Abstract
Peripheral nerve injuries caused by accidents may lead to paralysis, sensory disturbances, anaesthesia, and lack of autonomic functions. Functional recovery after disconnection of the motoneuronal soma from target tissue with proximal rupture of axons is determined by several factors: motoneuronal soma viability, proper axonal sprouting across inhibitory zones and elongation toward specific muscle, effective synapse contact rebuilding, and prevention of muscle atrophy. Therapies, such as adjuvant drugs with pleiotropic effects, that promote functional recovery after peripheral nerve injury are needed. Toward this aim, we designed a drug discovery workflow based on a network-centric molecular vision using unbiased proteomic data and neural artificial computational tools. Our focus is on boosting intrinsic capabilities of neurons for neuroprotection; this is in contrast to the common approach based on suppression of a pathobiological pathway known to be associated with disease condition. Using our workflow, we discovered neuroheal, a combination of two repurposed drugs that promotes motoneuronal soma neuroprotection, is anti-inflammatory, enhances axonal regeneration after axotomy, and reduces muscle atrophy. This drug discovery workflow has thus yielded a therapy that is close to its clinical application.
Keywords: motoneuron, neurodegeneration, axonal regeneration, muscle atrophy, systems biology, neuroheal, PI3K/AKT, sirtuin 1, motor function recovery
Introduction
Recent advances in systems-oriented approaches and ‘omics technologies have led to an assortment of new computational tools that promise to enable a more informed and successful implementation of the drug discovery workflow than the reductionist “one drug for one target for one disease” approach (Westerhoff and Palsson, 2004). Computational tools for the analysis of biological data can help decipher the mechanism or mechanisms that underlie a disease state or the mechanism of action of a drug by offering a holistic view of the system. These tools enable identification of promising pathways for modulation and selection of potential inhibitors (Fotis et al., 2018). In this review, we focus on the recent discovery of neuroheal (NH) through a systems biology approach and the use of artificial intelligence-based tools. NH is neuroprotective and pro-regenerative and pre-clinical data indicate that it holds promise as a co-adjuvant treatment to facilitate recovery of nervous system function after traumatic injury to the peripheral nerves.
There is a medical need for function recovery after a disruption of the neuron-target connection resulting from trauma-associated peripheral nerve injury (PNI) due to traffic, laboral or sportive accidents. These injuries usually occur at working ages and its consequences range from mild discomfort to life-long impairment accompanied of paralysis, sensory disturbances, anaesthesia, lack of autonomic functions in the affected body areas and neuropathic pain. PNI has a prevalence of 1.3–2.8% (Noble et al., 1998; Taylor et al., 2008; Castillo-Galván et al., 2014) and surgical repair is the best therapeutical option depending on the PNI degree. This, together with sick leave spending time, increases substantially the economic and social burden being the estimated annual cost in USA health care range from of USD 5.13 Billion in 2016 and the compound annual growth rate of 12.3% (https://www.marketsandmarkets.com). The prognosis and functional recovery after PNI depends on the severity of the injury, the gap distance between disrupted nerve stumps, the length of the nerve to be regenerated and the extend of target reconnection. Moreover, axonal integrity disruption or axonal retraction may be provoked by non-traumatic disorders such as peripheral neuropathies acquired during lifetime such as those induced by diabetes mellitus, antineoplastic agents or infiltrating tumours, or those relates with genetical disorders such as Charcot-Marie-tooth or amyotrophic lateral sclerosis disease (England and Asbury, 2004; Taylor et al., 2011; Laing, 2012; Robberecht and Philips, 2013; Ale et al., 2014).
Injuries affecting the nerve roots, close to the interface between central and peripheral nervous systems, are the most devastating types of PNI. Nerve roots can be transected or separated by mechanical traction, which is commonly termed root avulsion (RA). RA is often caused by high-energy traffic accidents (mainly motor vehicle) or sport-related incidents (e.g., American football), by iatrogenic injuries, and during obstetric interventions (Pondaag et al., 2004; Buitenhuis et al., 2012; Daly et al., 2016; Kachramanoglou et al., 2017). RA can affect single roots or nerve plexus from brachial, lumbar, and facial zones (Htut et al., 2007). Adult brachial plexus injury is present in 1.2% of the multi-trauma admission patients in Canada, and 450–500 supraclavicular injuries with root affectation occur every year in UK (Goldie and Coates, 1992). Neonatal brachial plexus injury affects 2 or 3 of 1000 births (Pondaag et al., 2004). Currently, the only treatment for brachial plexus injury is surgical nerve root reimplantation. The timing for surgical repairing is critical mainly due to the progressive motoneuronal soma (MN) death triggered after injury (Htut et al., 2007).
Complete motor functional recovery after PNI, and particularly after RA, requires five main steps: i) sustained survival of axotomized MNs; ii) promotion of axonal regrowth across the glial scar formed at the ventral-root intermediate zone; iii) axonal elongation through the degenerated distal nerve stump to the target muscle; iv) establishment of functional connections to denervated muscle, and v) prevention of muscle atrophy. Whether or not these steps occur depends on factors such as the patient’s age, the type of lesion, the distance from the injury to the target organ, and the timing and type of surgical repair (Navarro et al., 2007). We have performed a PubMed literature search of articles published with the search term “neuroheal”, “nerve root avulsion”, and “peripheral nerve injury”.
Experimental Models to Study Peripheral Nerve Lesions
Successful discovery of drugs that can be used as adjuvants to surgical intervention after PNI requires first the existence of good models. In vivo models are widely used to mimic the PNI and have been used to identify factors that modulate axonal regeneration and MN death. MN survival depends on the severity and type of the injury, the distance from the neuronal soma, and the age of the animals. Rat spinal MNs are extremely susceptible to the transection of the nerve by avulsion from the spinal cord affecting the roots and is defined as preganglionic (proximal to the dorsal root ganglia). Usually affecting roots from brachial or lumbosacral plexus (Penas et al., 2009). But adult MNs are resistant to postganglionic transection (Vanden Noven et al., 1993; Valero-Cabre et al., 2004) with the exception of immature MNs still in development (Snider et al., 1992; Lowrie et al., 1994; Sun and Oppenheim, 2003). There are also differences among organisms since, for instance, cranial hypoglossal MNs are susceptible to the transection of the hypoglossal nerve at distal level in mice but not in rats (Kiryu-Seo et al., 2005). Models based on nerve crush produced by compression that lead to the axonal continuity breakdown (axonotmesis) allow a reasonably good recovery, whereas those in which neurotmesis is produced by complete transection of the nerve leads to a poor functional recovery of the animals, although the latter model better recapitulates the clinical features of PNI (Lago and Navarro, 2006).
