Abstract
The World Health Organization guideline to use less sugar may be an opportunity and support for dentistry in its goal to get the message of using less sugar across to the public. Two ways (with all the combinations of these) to achieve a reduction of sugar consumption are the reduction of the amount of sugar in products or the reduction of the frequency of consumption of sugar-containing products. Which sugar-reducing strategy is best for caries prevention? To answer this question, this manuscript discusses the shape of the dose-response association between sugar intake and caries, the influence of fluoridated toothpaste on the association of sugar intake and caries and the relative contribution of frequency and amount of sugar intake to caries levels. The results suggest that when fluoride is appropriately used, the relation between sugar consumption and caries is very low or absent. The high correlation between amount and frequency hampers the decision related to which of both is of more importance, but frequency (and stickiness) fits better in our understanding of the caries process. Reducing the amount without reducing the frequency does not seem to be an effective caries preventive approach in contrast to the reciprocity. Goals set in terms of frequency may also be more tangible for patients to follow than goals set in amount. Yet, in sessions of dietary counselling to prevent dental caries, the counsellor should not forget the importance of quality tooth brushing with fluoride toothpaste.
Key Words: Sugar restriction, Dental caries
Introduction
It is beyond debate that the consumption of sugars containing foods imposes a risk on the integrity of our teeth. The actual risk of a certain food is modulated by many factors that are divided in food-related factors and consumer-related factors. Food-related factors involve the release of the sugars, the stickiness of the product although this may be less important at sites where food is impacted, and to a lesser extent, the type and concentration of the sugar. Consumer-related factors are the frequency of sugar consumption, the drinking and chewing habits, the chewing and swallowing efficiency, salivary flow and composition, the presence of cariogenic dental plaque and the use of fluorides. It is a common observation that with the comparable number of sugar-containing products, some people are able to manage the risk and will not develop caries, while others develop significant amounts of dental caries.
Reducing sugar consumption seems to be an important preventive measure to reduce caries risk. The new World Health Organization (WHO) guideline advocates to reduce free sugar consumption below 10% of the energy intake (10 E%) or even below 5 E% of the diet. Free sugars are defined as all monosaccharides and disaccharides added to foods by manufacturer, cook or consumer and sugars naturally present in honey, syrups, fruit juices and fruit juice concentrates [WHO, 2015]. Assuming a daily energy intake of 2,000 kcal, 10 E% equals 50 g of sugar a day and 5 E% equals 25 g a day. At the moment, for instance in the Netherlands, the average daily energy intake from free sugars is 14% for the whole population but varies from 20% for children and adolescents to 11% for those over the age of 50 [Sluik et al., 2016]. Added sugars constitute approximately 80–90% of this energy intake from free sugars. Significant contributors, for approximately 80%, are non-alcoholic beverages (sugar sweetened beverages and fruit drinks), sweets and candy and dairy products (with the exception of milk) [Sluik et al., 2016].
The WHO guideline to use less sugar may be an opportunity and support for dentistry in its goal to get the message of using less sugar across to the public. Two ways (of course with all the combinations of these) to achieve a reduction of sugar consumption are reduction of the amount of sugar in products or reduction of the number of consumptions of sugar containing products, which may or may not result in a reduction of the frequency of consumption. The main question is: Which sugar-reducing strategy is best for caries prevention? To answer this question, 3 issues are of importance: (1) the shape of the dose-response association between sugar intake and caries, (2) the influence of fluoridated toothpaste usage on the association of sugar intake and caries and (3) the relative contribution of frequency and amount of sugar intake to caries levels [Bernabé et al., 2016].
In order to be able to advice properly, the quality of evidence has to be taken into account. Normally, high-quality evidence is necessary to support preventive measures. Unfortunately, there are no high-quality randomized controlled trials that study the relationship between sugar intake and dental caries. So we have to rely on a few clinical and in situ intervention studies, prospective cohort studies, retrospective cohort and retrospective case control studies, and cross-sectional studies. Conclusions of these types of studies should well fit in the bio-chemical model of caries development, which predicts that more demineralization time and periods and less remineralization time and periods increase caries risk.
