Abstract
Inherited photoreceptor degeneration in humans constitutes a major cause of irreversible blindness in the world. They comprise various diseases, but retinitis pigmentosa is the most frequently observed. Retinitis pigmentosa is commonly limited to the eye, where there is progressive photoreceptor degeneration, rods and secondarily cones. The mechanisms of cone and rod degeneration continue to be investigated, since most of the mutations causing retinitis pigmentosa affect rods and thus, the secondary death of cones is an intriguing question but, ultimately, the cause of blindness. Understanding the mechanisms of rod and cone degeneration could help us to develop therapies to stop or, at least, slow down the degeneration process. Secondary cone degeneration has been attributed to the trophic dependence between rods and cones, but microglial cell activation could also have a role. In this review, based on previous work carried out in our laboratory in early stages of photoreceptor degeneration in two animal models of retinitis pigmentosa, we show that microglial cell activation is observed prior to the the initiation of photoreceptor death. We also show that there is an increase of the retinal microglial cell densities and invasion of the outer retinal layers by microglial cells. The inhibition of the microglial cells improves photoreceptor survival and morphology, documenting a role for microglial cells in photoreceptor degeneration. Furthermore, these results indicate that the modulation of microglial cell reactivity can be used to prevent or diminish photoreceptor death in inherited photoreceptor degenerations.
Keywords: Royal College of Surgeons, RCS, P23H-1, microglia, minocycline, photoreceptor, retinal degeneration, retinitis pigmentosa
Introduction
Inherited photoreceptor degenerations represent a major cause of irreversible blindness in the world. There are several genotypic and phenotypic forms of these diseases (OMIM; http://omim.org/), but the most frequent in humans is retinitis pigmentosa (RP). Photoreceptor degeneration is caused by diverse mutations in genes expressed mainly in photoreceptors or the retinal pigment epithelial cells (Hartong et al., 2006; Silverman and Wong, 2018), with more than 300 genes and loci identified (RetNet; https://sph.uth.edu/retnet/), although it is believed that there are probably more yet unidentified genes. Typical RP is caused by 71 mutations (RetNet; https://sph.uth.edu/retnet/) that affect diverse cellular functions, such as phototransduction, transcription, metabolism or ciliary structure and function among others, and this makes RP a highly variable disorder (Hartong et al., 2006; Sullivan et al., 2006; Dias et al., 2018). Thus, the age of onset, that can range from early childhood to adulthood, and the rate of degeneration, that differs between the forms of the disease, depend on the mutation and are very variable too (Hartong et al., 2006; Dias et al., 2018). The worldwide prevalence of RP is about 1 in 4000 individuals, and this disease is usually limited to the eye and characterized by the degeneration, first of rods and later of cones (Hartong et al., 2006; Lin et al., 2009). Cones and rods, known as classical photoreceptors, are the first order neurons of the visual system, hyperpolarize in response to light and pass the electrical signal to other neurons in the retina, being thus essential for vision. While the term RP includes many genotypic and phenotypic variations, most RP patients experience, at the beginning, night blindness due to the primary loss of rods. However, the most severe consequences of RP are caused later by cone loss (Hartong et al., 2006), manifested by tunnel vision and, ultimately, blindness. It is thus important to understand the events that occur at the onset of the disease that influence rod and cone degeneration in order to develop targeted therapies to slow or halt the degeneration process.
