Abstract
Toll-like receptor 4 (TLR4) and protease-activated receptor 2 (PAR2) play pivotal roles in the mammalian innate immune response. Notably, in addition to their involvement in detection of invading pathogens, PAR2 and TLR4 modulate the levels of cell death-induced sterile inflammation by activating pro- or anti-inflammatory downstream signaling cascades. Within the central nervous system, there is emerging evidence that both receptors are involved in synaptic transmission and brain plasticity. Furthermore, due to their prominent role in mediating neuroinflammation, PAR2 and TLR4 are associated with development and progression of neurodegenerative disorders including but not limited to Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. In this article, we summarise the current knowledge on the cooperation between PAR2 and TLR4, discuss the potential cross-talk levels and highlight the impact of the cross-coupling on neuroinflammation.
Keywords: signaling, inflammation, proteases, myeloid of differentiation primary response gene 88, TIR-domain containing adaptor inducing interferon, lipopolysaccharide, TLR4, PAR2
Controversial Roles of Toll-Like Receptor 4 and Protease-Activated Receptor 2 in Neurodegenerative Disorders
Toll-like receptor 4 (TLR4) and protease-activated receptor 2 (PAR2) are expressed in neuronal and glial cells and are involved in development and progression of neurodegenerative diseases with an inflammatory component. Notably, depending on the context, both receptors can have either neuroprotective roles or contribute to neurodegeneration. This correlates with the intrinsic ability of PAR2 and TLR4 to mediate both pro- and anti-inflammation.
In this respect, neuronal activation of PAR2 has been shown to protect against neuroinflammation and Amyloid β neurotoxicity in mouse models of Alzheimer’s disease (AD) (Afkhami-Goli et al., 2007). On the other hand, activation of PAR2 in microglia leads to increased production and secretion of inflammatory mediators contributing to the ongoing neurodegeneration [(reviewed in Ramachandran et al. (2012)]. Overall, PAR2 activation in AD can have either neuroprotective or neurodegenerative effects depending on the cell type and the stage of disease progression (Afkhami-Goli et al., 2007). Similarly, the role of TLR4 in AD is not well understood. We and others have demonstrated that amyloid β activates TLR4, induces neuroinflammation, and thereby drives neuronal apoptosis (Tang et al., 2008; Zeuner et al., 2017). In contrast, activation of TLR4 with a detoxified moiety of Salmonella minnesota-derived lipopolysaccharide has been demonstrated to reduce the levels of neuroinflammation and improve AD-related pathology in vivo (Michaud et al., 2013). In a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine rat model of Parkinson’s disease (PD) (another neurodegenerative disease with a strong link to ongoing neuroinflammation), PAR2 blockade has been shown to reduce the levels of α-synuclein and the pro-inflammatory cytokines interleukin-1 beta and tumor necrosis factor-alpha in the substantia nigra, suggesting a pro-inflammatory role of the receptor. Of note, in addition to the reduction of neuroinflammation, antagonism of PAR2 reversed behavioural deficits (Liu et al., 2014). However, more recent reports studying post mortem brain tissue of patients with PD, demonstrated altered expression levels of both, PAR2-activator serine proteases and serine protease inhibitors (Hurley et al., 2015) making the role of PAR2 in PD less clear. In this context, TLR4 has been shown to promote neuronal cell death in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of PD (Noelker et al., 2013). However, TLR4 has also been shown to mediate the clearance of extracellular α-synuclein deposits by microglia and TLR4 ablation increased the progressive loss of dopaminergic neurons and the resultant motor disability (reviewed in Trotta et al. (2014)). We have performed a detailed PubMed literature analysis on the current knowledge on the cooperation and potential cross-coupling between TLR4 and PAR2.
Signaling via Toll-Like Receptor 4
TLR4 is a pattern recognition receptor and a member of the toll-like receptor (TLR) family. TLRs are involved in nonspecific immune responses via recognition of Gram-positive and Gram-negative bacteria, DNA, RNA viruses, fungi, and protozoa (pathogen-associated molecular pattern) with bacterial lipopolysaccharides (LPSs) acting as specific pathogen-associated molecular patterns for TLR4.
In addition to their respective specific pathogen-associated molecular patterns, TLRs recognise endogenous signals of cell death and tissue damage (danger-associated molecular patterns), thereby driving sterile inflammation.
At the molecular level, binding of different LPS chemotypes or endogenous protein ligands to TLR4 can activate the prototypic pro-inflammatory transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), the antiviral and anti-inflammatory transcription factor interferon regulatory transcription factor 3 (IRF3) and the kinases c-Jun N-terminal kinase and extracellular-regulated protein kinases (ERKs). Importantly, all LPS chemotypes and danger-associated molecular patterns described to date activate NF-κB and IRF3, whereas ERK and c-Jun N-terminal kinase are only influenced by certain ligands (Palsson-McDermott and O’Neill, 2004). In this context, the balance between the levels of NF-κB and IRF3 activation depends on the nature of the ligand and can at least partly be attributed to the biased agonism via TLR4 (Zeuner et al., 2016).