The rational design of neuroprotective agents is often based on knowledge of the underlying mechanisms of neurodegeneration. In this regard, it is important to determine which process of cell death affects disconnected MNs. After axotomy, a neuronal retrograde response is initiated whose intensity and time course is influenced by maturational stage and MN subset as well as by the site and type of axonal injury and the age and species of animal. In neonates, axotomized MNs undergo apoptosis as a result of the lack of muscle-derived trophic supports (Snider et al., 1992). The aggressiveness of the process after RA is characterized by a drastic reduction of MN survival, which results in death of around 80% of MNs by 4 weeks post injury (Penas et al., 2009). Models obtained either through sciatic root or facial nerve avulsion result in similar losses of MNs but with some mechanistic differences (Watabe et al., 2005).
We have recently shed light on the mechanisms of MN death after RA in adult rats using unbiased comparative proteomic analysis combined with a systems biology approach for data mining (Casas et al., 2015). Our study showed that several programs, such as apoptosis and anti-apoptosis pathways, run in parallel and counterbalance to result in an overall non-apoptotic degenerative process (Li et al., 1998; Penas et al., 2011a) and anoikis. In contrast, when nerve avulsion is milder, not performed at the root but distally, the process may shift towards an apoptotic process, which upon sciatic nerve avulsion leads rapid loss of about 50% of MNs by two weeks post injury ((Martin et al., 1999, 2005) and personal observations). These observations reveal that to be clinically effective a neuroprotective agent should be plastic enough to protect against both forms of neuronal death.
Although these models of RA allow study of the mechanisms of degeneration and target discovery, more sophisticated models have been also characterized that better mimic clinical issues after surgical repair. Some authors have performed RA of ventral or brachial roots combined with an immediate reparative surgery (Eggers et al., 2010; Pintér et al., 2010; Torres-Espín et al., 2013). However, immediate repair cannot be carried out clinically because, among other reasons, patients need to be stabilized after the accident. Gu et al. (2005) were the first to evaluate delayed repair of the avulsed roots, and recently we set up a new in vivo rat model that combines RA of L3–6 ventral roots with delayed surgical repair (Romeo-Guitart et al., 2017). This model can be used to test new therapies against MN degeneration in the context of post-injury axonal regrowth. Importantly, this model reproduces long-term denervated muscle atrophy, which is essential for functional recovery (Romeo-Guitart et al., 2017).
Network-Centric Discovery of a New Neuroprotective Agent: Neuroheal
Many observations indicate the existence of multiple endogenous mechanisms of neuroprotection. Endogenous defence of the nervous system is a continuous process that simultaneously performs and integrates the neurobiological processes of tropism, neuroprotection, neuroplasticity, and neurogenesis. Clinical activation of endogenous protective strategies dates back more than six decades when Noble and collaborators observed that short periods of global hypoxia can protect the entire mammalian organism and preserve brain energy metabolism when re-exposed to longer periods of hypoxia (Noble, 1943). These early studies were the foundation for subsequent intensive research on the mechanisms involved in this type of protective pre-conditioning. However, drug discovery efforts have focused on identification of agents that suppress pathobiological processes rather than on discovery of drugs that enhance intrinsic neuroprotective pathways. This reductionist vision has not led to many successful therapies for neurodegeneration.
To identify a neuroprotective agent capable of boosting the endogenous mechanisms normally triggered after trauma, we took advantage of the existence of solid molecular knowledge on the mechanisms triggered after trauma and computational tools for handling and extracting meaning from biological data. We realized that dissimilar reactions occur in MNs after proximal and distal axotomy that lead to opposite readouts: MN degeneration vs. MN survival, respectively. We wondered which mechanisms triggered by distally axotomized MNs allowed them to recover from damage and even regenerate. We envisaged that mimicking these endogenous mechanisms might be neuroprotective for proximal axotomized degenerating MNs as well. In support of this hypothesis, we observed that although similar molecular mechanisms were initially triggered by both distal and proximal RA axotomy, there are differences in extent of neurodegeneration at seven days post injury (Penas et al., 2011a; Casas et al., 2015). Hence, we had identified the precise time to investigate and dissect out pro-survival mechanisms from pro-degenerative ones. We used proteomics and a systems biology approach to perform unbiased analysis of both models. We discovered that MN degeneration after RA was characterized by concurrent programs such as apoptosis and anti-apoptosis, anoikis and cytoskeletal rearrangements, among others, and that distally axotomized MNs survive because these MNs differently engage the anti-anoikis program and selective autophagy (Casas et al., 2015). Although not fully understood, this divergence in the molecular mechanisms between MN degeneration or regeneration may be due to the proximity of the injury to the soma. This provokes a more severe disturbance of the cell organelles after preganglionic injury and a harmful response to stress which leads to the opposite molecular responses (Penas et al., 2011b).
These descriptions were not enough to identify specific targets for a neuroprotective therapeutical strategy. We did not want to implement the reductionist vision – one drug for one target for one disease – approach. Instead, we wanted to take advantage of breakthroughs in systems biology and pharmacology approaches as well as in computational tools for big data analysis to design a network-centric-based drug discovery process. Network analysis can be used to elucidate the intricate connections that form the biological systems and how they are disturbed in pathological conditions (Barabási, 2007). The increasing amount of biological data from a variety of ‘omics platforms (genomics, transcriptomics, proteomics, and metabolomics) have assisted in the development of various in silico approaches that connect molecular data to their biological functions using knowledge bases as templates to build associations between them. Recently developed computational tools allow unique virtual screening strategies for polypharmacology and prediction of adverse and therapeutic polypharmacological effects and supervised machine-learning algorithms that enable prediction of drug-target interactions (Lavecchia, 2015) have revolutionized the workflow for drug discovery. Network models enable analysis of complex interaction networks between drugs and their targets aiding in elucidation of mechanisms of action (Cheng et al., 2012; Woo et al., 2015). Statistical models that make use of a data integration framework have been effectively applied both to predict novel drug indications and to identify potent drug combination strategies (Keiser et al., 2007). Computational/experimental frameworks have also been implemented for the systematic design of multi-targeted drugs (Bottegoni et al., 2012).