Some of the rodent models of RP share similar features to those of the human disease. Particularly noteworthy are the P23H and the Royal College of Surgeons (RCS) rats, the genetic mutations suffered by these animals are also observed in some forms of the human disease, and the progression of the degeneration is similar too. Thus, these models mimic faithfully the human disease and have been widely used to investigate it (García-Ayuso et al., 2010, 2013, 2014, 2015; Di Pierdomenico et al., 2017, 2018; Dias et al., 2018; LaVail et al., 2018). There are some differences between these two models: while in the P23H rats the rhodopsin gene bears the human mutation most commonly observed in RP (Hartong et al., 2006), the RCS strain suffers a mutation of the MERKT gene that inhibits retinal pigment epithelium phagocytosis (Hartong et al., 2006). Also, the onset and chronology of the degeneration is different, because in P23H rats the degeneration starts early (post-natal day 21; P21) and causes first rod loss and secondarily cone loss, and in RCS rats the degeneration is belated (starts around P45) and there is loss of both rods and cones from the beginning. The mechanisms of cone and rod degeneration in these models are thus different, but the question that remains unanswered is how both these mutations cause rod and cone loss, since one of them (P23H rat) affects only rods. There are several theories (Narayan et al., 2016; Sahel and Léveillard, 2018), and some attribute it to the dependency between rods and cones: cones may be dependent on trophic factors produced and secreted by normal rods (Léveillard et al., 2004; Kolomeyer and Zarbin, 2014). Another one suggests that cone degeneration is a result to nutrient deficiency, causing cone starvation (Punzo et al., 2009). A role for accumulated oxidative stress in cone degeneration has also been suggested (Shen et al., 2005). A theory supported by experimental data, is that cone loss is caused by toxic substances released by dying rods (Ripps, 2002). The abovementioned dependence between rods and cones may, perhaps, explain the appearance of ring-shaped areas lacking both rods and cones during retinal degeneration in the P23H rat (García-Ayuso et al., 2014). Finally, microglial activation has been suggested to cause photoreceptor degeneration (see below).
Microglial cells are the major resident immune cells in the retina, but their role and function in RP progression is not yet fully understood. The retina, as an extension of the central nervous system is an easily accessible tissue to study the dynamics of the activation and migration of the microglial cells in different diseases (Silverman and Wong, 2018). In various animal models of retinal degeneration (i.e., RP and age-related macular degeneration), it has been shown that retinal microglial cells change their morphology, become activated and travel within the retina (Langmann, 2007; Sobrado-Calvo et al., 2007; Galindo-Romero et al., 2013; Di Pierdomenico et al., 2016, 2017; Dannhausen et al., 2018; Silverman and Wong, 2018). Activated microglial cells have been shown to perform multiple functions that can be either neuroprotective or neurodestructive. Indeed, microglia and neuroinflammation have been implicated in the pathology of many neurodegenerative diseases (Glass et al., 2010; Silverman and Wong, 2018). However, it is not clear whether activated microglial cells may increase or decrease photoreceptor loss in inherited retinal degeneration. If this was the case, their inhibition could slow down photoreceptor death. Here we will review the pathogenic role of microglial cells in inherited photoreceptor degenerations, based on previous work conducted in our laboratory. We have performed a literature search of articles published on microglia and minocycline in retinal degeneration.
Microglial Cell Behavior in P23H-1 and Royal College of Surgeons Rats
To investigate the role of microglial cells in inherited photoreceptor degenerations we used two animal models with different genetic and pathogenic mechanisms of retinal degeneration: the P23H and the RCS rats (see above; Di Pierdomenico et al., 2017, 2018). Using antibodies against microglial and cone cell antigens and nuclear stainings, we studied the microglial cell reaction and the course of photoreceptor degeneration in retinal cross-sections.
In both albino and pigmented control animals (Sprague-Dawley and PVG rats for P23H and RCS rats, respectively), we found that the mean numbers of microglial cells per animal (three sections) were similar (Figures 1A, 1I, 2A and 2I). The microglial cells were found in four distinct retinal layers, but no microglial cells were found in the outer nuclear layer or in the photoreceptor outer segments layer (Figures 1A and 2A).
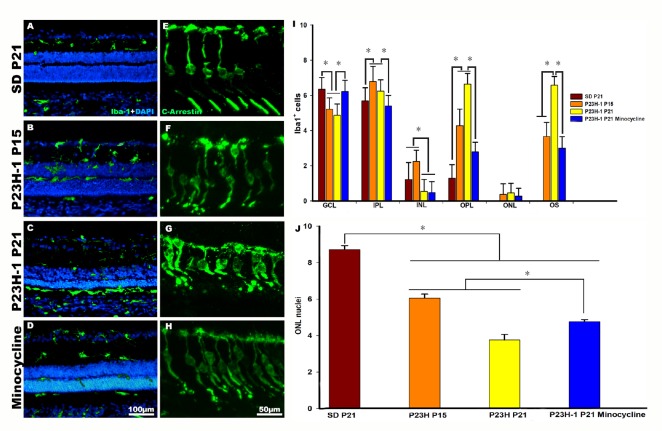
Figure 1.