Dose Response Curve between Sugar Consumption and Caries
In the late 1970s, before fluoride was widely used and when the quality of oral hygiene was generally poor, Sreebny (1982) compared caries prevalence among 12-year-old children in 47 nations with the availability of sugar per capita. Of the 47 nations, 21 had sucrose availability below 18 kg (approximately 10 E%) per person per year, 19 had availabilities of 18–44 kg (approximately 10–24 E%) per person per year and seven nations had availabilities of over 44 kg (> 24 E%) per person per year. The mean number of decayed, missing or filled permanent teeth (DMFT) of the 21 countries with a sugar supply below 18 kg per capita per year was 1.2 ± 0.6. For 9 of the 19 countries, with an average sugar supply between 18 and 44 kg per person per year, the mean DMFT was 2 ± 0.7, while for the other 10 of these countries the mean DMFT was 4 ± 0.9. In the 7 countries where sugar supply exceeded 44 kg per capita per year, the mean DMFT was 8 ± 2.4. A regression calculation with these data revealed an increase of 1 DMFT at the age of 12 for every 25 g sugar availability in a day. A similar comparison for 6-year-old children in 23 nations showed a less strong correlation: 1 dmfs for every 50 g of sugar availability a day [Sreebny, 1982]. Sreebny (1982) considered that 18.25 kg (approximately 10 E%) per person per year may represent an upper limit of safe, or at least “acceptable,” sugar consumption form the perspective of caries activity. Based on the data of Sreebny (1982) and the effect of the wartime diets [Tacheuki, 1962], Sheiham (1983; 1991) suggested that the relationship between sugar intake and caries levels is sigmoid. Below approximately 15 kg/person/year most of the population will not develop dental caries. Between 15 and 35 kg there is a steep increase in the rate of caries. Beyond 35 kg, the dose-response curve flattens. In many western societies, the sugar availability is around or above 40 kg/person/year. The sigmoid relationship would explain why the relative small differences in sugar consumption between persons in these societies are not necessarily reflected in differences in caries experience. No other studies, however, confirm the sigmoid curve. Most studies found no, a linear or a log-linear relationship between sugar consumption and caries.
Effect of Fluoride on the Relationship between Sugars and Caries
In more recent studies in countries where fluoride supplements are widely used, the relationship between sugar availability and caries was less clearly observed. Woodward and Walker (1994) studied the relationship in 61 developing countries and 29 industrialised countries. In the developing countries, approximately 26% of the variation in the caries data was explained by sugar availability. In the industrialized countries, less than 1% was explained, suggesting that, where fluoride is available, variation in the availability of sugar may be of lesser importance as determinant of caries prevalence and severity. Ruxton et al. (1999) used data from Sreebny (1982) and Woodward and Walker (1994) to inventory sugar availability and dental caries in more than 60 countries in the 1970s and 1980s to assess the relation between caries rates and the sugar supply. In 18 countries, both DMFT and the sugar supply declined, whereas in 25 countries, DMFT declined and sugar supply increased. In another 18 countries, the incidence of caries and the sugar supply increased. The authors concluded that the relationship between sugar reduction and caries on a nation-wide basis was clearly unreliable. In 2008, Downer et al. (2008) reported the relationships between dental caries experience of 12-year-old children in 29 countries of Europe and four independent variables: national wealth (GDP), population per active dentist; sugar disappearance (kg/capita/year); and volume sales of toothpaste (L/capita/year). Mean DMFT showed a strong negative association with national GDP (r = −0.729, p < 0.01), while toothpaste sales showed a statistically significant positive association with GDP (r = 0.599, p < 0.05) as did sugar disappearance (r = 0.575, p < 0.01). Paradoxically, caries experience yielded a strong negative correlation with sugar disappearance (r = −0.561, p < 0.01). The authors suggested as possible explanation for the anomalous association of low mean DMFT with high sugar disappearance in Western Europe that the extensive use of, mainly fluoride-containing, toothpastes neutralise the potential damage from high sugar consumption [Downer et al., 2008]. A recent global evaluation [Masood et al., 2012] confirmed that among high-income countries, there is a negative correlation between sugar disappearance (kg/capita/year) and dental caries level, while in low-income countries, this correlation is a positive one. In the ensuing discussion, (Masood et al. 2012) explain their findings by the accessibility to fluoride.
The low correlation between sugar consumption (disappearance/availability) and caries prevalence when fluoride is used indicates that proper use of fluoridated toothpaste has a major preventive effect on caries prevalence, although this is not easily achieved at every site in the mouth. This low correlation is no licence not to regard the reduction of sugar intake as a caries-preventive measure. But at least, even during dietary counselling the paramount importance of fluoride should always be stressed.