In the discovery of NH, we used the technology known as the therapeutic performance mapping system, which allows generation of disease-specific mathematical models for drug screening using neural artificial intelligence-based methods for machine learning (Pujol et al., 2010; Herrando-Grabulosa et al., 2016; Romeo-Guitart et al., 2017, 2018). Therapeutic performance mapping system is based on the building of the molecular networks characteristic of an specific health condition (either normal or pathological) using network and systems biology approaches (Pujol et al., 2010). Therapeutic performance mapping system facilitates the screening of drugs for their capacity to shift the profile of topological molecular maps from pathological to beneficial or normal (Herrando-Grabulosa et al., 2016). For this purpose, maps are converted into mathematical models using artificial artificial neural networks and sampling methods as computational tools, and machine learning allow the screening and choice of thousands of drug combinations with a desirable effect (e.g., neuroprotection) (Badiola et al., 2013). In our work, we sought to identify a combination of clinically used drugs that would be capable of converting the degeneration-associated map that describes the RA condition into the neuroprotective and regenerative-associated map described by the distal axotomy condition. Our therapeutic performance mapping system analysis resulted in a list of putative neuroprotective combinations of US Food and Drug Administration-approved drugs. Thus, computational tools allowed us to focus on the network and to select pharmaceutical combinations putatively capable of boosting endogenous neuroprotective mechanisms. Experimental investigations led us to the combination now known as NH.
We validated that NH increases MN survival after RA, reduces the inflammatory response, protects against endoplasmic reticulum stress in vitro, and induces pro-regenerative profiles in MN tracks in spinal cords; the NH components act synergistically (Romeo-Guitart et al., 2018). In addition, we demonstrated that NH treatment accelerates long-term motor function recovery after PNI and root reimplantation (Romeo-Guitart et al., 2017).
It is important to highlight that NH is a combination of two repurposed drugs: acamprosate and ribavirin. Drug repurposing facilitates clinical translation because it drastically reduces the need for preclinical toxicity and efficacy assays and for safety profiling in human volunteers (Ashburn and Thor, 2004). Some successful examples of drug repurposing include a glycogen synthase kinase- 3β-targeted therapy for the treatment of glioblastoma (Furuta et al., 2017) and the promising combination of two anti-diabetic drugs that exert neuroprotection in a mouse model of Alzheimer´s disease and in patients with Parkinson disease (Aviles-Olmos et al., 2013; Hettich et al., 2014).
Neuroheal’s Mechanism of Action in Neuroprotection and Nerve Regeneration
As evidenced by the increasing number of publications in the recent years, drug discovery is undergoing a steady paradigm shift from the ideology of selective agents (‘magic bullets’) toward selectively promiscuous multi-targeting drugs (‘magic shotguns’) (Roth et al., 2004; Strebhardt and Ullrich, 2008). The polypharmacology-based “one drug, multiple targets” paradigm has benefits relative to the conventional de novo approaches to drug discovery (DiMasi, 2001; Dickson and Gagnon, 2004). In the case of nerve regeneration, multi-targeting gives the opportunity to act through several pathways in parallel to support the neuroprotective and pro-regenerative phenotype. Analysis with computational tools allowed us to identify putative proteins (nodes) that mediate the synergistic mechanism of action of NH. Important nodes belong to the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (AKT) axis and anti-apoptotic pathways (p53, the apoptosis blocker X-linked inhibitor of apoptosis protein, cytoskeletal motor protein kinesin heavy chain isoform 5 (KIF5), and dynactin subunit 1 (DCNT1)), and the Src-integrin axis (the integrin subunit integrin beta-1 and the nicotinamide adenine dinucleotide-dependent deacetylase sirtuin 1 (SIRT1)) (Romeo-Guitart et al., 2018) (Figure 1).
Figure 1.
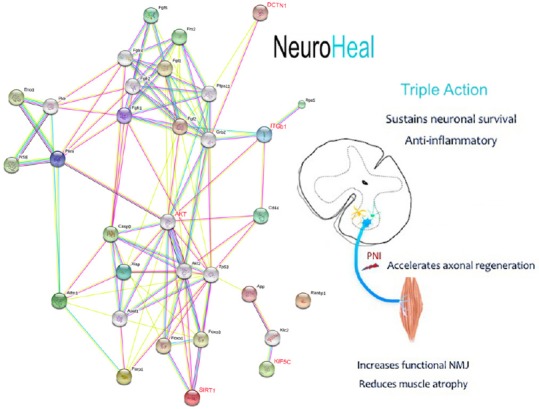
Neuroheal exerts neuroprotective and regenerative effects after peripheral nerve injury.
Left, String protein network representation of minimal mechanism of action of NeuroHeal. In red are highlighted key nodes experimentally proven to be modulated by neuroheal: an increased activity of nicotinamide adenine dinucleotide-dependent deacetylase sirtuin 1 (SIRT1) and serine/threonine-protein kinase protein kinase B (AKT), and an augmented presence of integrin subunit integrin beta-1 (ITGB1), kinesin-related protein KIF5 and dynactin subunit 1 (DCNT1). Right, pleiotropic effects promoted by neuroheal at different levels: the spinal cord motoneuronal soma (MNs) (blue), astrocytes (yellow), and microglia (green), the motor nerve at the peripheral nerve system (blue line), and the muscle (orange). NMJ: Neuromuscular junctions.
