Abstract
Spinal cord injury (SCI) causes disturbances of motor skills. Free radicals have been shown to be essential for the development of spinal cord trauma. Despite some progress, until now no effective pharmacological therapies against SCI have been verified. The purpose of our experiment was to investigate the neuroprotective effects of ebselen on experimental SCI. Twenty-two rats subjected to SCI were randomly subjected to SCI with no further treatment (n = 10) or intragastric administration of ebselen (10 mg/kg) immediately and 24 hours after SCI. Behavioral changes were assessed using the Basso, Beattie, and Bresnahan locomotor scale and footprint test during 12 weeks after SCI. Histopathological and immunohistochemical analyses of spinal cords and brains were performed at 12 weeks after SCI. Magnetic resonance imaging analysis of spinal cords was also performed at 12 weeks after SCI. Rats treated with ebselen presented only limited neurobehavioral progress as well as reduced spinal cord injuries compared with the control group, namely length of lesions (cysts/scars) visualized histopathologically in the spinal cord sections was less but cavity area was very similar. The same pattern was found in T2-weighted magnetic resonance images (cavities) and diffusion-weighted images (scars). The number of FluoroGold retrogradely labeled neurons in brain stem and motor cortex was several-fold higher in ebselen-treated rats than in the control group. The findings suggest that ebselen has only limited neuroprotective effects on injured spinal cord. All exprimental procedures were approved by the Local Animal Ethics Committee for Experiments on Animals in Katowice (Katowice, Poland) (approval No. 19/2009).
Keywords: ebselen, antioxidant, neuroprotection, neuroregeneration, spinal cord injury, spinal cord repair, functional recovery
Chinese Library Classification No. R453; R361; R741
Introduction
Injury of the spinal cord causes disturbances of both sensory and motor function (Blight, 1983; Basso et al., 1996). Besides devastating loss of neurons, spinal cord injury (SCI) activates a secondary cascade of events that induce rapid oxidative stress and a progressive degeneration of spinal cord (Kalayci et al., 2005; Baptiste and Fehlings, 2006). It is still one of the leading causes of disability in Western countries, especially in young people. The degree of dysfunction after SCI depends on various factors, such as severity of trauma, location of the injury, and subsequent degenerative response. The secondary response induces apoptosis, lipid peroxidation, demyelination, and neuronal loss. Besides the mechanical damage of the spinal cord structure, free radical species have been shown to be involved in the pathogenesis of spinal cord trauma (Demopoulos et al., 1982; Sekhon and Fehlings, 2001; Beattie et al., 2002; Xiong et al., 2007).
Ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one), a synthetic seleno-organic compound, has been demonstrated to have antioxidant abilities (Fujisawa and Kadoma, 2005). Ebselen catalyzes the reduction of reactive oxygen species (ROS) in a manner similar to glutathione peroxidase which degrades hydrogen peroxide and organic peroxides in the brain to the greatest extent (Müller et al., 1984; Parnham and Sies, 2013; Ozyigit et al., 2015).
Ebselen can protect cellular components from the oxidative damage via modulation of metalloproteins, enzymatic cofactors, gene expression, antioxidant and immune mechanisms. Also, cell functioning can be altered by ebselen by changing the thioredoxin pathways (Zhao et al., 2002). Additionally, it has low toxicity because ebselen molecule does not release selenium (Noguchi, 2016). Ebselen attenuates oxidative DNA damage (He et al., 2007).
Ebselen is lipid-soluble and acts mainly through reaction with cysteine thiol groups in proteins. That is why clinical trials are currently led to check ebselen applicability in prevention and treatment of a variety of disorders, such as stroke, brain injury, cardiovascular diseases, atherosclerosis, and cancer (Azad and Tomar, 2014). Ebselen may have potential neuroprotective effects in animal models of SCI (Unlu et al., 2002; Kalayci et al., 2005; Jia et al., 2018). Seo et al. (2009) observed the neuroprotective effects of ebselen against ischemic in the hippocampal CA1 region after transient forebrain ischemia.
The aim of our experiment was to study the possible neuroprotective effects of ebselen on experimental SCI in rats.
Materials and Methods
Ethics statement
All experimental procedures were performed in the Center for Experimental Medicine of Medical University of Silesia in Katowice in accordance with the European Union animal protection law and approved by the Local Animal Ethics Committee for Experiments on Animals in Katowice (Katowice, Poland) (approval No. 19/2009).
Animals
Twenty-five adult (3 months old, weighing 300 g) male Wistar rats (the Center for Experimental Medicine of Medical University of Silesia in Katowice, Poland) were used. Three animals were subjected to magnetic resonance imaging (MRI) to obtain reference images of healthy spinal cords. Remaining twenty-two animals were subjected to SCI and randomly divided into two groups: control group (n = 10): no additional treatment was given after SCI; ebselen group (n = 12): ebselen treatment was given after SCI.
SCI models
The focal spinal cord injury was produced by means of pressure impactor; this apparatus allows for achieving a precisely controlled air blast as described previously (Marcol et al., 2012).
Briefly, animals were anesthetized intraperitoneally with ketamine (100 mg/kg) and xylazine (10 mg/kg) and after immobilization; T9–11 spinal cord segments were exposed. A 2-mm hole in the right T10 vertebral arch was carefully drilled under the stereomicroscope (Nikon, Tokyo, Japan) to spare the dura. To avoid trepan overheating, cooling with ice-cold phosphate-buffered saline (PBS) was performed. Then, impactor tip was carefully positioned inside the hole, only touching the dura mater but not pressing it and an isolated air blast (150 kPa pressure, 0.1 seconds duration) was applied. The whole process was recorded by the digital camera paired to the stereomicroscope (Nikon, Tokyo, Japan). After removal of the tip from the hole, it was sealed with a bone wax, tissues were sutured in layers, and the wound was protected with a sterile bandage.