Another token of the importance of fluoride use on the relationship between caries is the fact that the dramatic decline in dental caries prevalence that occurred over the past thirty years in most Western industrialized countries cannot be attributed to reduction of the availability of sugars. Ecological observations in many countries confirm that sugar consumption remained virtually unchanged high in this period of caries decline [Nyvad, 2003; Einarsdottir and Bratthall, 1996]. In a special issue of the European Journal of Oral Sciences, experts from all over the world attributed the decline in caries prevalence to the widespread use of fluoride toothpaste [Bratthall et al., 1996].
Seventeen primary studies on the association between sugars and caries published between 1995 and 2006 were reviewed by Ruxton et al. (2010). Ten of them were epidemiological studies either cross-sectional or longitudinal and 7 of them were experimental with surrogate outcomes. The outcomes were classified as no association (5 out of the 10 epidemiological studies), a positive association (1 out of the 10 epidemiological studies) or a complex association meaning statistically significant association in certain subgroups (4 out of the 10 epidemiological studies). The subgroups in which the association between the use of sugars and caries was demonstrated were the groups that brushed the teeth once a day or less [Ruxton et al., 2010]. An example of a study with a complex outcome was the cross-sectional National Diet and Nutrition Survey of children aged 1.5–4.5 years [Gibson and Williams, 1999]. Caries was associated with sugar confectionary (amount and frequency) but only in children whose teeth were brushed less than twice a day.
Out of the 7 experimental studies, 3 were classified as having no association, 2 showing a positive association and another 2 showing a complex association. A positive association was found in a randomised controlled trial where subjects exposed enamel attached to prosthetic devices to sugar (10%), starch (2%) or the combination. The solutions were dripped onto the blocks 8 times per day. Greatest demineralisation was found with starch/sucrose combination > sucrose > starch = water [Ribeiro et al., 2005]. In the other positive experiment, lactic acid production in saliva was examined while rinsing with solutions containing 5, 10, 15, 20 and 30% sucrose (w/v). Lactic acid in saliva was significantly increased with increasing sucrose concentrations up to 15% sucrose then levelled out at higher concentration [Linke and Birchmeier, 2000].
The latter 2 studies are relevant as the first one suggests that starch is an important co-determinant of cariogenicity, while the second one touches upon the topic to what extent sugar should be reduced in products to make these less acidogenic.
At the 2001 National Institutes of Health Consensus Development Conference on Caries, Burt and Pai (2001) reported that of the 69 studies on diet and caries published between January 1980 and July 2000, only 2 showed a strong diet-caries relationship. Of the other studies, 16 showed a moderate relationship and 18 showed a weak relationship. Burt and Pai (2001) emphasized that the findings of their review differed from sugar-caries studies published in the decades before fluoride use. Although the papers reviewed indicated a decline in caries risk in relation to sugar intake, Burt and Pai (2001) attributed the decrease to fluoride use. They concluded that sugar consumption is likely to be a more powerful indicator for risk of caries infection in persons who do not have regular exposure to fluoride [Burt and Pai, 2001].
Amount versus Frequency of Sugars Consumption
Several studies examined the relationship between caries and sugar consumption expressed in multiple ways based on the same data collection being, for example, a diet registration, prospective or retrospective for a varying number of days, or a food frequency questionnaire. The relationship between various expressions of sugar consumption is logically high and, for instance, (Bernabé et al. 2016) showed that amount and frequency of consumption correlated positively with r = 0.64. This predicts that the logistic regression coefficients for the various expressions of sugar consumption vary within a limited range. When calculating these coefficients, the number of values of the independent variable (sugar consumption) may be important for the statistical significance. Many values would spread the participants in a veil cloud, while fewer values would compress them in a thick cloud resulting in a larger deviation from the mean.