We have shown that activation of SIRT1 mediated by NH promotes neuroprotection (Romeo-Guitart et al., 2017, 2018). It has been extensively reported that activation of SIRT1 can exert neuroprotection in different neurodegenerative diseases such as amyotrophic lateral sclerosis, Alzheimer and Huntington diseases and in experimental autoimmune encephalomyelitis induced by pharmacological or genetic activation (Kim et al., 2007; Jiang et al., 2012; Nimmagadda et al., 2013; Watanabe et al., 2014). In the context of trauma, SIRT1 activation reduces functional impairments after spinal cord injury (Chen et al., 2017). Although some reports indicate that SIRT1 activation has beneficial effects and improves functional recovery after traumatic brain injury (Wu et al., 2007), its role after stroke remains controversial (Petegnief and Planas, 2013). Mice that overexpress SIRT1 have ameliorated amyotrophic lateral sclerosis pathophysiology (Watanabe et al., 2014). We found that co-treatment of the RA-injured animals with NH and the selective SIRT1-activity inhibitor Ex-527 blocked NH pro-survival effects (Romeo-Guitart et al., 2017, 2018). Indeed, the overexpression of SIRT1 in spinal MNs using an adeno-associated viral vector increased MN survival after RA. Although SIRT1 activation is essential for NH neuroprotection, SIRT1 activation alone is not responsible for the beneficial spectrum of neuroprotection that NH exerts revealing the importance of its multi-target nature (Romeo-Guitart et al., 2018). Moreover, NH normalized the location or abundance of cytoskeletal motor proteins KIF5C and DCNT1, two transport proteins related with neurodegeneration or axonal disturbances. The kinesin family is formed by the kinesin-1 (historically named kif5c) and the kinesin-3 (KIF1A, KIF1Bα and KIF1Bβ) members (De Vos and Hafezparast, 2017). KIF5C is enriched in MNs (Kanai et al., 2000) and its genetic ablation or spontaneous mutation are linked with MN diseases and paralysis (Xia et al., 2003; De Vos and Hafezparast, 2017). Besides, a dysfunctional dynein/DCTN1 has been used as an ALS mice model. Its mutation causes a defective axonal transport that leads to an amyotrophic lateral sclerosis-like phenotype in mice and to MN degeneration (LaMonte et al., 2002; Heiman-Patterson et al., 2015). Finally, the increase on β-1 integrin can be yielding anti-anoikis effects to the MNs. In fact, the concomitant activation of AKT axis with an increase of β-1 integrin may be inhibiting anoikis in our model (Bouchard et al., 2007).
Although maintenance of MNs can be achieved after RA experimentally by different pharmacological treatments, translation to the human healthcare has never been performed. For example, the Pre084 compound, an agonist of the sigma receptor 1, is a good neuroprotectant but use systemically causes neuropathic pain, precluding clinical application (Penas et al., 2011b; Roh et al., 2011; Pyun et al., 2014). Other compounds such as N-acetyl-cysteine, GM-1 ganglioside, and valproic acid maintain MN pool, but their ability to enhance nerve regeneration has not been reported (Oliveira and Langone, 2000; Zhang et al., 2005; Wu et al., 2013). Riluzole, lithium, and an inhibitor of the proteoglycan receptor are neuroprotective in a model of RA with the immediate reimplantation of roots (Bergerot et al., 2004; Li et al., 2015; Fang et al., 2016). These compounds promote long-term survival of MN, as does NH, after injury, but in tests of these compounds, reparative surgery was performed immediately after RA, so it is unclear if they also protect MNs after a delayed root reimplantation. We showed that NH sustains MN survival for two weeks after RA in the absence of reparative surgery (Romeo-Guitart et al., 2017).
Although mammalian neurons have intrinsic capabilities to replace and repair axons by regeneration, in the best clinical scenario only 10% of axons regenerate to target organs after nerve transection (Janjic and Gorantla, 2017). The formation of the inhibitory glial scar at the central nervous system-peripheral nervous system transition zone after root injury make more difficult axon regrowth in this case than in distal axotomy (Silver and Miller, 2004; Silver et al., 2014). Different molecular pathways have been reported to mediate axonal regrowth within central nervous system and peripheral nervous system (Tedeschi, 2012). Among them, the PI3K/AKT axis, which is activated by neurotrophic factors, is the best characterized (Caporali et al., 2008). PI3K is very sensitive to PTEN (gene of phosphate and tension homology deleted on chromsome ten), which blocks PI3K and reduces the pro-regenerative effect of the axis (Berry et al., 2016). Several approaches, including PTEN deletion (Park et al., 2008) and its pharmacological inhibition, enhance axonal regrowth through the glial scar after injury to the central nervous system (Ohtake et al., 2014). However, the suppression of PTEN activity cannot be translated to the clinic due to its tumour-suppressor role (Tolkacheva and Chan, 2000). Thus, discovering new drug therapies that increase axonal regrowth by AKT activation without affecting PTEN has raised much interest (Tolkacheva and Chan, 2000). We demonstrated that NH reduces glial scar formation and enhances axonal regrowth after distal or proximal injury (Romeo-Guitart et al., 2017, 2018). Acceleration of nerve regeneration resulting from NH treatment is accompanied by an increase in compound muscle action potential at the muscles innervated by the L4–6 MNs. NH probably enhances nerve regeneration because it increases PI3K/AKT axis activity and growth associated protein 43 expression, a pro-regenerative factor also called neuromodulin (Denny, 2006; Holahan, 2017).
Very few drugs that enhance axonal regeneration after PNI have been described in the literature (Al-Majed et al., 2000; Elzinga et al., 2015). These are FK506, geldanamycin, N-acetylcysteine, acetyl-L-carnitine, IGF, chondroitinase ABC, and neurotrophic factors (Navarro et al., 2001; Welin et al., 2009; Sun et al., 2012; Farahpour and Ghayour, 2014; Janjic and Gorantla, 2017). All have undesired side effects such as immunosuppression in the case of FK-506 and tumorigenesis mediated by neurotrophic factors (Tannemaat et al., 2008). Some compounds, including riluzole, lithium, and an inhibitor of the proteoglycan receptor, have been demonstrated to increase axonal regeneration after RA with immediate root reimplantation (Bergerot et al., 2004; Li et al., 2015; Fang et al., 2016). However, in this model is difficult to distinguish whether functional recovery is mediated by an increase in MNs survival or by a specific effect on axonal regeneration. NH, as a combination of two repurposed drugs, has clear advantages in terms of clinical translation.
