Abstract
Stem cell transplantation has brought new hope for the treatment of neurological diseases. The key to stem cell therapy lies in inducing the specific differentiation of stem cells into nerve cells. Because the differentiation of stem cells in vitro and in vivo is affected by multiple factors, the final differentiation outcome is strongly associated with the microenvironment in which the stem cells are located. Accordingly, the optimal microenvironment for inducing stem cell differentiation is a hot topic. EGb761 is extracted from the leaves of the Ginkgo biloba tree. It is used worldwide and is becoming one of the focuses of stem cell research. Studies have shown that EGb761 can antagonize oxygen free radicals, stabilize cell membranes, promote neurogenesis and synaptogenesis, increase the level of brain-derived neurotrophic factors, and replicate the environment required during the differentiation of stem cells into nerve cells. This offers the possibility of using EGb761 to induce the differentiation of stem cells, facilitating stem cell transplantation. To provide a comprehensive reference for the future application of EGb761 in stem cell therapy, we reviewed studies investigating the influence of EGb761 on stem cells. These started with the composition and neuropharmacology of EGb761, and eventually led to the finding that EGb761 and some of its important components play important roles in the differentiation of stem cells and the protection of a beneficial microenvironment for stem cell transplantation.
Keywords: nerve regeneration, Ginkgo biloba extract, Ginkgolide B, traditional Chinese medicine, stem cells, induction of differentiation, stem cell transplantation, synaptic plasticity, pharmacological effect, neurological diseases, nervous systems, neural regeneration
Introduction
Nerve cells, once damaged or degenerated, cannot self-repair. Can the loss of neural function caused by neurological disorders be clinically improved or even cured by nerve regeneration or functional replacement by adjacent nerves? Neurons and glial cells differentiated from stem cells from different sources have brought hope for this issue (Chao et al., 2018). The clinical application of endogenous neural stem cells (NSCs) has been restricted by their specialized location and limited quantities. The successful differentiation of exogenous stem cells from other tissues into neural cells has provided a new source of NSCs (Zhang et al., 2018). However, induced differentiation in vitro is influenced by many factors and how a suitable microenvironment that favors neuronal differentiation and provides effective nutritional factors to promote neuronal growth can be created in vitro remains a problem (Wei et al., 2015). Although there are many ways to induce stem cells in vitro, there is still no single way to accurately induce the differentiation of exogenous stem cells into neurons. Hence, it is a focus of current research to find a controllable induction method for stem cell differentiation into neurons, bringing hope to patients suffering neurological disorders.
At present, the main induction methods include: (1) Cell growth factor induction by epidermal growth factor, basic fibroblast growth factor and nerve growth factor (Low et al., 2010); (2) chemical induction by β-mercaptoethanol, dimethyl sulfoxide, and butylated hydroxyanisole (Kang et al., 2006); and (3) growth factor and chemical combined induction: Woodbury et al. (2001) used β-mercaptoethanol, dimethyl sulfoxide, and basic fibroblast growth factor combined induction, while Gesine et al. (2004) utilized nerve growth factor, basic fibroblast growth factor, dibutyryl cyclic AMP, isobutylmethyl xanthine and all-trans-retinoic acid for combined induction of bone marrow mesenchymal stem cells (MSCs) into nerve cells in vitro; (4) other methods (Ren et al., 2014) such as traumatic brain tissue homogenate, gene transfection, traditional Chinese medicine (Baicalin and Salvia miltiorrhiza), co-culture, and conditioned growth medium close to the physiological state.
Cytokines are currently commonly used inducers due to their extensive function in neural nutrition, anti-free radical activity, calcium overload reducing, and inhibition of nitric oxide synthase expression. Among them, epidermal growth factor and basic fibroblast growth factor are most representative as they are not only strong polypeptide factors for promoting cell growth, but also important mitogens, which promote the proliferation and differentiation of stem cells into nerve cells through corresponding receptors on the cell surface (Türeyen et al., 2005). However, epidermal growth factor and basic fibroblast growth factor not only are expensive, they also promote excessive cell growth, which potentially increases tumorigenic risks. Hence, epidermal growth factor and basic fibroblast growth factor are infeasible for clinically use as inducers to obtain the target cells and are mainly used in basic laboratory research. However, it would kill two birds with one stone if currently approved drugs could be utilized to induce the differentiation of stem cells into nerve cells.