To avoid dehydration, all animals were subcutaneously injected with 2 mL of sterile saline. Because the autonomic function of urinary bladder was impaired due to the spinal shock, it was emptied manually twice a day until the recurrence of bladder function. To prevent pain, 400 mg of paracetamol (suspension 125 mg/5 mL) was dissolved in 100 mL drinking water (average drug dosage 200 mg/kg of body weight daily).
Drug administration
Immediately and 24 hours after SCI, a 0.05% ebselen (Sigma-Aldrich, St. Louis, MO, USA) solution was intragastrically administered. Prior to administration, ebselen was dissolved in distilled water to obtain a drug dosage of 10 mg/kg.
Behavioral assessment
All behavioral changes of rats were assessed during 12 weeks after SCI performed by observers blinded to the experimental groups. The assessment consisted of gait analysis (footprint test) and open field test according to the Basso, Beattie, and Bresnahan (BBB) locomotor scale method (Basso et al., 1996).
Footprint test
Footprint test was carried out at 1, 4, 7, and 12 weeks after SCI. Animals were examined on a 100-cm long and 7-cm wide u-shaped track with transparent floor. Footprints obtained in ink tracking tunnels were recorded with digital camera and automatically analyzed frame by frame (Catwalk XT 8.1, Noldus, The Netherlands). One session consisted of 3 consecutive tests per animal. Analysis covered foot rotation angle (the angle made by two lines connecting the third toe and the stride line at the center of the paw) of the right hindpaw (ipsilateral to the injury site), and interlimb coordination (smallest distance between the middle point of the hind paw and forepaw on the same side).
Open field test
This test was carried out on the plexiglas plate. Stepping ability of each rat was assessed by analyzing the motor function of joints. In addition, general coordination and stability of the body were evaluated. The obtained BBB locomotor rating scale scores ranged from 0 to 22, where 0 meant total lack of motor abilities and 22 referred to unimpaired locomotion (Basso et al., 1995; Karimi-Abdolrezaee et al., 2006).
Neuroanatomical tracing
To check whether and to what extent supraspinal neurons have regrown their axons into the spinal cord caudally beyond the injury site, retrograde labeling was applied at 11 week postoperatively. Four animals in each group were randomly selected (due to limitation of the Ethics Committee) for this procedure. Ketamine (100 mg/kg) and xylazine (10 mg/kg) (POLFA Lodz, Warszawa, Poland) were injected intraperitoneally to anesthetize the animals. Spinal cord segments below the injury site were exposed by laminectomy. Ten mm caudally from the injury site, microcrystal of FluoroGold (FG) (Fluorochrome Inc., Englewood, NJ, USA) was inserted into the tissue. To compare injured and non-injured spinal tracts, tracer was applied bilaterally. Week later, deeply anesthetized rats were rapidly perfused with a bolus of cold PBS and brains were collected. After dehydration in 15% sucrose, brains were embedded in TissueTek (Sakura, Tokyo, Japan) (Equimed, Krakow, Poland) and stored in –80°C. Coronal 10-µm sections were cut by means of microtome (ThermoScientific, Waltham, MA, USA) and mounted on slides. Brain stem (red nucleus) and primary motor cortex were examined under the fluorescent microscope (365 nm, Labophot 2, Nikon, Tokyo, Japan), in every-sixth section (average 20 sections/brain) and photographed. Digitally stored images were subjected to double-blinded counting of FG-positive neurons in five areas: four at the corners and one in the center of the area with highest density of labeled cells. Total number of cells was counted and the number of cells per mm2 for each brain was calculated, and finally averaged for whole – control or experimental, respectively – group.
Magnetic resonance imaging determination
Three randomly assigned rats from each SCI group, excluding rats subjected to FG labeling, were subjected to magnetic resonance imaging (MRI) in the last week of experiment. At the end of week 12, the spinal cords of rats were collected as described in next point.
Twelve weeks after SCI, MR images were obtained at the Institute of Nuclear Physics Polish Academy of Sciences (Cracow, Poland) using research MRI scanner, based on 4.7 T horizontal bore magnet (Bruker, Ettlingen, Germany), and controlled with MARAN DRX digital console (Resonance Instruments Ltd., Tubney Woods, Abingdon, UK). Locally built measuring probe-head, with surface RF coil geometrically optimised for shape and size of rat spinal cord in vivo was used during experiment. Animals were anesthetized with halothane (2%) mixed with air (60%/1.2 L/min) and oxygen (40%/0.8 L/min). Physiological conditions of the animal (temperature, respiratory rate and heart rate) were continuously monitored during examination using MR-compatible Small Animal Monitoring & Gating System (SA Instruments, Inc., Stony Brook, NY, USA).
Spin echo pulse sequence was used for acquiring T2-weighted and diffusion weighted MR images in sagittal and axial projections. The following parameters of the spin echo sequence were applied: echo time (TE = 32 ms), repetition time (TR = 1.8 seconds), field of view (FOV = 2.5 cm), image matrix size 128 × 128, slice thickness of 1.5 mm. The signal was accumulated (NA = 6) in order to improve signal-to-noise ratio.
Diffusion weighted images were obtained with additional diffusion sensitive gradients (δ = 5 ms and Δ = 23 ms) added to the spin echo sequence in transversal and longitudinal directions to the spinal cord axis. In order to optimize signal-to-noise ratio and thus minimize the examination time, different values of b-factor were used to assess effect of diffusion on echo signal: 750 s/mm2 and 1100 s/mm2 for longitudinal and transversal directions, respectively (Minati and Weglarz, 2007). The acquisition was both cardiac- and respiratory-gated to eliminate motion artifacts.