Microglial cell activation and photoreceptor degeneration in P23H-1 rats.
(A–H) Photomicrographs of representative retinal cross sections taken from control SD rats (A, E), post-natal day (P)15 and P21 P23H-1 rats (B, C, F, G) and P21 P23H-1 rats treated with minocycline (D, H). (A, E) In control rats, microglial cells are mainly found quiescent in the inner retinal layers, and cone outer segments show their typical elongated shape. (B, C, F, G) In P23H rats, microglial cells show signs of activation and migrated from the inner to the outer retinal layers (B, C), and cone outer segments showed morphological signs of degeneration (F, G). (D, H) Treatment with minocycline reduced microglial activation and migration (D), and improved the cone outer segments morphology (H). (I) Graph showing the mean ± SD of Iba-1 labelled microglial cells counted in different retinal layers in control, P23H-1 rats and in P23H-1 rats treated with minocycline. The mean numbers of microglial cells in the outer retinal layers were significantly lower in the P23H-1 rats treated with minocycline than in non-treated P23H-1 rats. (J) Graphs showing mean number of nuclei rows in the outer nuclear layer (ONL) of control, P23H-1 rats and P23H-1 rats treated with minocycline. The numbers of nuclei rows decrease significantly in dystrophic animals. Minocycline treatment increased significantly the number of nuclei rows in the ONL. *P < 0.001. Data in Figure 1 are from Di Pierdomenico et al. (2017).
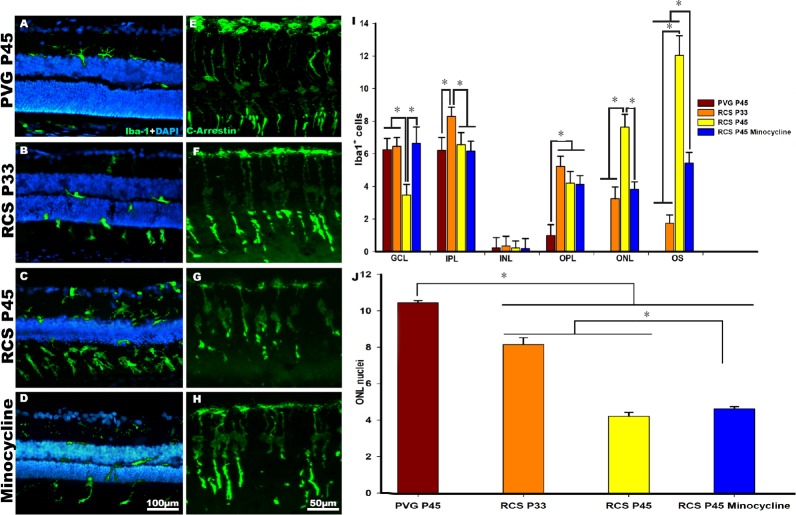
Figure 2.
Microglial cell activation and photoreceptor degeneration in Royal College of Surgeons (RCS) rats.
(A–H) Photomicrographs of representative retinal cross sections taken from control PVG rats (A, E), post-natal day (P)33 and P45 RCS rats (B, C, F, G) and P45 RCS rats treated with minocycline (D, H). (A, E) In control rats, microglial cells are mainly found quiescent in the inner retinal layers, and cone outer segments showed their typical elongated shape. (B, C, F, G) In RCS rats, microglial cells show signs of activation and migrated from the inner to the outer retinal layers (B, C), and cone outer segments showed morphological signs of degeneration (F, G). (D, H) Treatment with minocycline reduced microglial activation and migration (D) and improved the cone outer segments morphology (H). (I) Graph showing the mean ± SD of Iba-1 labelled microglial cells counted in different retinal layers in control, RCS rats and in RCS rats treated with minocycline. The mean number of microglial cells in the outer retinal layers were significantly lower in the RCS rats treated with minocycline compared to non-treated RCS rats. (J) Graphs showing the mean numbers of nuclei rows in the outer nuclear layer (ONL) of control (PVG), RCS and RCS rats treated with minocycline. The number of nuclei rows decrease significantly in dystrophic animals. Minocycline treatment increased significantly the number of nuclei rows in the ONL. *P < 0.001. Data in Figure 2 are from Di Pierdomenico et al. (2018).