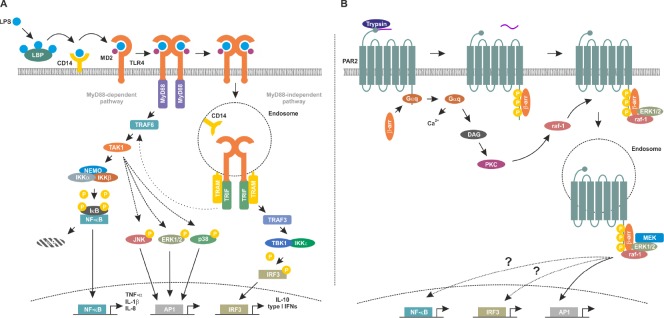
LPS is transported to TLR4 by LPS-binding protein and depending on the cell type, soluble or membrane bound CD14 is then recruited to this complex (Figure 1A) (Hailman et al., 1994). In the presence of LPS, myeloid of differentiation protein 2 forms a heterotetramer with TLR4 and regulates the responsiveness to different LPS chemotypes (Casella and Mitchell, 2013). If the TLR4 complex internalises by endocytosis, it activates mainly IRF3. In contrast, plasma membrane initiated TLR4 signaling promotes NF-κB activation.
Figure 1.
Toll-like receptor 4 (TLR4) and protease-activated receptor 2 (PAR2) signaling pathways.
(A) Lipopolysaccharide (LPS) is transferred to TLR4 by CD14 followed by formation of TLR4/myeloid of differentiation protein 2 (MD2) heterotetramers. Membrane-bound TLR4 signaling complex induces the myeloid of differentiation primary response gene 88 (MyD88)-dependent signaling cascade by recruiting MyD88 and tumor necrosis factor receptor associated factor (TRAF)6 which in turn activates transforming growth factor activated kinase-1 (TAK1) and the inhibitor of nuclear factor kappa-B kinase (IKK) complex composed of IKKα, IKKβ and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) essential modulator (NEMO). IKKβ phosphorylates inhibitory inhibitor of κB (IκB) proteins leading to their polyubiquitination and proteasomal degradation. This frees the DNA binding NF-κB subunits which in turn translocate into the nucleus and modulate expression of the pro-inflammatory target genes including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β and IL-8. In contrast, internalised, endosomal TLR4 complex recruits Toll/IL-1 receptor (TIR)-domain containing adaptor inducing interferon (TRIF) and TRIF-related adapter molecule (TRAM) activating thereby the MyD88 independent signaling cascade. Subsequently, TRAF3 activates the TRAF family member associated NF-κB activator (TANK)-binding kinase (TBK)1/IKKε which in turn phosphorylates interferon regulatory transcription factor 3 (IRF3) followed by its nuclear translocation and transcription of anti-inflammatory targets including type I interferons and IL-10. Notably, the MyD88 independent signaling cascade also cross-activates NF-κB via interaction between TRIF/TRAM and TRAF6 leading to a weak and late NF- κB activation. (B) Trypsin cleaves PAR2 to expose a new N-terminus which acts as an intramolecular ligand, activating the receptor. Gαq-coupling promotes the mobilization of intracellular Ca2+ and production of diacylglycerol (DAG), the latter activating protein kinase C (PKC). Raf-1 is in turn activated by PKC. Activated PAR2 is phosphorylated increasing its affinity for cytosolic β-arrestins (β-arr), which translocate to the cell surface to interact with the receptor. PAR2-boundβ-arrs act as a molecular scaffold recruiting signaling molecules including rapidly accelerated fibrosarcoma-1 (raf-1), mitogen-activated protein kinase kinase (MEK) and extracellular-regulated protein kinases (ERK1/2) at the plasma membrane and in endosomes. Further activation of NF-κB and IRF3 and activating peptide1 (AP1) occurs via as yet undefined mechanisms. Endosomal-activated ERK1/2 promotes AP1 activity. LBP: LPS-binding protein; JNK: c-Jun N-terminal kinase.
