- 24.Kent-Braun JA, Ng AV. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol (1985) 1999;87:22–9. doi: 10.1152/jappl.1999.87.1.22. [DOI] [PubMed] [Google Scholar]
- 25.Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol (1985) 1999;86:188–94. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZM, Messi ML, Delbono O. L-type ca(2+) channel charge movement and intracellular ca(2+) in skeletal muscle fibers from aging mice. Biophys J. 2000;78:1947–54. doi: 10.1016/S0006-3495(00)76742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe DA, Thomas DD, Thompson LV. Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers. Am J Physiol Cell Physiol. 2002;283:C187–92. doi: 10.1152/ajpcell.00008.2002. [DOI] [PubMed] [Google Scholar]
- 28.Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc. 2011;43:1177–87. doi: 10.1249/MSS.0b013e318207c15d. [DOI] [PubMed] [Google Scholar]
- 29.Walker S, Häkkinen K. Similar increases in strength after short-term resistance training due to different neuromuscular adaptations in young and older men. J Strength Cond Res. 2014;28:3041–8. doi: 10.1519/JSC.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 30.Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275:40235–43. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 31.Szulc P, Schoppet M, Goettsch C, Rauner M, Dschietzig T, Chapurlat R, et al. Endocrine and clinical correlates of myostatin serum concentration in men – The STRAMBO study. J Clin Endocrinol Metab. 2012;97:3700–8. doi: 10.1210/jc.2012-1273. [DOI] [PubMed] [Google Scholar]
- 32.Leiter JR, Peeler J, Anderson JE. Exercise-induced muscle growth is muscle-specific and age-dependent. Muscle Nerve. 2011;43:828–38. doi: 10.1002/mus.21965. [DOI] [PubMed] [Google Scholar]
- 33.Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA, et al. Myostatin gene expression is reduced in humans with heavy-resistance strength training: A brief communication. Exp Biol Med (Maywood) 2003;228:706–9. doi: 10.1177/153537020322800609. [DOI] [PubMed] [Google Scholar]
- 34.Willoughby DS. Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc. 2004;36:574–82. doi: 10.1249/01.mss.0000121952.71533.ea. [DOI] [PubMed] [Google Scholar]
- 35.Jespersen JG, Nedergaard A, Andersen LL, Schjerling P, Andersen JL. Myostatin expression during human muscle hypertrophy and subsequent atrophy: Increased myostatin with detraining. Scand J Med Sci Sports. 2011;21:215–23. doi: 10.1111/j.1600-0838.2009.01044.x. [DOI] [PubMed] [Google Scholar]
- 36.Willoughby DS, Wilborn CD. Estradiol in females may negate skeletal muscle myostatin mRNA expression and serum myostatin propeptide levels after eccentric muscle contractions. J Sports Sci Med. 2006;5:672–81. [PMC free article] [PubMed] [Google Scholar]
- 37.Bhasin S, Tenover JS. Age-associated sarcopenia – Issues in the use of testosterone as an anabolic agent in older men. J Clin Endocrinol Metab. 1997;82:1659–60. doi: 10.1210/jcem.82.6.4061. [DOI] [PubMed] [Google Scholar]
- 38.Braga M, Bhasin S, Jasuja R, Pervin S, Singh R. Testosterone inhibits transforming growth factor-β signaling during myogenic differentiation and proliferation of mouse satellite cells: Potential role of follistatin in mediating testosterone action. Mol Cell Endocrinol. 2012;350:39–52. doi: 10.1016/j.mce.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hulmi JJ, Ahtiainen JP, Kaasalainen T, Pöllänen E, Häkkinen K, Alen M, et al. Postexercise myostatin and activin IIb mRNA levels: Effects of strength training. Med Sci Sports Exerc. 2007;39:289–97. doi: 10.1249/01.mss.0000241650.15006.6e. [DOI] [PubMed] [Google Scholar]
- 40.Hansen J, Brandt C, Nielsen AR, Hojman P, Whitham M, Febbraio MA, et al. Exercise induces a marked increase in plasma follistatin: Evidence that follistatin is a contraction-induced hepatokine. Endocrinology. 2011;152:164–71. doi: 10.1210/en.2010-0868. [DOI] [PubMed] [Google Scholar]
- 41.Barbé C, Kalista S, Loumaye A, Ritvos O, Lause P, Ferracin B, et al. Role of IGF-I in follistatin-induced skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab. 2015;309:E557–67. doi: 10.1152/ajpendo.00098.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]