After traumatic injuries to the nervous system, such as PNI, neurodegenerative diseases, or intensive health-care periods, muscle is considerably atrophied (Powers et al., 2016). Skeletal muscle atrophy is correlated with a high mortality and morbidity risk (Calvani et al., 2013). The importance of muscle in recovery after PNI has recently been acknowledged. Recovery depends on efficient reconnection of axons with neuromuscular junctions (NMJs) from muscle, and this muscle must be minimally atrophied. We have observed that NH treatment reduces muscle-denervated atrophy and increases the number of functional NMJs (Figure 1). Hence, NH treatment shows clear positive combined effects on neuroprotection and axonal regeneration, and its activity shows that mimicking endogenous mechanisms can lead to effective therapeutic designs.
Conclusion and Summary
An optimal therapeutic strategy after PNI must sustain long-lasting neuronal viability, enhance axonal regrowth, favour functional reinnervation of NMJs, and reduce muscle atrophy. Covering all these aspects by influencing a single target is likely impossible, and, therefore, multi-targeting drugs are desired. The nervous system, like other systems in the body, has the availability to engage endogenous mechanisms in the face of injury that allow neurons to survive, axons to speedily regenerate, and healthy denervated muscle to be sustained. We reasoned that potentiating these mechanisms might result in effective neuroprotection and repair of the damaged nervous system. NH was discovered using a systems biology and neural artificial intelligence-based computational tools in a network-centric vision for drug discovery. In animal models, NH boosts endogenous mechanisms and its multi-targeting nature activates multiple factors necessary for functional recovery after PNI, resulting in neuronal survival, axonal regrowth, and limited denervated muscle atrophy.
Additional file: Open peer review reports 1 (76.1KB, pdf) and 2 (70.6KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no competing interests. Neuroheal is currently under patent review.
Financial support: This work was mainly supported by the Ministerio de Economía y Competitividad of Spain (#SAF 2014-59701) (to CC).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Daoli Zhu, Nantong University, China; Shanji Nan, The Second Hospital of Jilin University, China.
Funding: This work was mainly supported by the Ministerio de Economía y Competitividad of Spain (#SAF 2014-59701) (to CC).
P-Reviewers: Zhu D, Nan S; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ale A, Bruna J, Navarro X, Udina E. Neurotoxicity induced by antineoplastic proteasome inhibitors. Neurotoxicology. 2014;43:28–35. doi: 10.1016/j.neuro.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 4.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund P, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123:2370–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badiola N, Alcalde V, Pujol A, Münter LM, Multhaup G, Lleó A, Coma M, Soler-López M, Aloy P. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8:e58837. doi: 10.1371/journal.pone.0058837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barabási AL. Network medicine--from obesity to the “diseasome”. N Engl J Med. 2007;357:404–407. doi: 10.1056/NEJMe078114. [DOI] [PubMed] [Google Scholar]
- 7.Bergerot A, Shortland PJ, Anand P, Hunt SP, Carlstedt T. Co-treatment with riluzole and GDNF is necessary for functional recovery after ventral root avulsion injury. Exp Neurol. 2004;187:359–366. doi: 10.1016/j.expneurol.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Berry M, Ahmed Z, Morgan-Warren P, Fulton D, Logan A. Prospects for mTOR-mediated functional repair after central nervous system trauma. Neurobiol Dis. 2016;85:99–110. doi: 10.1016/j.nbd.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Bottegoni G, Favia AD, Recanatini M, Cavalli A. The role of fragment-based and computational methods in polypharmacology. Drug Discov Today. 2012;17:23–34. doi: 10.1016/j.drudis.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard V, Demers MJ, Thibodeau S, Laquerre V, Fujita N, Tsuruo T, Beaulieu JF, Gauthier R, Vezina A, Villeneuve L, Vachon PH. Fak/Src signaling in human intestinal epithelial cell survival and anoikis: differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways. J Cell Physiol. 2007;212:717–728. doi: 10.1002/jcp.21096. [DOI] [PubMed] [Google Scholar]
- 11.Buitenhuis S, van Wijlen-Hempel RS, Pondaag W, Malessy MJA. Obstetric brachial plexus lesions and central developmental disability. Early Hum Dev. 2012;88:731–734. doi: 10.1016/j.earlhumdev.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, Bernabei R, Marzetti E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393–414. doi: 10.1515/hsz-2012-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, Newby AC, Madeddu P, Emanueli C. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casas C, Isus L, Herrando-Grabulosa M, Mancuso FM, Borrás E, Sabidó E, Forés J, Aloy P. Network-based proteomic approaches reveal the neurodegenerative, neuroprotective and pain-related mechanisms involved after retrograde axonal damage. Sci Rep. 2015;5:9185. doi: 10.1038/srep09185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo-Galván ML, Martínez-Ruiz FM, de la Garza-Castro O, Elizondo-Omaña RE, Guzmán-López S. Study of peripheral nerve injury in trauma patients. Gac Med Mex. 2014;150:527–532. [PubMed] [Google Scholar]
- 16.Chen H, Ji H, Zhang M, Liu Z, Lao L, Deng C, Chen J, Zhong G. An agonist of the protective factor SIRT1 improves functional recovery and promotes neuronal survival by attenuating inflammation after spinal cord injury. J Neurosci. 2017;37:2916–2930. doi: 10.1523/JNEUROSCI.3046-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng F, Liu C, Jiang J, Lu W, Li W, Liu G, Zhou W, Huang J, Tang Y. Prediction of drug-target interactions and drug repositioning via network-based inference. PLoS Comput Biol. 2012;8:e1002503. doi: 10.1371/journal.pcbi.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daly CA, Payne SH, Seiler JG. Severe brachial plexus injuries in American football. Orthopedics. 2016;39:e1188–1192. doi: 10.3928/01477447-20160721-03. [DOI] [PubMed] [Google Scholar]