In addition to the subcellular localisation, TLR4 downstream signaling depends on the adapter molecule recruited to the intracellular Toll/interleukin-1 receptor (TIR)-domain of TLR4. The recruited adaptor proteins are either the myeloid of differentiation primary response gene 88 (MyD88) or the TIR-domain containing adaptor inducing interferon (TRIF). Thus, signaling downstream of TLR4 can be either MyD88-dependent or MyD88-independent. TRIF and TRIF-related adapter molecule (TRAM) initiate activation of tumour necrosis factor receptor-associated factor (TRAF)3 (Fitzgerald et al., 2003). Further downstream, TRAF family member associated NF-κB activator-binding kinase 1 associates with the inhibitor of κB (IκB) kinase (IKK)ε to phosphorylate the transcription factor interferon regulatory factor (IRF)3 promoting its nuclear translocation (Fitzgerald et al., 2003). Phosphorylated and thus activated IRF3 has both anti-viral and anti-apoptotic properties and is considered anti-inflammatory (Tarassishin et al., 2013).
In contrast, recruitment of MyD88 to the TIR-domain of TLR4 at the plasma membrane initiates the formation of the so called Myddosome containing MyD88, MyD88 adapter like (Mal or TIR-domain adapter protein) and interleukin-1 receptor-associated kinase 1/2 and 4 (Dossang et al., 2016). This protein complex promotes further downstream signaling, eventually activating the IKK complex. The IKK complex is composed of the catalytic subunits IKKα and β, and the regulatory subunit NF-κB essential modulator (NEMO, also referred to as IKKγ). Activated IKKβ phosphorylates the IκBα, in turn leading to its polyubiqitination and proteasomal degradation. As a cytosolic inhibitor protein, IκBα masks the nuclear localisation signal of the pro-inflammatory transcription factor NF-κB. Thus, its proteasomal degradation frees NF-κB and allows its nuclear translocation. Subsequently, NF-κB binds to its consensus sequences and positively modulates the transcription of its pro-inflammatory target genes (e.g., tumor necrosis factor-alpha) (Kawai and Akira, 2007).
Importantly, TLR4 signaling is tremendously sensitive to the nature, purity and concentration of the ligand and other parameters. Specifically, common technical and conceptual pitfalls in studying TLR4 signaling include the use ligands of unspecified chemotype and purity, inappropriate culture conditions (e.g., serum-free medium for cells lacking CD14) or the choice of inappropriate cellular model (e.g., unmodified human embryonic kidney (HEK) cells for the that lack adapters required to fully activate the MyD88-independent signaling) (Zeuner et al., 2015).
Protease-Activated Receptor 2 Signaling
PAR2 is a member of a small family of G protein-coupled receptors that are activated by an ever-growing repertoire of proteases. Activating proteases cleave PAR2 within its N-terminus to create a new N-terminus that binds to and activates the receptor (reviewed in Ossovskaya and Bunnett (2004)). Once activated PAR2 couples to Gαq proteins to promote the mobilization of intracellular Ca2+ and there are also reports of PAR2-dependent cyclic adenosine monophosphate (cAMP) accumulation, presumably via Gαs proteins (Figure 1B). In addition to G protein-dependent signaling cascades, similar to other G protein-coupled receptors, PAR2 also activates mitogenic signaling pathways. Extracellular-regulated protein kinases are activated via cell surface-dependent signaling molecules and also from internalized PAR2 in endosomes (DeFea et al., 2000). In common with TLR4, PAR2 activates the pro-inflammatory NF-κB signaling cascade. PAR2-dependent activation of NF-κB has been reported in bovine coronary artery smooth muscle cells (Bretschneider et al., 1999), human dermal microvascular endothelial cells (Buddenkotte et al., 2005) and keratinocytes (Macfarlane et al., 2005).
As with other GPCRs, it is now clear that different agonists of PAR2 can activate distinct signaling pathways or activate the same pathways in a biased manner (reviewed in Hollenberg et al. (2014) and Zhao et al. (2014a)). For example, trypsin activates human PAR2 revealing a new N-terminus of S37LIGKV (Knecht et al., 2007), whereas human neutrophil elastase cleaves PAR2 creating a new N-terminus of V68LTGKL (Ramachandran et al., 2011). These unique intra-molecular ligands activate diverse signaling pathways, with trypsin promoting both Gαq-coupling and ERK1/2 activation, whereas neutrophil elastase only activates ERK1/2 (Ramachandran et al., 2011). Another neutrophil-derived protease, cathepsin G cleaves PAR2 in yet another position of its N-terminus, but no known signaling cascades have yet been elucidated following this cleavage and thus, cathepsin G is currently considered as a disabling PAR2 protease (Ramachandran et al., 2011). Synthetic peptides that mimic the proteolytically-derived N-terminus are widely used as specific pharmacological tools to activate PARs. However, it is also clear that these are also biased agonists (Ramachandran et al., 2009) and thus, nothing truly recapitulates the activation via proteases and care must be taken when interpreting the observations when using these tools both in vitro and in vivo.