Rugg-Gunn et al. (1984) showed that the bivariate correlations with caries were higher when the sugar variables were calculated for snacks alone than for all intakes. The differences were smaller when only fissure caries was assessed. Burt et al. (1988) showed the energy from total sugars and meal sugars was not significant different between low caries children compared to high caries children, but the energy from snacks, snack carbohydrate and snack sugars was significant. Bernabé et al. (2016) showed in Finnish adults that DMFT increased over a period of 4–11 years by 0.15 and 0.1 units for every additional occasion of sugars consumption and every 10 g of sugars consumed respectively. In the mutually adjusted model, only the amount of sugars intake remained significantly associated with DMFT levels although the coefficient reduced to 0.09. When the population was divided in those who used fluoride daily versus those who used fluoride less frequently the coefficient for amount was 0.08 and for frequency 0.12 for the frequent F-users and 0.26 and 0.43 at infrequent fluoride use. This is again a strong indication of the importance of the daily use of fluorides. Dusseldorp et al. (2015) operationalised the number of eating moments in more than 7 a day versus less than 7 a day, which is the nationally recommended maximum frequency of food and drinks consumption per day in the Netherlands. With this operationalisation, the number of foods and drinks consumed per day had impact on caries prevalence in the primary teeth of 9 year olds (OR 2.78, 95% CI 1.21–6.40), but not on the caries experience in the primary teeth of 9 year olds (OR 0.97, 95% CI 0.65–1.44) or prevalence and experience in the permanent dentition of 15- or 21-year-olds. The authors concluded that the used number of eating moments is an appropriate cut-off point for recommendations. In this study, caries prevalence was approximately 52 and 78% for the 15-year-olds and 21-year-olds respectively.
A study conducted in nursery homes showed that 3-year-old low socio-economic schoolchildren with the highest frequency of sugar consumption (4 ± 5 times per day) at the nursery were 4.7 times more likely to have a high caries increment over 1 year, compared to those with the lowest frequency (1 ± 2.9 times per day; OR 4.7, 95% CI 2.7–8.2; p < 0.001). Daily frequency of sugar intake at the nursery showed a dose-response trend with the risk of having high caries increment. Children having more than 32.6 g of sugar daily at the nursery were 3 times more likely to have high caries increment than those having less than that amount (OR 2.99, 95% CI 1.82–4.91; p < 0.001) [Rodrigues and Sheiham, 2000]. (Feldens et al. 2010) studied the relationship between feeding practices in the first year of life and the occurrence of severe early childhood caries at 4 years of age. A total of 340 children were examined. The multivariable model showed a higher adjusted risk of severe early childhood caries for the following dietary practices at 12 months: daily breastfeeding frequency at 12 months 0–2, 3–6, or ≥7 times showed RR values of 1.00, 2.04 (95% CI 1.22–3.39), 1.97 (95% CI 1.45–2.68; p < 0.001) respectively; number of daily meals and snacks at 12 months < 7, 7–8, > 8 showed RR values of 1.00, 0.99 (95% CI 0.70–1.39) and 1.42 (95% CI 1.02–1.97; p = 0.025) respectively, bottle use for fruit juices/soft drinks at 12 months (No/Yes) demonstrated RR 1.41 (95% CI1.08–1.86) for the users (p = 0.025); high density sugar foods at 12 months (> 50% of simple carbohydrates in unit of food) (No/Yes) gave RR of 1.43 (95% CI 1.08–1.89; p = 0.003); bottle use for liquids other than milk gave a RR 1.41 (95% CI 1.08–1.86). The studies of Rodrigues and Shieham (2000) and Feldens et al. (2010) strongly indicate that the increased frequency of sugary foods impose caries risk in the low socio-economic populations studied.
When considering the amount versus frequency issue, the findings of the Vipeholm study are still relevant. As much as 300 g additional sugar during the mean meals did not increase caries risk, while the addition of sugary snacks between meals did so significantly [Gustafsson et al., 1954].
These data still support the scientific basis for dental health professionals to focus their dietary advice on reducing the frequency of intake of sugars. Also the type of products, sugar sweetened beverages and fruit drinks, sweets and candy, and sweetened diary products (milk excepted) contributing to the intake of sugar [Sluik et al., 2016] lend themselves to skip, to combine or to reduce the moments of intake.
When Do Two Occasions Count for Two or One
It is generally accepted that a pH drop after a sugary intake takes 30 min, implying that a second intake within this 30 min is less harmful than a second intake after these 30 min. Probably, this time span was concluded from the original experiments of Stephan and Miller (1940). PH-telemetry experiments, however, showed that the pH drop in dental plaque can continue far beyond these 30 min [Imfeld, 1977]. Obviously stickiness of the product may be a determinant of this. (Arcella et al. 2002) assessed the relationship between the frequency of sugars and starch intake and dental caries in 16-year-olds. Once lunch and dinner were excluded, the mean number of separate eating events was counted with no separating time interval (i.e. every eating event was separately counted) up to a separating interval of 60 min. The correlation coefficients between DMFT and the frequency of eating occasions thus established varied from 0.3 with no separating interval time to 0.31 for the 45 min interval to separate the eating occasions. These results suggest that not all separate eating events within the 45 min intervals necessarily contribute to an increased caries risk. The authors suggest that characterisation of eating events in real-life conditions deserves more attention. An interesting observation by (Arcella et al. 2002) was that the correlation between the number of intakes of sugars and starches and DMFT was significantly higher for boys than for girls, which the authors ascribed to poorer oral hygiene and thus fluoride use of boys.