Histopathological procedures
Twelve weeks after SCI, rats were deeply anesthetized with pentobarbitone sodium (50 mg/kg) intraperitoneally (Biowet, Pulawy, Poland) and perfused transcardially with 100 mL of PBS (pH 7.4) followed by 100 mL of 4% paraformaldehyde solution in the same buffer. Spinal cords were dissected and 2-cm segments covering the injured area were cut out. After 24 hours, tissue was dehydrated (20% sucrose in PBS) at 4°C, embedded in TissueTek (Sakura, Japan) and then stored in –80°C. Sagittal or transverse 10 µm sections were cut and mounted on SuperFrost Plus slides (Menzel Glaser, Braunschweig, Germany). Slides were stained with 1% toluidine blue for routine histopathological examination or labeled immunohistochemically to visualize astrocytes. Slides were incubated overnight at 4°C with rabbit anti-rat glial fibrillary acidic portein primary antibody (dilution 1:500; Molecular Probes - Thermo Fisher Scientific, Waltham, MA, USA). After removal of unbound antibodies by triple rinsing in cold PBS, they were treated with the secondary antibody goat anti-rabbit IgG conjugated with Alexa Fluor® 488 (dilution 1:1000, Molecular Probes, USA). Sections were cover-slipped in VectaShield with DAPI (Vector, Burlingame, CA, USA) and examined under confocal laser scanning microscope FluoView (Olympus, Tokyo, Japan). Digitally stored images were analyzed. In longitudinal or transverse sections, respectively, the total average length and the sizes of main lesions (cavity/scar area) were measured using Image Pro Plus software (Media Cybernetic, Rockville, MD, USA) calculating automatically manually outlined contour (delineated by glial fibrillary acidic portein primary immunostaining) of cavity/ scar lesions located in injured fragment of the spinal cord.
Statistical analysis
Statistical analysis was carried out with SPSS 23.0 software (IBM, Armonk, NY, USA). Statistical significance was determined by the Student’s t-test (white/grey matter sparing as well as number of FG-positive cells) or one-way analysis of variance followed by Tukey’s test (behavioral/MRI studies). A value of P < 0.05 was considered statistically significant. All data are presented as the mean ± SD.
Results
Behavioral findings
Injury of spinal cord resulted in immediate monoplegia in all animals, with no visible hindlimb movements. The footprint test revealed deterioration in the angle of rotation in hindlimb in both groups of animals in the 1st week after SCI. At the end of experiment we observed continuous deterioration of motor skills in the control group. In the ebselen group at 12 weeks, this parameter decreased only slightly, showing no statistical significance (Figure 1).
Figure 1.
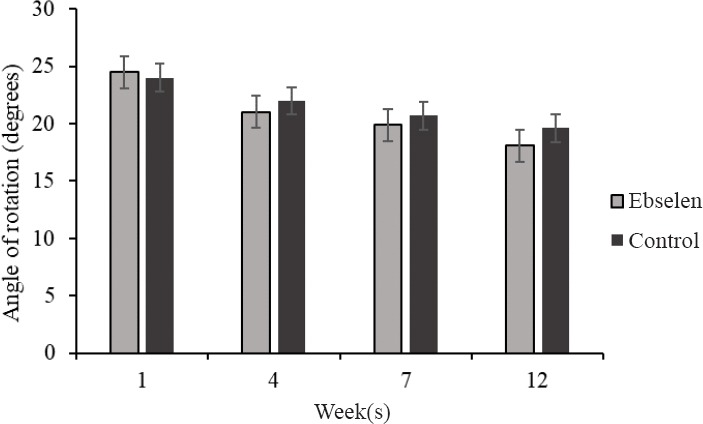
Angle of rotation of the hind paw in spinal cord injury rats treated (n = 12; at 12 weeks n = 8) or not (n = 10; at 12 weeks n = 6) with ebselen.
All data are presented as the mean ± SD.
Interlimb coordination in rats treated with ebselen improved significantly at 4, 7, and 12 weeks and was significantly better than that in the control group (P < 0.05; Figure 2).
Figure 2.
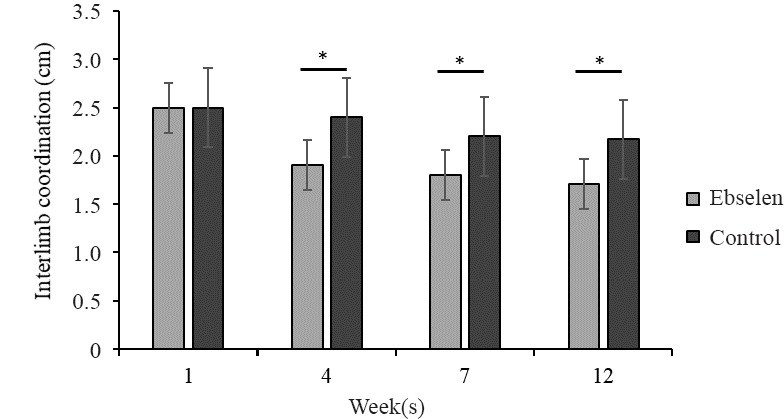
Inter-limb coordination (cm) in spinal cord injury rats treated or not with ebselen.
All data are presented as the mean ± SD. *P < 0.05 (Student’s t-test). n = 12 and 10 in the ebselen and control groups, respectively, at 1–7 weeks, and n = 8 and 6 in both groups at 12 weeks.