- 19.De Vos KJ, Hafezparast M. Neurobiology of axonal transport defects in motor neuron diseases: Opportunities for translational research? Neurobiol Dis. 2017;105:283–299. doi: 10.1016/j.nbd.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denny JB. Molecular mechanisms, biological actions, and neuropharmacology of the growth-associated protein GAP-43. Curr Neuropharmacol. 2006;4:293–304. doi: 10.2174/157015906778520782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickson M, Gagnon JP. Key factors in the rising cost of new drug discovery and development. Nat Rev Drug Discov. 2004;3:417–429. doi: 10.1038/nrd1382. [DOI] [PubMed] [Google Scholar]
- 22.DiMasi JA. Risks in new drug development: approval success rates for investigational drugs. Clin Pharmacol Ther. 2001;69:297–307. doi: 10.1067/mcp.2001.115446. [DOI] [PubMed] [Google Scholar]
- 23.Eggers R, Tannemaat MR, Ehlert EM, Verhaagen J. A spatio-temporal analysis of motoneuron survival, axonal regeneration and neurotrophic factor expression after lumbar ventral root avulsion and implantation. Exp Neurol. 2010;223:207–220. doi: 10.1016/j.expneurol.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Elzinga K, Tyreman N, Ladak A, Savaryn B, Olson J, Gordon T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp Neurol. 2015;269:142–153. doi: 10.1016/j.expneurol.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 25.England JD, Asbury AK. Peripheral neuropathy. Lancet. 2004;363:2151–2161. doi: 10.1016/S0140-6736(04)16508-2. [DOI] [PubMed] [Google Scholar]
- 26.Fang XY, Zhang WM, Zhang CF, Wong WM, Li W, Wu W, Lin JH. Lithium accelerates functional motor recovery by improving remyelination of regenerating axons following ventral root avulsion and reimplantation. Neuroscience. 2016;329:213–225. doi: 10.1016/j.neuroscience.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Farahpour MR, Ghayour SJ. Effect of in situ delivery of acetyl-L-carnitine on peripheral nerve regeneration and functional recovery in transected sciatic nerve in rat. Int J Surg. 2014;12:1409–1415. doi: 10.1016/j.ijsu.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Fotis C, Antoranz A, Hatziavramidis D, Sakellaropoulos T, Alexopoulos LG. Pathway-based technologies for early drug discovery. Drug Discov Today. 2018;23:626–635. doi: 10.1016/j.drudis.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Furuta T, Sabit H, Dong Y, Miyashita K, Kinoshita M, Uchiyama N, Hayashi Y, Hayashi Y, Minamoto T, Nakada M. Biological basis and clinical study of glycogen synthase kinase- 3β-targeted therapy by drug repositioning for glioblastoma. Oncotarget. 2017;8:22811–22824. doi: 10.18632/oncotarget.15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldie BS, Coates CJ. Brachial plexus injury: a survey of incidence and referral pattern. J Hand Surg Br. 1992;17:86–88. doi: 10.1016/0266-7681(92)90018-w. [DOI] [PubMed] [Google Scholar]
- 31.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu HY, Chai H, Zhang JY, Yao ZB, Zhou LH, Wong WM, Bruce IC, Wu WT. Survival, regeneration and functional recovery of motoneurons after delayed reimplantation of avulsed spinal root in adult rat. Exp Neurol. 2005;192:89–99. doi: 10.1016/j.expneurol.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Heiman-Patterson TD, Blankenhorn EP, Sher RB, Jiang J, Welsh P, Dixon MC, Jeffrey JI, Wong P, Cox GA, Alexander GM. Genetic background effects on disease onset and lifespan of the mutant dynactin p150glued mouse model of motor neuron disease. PLoS One. 2015;10:e0117848. doi: 10.1371/journal.pone.0117848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrando-Grabulosa M, Mulet R, Pujol A, Mas JM, Navarro X, Aloy P, Coma M, Casas C. Novel neuroprotective multicomponent therapy for amyotrophic lateral sclerosis designed by networked systems. PLoS One. 2016;11:e0147626. doi: 10.1371/journal.pone.0147626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hettich MM, Matthes F, Ryan DP, Griesche N, Schröder S, Dorn S, Krauβ S, Ehninger D. The anti-diabetic drug metformin reduces BACE1 protein level by interfering with the MID1 complex. PLoS One. 2014;9:e102420. doi: 10.1371/journal.pone.0102420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holahan MR. A shift from a pivotal to supporting role for the growth-associated protein (GAP-43) in the coordination of axonal structural and functional plasticity. Front Cell Neurosci. 2017;11:266. doi: 10.3389/fncel.2017.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Htut M, Misra VP, Anand P, Birch R, Carlstedt T. Motor recovery and the breathing arm after brachial plexus surgical repairs, including re-implantation of avulsed spinal roots into the spinal cord. J Hand Surg Am. 2007;32:170–178. doi: 10.1016/J.JHSB.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Janjic JM, Gorantla VS. Peripheral nerve nanoimaging: monitoring treatment and regeneration. AAPS J. 2017;19:1304–1316. doi: 10.1208/s12248-017-0129-x. [DOI] [PubMed] [Google Scholar]
- 39.Jiang M, Wang J, Fu J, Du L, Jeong H, West T, Xiang L, Peng Q, Hou Z, Cai H, Seredenina T, Arbez N, Zhu S, Sommers K, Qian J, Zhang J, Mori S, Yang XW, Tamashiro KL, Aja S, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med. 2012;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kachramanoglou C, Carlstedt T, Koltzenburg M, Choi D. Long-term outcome of brachial plexus reimplantation after complete brachial plexus avulsion injury. World Neurosurg. 2017;103:28–36. doi: 10.1016/j.wneu.2017.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanai Y, Okada Y, Tanaka Y, Harada a, Terada S, Hirokawa N. KIF5C, a novel neuronal kinesin enriched in motor neurons. J Neurosci. 2000;20:6374–6384. doi: 10.1523/JNEUROSCI.20-17-06374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, Shoichet BK. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25:197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- 43.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiryu-Seo S, Hirayama T, Kato R, Kiyama H. Noxa is a critical mediator of p53-dependent motor neuron death after nerve injury in adult mouse. J Neurosci. 2005;25:1442–1447. doi: 10.1523/JNEUROSCI.4041-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lago N, Navarro X. Correlation between target reinnervation and distribution. J Neurotrauma. 2006;23:227–240. doi: 10.1089/neu.2006.23.227. [DOI] [PubMed] [Google Scholar]