Breakfast
When advising patients, dental professionals should not only convey the rules but also make them realize why sugar is consumed so frequently. Besides the nice taste, an important reason might be having feelings of appetite or being hungry. This may be the result of insufficient quality or skipping of the main meals, which has an effect on blood glucose levels and ghrelin; both are involved in regulating this hunger feeling. (Dusseldorp et al. 2015) showed for 9 and 15-year olds that a low breakfast frequency per week was related with having caries experience, while the frequency of brushing teeth per day was related with the degree of caries experience. Also, other studies [Nagel et al., 2009; Cinar et al., 2011; Bruno-Ambrosius et al., 2005] confirmed a relationship between caries and skipping breakfast. Having breakfast has also been associated with tooth brushing twice a day [Macgregor et al., 1996; Levin and Currie, 2010]. An explanation for this might be that both behaviours are symptoms of lifestyle structure and regularity. (Dusseldorp et al. 2015) observed that the lifestyle factors were not significant determinants of caries experience or the degree of caries experience for the 21-year-olds. This indicates that life style factors may become less significant at an older age. It is of importance to recognize that skipping breakfast is also suggested to contribute to childhood obesity [Szajewska and Ruszczynski, 2010].
Reducing the Amounts of Free Sugar in Products
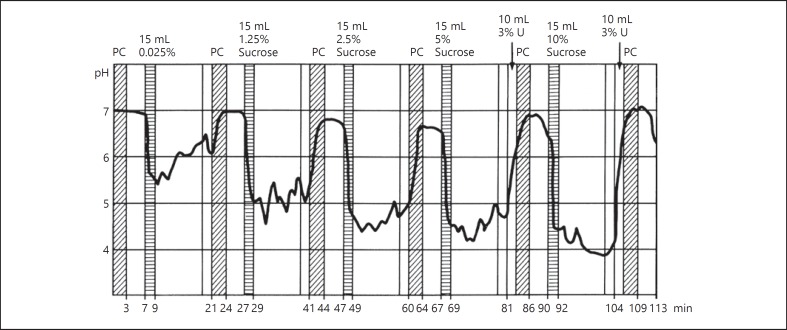
Several manufacturers and retailers assist clients to reduce the intake of free sugars by clearly indicating whether products are high in sugars > 29 g per 100 g, middle to high in sugars (17–29 g per 100 g) low to middle in sugars (5–17 g per 100 g) and low in sugars (0–5 g per 100 g) [Heijn, 2016]. It would be interesting to compare the cariogenicity of products from these various categories. PH telemetric measurements showed the pH curve in dental plaque upon sucrose challenges with increasing concentrations (Fig. 1) [Imfeld, 1977]. These data suggest that maximum acid production is reached with concentrations at or just above 10% sucrose. This would imply that differences of cariogenicity of the products in the upper 3 categories of the manufactures/retailers “health” list cannot be discerned, while products in the lowest category may not be or may be cariogenic. Furthermore, it has been claimed that the relative cariogenicity of a food is not correlated with the amount of carbohydrates it contains and it is not possible to estimate the minimal cariogenic concentration of sugars in foods, since this threshold varies with too many factors [Kandelman, 1997].
Fig. 1.
pH curves in dental plaque after consecutive rinses with solutions of increasing sucrose concentrations [Imfeld, 1977].
Intrinsic Sugars
The dietary advice for the reduction of caries is complicated by the claim that so-called intrinsic sugars are not cariogenic. Intrinsic sugars are sugars within the structure of fruits and vegetables. Indeed, when not released in the oral cavity, these sugars may not contribute to caries. But it is probably unrealistic to consume fruits without releasing the intrinsic sugars, and experimental studies indicate that consuming fruits maybe as cariogenic as consuming fruit juices [Issa et al., 2011; Zaura et al., 2005]. There are multiple arguments that it is better to replace foods high in free sugars with fresh fruit and the dental health professionals should encourage people to do so, but they should still be alert not to increase the frequency of intake.