Analysis of BBB test revealed comparable dynamics of recovery of motor skills in both groups. One week after SCI, rats with BBB score lower than 10 showed no sufficient motor functional recovery. This value did not improve at 12 week after SCI in both groups (Figure 3).
Figure 3.
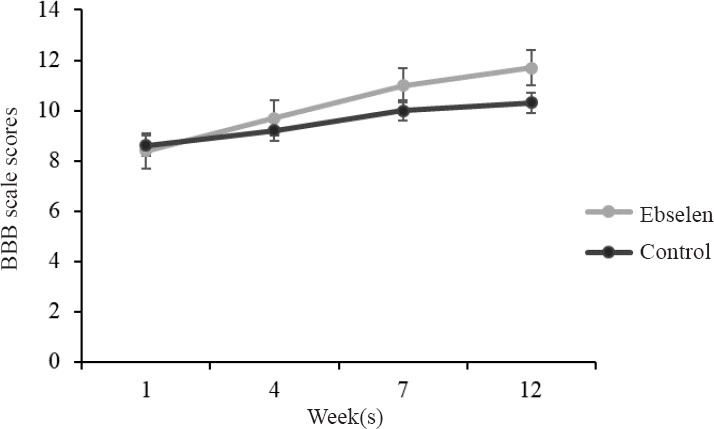
Basso, Beattie, and Bresnahan (BBB) locomotor scale scores after spinal cord injury in rats treated or not with ebselen.
All data are presented as the mean ± SD. n = 12 and 10 in the ebselen and control groups, respectively, at 1–7 weeks, and n = 8 and 6 in both groups at 12 weeks.
MRI findings
Examples of the MR images of the injured spinal cord are presented in Figure 4. There were two major types of pathological changes in injured spinal cord white matter: cysts (a similar size in both groups) appearing as increased signal intensity in the T2-weighted images and reduced signal intensity in the diffusion-weighted images as compared to the surrounding tissues, and much less visible scars producing reduced signal intensity both in T2-weighted and diffusion-weighted images as presented in Figure 4. Quantitative diffusion tensor imaging can provide information about the microstructure of nerve tissue and can quantify the pathological damage of spinal cord white matter and gray matter (Zhang et al., 2015; Liu et al., 2018).
Figure 4.
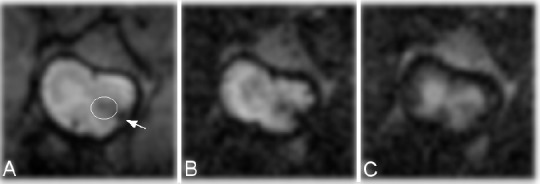
Magnetic resonance images from the representative injured spinal cord after ebselen treatment at 12 weeks.
(A) T2-weighted image. Diffusion-weighted image with diffusion gradient applied along phase direction (B) and (C) slice direction. Scar/hemorrhage pathological change present in the white matter (indicted by arrow) and at the border between the white and gray matter of lateral funiculus of spinal cord (marked with the circle).
Average value of the longitudinal diffusion coefficient in spinal cord white matter (outside of macroscopic changes) in ebselen-treated spinal cord was equal to 1.85 ± 0.05 × 10−3 mm2/s as compared to 1.92 ± 0.03 × 10–3 mm2/s for healthy spinal cord and 1.57 ± 0.04 × 10–3 mm2/s for injured and not treated one (P < 0.05; Figure 5).
Figure 5.
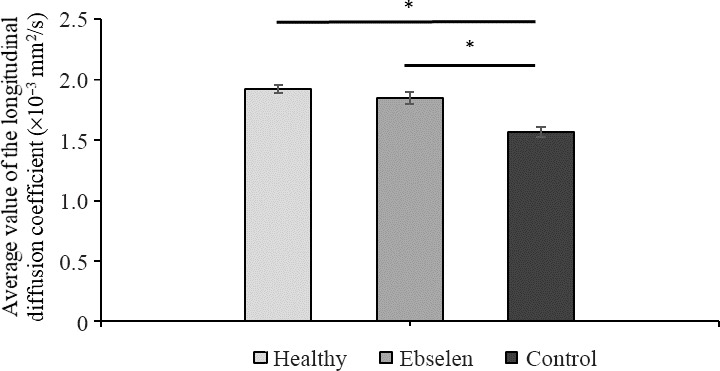
Magnetic resonance imaging – average value of the longitudinal diffusion coefficient in spinal cord white matter, respectively, in healthy, ebselen, and control groups.
All data are presented as the mean ± SD from n = 3 per group. *P < 0.05 (one-way analysis of variance followed by Tukey’s test).
Histopathological findings
Typical pathological changes observed in both groups were cysts resembling post-traumatic syringomyelia. These changes were randomly dispersed in the white matter and at the border of white and gray matter of the spinal cord (Figure 6). Their number ranged from one to several. In many cases, only the formation of cystic cavitations in the spinal cord white matter was found which was confirmed by the diffusion-weighted image MRI with diffusion gradient applied along slice direction. Sporadically, the presence of scars in the injured spinal cord was observed. Microcysts with astrogliosis were observed (Takazawa et al., 2018). The average areas of cavities in spinal cord were similar in both groups. This value only slightly decreased in the ebselen group compared with the control group (Figure 7). The total length of lesion in the spinal cord in the control group was significantly higher than the ebselen group (P < 0.05; Figure 8).
Figure 6.
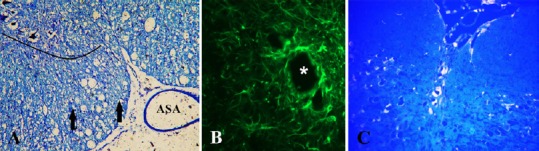
Microphotographs obtained 12 weeks after spinal cord injury in rats treated with ebselen.