- 46.Laing NG. Genetics of neuromuscular disorders. Crit Rev Clin Lab Sci. 2012;49:33–48. doi: 10.3109/10408363.2012.658906. [DOI] [PubMed] [Google Scholar]
- 47.LaMonte BH, Wallace KE, Holloway BA, Shelly SS, Ascaño J, Tokito M, Van Winkle T, Howland DS, Holzbaur ELF. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 48.Lavecchia A. Machine-learning approaches in drug discovery: Methods and applications. Drug Discov Today. 2015;20:318–331. doi: 10.1016/j.drudis.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Wong C, Li W, Ruven C, He L, Wu X, Lang BT. Enhanced regeneration and functional recovery after spinal root avulsion by manipulation of the proteoglycan receptor PTP σ. Nat Publ Gr. 2015:1–14. doi: 10.1038/srep14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L, Houenou LJ, Wu W, Lei M, Prevette DM, Oppenheim RW. Characterization of spinal motoneuron degeneration following different types of peripheral nerve injury in neonatal and adult mice. J Comp Neurol. 1998;396:158–168. [PubMed] [Google Scholar]
- 51.Lowrie MB, Lavalette D, Davies CE. Time course of motoneurone death after neonatal sciatic nerve crush in the rat. Dev Neurosci. 1994;16:279–284. doi: 10.1159/000112120. [DOI] [PubMed] [Google Scholar]
- 52.Martin LJ, Chen K, Liu Z. Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. J Neurosci. 2005;25:6449–6459. doi: 10.1523/JNEUROSCI.0911-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin LJ, Kaiser A, Price AC. Motor neuron degeneration after sciatic nerve avulsion in adult rat evolves with oxidative stress and is apoptosis. J Neurobiol. 1999;40:185–201. [PubMed] [Google Scholar]
- 54.Navarro X, Udina E, Ceballos D, Gold BG. Effects of FK506 on nerve regeneration and reinnervation after graft or tube repair of long nerve gaps. Muscle Nerve. 2001;24:905–915. doi: 10.1002/mus.1088. [DOI] [PubMed] [Google Scholar]
- 55.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Nimmagadda VK, Bever CT, Vattikunta NR, Talat S, Ahmad V, Nagalla NK, Trisler D, Judge SI V, Royal W, 3rd, Chandrasekaran K, Russell JW, Makar TK. Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 targets. J Immunol. 2013;190:4595–4607. doi: 10.4049/jimmunol.1202584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 58.Ohtake Y, Park D, Abdul-Muneer PM, Li H, Xu B, Sharma K, Smith GM, Selzer ME, Li S. The effect of systemic PTEN antagonist peptides on axon growth and functional recovery after spinal cord injury. Biomaterials. 2014;35:4610–4626. doi: 10.1016/j.biomaterials.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oliveira a. LR, Langone F. GM-1 ganglioside treatment reduces motoneuron death after ventral root avulsion in adult rats. Neurosci Lett. 2000;293:131–134. doi: 10.1016/s0304-3940(00)01506-8. [DOI] [PubMed] [Google Scholar]
- 60.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Penas C, Casas C, Robert I, Forés J, Navarro X. Cytoskeletal and activity-related changes in spinal motoneurons after root avulsion. J Neurotrauma. 2009;26:763–779. doi: 10.1089/neu.2008.0661. [DOI] [PubMed] [Google Scholar]
- 62.Penas C, Font-Nieves M, Forés J, Petegnief V, Planas a, Navarro X, Casas C. Autophagy, and BiP level decrease are early key events in retrograde degeneration of motoneurons. Cell Death Differ. 2011a;18:1617–1627. doi: 10.1038/cdd.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Penas C, Pascual-Font A, Mancuso R, Forés J, Casas C, Navarro X. Sigma receptor agonist 2-(4-morpholinethyl)1 phenylcyclohexanecarboxylate (Pre084) increases GDNF and BiP expression and promotes neuroprotection after root avulsion injury. J Neurotrauma. 2011b;28:831–840. doi: 10.1089/neu.2010.1674. [DOI] [PubMed] [Google Scholar]
- 64.Petegnief V, Planas AM. SIRT1 regulation modulates stroke outcome. Transl Stroke Res. 2013;4:663–671. doi: 10.1007/s12975-013-0277-y. [DOI] [PubMed] [Google Scholar]
- 65.Pintér S, Gloviczki B, Szabó A, Márton G, Nógrádi A. Increased survival and reinnervation of cervical motoneurons by riluzole after avulsion of the C7 ventral root. J Neurotrauma. 2010;27:2273–2282. doi: 10.1089/neu.2010.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pondaag W, Malessy MJ, van Dijk JG, Thomeer RT. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46:138–144. doi: 10.1017/s0012162204000258. [DOI] [PubMed] [Google Scholar]
- 67.Powers SK, Lynch GS, Murphy KT, Reid MB, Zijdewind I. Disease-induced skeletal muscle atrophy and fatigue. Med Sci Sports Exerc. 2016;48:2307–2319. doi: 10.1249/MSS.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pujol A, Mosca R, Farrés J, Aloy P. Unveiling the role of network and systems biology in drug discovery. Trends Pharmacol Sci. 2010;31:115–123. doi: 10.1016/j.tips.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Pyun K, Son JS, Kwon YB. Chronic activation of sigma-1 receptor evokes nociceptive activation of trigeminal nucleus caudalis in rats. Pharmacol Biochem Behav. 2014;124C:278–283. doi: 10.1016/j.pbb.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 70.Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14:1–17. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 71.Roh DH, Choi SR, Yoon SY, Kang SY, Moon JY, Kwon SG, Han HJ, Beitz AJ, Lee JH. Spinal nNOS activation mediates sigma-1 receptor-induced mechanical and thermal hypersensitivity in mice: involvement of PKC-dependent NR1 phosphorylation. Br J Pharmacol. 2011;163:1707–1720. doi: 10.1111/j.1476-5381.2011.