As well as differences in signaling cascades, biased agonists of PAR2 have major differences in the agonist-induced trafficking of the receptor. Trypsin-dependent cleavage of PAR2 promotes dynamin-, clathrin- and β-arrestin-dependent internalization to endosomes and ultimately ubiquitin-dependent downregulation of the receptor in lysosomes (Bohm et al., 1996; Dery et al., 1999; Roosterman et al., 2003; Jacob et al., 2005). In stark contrast, PAR2 remains on the cell surface following cleavage with neutrophil elastase and cathepsin S (Zhao et al., 2014b, 2015). As receptor signaling is intimately linked to receptor trafficking (reviewed in Villasenor et al. (2016)), indeed signaling from endosomes clearly has different properties to that elicited from the cell surface, it is important that we not only define the biased signaling pathways, but also how they relate to biased trafficking of receptors. Only then can we define new therapeutic targets and interventions.
Bacteria secrete many proteases, but there have been surprisingly few reports of direct cleavage of PARs by bacterial proteases. Pseudomonas aeruginosa elastase cleaves PAR2, but does not promote internalization, leaving PAR2 at the plasma membrane. Furthermore, intracellular Ca2+ release was not promoted (Dulon et al., 2005). A protease from Group A Streptococcus, a cysteine protease streptococcal pyrogenic exotoxin B was similarly found to disarm PAR1, preventing thrombin-induced ERK1/2 activation and platelet aggregation (Ender et al., 2013). The only reported activator of PAR2 is gingipain-R derived from Porphyromonas gingivalis (Lourbakos et al., 1998). Challenge of human neutrophils with purified gingipain-R, promoted intracellular Ca2+ mobilization in a PAR2-dependent mechanism. Given the large number of potential activating and disabling proteases secreted by invading bacteria and other pathogens, this is an area that warrants further investigation.
Potential Interaction Between Toll-Like Receptor 4 and Protease-Activated Receptor 2 and Controversy on the Downstream Signaling
Although both TLR and PAR2 are expressed in the central nervous system and play similar roles in several brain pathologies, little is known about their interaction in brain cells. In other systems, a receptor cooperation and even a direct interaction has been postulated. However, the downstream signaling pathways mediated by the receptor cooperation are still controversially discussed. In particular, a synergistic PAR2 and TLR4-mediated nuclear translocation of NF-κB (assessed using an electrophoretic mobility shift assay) along with an increased production of the pro-inflammatory NF-κB target interleukin 6 have been reported in endothelial human umbilical vein endothelial cells exposed to synthetic PAR2 activating peptides (APs) and Escherichia coli (unspecified strain)-derived LPS of unknown purity (Chi et al., 2001). These results could suggest that PAR2 is cooperating with the MyD88-dependent TLR4 signaling. This view is supported by a report by Zhou et al. (2011) where synergistic up-regulation of the pro-inflammatory NF-κB target gene interleukin-8 has been observed after co-stimulation of the double positive SW620 cells with an AP and LPS of unspecified chemotype and purity.
Over a decade ago, in an attempt to study a potential interaction between PAR2 and TLR4, Rallabhandi and colleagues used TLR4/MD-2/CD14-transduced and PAR2 overexpressing HEK293T (endogenously negative for TLR4, myeloid of differentiation protein 2, and CD14) (Rallabhandi et al., 2008). In their study, they demonstrated that in the absence of TLR4, PAR2-induced NF-κB activation was inhibited by dominant negative TRIF and TRAM constructs. Notably, no NF-κB inhibition was seen if dominant negative MyD88 constructs were used. In presence of TLR4, PAR2 AP showed a synergistic increase of NF-κB activity that was MyD88 dependent in both, TLR4/MD-2/CD14-transduced and PAR2 overexpressing HEK293T and double positive SW620 colonic epithelial cells. In this study, PAR2 APs showed no direct TLR4 agonist activity. Control experiments in macrophages derived from TLR4–/– and PAR2–/– mice revealed that PAR2 APs lead to lower levels of interleukin-1 beta mRNA in TLR4–/– cells, while PAR2–/– macrophages have reduced ability to produce the TLR4 downstream target inducible nitric oxide synthase. Interestingly both activation of interleukin-1 beta and inducible nitric oxide synthase are known to require MyD88-dependent signaling axis of TLR4 downstream cascade.