Dietary Advice
Dietary advice by dental health professionals should be consistent and not conflict with advices from other health professionals, based on the evidence in the various professional fields and based on the national dietary guidelines. In this respect, it has to be remarked that not all national boards of health or nutrition have (yet) adapted the WHO-guidelines and that national guidelines may be less strict on the sugar intake. Furthermore, when people receive dietary advices from the dental health professional, the advices may be more readily accepted when the professional can make unequivocally clear that the advice benefits caries prevention. If not, the person may not understand why the dental professional interferes with his diet and not accept the advices. This does not dismiss the dental professional from also explaining the benefits for general health on limiting or reducing the intake of sugars. There is a risk that an overzealous dental professional may suggest alternatives for sugar take that increase the intake of fats and salt. Under the premise that it benefits oral health, the dental health professional can make stronger restrictions than the general guidelines as long as they do not harm general health. If so, dentistry even has the responsibility to claim at the national boards of health and nutrition to accept these restrictions. The dental professional should recognize the role of diet in patients with non-communicable diseases and encourage these patients to seek for adequate professional guidance. In general, a diet that is beneficial to both general and dental health is one that is low in free sugars, saturated fat and salts, high in fresh fruits, vegetables, nuts and seeds, wholegrain carbohydrates with modest amounts of legumes, fish, poultry and lean meat and plenty of fluids preferably water and milk and thus, modest with sugar sweetened beverages [Moynihan et al., 2017].
Conclusion
This manuscript discussed (1) the shape of the dose-response association between sugar intake and caries, (2) the association between sugar intake and caries at exposure to fluoride toothpaste and (3) the relative contribution of frequency and amount of sugar intake to caries levels. Most studies on the association between sugar intake and the amount of caries showed a no, a linear or log-linear association with relatively low correlation coefficients. When applying fluoride by appropriate toothbrushing twice a day, the association reduces significantly or is virtually absent. The relative contribution of the frequency versus the amount of sugar intake is difficult to discriminate because of the high correlation between frequency and amount. It is clear that the relative cariogenicity of a food is not directly correlated with the amount of sugar it contains, unless the amount is very low and it is clear that it is not possible to estimate the minimal cariogenic concentration of sugars in foods, since this threshold varies with too many factors. A model where frequency is more important fits better in the biological knowledge of the caries process. Also, the types of products contributing to the intake of free and added sugars lend themselves to be skipped or to be combined to reduce the numbers of intake. Goals set in terms of frequency may also be more tangible for patients to follow. Yet, in sessions of dietary counselling to prevent dental caries, the counsellor should not forget to highlight the importance of quality tooth brushing with fluoride toothpaste and strongly support this.
Disclosure Statement
The author declares no conflicts of interest.
References
- Arcella D, Ottolenghi L, Polimeni A, Leclercq C. The relationship between frequency of carbohydrates intake and dental caries: a cross-sectional study in Italian teenagers. Public Health Nutr. 2002;4:553–560. doi: 10.1079/PHN2001319. [DOI] [PubMed] [Google Scholar]
- Bernabé E, Vehkalahti MM, Sheiham A, Lundqvist A, Suominen AL. The shape of the dose-response relationship between sugars and caries in adults. J Dent Res. 2016;95:167–172. doi: 10.1177/0022034515616572. [DOI] [PubMed] [Google Scholar]