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romeo-Guitart D, Forés J, Herrando-Grabulosa M, Valls R, Leiva-Rodríguez T, Galea E, González-Pérez F, Navarro X, Petegnief V, Bosch A, Coma M, Mas JM, Casas C. Neuroprotective drug for nerve trauma revealed using artificial intelligence. Sci Rep. 2018;8:1879. doi: 10.1038/s41598-018-19767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romeo-Guitart D, Forés J, Navarro X, Casas C. Boosted regeneration and reduced denervated muscle atrophy by neuroheal in a pre-clinical model of lumbar root avulsion with delayed reimplantation. Sci Rep. 2017c;7:12028. doi: 10.1038/s41598-017-11086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roth BL, Sheffer DJ, Kroeze WK. Magic shotguns versus magic bullets: Selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 75.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 76.Silver J, Schwab ME, Popovich PG. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol. 2014;7:a020602. doi: 10.1101/cshperspect.a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Snider WD, Elliott JL, Yan Q. Axotomy-induced neuronal death during development. J Neurobiol. 1992;23:1231–1246. doi: 10.1002/neu.480230913. [DOI] [PubMed] [Google Scholar]
- 78.Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 79.Sun HH, Saheb-Al-Zamani M, Yan Y, Hunter DA, MacKinnon SE, Johnson PJ. Geldanamycin accelerated peripheral nerve regeneration in comparison to FK-506 in vivo. Neuroscience. 2012;223:114–123. doi: 10.1016/j.neuroscience.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 80.Sun W, Oppenheim RW. Response of motoneurons to neonatal sciatic nerve axotomy in Bax-knockout mice. Mol Cell Neurosci. 2003;24:875–886. doi: 10.1016/s1044-7431(03)00219-7. [DOI] [PubMed] [Google Scholar]
- 81.Tannemaat MR, Eggers R, Hendriks WT, de Ruiter GCW, van Heerikhuize JJ, Pool CW, Malessy MJA, Boer GJ, Verhaagen J. Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur J Neurosci. 2008;28:1467–1479. doi: 10.1111/j.1460-9568.2008.06452.x. [DOI] [PubMed] [Google Scholar]
- 82.Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87:381–385. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- 83.Taylor DM, Balabadra U, Xiang Z, Woodman B, Meade S, Amore A, Maxwell MM, Reeves S, Bates GP, Luthi-Carter R, Lowden PAS, Kazantsev AG. A brain-permeable small molecule reduces neuronal cholesterol by inhibiting activity of sirtuin 2 deacetylase. ACS Chem Biol. 2011;6:540–546. doi: 10.1021/cb100376q. [DOI] [PubMed] [Google Scholar]
- 84.Taylor JP, Brown RH, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tedeschi A. Tuning the orchestra: transcriptional pathways controlling axon regeneration. Front Mol Neurosci. 2012;4:1–12. doi: 10.3389/fnmol.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tolkacheva T, Chan a M. Inhibition of H-Ras transformation by the PTEN/MMAC1/TEP1 tumor suppressor gene. Oncogene. 2000;19:680–689. doi: 10.1038/sj.onc.1203331. [DOI] [PubMed] [Google Scholar]
- 87.Torres-Espín A, Corona-Quintanilla DL, Forés J, Allodi I, González F, Udina E, Navarro X. Neuroprotection and axonal regeneration after lumbar ventral root avulsion by re-implantation and mesenchymal stem cells transplant combined therapy. Neurotherapeutics. 2013;10:354–368. doi: 10.1007/s13311-013-0178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valero-Cabre A, Fores J, Navarro X. Reorganization of reflex responses mediated by different afferent sensory fibers after spinal cord transection. J Neurophysiol. 2004;91:2838–2848. doi: 10.1152/jn.01177.2003. [DOI] [PubMed] [Google Scholar]
- 89.Vanden Noven S, Wallace N, Muccio D, Turtz A, Pinter MJ. Adult spinal motoneurons remain viable despite prolonged absence of functional synaptic contact with muscle. Exp Neurol. 1993;123:147–156. doi: 10.1006/exnr.1993.1147. [DOI] [PubMed] [Google Scholar]
- 90.Watabe K, Hayashi Y, Kawazoe Y. Peripheral nerve avulsion injuries as experimental models for adult motoneuron degeneration. Neuropathology. 2005;25:371–380. doi: 10.1111/j.1440-1789.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 91.Watanabe S, Ageta-Ishihara N, Nagatsu S, Takao K, Komine O, Endo F, Miyakawa T, Misawa H, Takahashi R, Kinoshita M, Yamanaka K. SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system. Mol Brain. 2014;7:62. doi: 10.1186/s13041-014-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Welin D, Novikova LN, Wiberg M, Kellerth J-O, Novikov LN. Effects of N-acetyl-cysteine on the survival and regeneration of sural sensory neurons in adult rats. Brain Res. 2009;1287:58–66. doi: 10.1016/j.brainres.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 93.Westerhoff H V, Palsson BO. The evolution of molecular biology into systems biology. Nat Biotechnol. 2004;22:1249–1252. doi: 10.1038/nbt1020. [DOI] [PubMed] [Google Scholar]
- 94.Woo JH, Shimoni Y, Yang WS, Subramaniam P, Iyer A, Nicoletti P, Rodríguez Martínez M, López G, Mattioli M, Realubit R, Karan C, Stockwell BR, Bansal M, Califano A. Elucidating compound mechanism of action by network perturbation analysis. Cell. 2015;162:441–451. doi: 10.1016/j.cell.2015.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu A, Ying Z, Gomez-Pinilla F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J Neurotrauma. 2007;24:1587–1595. doi: 10.1089/neu.2007.0313. [DOI] [PubMed] [Google Scholar]
- 96.Wu D, Li Q, Zhu X, Wu G, Cui S. Valproic acid protection against the brachial plexus root avulsion-induced death of motoneurons in rats. Microsurgery. 2013;33:551–559. doi: 10.1002/micr.22130. [DOI] [PubMed] [Google Scholar]
- 97.Xia CH, Roberts EA, Her LS, Liu X, Williams DS, Cleveland DW, Goldstein LSB. Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J Cell Biol. 2003;161:55–66. doi: 10.1083/jcb.200301026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang CG, Welin D, Novikov L, Kellerth JO, Wiberg M, Hart a. M. Motorneuron protection by N-acetyl-cysteine after ventral root avulsion and ventral rhizotomy. Br J Plast Surg. 2005;58:765–773. doi: 10.1016/j.bjps.2005.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