(A) Spinal cord, toluidine blue staining; numerous cystic cavitations (vacuolar structures). Lined area of anterior horn of spinal cord; ASA: anterior spinal artery; arrows: axon spheroids (swollen axons). (B) Formation of glial fibrillary acidic portein primary-labeled cavity in the injured spinal cord ventral white column. Asterisk: microcyst with astrogliosis. (C) FluoroGold retrograde labeling of regenerating neurons. Neuronal cytoplasm is labeled by fluorogold in transverse sections of the raphe nuclei in the brain stem.
Figure 7.
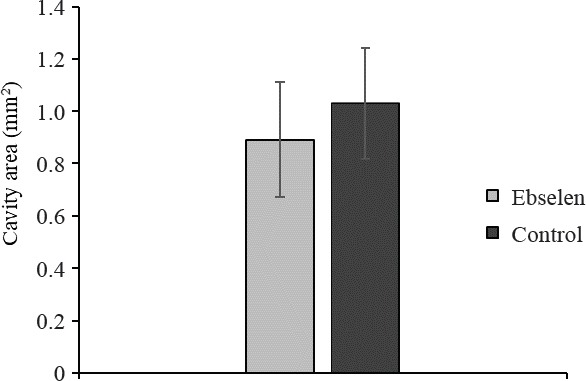
Area of lesion cavity 12 weeks after spinal cord injury in rats treated or not with ebselen.
All data are presented as the mean ± SD. n = 8 and 6 in the ebselen and control groups, respectively.
Figure 8.
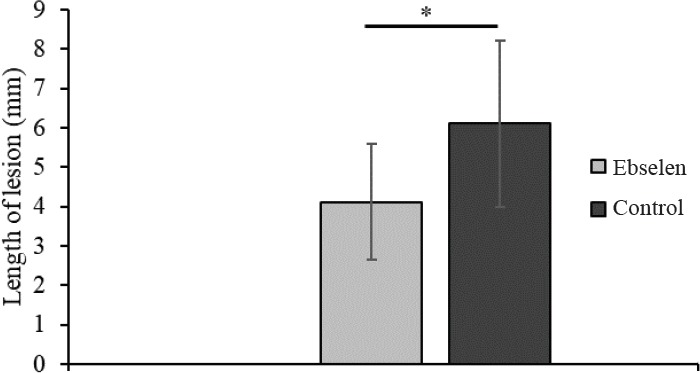
Measurement of the length of the spinal cord lesion in rats treated or not with ebselen.
All data are presented as the mean ± SD. *P < 0.05 (Student’s t-test). n = 8 and 6 in the ebselen and control groups, respectively.
Neuronal tracing findings
Number of FG-positive cells in the brain stem and primary motor cortex, proving survival of long-tract neurons, was significantly higher in the ebselen group than the control group (P < 0.05) (Figure 9).
Figure 9.
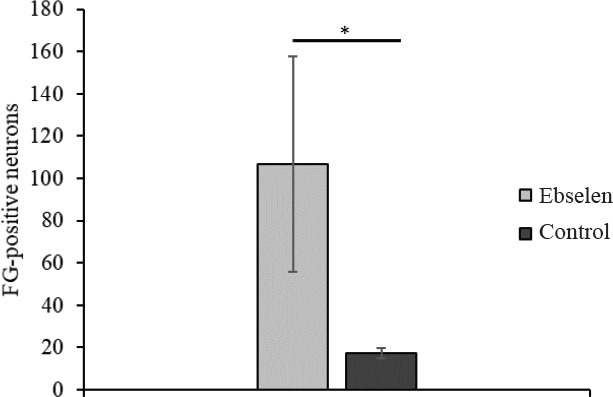
Number of FluoroGold (FG)-positive brain stem and motor cortex neurons 12 weeks after spinal cord injury in rats treated or not with ebselen.
All data are presented as the mean ± SD from 4 rats per group. *P < 0.05 (Student’s t-test).
Discussion
Our study aimed to investigate the long-term effects of ebselen on experimental injury of spinal cord in rats. We observed some improvements in morphological parameters of lesions in the spinal cord white matter as limiting the length of lesions without affecting the size. Such damage characterization suggests the occurrence of lesions limited to one segment but with a larger volume, which would explain the lack of functional recovery effect expected in the ebselen group. The explanation may be that the interruption of long projection tract (e.g. rubrospinal tract) from the brain to the spinal cord and vice versa at just one spinal cord segment may result in complete lack of motor recovery. Maintaining long projection tracts functioning, especially in humans, is fundamental to a correct gait pattern. It is important to consider the major differences between the sensorimotor pathways of rats and humans (Filipp et al., 2019). In addition, what is surprising, in our study we have demonstrated an increased survival the brain stem as well as motor cortex neurons, however without restoration of motor function, which is in opposition to a previous report (Marcol et al., 2017). It might indicate that motor defects following spinal cord injury do not involve axons of supraspinal neurons but are rather the effect of damage of other, more local, nerve circuits. The location of microcysts in our study was accidental and concerned very different nerve pathways. Based on our study, we can only speculate about neuroprotective effects of ebselen on oligodendrocyte whose increased survival could have some impact on the limitation of secondary destruction of axons as well as spinal cord cavitation. The only clearly visible improvement seen in neurobehavioral tests referred to interlimb coordination which is very complex parameter and may be rather related to the increase in neuronal plasticity of spinal cord or/and cerebral neurons. These unsatisfactory results of functional recovery may be also caused by a relatively weak permeability of ebselen and a time of the drug administration with respect to the long-term observation. Additionally, application of this unique experimental method does not allow us to precisely compare our findings with results of other experiments in SCI.