In the P23H-1 (Figure 1) and RCS (Figure 2) rats, we observed that starting at P15 in the P23H-1 rat and at P21 in the RCS rat, the microglial cells changed their morphology and became activated (Figures 1B and 2B; Di Pierdomenico et al., 2017). At these ages, there was an increase of microglial cells in the retina and the microglial cells invaded the outer retinal layers in both strains (Figures 1B, 1I, 2B and 2I; Di Pierdomenico et al., 2017). Microglial cell migration to the outer retinal layers continued because at P21 and P45 in the P23H-1 and RCS strain, respectively, the mean numbers of microglial cells in the outer nuclear layer and outer segments layer was significantly increased compared to the observed at the earlier ages (Figures 1B, 1I, 2B and 2I; Di Pierdomenico et al., 2017). In dystrophic animals, we also observed that cones showed shortened outer segments, between P15 and P21 in the P23H-1 rat (Figure 2F and G; Di Pierdomenico et al., 2017) and between P33 and P45 in the RCS strain (Figure 2F and G; Di Pierdomenico et al., 2017). However, at these time points, we also found a significant decrease in the mean number of nuclei rows in the outer nuclear layer in both strains, from 8–10 nuclei to 4–5 (43.5% decrease) in the P23H-1 (P21) and 3–4 (40.6% decrease) in RCS (P45) rats (Figures 1J and 2J; Di Pierdomenico et al., 2017) indicating rod loss during that period (the outer nuclear layer is rod-dominated in the rat retina). Furthermore, we found that the increase of the mean number of microglial cells found in degenerate retinas, and particularly in the RCS rat, could not be explained only by intraretinal migration because the numbers of microglial cells in all the retinal layers outnumbered the numbers found in control animals and thus, could be the result of monocyte infiltration or cell proliferation. So, we studied cellular proliferation using immunodetection of the proliferating cell nuclear antigen and we showed that at P21 in P23H-1 and at P45 in RCS rats there was microglial cell proliferation, so the observed increase is also due to, at least in part, cellular division of microglial cells (Di Pierdomenico et al., 2017).
We have concluded from these studies that microglial cell activation and migration occurs simultaneously with the beginning of photoreceptor degeneration, as shown also in other studies (Noailles et al., 2014; Zhao et al., 2015; Dannhausen et al., 2018), but before the loss of the vast majority of photoreceptors and that there is also microglial cell proliferation in both models (Di Pierdomenico et al., 2017). In dystrophic animals, and in contrast with healthy animals, microglial cells reached the outer nuclear and outer segment layers. The signals initiating early microglial responses and attracting microglial cells to the outer retinal layers, is still unclear (Silverman and Wong, 2018). But because in our studies microglial cell migration occurs at the same time as the photoreceptor death is initiated, our results suggest that photoreceptor death triggers microglial cell activation and migration to the outer retinal layers to phagocytose dying photoreceptors and eliminate cellular debris (Sobrado-Calvo et al., 2007; Galindo-Romero et al., 2013; Zhao et al., 2015; Nadal-Nicolás et al., 2017, 2019). In performing these functions, retinal microglial cells may perhaps influence photoreceptor survival or death, by eventually phagocytizing stressed but still viable photoreceptors (Zhao et al., 2015; Silverman and Wong, 2018; Nadal-Nicolás et al., 2019).
