Neurodegenerative diseases are a heterogeneous group of disorders characterized by a progressive dysfunction and death of neural cells which lead to compromised motor or cognitive function. Morphologically, the loss of neuron is associated with both gliosis and, frequently, with abnormal accumulation of extracellular and intracellular filamentous deposit in specific cell types.
With a rapidly increasing aging population, these diseases are becoming a primary health problem (Albers and Beal, 2000). Neurodegenerative diseases have been considered to be interplay of a number of factors, however mounting evidence suggests that a rise in oxidative stress may play a critical role in the pathogenesis of many of these disorders like Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease (Li et al., 2013). It has been observed that the abnormal protein aggregation that characterizes many neurodegenerative diseases can in turn activate inflammation in the brain, which triggers a strong release of reactive oxygen species and reactive nitrogen species, subsequent oxidative and nitrosative stress and, eventually, cell death. It has been observed that the principal mode of neuronal cell death in neurodegenerative diseases is via apoptosis (Liu et al., 2017). Reactive oxygen species are a heterogeneous chemical class that includes radicals, such as superoxide ion (O2 •–), hydroxyl radical (OH•), and non-radicals, such as hydrogen peroxide (H2O2), hypochlorous acid (HOl), singlet oxygen (1O2). Peroxynitrite (NO3 –) is the principal reactive nitrogen species that can coexist and interact with reactive oxygen species. Endogenously reactive oxygen species can be produced by different enzymes including nicotinamide adenine dinucleotide phosphate oxidase, xanthine oxidase, cytochrome P450 from endoplasmic reticulum, and flavin oxidases from peroxisomes. Reactive oxygen species are mainly produced in the mitochondria, where 1% to 5% O2 escapes the respiratory chain to form the superoxide anion, and by nicotinamide adenine dinucleotide phosphate oxidase systems. Reactive nitrogen species are produced by superoxide anion and nitric oxide. Superoxide dismutase competes with nitric oxide for O2 •– and if insufficient superoxide dismutase is available or there is an overproduction of nitric oxide, ONOO− is formed. To keep a physiological level of reactive oxygen species/reactive nitrogen species, cells possess an effective defense system of antioxidant enzymes like superoxide dismutase, catalase and glutathione peroxidase and small-molecule antioxidants such as glutathione (Gandhi and Abramov, 2012). When reactive oxygen species exceed the ability of the endogenous antioxidant defense system, a deleterious condition known as oxidative stress is established. Brain is particularly vulnerable to reactive oxygen species because of its high polyunsaturated fatty acids content, high oxygen consumption due to its large dependence on oxidative phosphorylation, elevated concentration of metal ions which accumulate in the brain as a function of age, and low levels of antioxidants and related enzymes (Liu et al., 2017). For these reasons, many efforts have been made to find agents able to counteract oxidative stress and potentially cure neurodegenerative diseases. Currently, there are no therapeutic approaches to treat or even halt the inexorable progression of these debilitating disorders. In this context, great attention has been paid to phytochemicals because of their broad spectrum of pharmacological and biological activities. These compounds have been recognized to possess health-promoting properties such as anti-inflammatory, anti-allergic, anti-atherogenic, anti-thrombotic, and anti-mutagenic and anti-oxidative effects (Ellis et al., 2011; Angeloni et al., 2014). Among phytochemicals, olive oil phenols are getting more scientific attention due to their interesting biological activities (Angeloni et al., 2017). The interest in olive oil phytochemicals arose from the observation that adherence to a typical Mediterranean diet with olive oil as main fat source is associated with improved health, lower mortality, increased longevity, and reduced incidence of chronic degenerative diseases like cardiovascular disease, cancer and neurodegenerative disorders (Angeloni et al., 2017). These benefits have been attributed not only to olive oil high monounsaturated fatty acid content but also to the biological activity of minor compounds like phenols. In particular, inside the secoiridoid family of olive oil constituents, oleocanthal, also known as (-)decarboxymethyl ligstroside aglycone (Figure 1), has been mainly investigated for its anti-inflammatory activity and its ability to counteract abnormal protein aggregation and deposition involved in Alzheimer’s disease. Few data are available on oleocanthal pharmacokinetic and bioavailability. Only one study addressed these aspect and identified oleocanthal and its metabolites in urines, suggesting that this phenolic compound is uptaken and metabolized by the human body (Angeloni et al., 2017). The anti-inflammatory activity of oleocanthal was initially investigated in relation to ibuprofen because these two compounds share similar chemical structure and cause a similar irritation in the oropharyngeal region (Beauchamp et al., 2005). Interestingly, oleocanthal dose dependently inhibited the inflammatory enzymes cyclooxygenase-1 and cyclooxygenase-2 and was more potent than ibuprofen in inhibiting these inflammatory enzymes at equimolar concentrations. Subsequent investigations demonstrated that oleocanthal fulfills its anti-inflammatory action also by other mechanisms such as the inhibition of 5-lipoxygenase activity, the reduction of lipopolysaccharide-mediated upregulation of inducible nitric oxide synthase, interleukin-1β, interleukin-6, macrophage inflammatory protein-1α, tumor necrosis factor-α, and granulocyte-macrophage colony stimulating factor (Pang and Chin, 2018).