Ebselen is the radical scavenger with strong antioxidant and anti-inflammatory properties (Satoh et al., 2004). Because of these effects, ebselen may be protective against secondary injury to the nervous system and not only neurons but also oligodendrocyte cells (Gilgun-Sherki et al., 2002; Santofimia-Castaño et al., 2016). The concept that a delayed wave of oligodendrocyte apoptosis occurs in the spinal cord white matter after traumatic insults suggests that poor myelination of axons can persist after spinal cord injury. Such axons can be functionally impaired for a very long time. Therefore, further intensive studies are needed to identify the mechanisms responsible for delayed oligodendrocyte death and to find a way for effective prevention of them (Liu et al, 1997). Only a limited number of studies on the application of ebselen in spinal cord injury have been published. The first article evaluating the influence of ebselen on regeneration of spinal cord was published in 2002 by Unlu et al. However, authors have not performed behavioral studies; they have only assessed the biochemical and morphological parameters. They demonstrated that application of a single dose of ebselen 1 or 12 hours after trauma increased the level of superoxide dismutase, but did not limit the lipid peroxidation which was essential for the preservation of cell membrane integrity (Unlu et al., 2002).
In another study, Kalayci et al. (2005) investigated the possible beneficial effects of ebselen in comparison with methylprednisolone in experimental rat models of SCI. SCI was performed by placement of an aneurysm clip, extradurally at the level of T11–12, and then rats received methylprednisolone (30 mg/kg) or ebselen (10 mg/kg) intraperitoneally. After 24 hours, all rats were neurologically examined and spinal cord tissue samples have been harvested for both biochemical and histopathological evaluation. The results showed that methylprednisolone and ebselen treatment decreased tissue levels of malondialdehyde and protein carbonyl, and prevented inhibition of the superoxide dismutase, glutathione peroxidase and catalase. The authors concluded that treatment with ebselen might have potential benefits in spinal cord damage.
The influence of ebselen on mitochondrial function 3 days after acute SCI was examined by Jia et al. (2018). They found that mitochondrial apoptosis was reduced, probably via antioxidant action of the drug, delivered in the same dose as in our experiment, daily three times after weight-drop induced SCI. Authors also have examined motor function using BBB scale and found significant improvement in the ebselen group 14 days after the injury. Our results showed no significant differences between groups, however the final maximal value in ebselen group is very similar to these reported by Jia et al. This may indicate that protective effects of ebselen are not persistent long time after its administration.
In our study, we used a quite different model of SCI. This novel method is less invasive than clip compression or weight-drop and does not require laminectomy. Additionally, a precise stream of air under pressure allows for graded white matter spinal cord injury (Marcol et al., 2012).
Ebselen was also administered in various experimental models of brain injury. Dawson et al. (1995) showed that oral or intravenosus ebselen administration before occlusion of middle cerebral artery in rats reduced the volume of ischemic damage in the cerebral hemisphere and cerebral cortex (Imai et al., 2001, 2003). Wei et al. (2014) investigated the neuroprotective effects of ebselen on traumatic brain injury in rats using a modified weight-drop model. Authors revealed that ebselen application significantly ameliorated the neurological deficit in rats after traumatic brain injury in dose-dependent manner, and might be effective in treatment of traumatic brain injuries. Takasago et al. (1997) showed that intragastric administration of ebselen at lower dose (10 mg/kg) significantly reduced the area of infarction in the hemisphere and cerebral cortex. The neuroprotective efficacy of the higher dose of ebselen (40 mg/kg), however, was less than that of the lower dose.
Watanabe et al. (1997) evaluated the efficacy of ebselen in protection of vasospasm after subarachnoid hemorrhage (SAH). Ebselen at high dose (50 mg/ kg) was administered twice a day for 7 days in dogs subjected to SAH. The authors showed that treatment with ebselen ameliorated delayed cerebral vasospasm detected angiographically. Similarly, Gul et al. (2010) investigated the neuroprotective effects of ebselen in comparison with nimodipine in rats after SAH. One hour after trauma, one group of animals received nimodipine at 6-hour intervals and the second group received ebselen twice a day for 48 hours. The results showed that ebselen in comparison with nimodipine diminished malonyldialdehyde concentration and superoxide dismutase activity and prevented the vasospasm of the basilar artery. The authors concluded that treatment with ebselen might have neuroprotective effects by preventing the occurrence of vasospasm. Also Aras et al. (2014) and Imai et al. (2001) investigated the effects of ebselen treatment on ischemia/reperfusion injury in rat brain. They revealed, by histopathological examination, that ebselen had a neuron-protective effect due to its antioxidant properties in brain tissue.
Similarly, Ozyigit et al. (2015) investigated the neuroprotective effects of pre-treatment with two doses of ebselen in rats subjected to experimental sciatic nerve ischemia/reperfusion injury. The authors revealed that a 15 mg/kg dose of ebselen administrated intraperitoneally 24 hours before the ischemia/reperfusion had remarkable protective effects against sciatic nerve injury proved by histopathologic and immunohistochemical examinations, while administration of ebselen at dose of 30 mg/kg had no effect in protecting the sciatic nerve against ischemia/reperfusion injury.
Based on encouraging evidence of the neuroprotective effects of ebselen in animal models, Saito et al. (1998) performed a randomized clinical study in 286 patients with SAH. The authors showed that oral administration of ebselen (150 mg, twice a day) did not affect the incidence of vasospasm but significantly ameliorated delayed ischemic neurological deficits, reduced volume of subsequent cerebral infarction, and improved clinical outcomes.