Microglial Cell Inhibition with Minocycline Improves Photoreceptor Survival
We wondered whether the inhibition of microglial cells could improve photoreceptor survival in both models of retinal degeneration. For this purpose, we treated the animals with minocycline, a broad-spectrum tetracycline derivative antibiotic, with known antiapoptotic, antimicrobial, anti-inflammatory and neuroprotective properties (Scholz et al., 2015; Silverman and Wong, 2018), that has been used clinically for the treatment of various diseases (Silverman and Wong, 2018). Minocycline was administered intraperitoneally, twice the first day and daily for the remaining days (Di Pierdomenico et al., 2018), starting before the onset of photoreceptor degeneration (at P9 in the P23H-1 rat and at P32 in the RCS rat) (Di Pierdomenico et al., 2018). In these experiments, we observed that minocycline treatment caused a significative reduction in the number of migrated microglial cells in the outer retinal layers in both strains (Figures 1D, 1I, 2D and 2I; Di Pierdomenico et al., 2018) and thus, an increase in the numbers of microglial cells in the inner retinal layers (Figures 1D, 1I, 2D and 2I; Di Pierdomenico et al., 2018). Also, in mynocicline treated animals, the cone outer segments appeared healthier, straighter, longer and more strongly labelled with arrestin in both strains (Figures 1H and 2H; Di Pierdomenico et al., 2018). In addition, minocycline treatment increased significantly the numbers of nuclei rows in the outer nuclear layer by approximately + 22% in P23H-1 rats and + 17% in RCS rats (Figures 1G and 2G; Di Pierdomenico et al., 2018).
Our results suggest that minocycline treatment inhibited microglial cell activation and migration during photoreceptor degeneration (Di Pierdomenico et al., 2018) resulting in improved cone outer segment morphology and increased photoreceptor survival (Di Pierdomenico et al., 2018).
Because photoreceptor death in inherited retinal degenerations is due to apoptosis (Di Pierdomenico et al., 2017), and may be increased by neuroinflammation (Zhao et al., 2015; Di Pierdomenico et al., 2018), there could be two main potential mechanisms through which minocycline neuroprotects the dying photoreceptors: through its anti-apoptotic properties (Scholz et al., 2015; Di Pierdomenico et al., 2018), and or through its anti-inflammatory (anti-phagocytic) effect (Scholz et al., 2015; Di Pierdomenico et al., 2018). In our opinion, because in both models of RP used, microglial cell activation and migration occurs at the initiation of the degeneration and minocycline inhibits them both, the neuroprotection observed is most probably due to an anti-inflammatory effect. However, further experiments will be needed to clarify this fact.
As a final conclusion, we would like to remark that microglial cell involvement in the two animal models was similar but took place with distinct timelines. However, in both models, microglial cell activation and migration occurred simultaneously with the initiation of photoreceptor cell death, although this occurred at different age in the two models. Finally, we showed that there was microglial cell proliferation in both models and that administration of minocycline decreased microglial activation and migration and enhanced photoreceptor survival in both models. Thus, we propose that anti-inflammatory drugs that modulate microglial reactivity may be used in the early stages of retinal degenerations to prevent or slow photoreceptor death.
Additional file: Open peer review reports 1 (64.1KB, pdf) and 2 (59KB, pdf) .
Acknowledgments
The authors thank Ana Martínez-Vacas and Jose Manuel Bernal-Garro (both from Departamento de Oftalmología, Facultad de Medicina, Universidad de Murcia, and Instituto Murciano de Investigación Biosanitaria Hospital Virgen de la Arrixaca (IMIB-Virgen de la Arrixaca), Murcia, Spain) for their excellent help.
Footnotes
Conflicts of interest: None declared.
Financial support: This work was supported by grants from Fundación Séneca, Agencia de Ciencia y Tecnología Región de Murcia, No. 19881/GERM/15 (to MVS), Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III, Fondo Europeo de Desarrollo Regional ‘‘Una 30 Manera de Hacer Europa’’, No. SAF2015-67643-P (to MVS), PI16/00380 (to MPVP), RD16/0008/0026 (to MPVP), PI16/00031 (to MAB).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Kin-Sang Cho, Schepens Eye Research Institute, USA; Annagrazia Adornetto, University of Calabria, Italy.
Funding: This work was supported by grants from Fundación Séneca, Agencia de Ciencia y Tecnología Región de Murcia, No. 19881/GERM/15 (to MVS), Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III, Fondo Europeo de Desarrollo Regional ‘‘Una 30 Manera de Hacer Europa’’, No. SAF2015-67643-P (to MVS), PI16/00380 (to MPVP), RD16/0008/0026 (to MPVP), PI16/00031 (to MAB).