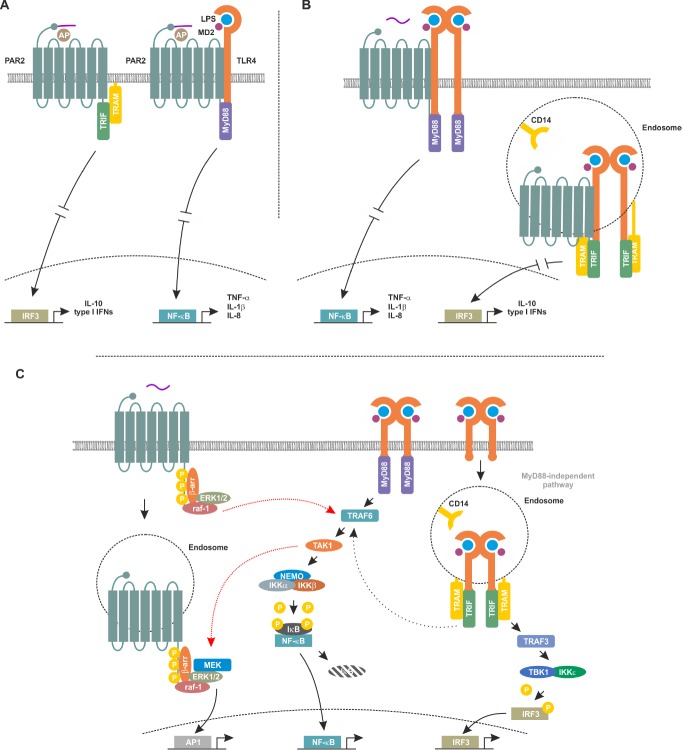
Finally, using co-immunoprecipitation, Rallabhandi et al. suggested a direct association between TLR4 and PAR2. However, as the claim of direct interaction (Figure 2A) has been made based on co-immunoprecipitation alone, a non-direct interaction with involvement of intermediate adapter proteins (e.g., MyD88, TRIF or TRAM) cannot be excluded. In order to address this open question, other modern methodical approaches including but not limited to proximity ligation assays, two-colour super-resolution microscopy, split-yellow fluorescent protein, fluorescence recovery after photobleaching or fluorescence lifetime imaging are required. Moreover, this study utilised lab-made phenol/water-extracted Escherichia coli K235-derived LPS without information on the purity potentially biasing the results by co-stimulation of other receptors.
Figure 2.
Models for potential interaction between Toll-like receptor 4 (TLR4) and protease-activated receptor 2 (PAR2).
(A) Direct interaction at the plasma membrane model. This model proposes that in absence of TLR4, PAR2 activates the myeloid of differentiation primary response gene 88 (MyD88) independent signaling culminating in expression of its anti-inflammatory target genes. However, this model is based on data from experiment involving synthetic activating peptides and does not consider the significant differences between membrane-initiated and endosomal PAP2 and TLR4 signaling (Rallabhandi et al., 2008). (B) Alternative model of direct interaction in endosomes. In this model, PAR2 and TLR4 interact in the plasma membrane and, after internalisation, within endosomes. Membrane bound heterooligomers of PAR2 and TLR4 signal via the MyD88-dependent signaling cascade, whereas the endosomal heterooligomers activate the MyD88-independent signaling pathway. This model considers the proposed direct interaction between TLR4 and PAR2, recognises the differences between membrane initiated and endosomal signaling cascades and could explain the controversial role of the TLR4/PAR2 interaction in modulating pro- and anti-inflammation. (C) Signaling crosstalk model. PAR2 signaling cascade could cross-talk to the TLR4 signaling pathway through the ability of rapidly accelerated fibrosarcoma-1 (raf-1) to (cross-) activate tumor necrosis factor receptor associated factor 6 (TRAF6) which in turn could result in modulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activity. On the other hand, TLR4 could cross-activate the PAR2 signaling cascade via transforming growth factor activated kinase-1 (TAK1)-mediated activation of extracellular-regulated protein kinases (ERK1/2) and subsequent regulation of activating peptide1 (AP1) activity. Overall, this model suggests a signaling crosstalk between PAR2 and TLR4-mediated signaling without a direct interaction of both receptors. LPS: Lipopolysaccharide; MEK: mitogen-activated protein kinase kinase; IL: interleukin; β-arr: β-arrestins; TRIF: Toll/IL-1 receptor-domain containing adaptor inducing interferon; TRAM: TRIF-related adapter molecule; MD2: myeloid of differentiation protein 2; IRF3: interferon regulatory transcription factor 3; TNF-α: tumor necrosis factor-alpha; NEMO: NF-κB essential modulator; IKK: inhibitor of nuclear factor kappa-B kinase; IκB: inhibitor of κB.
Conversely, the same group reported that in peritoneal macrophages isolated from C57BL/6J mice exposed to PAR2 APs and lab-purified LPS from E. coli K235 (no data on purity provided), an upregulation of the anti-inflammatory IRF3 target gene interleukin-10 and suppression of the pro-inflammatory tumor necrosis factor-alpha, interleukin-6, and interleukin-12 has been reported (Nhu et al., 2012). In general accordance with these findings Liang et al. reported in 2015, that PAR2–/– mice showed reduced levels of IRF target gene activation after an in vivo challenge with E. coli O55:B LPS of unspecified purity (Liang et al., 2015). Similarly, PAR2–/– mice displayed more severe lung inflammation in response to P. aeruginosa infection compared to wild-type animals and showed higher levels of the NF-κB target gene tumor necrosis factor-alpha (Moraes et al., 2008). These results support the hypothesis that PAR2 is positively modulating the MyD88-independent signaling axis of the TLR4 signaling cascade and thus plays an anti-inflammatory role.