In recent years, the success of stem cell differentiation induced by traditional Chinese medicine compound preparations and/or monomers has given hope to stem cell researchers (Xu et al., 2013; Wei et al., 2017). A Ginkgo biloba extract, EGb761, is becoming one of the focuses of stem cell researchers (Schneider, 2008). It is classified as a western medicine and its clinical application has been accepted worldwide. The main chemical components of EGb761 are flavonoids, terpenoid esters, small amounts of polyphenols, alkaloids, long chain alcohols, ketones, and trace elements (Kleijnen and Knipschild, 1992; Chi et al., 1997; Yuan et al., 2010). Approximately 87% of the components have been identified (Lang et al., 2013), and their approximate proportions and possible functions are shown in Table 1.
Table 1.
Main components of EGb761 and their possible functions
| Component | Proportion | Function |
|---|---|---|
| Flavonoids: approximately 38 species, mainly Monoflavones, Flavonols and their glycosides, Biflavones and Latechines, which usually combine with glycosides. | 24% | Excellent natural antioxidants that can scavenge free radicals. |
| Terpenoids lactone: mainly including diterpenoids such as Ginkgolide A, B, C, M and J, and sesquiterpenoids such as Bilobalide. | 6% (Ginkgolides 3.1% and Bilobalide 2.9%, Bilobalide may be the metabolic intermediates of Ginkgolides) | The content of Ginkgolides is the key to evaluate EGb761 quality. Ginkgolides have a strong specific inhibitory effect on platelet activating factor (PAF) receptor, of which Ginkgolide B has the highest anti-PAF activity. |
| Others: polyisoenenol, fatty acids, hydroxy acids, shikimic acids, amino acids, 6-HKA, ginkgo acids (mainly Ginkgolic Acid, Hydroginkgolic Acid, Bilobo1 and Ginkgol). | Non flavonoid glycosides 20%, carboxylic acids 13%, proanthocyanidins 7%, catechin 2%, macromolecule compounds 4%, inorganic matter 5%, water 3%, other 3%. | Polyisopenyl alcohol is a type of polygene (or dolichol). It is a lipid compound in Ginkgo biloba leaves that has cis and trans forms, and plays an important role in maintaining liver function and promoting hematopoiesis. 6-HKA is a broad-spectrum central nervous amino acid antagonist. It acts on N-methyl-D-aspartate to reduce brain hypoxia. Ginkgo acids may be associated with allergy and mutagenesis, therefore are toxic and side effect components of Ginkgo biloba extract. Its content should be controlled below 5 μg/g. |
Studies have shown that EGb761 can antagonize oxygen free radicals, stabilize cell membranes, promote neurogenesis and synaptic plasticity, and improve the level of brain-derived neurotrophic factors, approximating the environment required during the differentiation of stem cells into nerve cells. This offers the possibility of using EGb761 to induce the differentiation of stem cells and provide cell protection after stem cell transplantation. Against this background, we reviewed world-wide studies of the influence of EGb761 on stem cells, in order to provide a comprehensive reference for the future application of EGb761 in stem cell therapy.
Literature Retrieval
We searched the PubMed and Wanfang electronic databases to obtain relevant articles in English and Chinese published up until August 2018 regarding the effects of Ginkgo biloba extracts on the nervous system and stem cells using the following retrieval criteria: Ginkgo biloba extract, stem cells, induction, differentiation, and nerve. The results were further screened by title and abstract to only present Ginkgo biloba extract, neurological diseases, neuropharmacology, and stem cells. Case reports were excluded, but review articles were retained. The articles retrieved are summarized here.
Background to EGb761
EGb761 is extracted from the leaves of the Ginkgo biloba, tree, which originated 200 million years ago, and is thus often termed a living fossil. Ginkgo biloba is mainly found in China and Japan. The leaves were first used as medicine in the Ming Dynasty of ancient China to treat senile cardiovascular diseases. However, as unprocessed Ginkgo biloba leaves contain hydrocyanic acid, which accelerates heart rates, unwelcome side effects occurred (Kumar and Kumar, 2018).
The pharmaceutical development of EGb761 was very active in the 1960s, and the most representative work was promoted by the German company Dr. Willmar Schwabe Pharmaceuticals, who used the leaves of Ginkgo biloba sourced from Japan, as raw material. Around the same time, Japanese scientists also tried to develop EGb761 as a drug. However, because natural extracts contain a variety of ingredients, it was difficult to obtain drug approval in Japan. As reported in a special issue of The Lancet (Kleijnen and Knipschild, 1992), the German team focused on basic research and clinical trials of EGb761. EGb761 was finally approved as a drug in Germany in 1967, and in France later. In the 1980s, more than ¥20 billion worth of EGb761 sold in Germany, becoming the best-selling drug in the field. In 1991, the German company Dr. Willmar Schwabe Pharmaceuticals produced the standardized Ginkgo biloba leaf extract, Ginaton, and patented it. Its quality indexes are: flavonoid glycosides ≥ 24%, terpene lactones ≥ 6%, ginkgolic acids ≤ 5 ppm, and this standard has been adopted in many countries.