Figure 1.
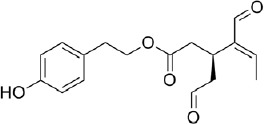
Oleocanthal chemical structure.
Oleocanthal has been suggested to be effective in counteracting two distinctive hallmarks of Alzheimer’s disease: neurofibrillary tangle and amyloid β deposition [reviewed in Pang and Chin (2018)]. In particular, it has been observed that oleocanthal inhibits the formation of hyperphosphorylated tau aggregates by interfering with the aggregation sites on tau protein, thanks to its ability to cross-link and abrogate fibrillization of tau T40 and K18 construct protein via covalent modification. Moreover, oleocanthal induces amyloid β efflux and clearance by increasing P-glycoprotein expression and activity and up-regulating low-density lipoprotein receptor-related protein. Oleocanthal also stimulates amyloid β degradation inducing monomer degradation via up-regulation of the amyloid β degradation enzyme, adenosine triphosphate-binding cassette transporter-A1. Another effect attributed to oleocanthal is its ability to modify the structure of amyloid β increasing its immunoreactivity. Only in the last years, oleocanthal has been demonstrated to act as an antioxidant agent. The first data on the antioxidant activity of oleocanthal were obtained by Rosignoli et al. (2013). In isolated human monocytes, they observed that oleocanthal inhibits nicotinamide adenine dinucleotide phosphate oxidase activity and reduces intracellular level of superoxide anion (Rosignoli et al., 2013). Interestingly, the main focus of this study was to investigate the anti-inflammatory activity of olive oil phenols and not their antioxidant activity. Very recently, we investigated the role of oleocanthal in counteracting oxidative stress in differentiated neuron-like SH-SY5Y cells by an omic approach (Giusti et al., 2018). In particular, we pre-treated cells with 1–10 µM oleocanthal for 24 hours before inducing oxidative stress by hydrogen peroxide exposure. Our data showed that 10 µM oleocanthal is able to protect cells against H2O2 as demonstrated by an increase of cell viability measured by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide assay, a decrease of intracellular reactive oxygen species level measured by dichloro-dihydro-fluorescein diacetate assay and an increase of reduced glutathione levels measured by monochlorobimane assay. The proteomic analysis indicated that 5 proteins were up-regulated and 7 proteins were down-regulated in cells treated with oleocanthal, and 46 spots were significantly modified by the addition of oleocanthal + H2O2, 14 were up-regulated and 32 down-regulated. In particular, the analysis of this data suggested that oleocanthal protection against peroxide could be due to different co-existing mechanisms among which the up-regulation of heat-shock protein 90 and the phosphorylation of the protein kinase B seem to play a key role. Among the other mechanisms suggested by proteomic analysis an important role in counteracting neurodegeneration could be played by the up-regulation of both pyruvate kinase 1 and 2. Pyruvate kinase is a glycolytic enzyme that catalyzes the conversion of phosphoenolpyruvate and adenosine diphosphate to pyruvate and adenosine triphosphate and plays a role in regulating cell energy metabolism. In mammalian tissues, there are 4 isoforms of pyruvate kinases. Most adult tissues express pyruvate kinase M2, and expression of the other three isoforms is restricted to distinct tissues and cell type. M1 isoenzyme is found in skeletal muscle, heart and brain, tissues with high catabolic demand. Pyruvate kinase L is the major isoform in the liver and pyruvate kinase R is found exclusively in red blood cells. A very recent paper demonstrated that in preclinical and very mildly symptomatic Alzheimer’s disease individuals, lower aerobic glycolysis is associated with higher tau deposition in vulnerable regions of the brain (Vlassenko et al., 2018). In this context, we believe that the ability of oleocanthal to upregulate both pyruvate kinase M1 and 2 should be further investigated as it could be important both in supporting energy cell metabolism and preventing tau deposition.
We also carried out the ingenuity pathways analysis on proteomic data and the results suggested the oleocanthal with high probability is involved in the activation of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) transcription factor, a member of the cap ‘n’ collar subfamily of basic region leucine zipper transcription factors. Under physiological conditions, Nrf2 is covalently bound to cysteine residues on its native repressor kelch-like ECH (enoyl-coenzyme A hydratase)-associated protein 1 in the cytoplasm; in the presence of oxidative stress, Nrf2 dissociates from kelch-like ECH-associated protein 1 and translocates into the nucleus, where it can bind to antioxidant responsive element initiating the transcription of various cytoprotective antioxidant enzymes such as nicotinamide adenine dinucleotide phosphate quinone oxidoreductase 1, superoxide dismutase, glutathione s-transferase, and heme oxygenase 1. Taking into account that we observed a reduction of oxidative stress and an increase of reduced glutathione by oleocanthal treatment, the up-regulation of some antioxidant enzymes is very likely.