Summary and Conclusions
As emphasized above, there is increasing evidence that both TLR4 and PAR2 play a significant role in modulating neuroinflammation in health and disease of the central nervous system. Importantly, both receptors can activate pro- and anti-inflammatory signaling pathways and can have either neuroprotective or neurodegenerative roles. Thus, both agonism and antagonism of TLR4 and PAR2 have therapeutic potential when targeting inflammatory diseases of the brain. Due to the reciprocal modulation of both receptors, developing compounds that modulate receptor interactions and selectively activate beneficial signaling pathway represents a promising strategy for targeting neurodegenerative disorders with an inflammatory component including but not limited to AD and PD. However, the exact nature of the interaction between PAR2 and TLR4 is still a matter of an ongoing scientific debate. In particular, open questions include if the reciprocal signaling cross-talk occurs at the level of a direct physical interaction (with or without involvement of adapter proteins, Figure 2A), if it involves endocytosis (Figure 2B) in a complex or if the interaction and cooperation is mainly a result of the use of shared downstream signaling elements (e.g., TRIF and TRAM, Figure 2C).
Additional file: Open peer review report 1 (89.9KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Leonardo Cocito, Universita degli Studi di Genova, Italy.
P-Reviewer: Cocito L; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Afkhami-Goli A, Noorbakhsh F, Keller AJ, Vergnolle N, Westaway D, Jhamandas JH, Andrade-Gordon P, Hollenberg MD, Arab H, Dyck RH, Power C. Proteinase-activated receptor-2 exerts protective and pathogenic cell type-specific effects in Alzheimer’s disease. J Immunol. 2007;179:5493–5503. doi: 10.4049/jimmunol.179.8.5493. [DOI] [PubMed] [Google Scholar]
- 2.Bohm SK, Khitin LM, Grady EF, Aponte G, Payan DG, Bunnett NW. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. J Biol Chem. 1996;271:22003–22016. doi: 10.1074/jbc.271.36.22003. [DOI] [PubMed] [Google Scholar]
- 3.Bretschneider E, Kaufmann R, Braun M, Wittpoth M, Glusa E, Nowak G, Schror K. Evidence for proteinase-activated receptor-2 (PAR-2)-mediated mitogenesis in coronary artery smooth muscle cells. Br J Pharmacol. 1999;126:1735–1740. doi: 10.1038/sj.bjp.0702509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buddenkotte J, Stroh C, Engels IH, Moormann C, Shpacovitch VM, Seeliger S, Vergnolle N, Vestweber D, Luger TA, Schulze-Osthoff K, Steinhoff M. Agonists of proteinase-activated receptor-2 stimulate upregulation of intercellular cell adhesion molecule-1 in primary human keratinocytes via activation of NF-kappa B. J Invest Dermatol. 2005;124:38–45. doi: 10.1111/j.0022-202X.2004.23539.x. [DOI] [PubMed] [Google Scholar]
- 5.Casella CR, Mitchell TC. Inefficient TLR4/MD-2 heterotetramerization by monophosphoryl lipid A. PLoS One. 2013;8:e62622. doi: 10.1371/journal.pone.0062622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi L, Li Y, Stehno-Bittel L, Gao J, Morrison DC, Stechschulte DJ, Dileepan KN. Interleukin-6 production by endothelial cells via stimulation of protease-activated receptors is amplified by endotoxin and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2001;21:231–240. doi: 10.1089/107999001750169871. [DOI] [PubMed] [Google Scholar]
- 7.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dery O, Thoma MS, Wong H, Grady EF, Bunnett NW. Trafficking of proteinase-activated receptor-2 and beta-arrestin-1 tagged with green fluorescent protein, beta-Arrestin-dependent endocytosis of a proteinase receptor. J Biol Chem. 1999;274:18524–18535. doi: 10.1074/jbc.274.26.18524. [DOI] [PubMed] [Google Scholar]
- 9.Dossang AC, Motshwene PG, Yang Y, Symmons MF, Bryant CE, Borman S, George J, Weber AN, Gay NJ. The N-terminal loop of IRAK-4 death domain regulates ordered assembly of the Myddosome signalling scaffold. Sci Rep. 2016;6:37267. doi: 10.1038/srep37267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulon S, Leduc D, Cottrell GS, D’Alayer J, Hansen KK, Bunnett NW, Hollenberg MD, Pidard D, Chignard M. Pseudomonas aeruginosa elastase disables proteinase-activated receptor 2 in respiratory epithelial cells. Am J Respir Cell Mol Biol. 2005;32:411–419. doi: 10.1165/rcmb.2004-0274OC. [DOI] [PubMed] [Google Scholar]