P-Reviewers: Cho KS, Adornetto A; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Dannhausen K, Rashid K, Langmann T. Microglia analysis in retinal degeneration mouse models. Methods Mol Biol. 2018;1753:159–166. doi: 10.1007/978-1-4939-7720-8_10. [DOI] [PubMed] [Google Scholar]
- 2.Di Pierdomenico J, García-Ayuso D, Jiménez-López M, Agudo-Barriuso M, Vidal-Sanz M, Villegas-Pérez MP. Different ipsi- and contralateral glial responses to anti-vegf and triamcinolone intravitreal injections in rats. Invest Ophthalmol Visual Sci. 2016;57:3533–3544. doi: 10.1167/iovs.16-19618. [DOI] [PubMed] [Google Scholar]
- 3.Di Pierdomenico J, García-Ayuso D, Pinilla I, Cuenca N, Vidal-Sanz M, Agudo-Barriuso M, Villegas-Pérez MP. Early events in retinal degeneration caused by rhodopsin mutation or pigment epithelium malfunction: differences and similarities. Front Neuroanat. 2017;11:14. doi: 10.3389/fnana.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Pierdomenico J, Scholz R, Valiente-Soriano FJ, Sánchez-Migallón MC, Vidal-Sanz M, Langmann T, Agudo-Barriuso M, García-Ayuso D, Villegas-Pérez MP. Neuroprotective effects of FGF2 and minocycline in two animal models of inherited retinal degeneration. Invest Ophthalmol Visual Sci. 2018;59:4392–4403. doi: 10.1167/iovs.18-24621. [DOI] [PubMed] [Google Scholar]
- 5.Dias MF, Joo K, Kemp JA, Fialho SL, da Silva Cunha A, Jr, Woo SJ, Kwon YJ. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog Retin Eye Res. 2018;63:107–131. doi: 10.1016/j.preteyeres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Galindo-Romero C, Valiente-Soriano FJ, Jiménez-López M, García-Ayuso D, Villegas-Pérez MP, Vidal-Sanz M, Agudo-Barriuso M. Effect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microglia. Invest Ophthalmol Visual Sci. 2013;54:974–985. doi: 10.1167/iovs.12-11207. [DOI] [PubMed] [Google Scholar]
- 7.García-Ayuso D, Salinas-Navarro M, Agudo M, Cuenca N, Pinilla I, Vidal-Sanz M, Villegas-Pérez MP. Retinal ganglion cell numbers and delayed retinal ganglion cell death in the P23H rat retina. Exp Eye Res. 2010;91:800–810. doi: 10.1016/j.exer.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 8.García-Ayuso D, Salinas-Navarro M, Nadal-Nicolás FM, Ortín-Martínez A, Agudo-Barriuso M, Vidal-Sanz M, Villegas-Pérez MP. Sectorial loss of retinal ganglion cells in inherited photoreceptor degeneration is due to RGC death. Br J Ophthalmol. 2014;98:396–401. doi: 10.1136/bjophthalmol-2013-303958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Ayuso D, Ortín-Martínez A, Jiménez-López M, Galindo-Romero C, Cuenca N, Pinilla I, Vidal-Sanz M, Agudo-Barriuso M, Villegas-Pérez MP. Changes in the photoreceptor mosaic of P23H-1 rats during retinal degeneration: implications for rod-cone dependent survival. Invest Ophthalmol Visual Sci. 2013;54:5888–5900. doi: 10.1167/iovs.13-12643. [DOI] [PubMed] [Google Scholar]
- 10.García-Ayuso D, Di Pierdomenico J, Esquiva G, Nadal-Nicolás FM, Pinilla I, Cuenca N, Vidal-Sanz M, Agudo-Barriuso M, Villegas-Pérez MP. Inherited photoreceptor degeneration causes the death of melanopsin-positive retinal ganglion cells and increases their coexpression of Brn3a. Invest Ophthalmol Visual Sci. 2015;56:4592–4604. doi: 10.1167/iovs.15-16808. [DOI] [PubMed] [Google Scholar]