Moreover, besides regulating phase 2 enzymes, Nrf2 acts also as a modulator of intermediary metabolism, so the neuroprotective effect of oleocanthal could be ascribed also to the pleiotropic action of Nrf2 in inducing detoxifying enzymes and supporting activation of enzymes that catalyze rate-limiting steps in glycolytic pathway allowing elevated energy utilization. Noteworthy, our studies indicate that the beneficial antioxidant effects of oleocanthal in neuronal like cells are achieved at micromolar concentrations corresponding approximately to 25 mL of extra virgin olive oil given that the median content of oleocanthal is 85 mg/kg of oil. In fact, the oleocanthal content in extra virgin olive oil is strictly dependent on olive’ cultivar, oil extraction method and ripening of the olive. Interestingly, this value of extra virgin olive oil intake is likely achieved in a Mediterranean diet. We think that further studies should be carried out to study the potential modulatory activity of oleocanthal on antioxidant and phase II enzymes and intermediary metabolism both in vitro and in vivo model of different neurodegenerative diseases.
Moreover, preliminary data from our lab suggest an involvement of oleocanthal in counteracting oxidative stress caused by methylglyoxal. This α-ketoaldehyde, formed endogenously by different pathways, is responsible of non-enzymatic protein glycation, an endogenous process in which reducing sugars react with amino groups in proteins leading to the production of advanced glycation end products. Methylglyoxal plays an important role in neurodegeneration as it induces cross-linking of proteins. In Alzheimer’s disease, it has been shown that advanced glycation end products accumulate in neurons and astroglia and are also found associated with neuritic amyloid plaques and neurofibrillary tangles, of note, extensive cross-link of Aβ deposits induced by advanced glycation end products makes them more insoluble, protease resistant, and more toxic (Angeloni et al., 2014).
In conclusion, oleocanthal demonstrated to possess a strong pleiotropic activity against neurodegeneration as, besides its known biological effects like its anti-inflammatory activity, and its ability to reduce Aβ and neurofibrillary tangles production, new mechanisms of action are emerging like its ability to counteract oxidative stress and to enhance neuron energy metabolism. Moreover, promising results suggest a potential role of this compounds in inhibiting the deleterious effect of protein glycation. Taking into account the multifactorial etiology of neurodegeneration, oleocanthal could play a role of primary importance in the arduous fight against these debilitating diseases. Moreover, unlike the currently approved therapies for neurodegenerative disorders that have the purpose to alleviate final clinical manifestations, oleocanthal is a natural compound with not side effects that can act long before the clinical manifestation of neurodegeneration.
This work was supported by MIUR-PRIN 2015 (N. 20152HKF3Z) (to SH).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl. 2000;59:133–154. doi: 10.1007/978-3-7091-6781-6_16. [DOI] [PubMed] [Google Scholar]
- 2.Angeloni C, Zambonin L, Hrelia S. Role of methylglyoxal in Alzheimer’s disease. Biomed Res Int. 2014;2014:238485. doi: 10.1155/2014/238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angeloni C, Malaguti M, Barbalace MC, Hrelia S. Bioactivity of olive oil phenols in neuroprotection. Int J Mol Sci. 2017;18:E2230. doi: 10.3390/ijms18112230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchamp GK, Keast RS, Morel D, Lin J, Pika J, Han Q, Lee CH, Smith AB, Breslin PA. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature. 2005;437:45–46. doi: 10.1038/437045a. [DOI] [PubMed] [Google Scholar]
- 5.Ellis LZ, Liu W, Luo Y, Okamoto M, Qu D, Dunn JH, Fujita M. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1beta secretion. Biochem Biophys Res Commun. 2011;414:551–556. doi: 10.1016/j.bbrc.2011.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev. 2012;2012:428010. doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giusti L, Angeloni C, Barbalace MC, Lacerenza S, Ciregia F, Ronci M, Urbani A, Manera C, Digiacomo M, Macchia M, Mazzoni MR, Lucacchini A, Hrelia S. A proteomic approach to uncover neuroprotective mechanisms of oleocanthal against oxidative stress. Int J Mol Sci. 2018;19:E2329. doi: 10.3390/ijms19082329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, O W, Li W, Jiang ZG, Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci. 2013;14:24438–24475. doi: 10.3390/ijms141224438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang KL, Chin KY. The biological activities of oleocanthal from a molecular perspective. Nutrients. 2018;10:E570. doi: 10.3390/nu10050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosignoli P, Fuccelli R, Fabiani R, Servili M, Morozzi G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J Nutr Biochem. 2013;24:1513–1519. doi: 10.1016/j.jnutbio.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Vlassenko AG, Gordon BA, Goyal MS, Su Y, Blazey TM, Durbin TJ, Couture LE, Christensen JJ, Jafri H, Morris JC, Raichle ME, Benzinger TL. Aerobic glycolysis and tau deposition in preclinical Alzheimer’s disease. Neurobiol Aging. 2018;67:95–98. doi: 10.1016/j.neurobiolaging.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