- 11.Ender M, Andreoni F, Zinkernagel AS, Schuepbach RA. Streptococcal SpeB cleaved PAR-1 suppresses ERK phosphorylation and blunts thrombin-induced platelet aggregation. PLoS One. 2013;8:e81298. doi: 10.1371/journal.pone.0081298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT. LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the Toll adapters TRAM and TRIF. J Biol Chem. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hailman E, Lichenstein HS, Wurfel MM, Miller DS, Johnson DA, Kelley M, Busse LA, Zukowski MM, Wright SD. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollenberg MD, Mihara K, Polley D, Suen JY, Han A, Fairlie DP, Ramachandran R. Biased signalling and proteinase-activated receptors (PARs): targeting inflammatory disease. Br J Pharmacol. 2014;171:1180–1194. doi: 10.1111/bph.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurley MJ, Durrenberger PF, Gentleman SM, Walls AF, Dexter DT. Altered expression of brain proteinase-activated receptor-2, Trypsin-2 and serpin proteinase inhibitors in Parkinson’s disease. J Mol Neurosci. 2015;57:48–62. doi: 10.1007/s12031-015-0576-8. [DOI] [PubMed] [Google Scholar]
- 16.Jacob C, Cottrell GS, Gehringer D, Schmidlin F, Grady EF, Bunnett NW. c-Cbl mediates ubiquitination, degradation, and down-regulation of human protease-activated receptor 2. J Biol Chem. 2005;280:16076–16087. doi: 10.1074/jbc.M500109200. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Knecht W, Cottrell GS, Amadesi S, Mohlin J, Skaregarde A, Gedda K, Peterson A, Chapman K, Hollenberg MD, Vergnolle N, Bunnett NW. Trypsin IV or mesotrypsin and p23 cleave protease-activated receptors 1 and 2 to induce inflammation and hyperalgesia. J Biol Chem. 2007;282:26089–26100. doi: 10.1074/jbc.M703840200. [DOI] [PubMed] [Google Scholar]
- 19.Liang HP, Kerschen EJ, Hernandez I, Basu S, Zogg M, Botros F, Jia S, Hessner MJ, Griffin JH, Ruf W, Weiler H. EPCR-dependent PAR2 activation by the blood coagulation initiation complex regulates LPS-triggered interferon responses in mice. Blood. 2015;125:2845–2854. doi: 10.1182/blood-2014-11-610717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu P, Sun L, Zhao XL, Zhang P, Zhao XM, Zhang J. PAR2-mediated epigenetic upregulation of alpha-synuclein contributes to the pathogenesis of Parkinsons disease. Brain Res. 2014;1565:82–89. doi: 10.1016/j.brainres.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Lourbakos A, Chinni C, Thompson P, Potempa J, Travis J, Mackie EJ, Pike RN. Cleavage and activation of proteinase-activated receptor-2 on human neutrophils by gingipain-R from Porphyromonas gingivalis. FEBS Lett. 1998;435:45–48. doi: 10.1016/s0014-5793(98)01036-9. [DOI] [PubMed] [Google Scholar]
- 22.Macfarlane SR, Sloss CM, Cameron P, Kanke T, McKenzie RC, Plevin R. The role of intracellular Ca2+ in the regulation of proteinase-activated receptor-2 mediated nuclear factor kappa B signalling in keratinocytes. Br J Pharmacol. 2005;145:535–544. doi: 10.1038/sj.bjp.0706204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaud JP, Halle M, Lampron A, Theriault P, Prefontaine P, Filali M, Tribout-Jover P, Lanteigne AM, Jodoin R, Cluff C, Brichard V, Palmantier R, Pilorget A, Larocque D, Rivest S. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proc Natl Acad Sci U S A. 2013;110:1941–1946. doi: 10.1073/pnas.1215165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moraes TJ, Martin R, Plumb JD, Vachon E, Cameron CM, Danesh A, Kelvin DJ, Ruf W, Downey GP. Role of PAR2 in murine pulmonary pseudomonal infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L368–377. doi: 10.1152/ajplung.00036.2007. [DOI] [PubMed] [Google Scholar]