We conclude that neuroprotective activity of ebselen had limited positive impact on regeneration of the injury in the spinal cord white matter in rats in our study. We applied the same dose of ebselen like other experimenters (Takasago et al., 1997; Kalayci et al., 2005; Gul et al., 2010; Aras et al., 2014; Jia et al., 2018), but further investigations should be performed to identify an optimal dose of ebselen as well as the time of administration that may provide the best neuroprotective effects on SCI.
Footnotes
Conflicts of interest: The authors declare that they have no competing interests.
Financial support: None.
Institutional review board statement: All experimental procedures were performed in the Center for Experimental Medicine of Medical University of Silesia in Katowice in accordance with the European Union animal protection law and approved by the Local Animal Ethics Committee for Experiments on Animals in Katowice (Katowice, Poland) (approval No. 19/2009).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editor: Zhao M; S-Editor: Li CH; T-Editor: Liu XL
References
- 1.Aras M, Altaş M, Meydan S, Nacar E, Karcıoğlu M, Ulutaş KT, Serarslan Y. Effects of ebselen on ischemia/reperfusion injury in rat brains. Int J Neurosci. 2014;124:771–776. doi: 10.3109/00207454.2013.879581. [DOI] [PubMed] [Google Scholar]
- 2.Azad GK, Tomar RS. Ebselen, a promising antioxidant drug: mechanisms of action and targets of biological pathways. Mol Biol Rep. 2014;41:4865–4879. doi: 10.1007/s11033-014-3417-x. [DOI] [PubMed] [Google Scholar]
- 3.Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 5.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 6.Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- 7.Blight AR. Cellular morphology of chronic spinal cord injury in the cat: analysis of myelinated axons by line-sampling. Neuroscience. 1983;10:521–543. doi: 10.1016/0306-4522(83)90150-1. [DOI] [PubMed] [Google Scholar]
- 8.Dawson DA, Masayasu H, Graham DI, Macrae IM. The neuroprotective efficacy of ebselen (a glutathione peroxidase mimic) on brain damage induced by transient focal cerebral ischaemia in the rat. Neurosci Lett. 1995;185:65–69. doi: 10.1016/0304-3940(94)11226-9. [DOI] [PubMed] [Google Scholar]
- 9.Demopoulos HB, Flamm ES, Seligman ML, Pietronigro DD, Tomasula J, DeCrescito V. Further studies on free-radical pathology in the major central nervous system disorders: effect of very high doses of methylprednisolone on the functional outcome, morphology, and chemistry of experimental spinal cord impact injury. Can J Physiol Pharmacol. 1982;60:1415–1424. doi: 10.1139/y82-210. [DOI] [PubMed] [Google Scholar]
- 10.Filipp ME, Travis BJ, Henry SS, Idzikowski EC, Magnuson SA, Loh MY, Hellenbrand DJ, Hanna AS. Differences in neuroplasticity after spinal cord injury in varying animal models and humans. Neural Regen Res. 2019;14:7–19. doi: 10.4103/1673-5374.243694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujisawa S, Kadoma Y. Kinetic studies of the radical-scavenging activity of ebselen, a seleno-organic compound. Anticancer Res. 2005;25:3989–3994. [PubMed] [Google Scholar]
- 12.Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54:271–284. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- 13.Gul S, Bahadir B, Hanci V, Acikgoz S, Bektas S, Ugurbas E, Ankarali H, Kalayci M, Acikgoz B. Effects of ebselen versus nimodipine on cerebral vasospasm subsequent to experimental subarachnoid hemorrhage in rats. J Clin Neurosci. 2010;17:608–611. doi: 10.1016/j.jocn.2009.07.115. [DOI] [PubMed] [Google Scholar]
- 14.He M, Xing S, Yang B, Zhao L, Hua H, Liang Z, Zhou W, Zeng J, Pei Z. Ebselen attenuates oxidative DNA damage and enhances its repair activity in the thalamus after focal cortical infarction in hypertensive rats. Brain Res. 2007;1181:83–92. doi: 10.1016/j.brainres.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 15.Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke. 2001;32:2149–2154. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- 16.Imai H, Graham DI, Masayasu H, Macrae IM. Antioxidant ebselen reduces oxidative damage in focal cerebral ischemia. Free Radic Biol Med. 2003;34:56–63. doi: 10.1016/s0891-5849(02)01180-2. [DOI] [PubMed] [Google Scholar]
- 17.Jia ZQ, Li SQ, Qiao WQ, Xu WZ, Xing JW, Liu JT, Song H, Gao ZY, Xing BW, He XJ. Ebselen protects mitochondrial function and oxidative stress while inhibiting the mitochondrial apoptosis pathway after acute spinal cord injury. Neurosci Lett. 2018;678:110–117. doi: 10.1016/j.neulet.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Kalayci M, Coskun O, Cagavi F, Kanter M, Armutcu F, Gul S, Acikgoz B. Neuroprotective effects of ebselen on experimental spinal cord injury in rats. Neurochem Res. 2005;30:403–410. doi: 10.1007/s11064-005-2615-2. [DOI] [PubMed] [Google Scholar]
- 19.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CB, Yang DG, Meng QR, Li DP, Yang ML, Sun W, Zhang WH, Cai C, Du LJ, Li J, Gao F, Yu Y, Zhang X, Zuo ZT, Li JJ. Dynamic correlation of diffusion tensor imaging and neurological function scores in beagles with spinal cord injury. Neural Regen Res. 2018;13:877–886. doi: 10.4103/1673-5374.232485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;15:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcol W, Slusarczyk W, Gzik M, Larysz-Brysz M, Bobrowski M, Grynkiewicz-Bylina B, Rosicka P, Kalita K, Węglarz W, Barski JJ, Kotulska K, Labuzek K, Lewin-Kowalik J. Air gun impactor--a novel model of graded white matter spinal cord injury in rodents. J Reconstr Microsurg. 2012;28:561–568. doi: 10.1055/s-0032-1315779. [DOI] [PubMed] [Google Scholar]