- Bratthall D, Hänsel-Petersson G, Sundberg H. Reasons for the caries decline: what do the experts believe? Eur J Oral Sci. 1996;104:416–422. doi: 10.1111/j.1600-0722.1996.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Bruno-Ambrosius K, Swanholm G, Twetman S. Eating habits, smoking and toothbrushing in relation to dental caries: a 3-year study in Swedish female teenagers. Int J Paediatr Dent. 2005;15:190–196. doi: 10.1111/j.1365-263X.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- Burt BA, Pai S. Sugar consumption and caries risk: a systematic review. J Dent Educ. 2001;65:1017–1023. [PubMed] [Google Scholar]
- Burt BA, Eklund SA, Morgan KJ, Larkin FE, Guire KE, Brown LO, Weintraub JA. The effects of sugars intake and frequency of ingestion on dental caries increment in a three-year longitudinal study. J Dent Res. 198;67:1422–1429. doi: 10.1177/00220345880670111201. [DOI] [PubMed] [Google Scholar]
- Cinar AB, Christensen LB, Hede B. Clustering of obesity and dental caries with lifestyle factors among Danish adolescents. Oral Health Prev Dent. 2011;9:123–130. [PubMed] [Google Scholar]
- Downer MC, Drugan CS, Blinkhorn AS. Correlates of dental caries in 12-year-old children in Europe: a cross-sectional analysis. Community Dent Health. 2008;25:70–78. [PubMed] [Google Scholar]
- Dusseldorp E, Kamphuis M, Schuller A. Impact of lifestyle factors on caries experience in three different age groups: 9, 15, and 21-year-olds. Community Dent Oral Epidemiol. 2015;43:9–16. doi: 10.1111/cdoe.12123. [DOI] [PubMed] [Google Scholar]
- Einarsdottir KG, Bratthall D. Restoring oral health. On the rise and fall of dental caries in Iceland. Eur J Oral Sci. 1996;104:459–469. doi: 10.1111/j.1600-0722.1996.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Feldens CA, Giugliani ER, Vigo Á, Vítolo MR. Early feeding practices and severe early childhood caries in four-year-old children from southern Brazil: a birth cohort study. Caries Res. 2010;44:445–452. doi: 10.1159/000319898. [DOI] [PubMed] [Google Scholar]
- Gibson S, Williams S. Dental caries in pre-school children: associations with social class, toothbrushing habit and consumption of sugars and sugar-containing foods. Further analysis of data from the National Diet and Nutrition Survey of children aged 1.5–4.5 years. Caries Res. 1999;33:101–113. doi: 10.1159/000016503. [DOI] [PubMed] [Google Scholar]
- Gustaffson BE, Quensel CE, Lanke LS, Lundqvist C, Grahnen H, Bonow BE, Krasse B. The Vipeholm dental caries study; the effect of different levels of carbohydrate intake on caries activity in 436 individuals observed for five years. Acta Odontol Scand. 1954;11:232–264. doi: 10.3109/00016355308993925. [DOI] [PubMed] [Google Scholar]
- Heijn A. https://www.ah.nl/over-ah/pers/persberichten/bericht?id=1572564 (accesed January 30, 2018)
- Imfeld T. Identification of low caries risk dietary components. Monogr Oral Sci. 1977;11:1–198. [PubMed] [Google Scholar]
- Issa AI, Toumba KJ, Preston AJ, Duggal MS. Comparison of the Effects of Whole and Juiced fruits and vegetables on enamel demineralisation in situ. Caries Res. 2011;45:448–452. doi: 10.1159/000330597. [DOI] [PubMed] [Google Scholar]
- Kandelman D. Sugar, alternative sweeteners and meal frequency in relation to caries prevention: new perspectives. Br J Nutr. 1997;77((suppl 1)):S121–S128. doi: 10.1079/bjn19970109. [DOI] [PubMed] [Google Scholar]
- Levin KA, Currie C. Adolescent toothbrushing and the home environment: sociodemographic factors, family relationships and mealtime routines and disorganisation. Community Dent Oral Epidemiol. 2010;38:10–18. doi: 10.1111/j.1600-0528.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- Linke HA, Birchmeier RI. Effect of increasing sucrose concentrations on oral lactic acid production. Ann Nutr Metab. 2000;44:121–124. doi: 10.1159/000012833. [DOI] [PubMed] [Google Scholar]
- Macgregor ID, Balding J, Regis D. Toothbrushing schedule, motivation and “lifestyle” behaviours in 7,770 young adolescents. Community Dent Health. 1996;13:232–237. [PubMed] [Google Scholar]
- Masood M, Masood Y, Newton T. Impact of national income and inequality on sugar and caries relationship. Caries Res. 2012;46:581–588. doi: 10.1159/000342170. [DOI] [PubMed] [Google Scholar]