- 25.Nhu QM, Shirey KA, Pennini ME, Stiltz J, Vogel SN. Proteinase-activated receptor 2 activation promotes an anti-inflammatory and alternatively activated phenotype in LPS-stimulated murine macrophages. Innate Immun. 2012;18:193–203. doi: 10.1177/1753425910395044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, Breloer M, Henze C, Depboylu C, Skrzydelski D, Michel PP, Dodel RC, Lu L, Hirsch EC, Hunot S, Hartmann A. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep. 2013;3:1393. doi: 10.1038/srep01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 28.Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rallabhandi P, Nhu QM, Toshchakov VY, Piao W, Medvedev AE, Hollenberg MD, Fasano A, Vogel SN. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J Biol Chem. 2008;283:24314–24325. doi: 10.1074/jbc.M804800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD. Targeting proteinase-activated receptors: therapeutic potential and challenges. Nat Rev Drug Discov. 2012;11:69–86. doi: 10.1038/nrd3615. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran R, Mihara K, Mathur M, Rochdi MD, Bouvier M, Defea K, Hollenberg MD. Agonist-biased signaling via proteinase activated receptor-2: differential activation of calcium and mitogen-activated protein kinase pathways. Mol Pharmacol. 2009;76:791–801. doi: 10.1124/mol.109.055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramachandran R, Mihara K, Chung H, Renaux B, Lau CS, Muruve DA, DeFea KA, Bouvier M, Hollenberg MD. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2) J Biol Chem. 2011;286:24638–24648. doi: 10.1074/jbc.M110.201988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roosterman D, Schmidlin F, Bunnett NW. Rab5a and rab11a mediate agonist-induced trafficking of protease-activated receptor 2. Am J Physiol Cell Physiol. 2003;284:C1319–1329. doi: 10.1152/ajpcell.00540.2002. [DOI] [PubMed] [Google Scholar]
- 34.Tang SC, Lathia JD, Selvaraj PK, Jo DG, Mughal MR, Cheng A, Siler DA, Markesbery WR, Arumugam TV, Mattson MP. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp Neurol. 2008;213:114–121. doi: 10.1016/j.expneurol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarassishin L, Bauman A, Suh HS, Lee SC. Anti-viral and anti-inflammatory mechanisms of the innate immune transcription factor interferon regulatory factor 3: relevance to human CNS diseases. J Neuroimmune Pharmacol. 2013;8:132–144. doi: 10.1007/s11481-012-9360-5. [DOI] [PubMed] [Google Scholar]
- 36.Trotta T, Porro C, Calvello R, Panaro MA. Biological role of Toll-like receptor-4 in the brain. J Neuroimmunol. 2014;268:1–12. doi: 10.1016/j.jneuroim.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Villasenor R, Kalaidzidis Y, Zerial M. Signal processing by the endosomal system. Curr Opin Cell Biol. 2016;39:53–60. doi: 10.1016/j.ceb.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Zeuner M, Bieback K, Widera D. Controversial role of Toll-like receptor 4 in adult stem cells. Stem Cell Rev. 2015;11:621–634. doi: 10.1007/s12015-015-9589-5. [DOI] [PubMed] [Google Scholar]
- 39.Zeuner MT, Vallance T, Vaiyapuri S, Cottrell GS, Widera D. Development and characterisation of a novel NF-κB reporter cell line for investigation of neuroinflammation. Mediators Inflamm. 2017;2017:6209865. doi: 10.1155/2017/6209865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeuner MT, Kruger CL, Volk K, Bieback K, Cottrell GS, Heilemann M, Widera D. Biased signalling is an essential feature of TLR4 in glioma cells. Biochim Biophys Acta. 2016;1863:3084–3095. doi: 10.1016/j.bbamcr.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Zhao P, Metcalf M, Bunnett NW. Biased signaling of protease-activated receptors. Front Endocrinol (Lausanne) 2014a;5:67. doi: 10.3389/fendo.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao P, Lieu T, Barlow N, Sostegni S, Haerteis S, Korbmacher C, Liedtke W, Jimenez-Vargas NN, Vanner SJ, Bunnett NW. Neutrophil elastase activates protease-activated receptor-2 (par2) and Transient Receptor Potential Vanilloid 4 (TRPV4) to cause inflammation and pain. J Biol Chem. 2015;290:13875–13887. doi: 10.1074/jbc.M115.642736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao P, Lieu T, Barlow N, Metcalf M, Veldhuis NA, Jensen DD, Kocan M, Sostegni S, Haerteis S, Baraznenok V, Henderson I, Lindstrom E, Guerrero-Alba R, Valdez-Morales EE, Liedtke W, McIntyre P, Vanner SJ, Korbmacher C, Bunnett NW. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem. 2014b;289:27215–27234. doi: 10.1074/jbc.M114.599712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou B, Zhou H, Ling S, Guo D, Yan Y, Zhou F, Wu Y. Activation of PAR2 or/and TLR4 promotes SW620 cell proliferation and migration via phosphorylation of ERK1/2. Oncol Rep. 2011;25:503–511. doi: 10.3892/or.2010.1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.