- 23.Marcol W, Slusarczyk W, Larysz-Brysz M, Łabuzek K, Kapustka B, Staszkiewicz R, Rosicka P, Kalika K, Węglarz W, Lewin-Kowalik K. Extended magnetic resonance imaging studies on the effect of classically activated microglia transplantation on white matter regeneration following spinal cord focal injury in adult rats. J Exp Ther Med. 2017;14:4869–4877. doi: 10.3892/etm.2017.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minati L, Weglarz WP. Physical foundations, models, and methods of diffusion magnetic resonance imaging of the brain: A review. Concepts Magn Reson Part A. 2007;30:278–307. [Google Scholar]
- 25.Müller A, Cadenas E, Graf P, Sies H. A novel biologically active seleno-organic compoun-1. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen) Biochem Pharmacol. 1984;33:3235–3239. doi: 10.1016/0006-2952(84)90083-2. [DOI] [PubMed] [Google Scholar]
- 26.Noguchi N. Ebselen, a useful tool for understanding cellular redox biology and a promising drug candidate for use in human diseases. Arch Biochem Biophys. 2016;595:109–112. doi: 10.1016/j.abb.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Ozyigit F, Kucuk A, Akcer S, Tosun M, Kocak FE, Kocak C, Kocak A, Metineren H, Genc O. Different dose-dependent effects of ebselen in sciatic nerve ischemia-reperfusion injury in rats. Bosn J Basic Med Sci. 2015;15:36–43. doi: 10.17305/bjbms.2015.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parnham MJ, Sies H. The early research and development of ebselen. Biochem Pharmacol. 2013;86:1248–1253. doi: 10.1016/j.bcp.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Saito I, Asano T, Sano K, Takakura K, Abe H, Yoshimoto T, Kikuchi H, Ohta T, Ishibashi S. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1998;42:269–277. doi: 10.1097/00006123-199802000-00038. [DOI] [PubMed] [Google Scholar]
- 30.Santofimia-Castaño P, Izquierdo-Alvarez A, de la Casa-Resino I, Martinez-Ruiz A, Perez-Lopez M, Portilla JC, Salido GM, Gonzalez A. Ebselen alters cellular oxidative status and induces endoplasmic reticulum stress in rat hippocampal astrocytes. Toxicology. 2016;357-358:74–84. doi: 10.1016/j.tox.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Satoh T, Ishige K, Sagara Y. Protective effects on neuronal cells of mouse afforded by ebselen against oxidative stress at multiple steps. Neurosci Lett. 2004;371:1–5. doi: 10.1016/j.neulet.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 32.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 33.Seo JY, Lee CH, Cho JH, Choi JH, Yoo KY, Kim DW, Park OK, Li H, Choi SY, Hwang IK, Won MH. Neuroprotection of ebselen against ischemia/reperfusion injury involves GABA shunt enzymes. J Neurol Sci. 2009;285:88–94. doi: 10.1016/j.jns.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Takasago T, Peters EE, Graham DI, Masayasu H, Macrae IM. Neuroprotective efficacy of ebselen, an anti-oxidant with anti-inflammatory actions, in a rodent model of permanent middle cerebral artery occlusion. Br J Pharmacol. 1997;122:1251–1256. doi: 10.1038/sj.bjp.0701426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takazawa A, Kamei N, Adachi N, Ochi M. Endoplasmic reticulum stress transducer old astrocyte specifically induced substance contributes to astrogliosis after spinal cord injury. Neural Regen Res. 2018;13:536–540. doi: 10.4103/1673-5374.228759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unlu YA, Kaya E, Uzun H, Barut S, Belce A, Oz B, Colak A. The neuroprotective effects of ebselen in experimental spinal cord injury. Turk Neurosurg. 2002;12:9–16. [Google Scholar]
- 37.Watanabe T, Nishiyama M, Hori T, Asano T, Shimizu T, Masayasu H. Ebselen (DR3305) ameliorates delayed cerebral vasospasm in a canine two-hemorrhage model. Neurol Res. 1997;19:563–565. doi: 10.1080/01616412.1997.11740859. [DOI] [PubMed] [Google Scholar]
- 38.Wei L, Zhang Y, Yang C, Wang Q, Zhuang Z, Sun Z. Neuroprotective effects of ebselen in traumatic brain injury model: involvement of nitric oxide and p38 mitogen-activated protein kinase signalling pathway. Clin Exp Pharmacol Physiol. 2014;41:134–138. doi: 10.1111/1440-1681.12186. [DOI] [PubMed] [Google Scholar]
- 39.Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem. 2007;100:639–649. doi: 10.1111/j.1471-4159.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang D, Li XH, Zhai X, He XJ. Feasibility of 3.0 T diffusion-weighted nuclear magnetic resonance imaging in the evaluation of functional recovery of rats with complete spinal cord injury. Neural Regen Res. 2015;10:412–418. doi: 10.4103/1673-5374.153689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao R, Masayasu H, Holmgren A. Ebselen: a substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc Natl Acad Sci U S A. 2002;99:8579–8584. doi: 10.1073/pnas.122061399. [DOI] [PMC free article] [PubMed] [Google Scholar]