Pharmacological Effect of EGB761 on the Nervous System
The pharmacological action of EGb761 mainly focuses on nervous system function, the anti-oxidation activity of clearing free radicals, anti-platelet aggregation, and improving hemorheology (Zhang et al., 2017b). Research on its function in the nervous system mainly focuses on the protection of the cerebral cortex, hippocampus, substantia nigra, and spinal cord (Defeudis and Drieu, 2000; Maclennan et al., 2002; Ponto and Schultz, 2003). It is widely used in the clinical treatment of nervous system diseases and its curative effect has been confirmed, particularly in cerebrovascular disease and Alzheimer’s disease (Chan et al., 2007; Zhang et al., 2013, 2017a). At present, the mechanisms of EGb761’s effect on the nervous system mainly include:
Influencing nerve cell apoptosis
Clearing free radicals and anti-oxidation: Oxidative stress is a common cause of neuronal apoptosis. The mechanism of EGb761’s free radical clearance and anti-oxidation activity has mostly been studied through oxidative stress-induced dynamic changes in oxidative stress and anti-oxidative stress substances (Pan, 2005) and through free radical induced alteration in gene expression (Xin et al., 2000). At present, it is believed that EGb761 contains flavonoids that can directly scavenge free radicals. Additionally, EGb761 can reduce the formation of oxygen free radicals and the release of superoxide anions by stimulating cytochrome P450 enzyme systems, thus exerting an indirect anti-oxidation effect (Logani et al., 2000).
Stabilizing biofilm and protecting the mitochondria: Mitochondrial damage is an important step in nerve cell apoptosis. In addition to scavenging free radicals and anti-oxidation functioning, EGb761 also protects mitochondria by stabilizing the cell membrane, antagonizing Ca2+ overload, regulating enzymes in the respiratory chain, and regulating mitochondrial membrane potential (Ni et al., 1996; Tendi et al., 2002; Chen et al., 2010).
Effect on neurotransmitters
Studies have found that EGb761 has an effect on neurotransmitter systems (Table 2) including the cholinergic systems (Nathan, 2000), monoamine transmitter systems (White et al., 1996; Pardon et al., 2004; Zhang and Cai, 2005), and amino acid neurotransmitters (Chandrasekaran et al., 2003; Wang and Chen, 2005), which is the basis for the increasingly extensive application of EGb761 in neuropsychiatry. Of these systems, EGb761 has the greatest effect on the cholinergic system, which is an important target for the clinical treatment of cognitive disorders such as Alzheimer’s disease.
Table 2.
EGb761 effects on neurotransmission
| References | Types of neurontransmission | Effects |
|---|---|---|
| Nathan (2000) | Cholinergic systems | Immediate actions: Upregulation of post-synaptic muscarnic receptors, modulation of pre-synaptic choline uptake and acetylcholine release, reduction of scopolamine-induced amnesia; Indirect effects: modulation of the serotonergic systems. |
| White et al. (1996) | Monoamine transmitter systems | Inhibit monoamine oxidase (MAO): Both MAO-A and -B types were inhibited to a similar extent; reduction 5-hydroxyindolacetic acid levels, which is metabolite of serotonin; actions on alpha-2-adrenoceptors. |
| Pardon et al. (2004) | ||
| Zhang and Cai (2005) | ||
| Chandrasekaran et al. (2003) | Amino acid neurotransmitters | Against glutamate-induced exitotoxicity by affecting glutamate release |
| Wang and Chen (2005) |
Effect on synaptic plasticity and nerve regeneration
EGb761’s effects on synaptic plasticity mainly relate to the synaptic excitatory activity of neurons in the hippocampus and the induction and formation of long-term potentiation (Smriga et al., 1997), which is another important target for its clinical application in the treatment of cognitive disorders such as Alzheimer’s disease. Recently, an increasing number of studies have found that bilobalide, a sesquiterpene in EGb761, can promote nerve regeneration and increase the level of brain derived neurotrophic factors (Tchantchou et al., 2007; Tchantchou and Pncao, 2009). This has become a point of focus for many researchers, and is also the focus of this review.
Others
EGb761 also has therapeutic effects in cerebrovascular diseases and some neurodegenerative diseases by antagonizing platelet activating factor, regulating the metabolism of arachidic acid, and improving brain circulation (Shi, 2009; Shi et al., 2010).