- Moynihan P, Makino Y, Petersen PE, Ogawa H. Implications of WHO Guideline on Sugars for dental health professionals. Community Dent Oral Epidemiol. 2018;46:1–7. doi: 10.1111/cdoe.12353. [DOI] [PubMed] [Google Scholar]
- Nagel G, Wabitsch M, Galm C, Berg S, Brandstetter S, Fritz M, Klenk J, Peter R, Prokopchuk D, Steiner R, Stroth S, Wartha O, Weiland SK, Steinacker J. Determinants of obesity in the Ulm research on metabolism, exercise and lifestyle in children (URMEL-ICE) Eur J Pediatr. 2009;168:1259–1267. doi: 10.1007/s00431-009-1016-y. [DOI] [PubMed] [Google Scholar]
- Nyvad B, Sukker og caries . Sukkers sundhedsmæssige betydning. In: Mølgaard C, Lyhne Andersen N, Barkholt V, Grunnet N, Hermansen K, Pedersen BK, Raben A, Stender S, editors. Copenhagen, Danish Board of Nutrition. 2003. pp. pp 59–67. [PubMed] [Google Scholar]
- Ribeiro CC, Tabchoury CP, Del Bel Cury AA, Tenuta LM, Rosalen PL, Cury JA. Effect of starch on the cariogenic potential of sucrose. Br J Nutr. 2005;94:44–50. doi: 10.1079/bjn20051452. [DOI] [PubMed] [Google Scholar]
- Rodrigues CS, Sheiham A. The relationships between dietary guidelines, sugar intake and caries in primary teeth in low income Brazilian 3-year-olds: a longitudinal study. Int J Paediatr Dent. 2000;10:47–55. doi: 10.1046/j.1365-263x.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn AJ, Hackett AF, Appleton DR, Jenkins GN, Eastoe JE. Relationship between dietary habits and caries increment assessed over two years in 405 English adolescent school children. Arch Oral Biol. 1984;29:983–992. doi: 10.1016/0003-9969(84)90145-6. [DOI] [PubMed] [Google Scholar]
- Ruxton CH, Garceau FJ, Cottrell RC. Guidelines for sugar consumption in Europe: is a quantitative approach justified? Eur J Clin Nutr. 1999;53:503–513. doi: 10.1038/sj.ejcn.1600831. [DOI] [PubMed] [Google Scholar]
- Ruxton CH, Gardner EJ, McNulty HM. Is sugar consumption detrimental to health? A review of the evidence 1995–2006. Crit Rev Food Sci Nutr. 2010;50:1–19. doi: 10.1080/10408390802248569. [DOI] [PubMed] [Google Scholar]
- Sheiham A. Sugars and dental decay. Lancet. 1983;1:282–284. doi: 10.1016/s0140-6736(83)91696-3. [DOI] [PubMed] [Google Scholar]
- Sheiham A. Why free sugars consumption should be below 15 kg per person per year in industrialised countries: the dental evidence. Br Dent J. 1991;171:63–65. doi: 10.1038/sj.bdj.4807606. [DOI] [PubMed] [Google Scholar]
- Sluik D, Van Lee L, Engelen AI, Feskens EJM. Total, free, and added sugar consumption and adherence to guidelines: the dutch national food consumption survey 2007–2010. Nutrients. 2016;8:70–84. doi: 10.3390/nu8020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan RM, Miller BF. A quantitative method for evaluating physical and chemical agents which modify production of acids in bacterial plaques on human teeth. J Dent Res. 1943;22:45–53. [Google Scholar]
- Sreebny LM. Sugar availability, sugar consumption and dental caries. Community Dent Oral Epidemiol. 1982;10:1–7. doi: 10.1111/j.1600-0528.1982.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Szajewska H, Ruszczynski M. Systematic review demonstrating that breakfast consumption influences body weight outcomes in children and adolescents in Europe. Crit Rev Food Sci Nutr. 2010;50:113–119. doi: 10.1080/10408390903467514. [DOI] [PubMed] [Google Scholar]
- Takeuchi M. Epidemiological study on dental caries in Japanese children before, during and after World War II. Int Dent J. 1961;11:443–457. [Google Scholar]
- WHO (World Health Organisation) Sugars Intake in Adults and Children. Geneva, WHO. 2015 [PubMed] [Google Scholar]
- Woodward M, Walker AR. Sugar consumption and dental caries: evidence from 90 countries. Br Dent J. 1994;176:297–302. doi: 10.1038/sj.bdj.4808437. [DOI] [PubMed] [Google Scholar]
- Zaura E, van Loveren C, ten Cate JM. Efficacy of fluoride toothpaste in preventing demineralization of smooth dentin surfaces and narrow grooves in situ under frequent exposures to sucrose or bananas. Caries Res. 2005;39:116–122. doi: 10.1159/000083156. [DOI] [PubMed] [Google Scholar]