- 11.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 13.Kolomeyer AM, Zarbin MA. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv Ophthalmol. 2014;59:134–165. doi: 10.1016/j.survophthal.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Léveillard T, Mohand-Saïd S, Lorentz O, Hicks D, Fintz AC, Clérin E, Simonutti M, Forster V, Cavusoglu N, Chalmel F, Dollé P, Poch O, Lambrou G, Sahel JA. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36:755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- 15.Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- 16.LaVail MM, Nishikawa S, Steinberg RH, Naash MI, Duncan JL, Trautmann N, Matthes MT, Yasumura D, Lau-Villacorta C, Chen J, Peterson WM, Yang H, Flannery JG. Phenotypic characterization of P23H and S334ter rhodopsin transgenic rat models of inherited retinal degeneration. Exp Eye Res. 2018;167:56–90. doi: 10.1016/j.exer.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin B, Masland RH, Strettoi E. Remodeling of cone photoreceptor cells after rod degeneration in rd mice. Exp Eye Res. 2009;88:589–599. doi: 10.1016/j.exer.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadal-Nicolás FM, Vidal-Sanz M, Agudo-Barriuso M. The senescent vision: dysfunction or neuronal loss? Aging (Albany NY) 2019;11:15–17. doi: 10.18632/aging.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadal-Nicolás FM, Jiménez-López M, Salinas-Navarro M, Sobrado-Calvo P, Vidal-Sanz M, Agudo-Barriuso M. Microglial dynamics after axotomy-induced retinal ganglion cell death. J Neuroinflammation. 2017;14:218. doi: 10.1186/s12974-017-0982-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayan DS, Wood JP, Chidlow G, Casson RJ. A review of the mechanisms of cone degeneration in retinitis pigmentosa. Acta Ophthalmol. 2016;94:748–754. doi: 10.1111/aos.13141. [DOI] [PubMed] [Google Scholar]
- 21.Noailles A, Fernández-Sánchez L, Lax P, Cuenca N. Microglia activation in a model of retinal degeneration and TUDCA neuroprotective effects. J Neuroinflammation. 2014;11:186. doi: 10.1186/s12974-014-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ripps H. Cell death in retinitis pigmentosa: gap junctions and the ‘bystander’ effect. Exp Eye Res. 2002;74:327–336. doi: 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- 24.Sahel JA, Léveillard T. Maintaining cone function in rod-cone dystrophies. Adv Exp Med Biol. 2018;1074:499–509. doi: 10.1007/978-3-319-75402-4_62. [DOI] [PubMed] [Google Scholar]
- 25.Scholz R, Sobotka M, Caramoy A, Stempfl T, Moehle C, Langmann T. Minocycline counter-regulates pro-inflammatory microglia responses in the retina and protects from degeneration. J Neuroinflammation. 2015;12:209. doi: 10.1186/s12974-015-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen J, Yang X, Dong A, Petters RM, Peng YW, Wong F, Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 27.Silverman SM, Wong WT. Microglia in the retina: roles in development, maturity, and disease. Annu Rev Vis Sci. 2018;4:45–77. doi: 10.1146/annurev-vision-091517-034425. [DOI] [PubMed] [Google Scholar]
- 28.Sobrado-Calvo P, Vidal-Sanz M, Villegas-Pérez MP. Rat retinal microglial cells under normal conditions, after optic nerve section, and after optic nerve section and intravitreal injection of trophic factors or macrophage inhibitory factor. J Comp Neurol. 2007;501:866–878. doi: 10.1002/cne.21279. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan LS, Bowne SJ, Birch DG, Hughbanks-Wheaton D, Heckenlively JR, Lewis RA, Garcia CA, Ruiz RS, Blanton SH, Northrup H, Gire AI, Seaman R, Duzkale H, Spellicy CJ, Zhu J, Shankar SP, Daiger SP. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: a screen of known genes in 200 families. Invest Ophthalmol Vis Sci. 2006;47:3052–3064. doi: 10.1167/iovs.05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao L, Zabel MK, Wang X, Ma W, Shah P, Fariss RN, Qian H, Parkhurst CN, Gan WB, Wong WT. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med. 2015;7:1179–1197. doi: 10.15252/emmm.201505298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.