EGB761 Effect on Stem Cells
As already mentioned, EGb761 was originally found to promote the regeneration of endogenous hippocampal neurons in animals (Osman et al., 2016). Recently, the influence of Ginkgolide B, a bioactive component of EGb761, on stem cells has been well reported. As no clinical grade Ginkgolide B has yet been developed, research on the therapeutic influence of EGb761 on stem cells is beginning to receive more attention.
Effect of EGb761 on neural stem cells
EGb761’s effect on NSCs has mostly been studied through the specific effects of Ginkgolide B on NSCs by Chinese researchers (Zheng et al., 2018). Wang et al. (2007a, b), Huang et al. (2003), and Ding et al. (2004) have studied the effects of Ginkgolide B on isolated NSCs in vitro, while Niu et al. (2014) and Wang et al. (2014) focused on its effect on endogenous NSCs in vivo. Each of these studies explored the correlation between Ginkgolide B dosage and the differentiation of NSCs, and suggested that different dose-dependent effects impact on differentiated neurons and gliocytes, which may be associated with dose-dependent gene regulation (Si et al., 2014). It was also found that flavonoids in EGb761 can promote the differentiation of NSCs, but a three-dimensional stem cell-derived neural model is needed to confirm this (Wu et al., 2016a).
There have been few studies on the effects of EGb761 itself on NSCs. The few studies reported in English investigated the effects on endogenous NSCs in rat models of vascular dementia (Wang et al., 2013) and NSCs in the mouse cochlear (Wang and Han, 2015; Wang and Wang, 2016). More studies have been published in Chinese but these have mostly focused on EGb761s effects on endogenous NSCs. The effects on isolated NSCs were better explored by Li et al. (2006, 2010), who also found that, like Ginkgolide B, the induction outcome of EGb761 was dosage dependent and the optimal dosage of EGb761 was needed for the highest yield of nerve cells (Note: this dosage cannot be too high or too low).
Effect of EGb761 on mesenchymal stem cells
Previous studies have usually focused on the induction of MSCs towards osteogenesis (Gu et al., 2015) and adipogenesis (Wu et al., 2016b) by EGb761. In recent years, scholars began to pay attention to the EGb761-induced differentiation of MSCs into nerve cells. Among them, Li et al. (2013) focused on adipose MSCs, while Su et al. (2007) and Lu et al. (2012) studied bone marrow MSCs, the latter two groups using Ginkgolide B. Whether EGb761 or Ginkgolide B was used, the effect on MSCs in inducing differentiation into nerve cells was time-sensitive, but not dosage-dependent. Additionally, when there was no other neural inducing factor in the induction scheme, EGb761 promoted the differentiation of MSCs into nerve cells rather than into glial cells. The mechanism of this requires further investigation. However, another study of the protective effect of EGb761 on MSCs (Wang et al., 2018) provided another idea for the future application of EGb761 in stem cell therapy, that is, acting as the “guard of MSCs” during MSCs transplantation (Hao et al., 2016).
Other effects
Ginkgolide B can induce NSCs to differentiate into dopaminergic neurons in vitro (Tan et al., 2016). Additionally, studies of the effects of EGb761 or Ginkgolide B on embryonic stem cells (Chan, 2006; Yander et al., 2010) and peripheral blood endothelial progenitor cells (Dong et al., 2007) have also brought new ideas for the application of stem cell therapy to nervous system diseases.
Problems and Perspectives
Numerous studies have shown that the interaction of EGb761 with stem cells offers hope for treating nervous system diseases. This is because EGb761 can promote neurogenesis and synaptic plasticity, and raise the level of brain-derived neurotrophic factors, thus replicating the environment required during the differentiation of stem cells into nerve cells. This makes possible the application of EGb761 to induce stem cell differentiation and cell protection after stem cell transplantation (Figure 1). Relevant patents have already been applied for, such as by the team of Wen-jie Zhao (patent No. CN101396355A) and Yuan-Shan Zeng (patent No. CN1446907A). Therefore, the use of EGb761 to promote the homing of stem cells and to enhance the ability of neural differentiation in stem cell transplantation is worthy of attention. We are currently exploring this field (a project from NSFC 81501185), but the study of EGb761 and the neural differentiation of stem cells is still in its initial stages. At present, most of the studies of the effects of EGb761 on the induction and transplantation of stem cells are conducted in animals. The regulatory mechanism of the neural differentiation of stem cells is very complicated and EGb761 might have a biphasic effect on the neural differentiation of stem cells (Li et al., 2011).
Figure 1.
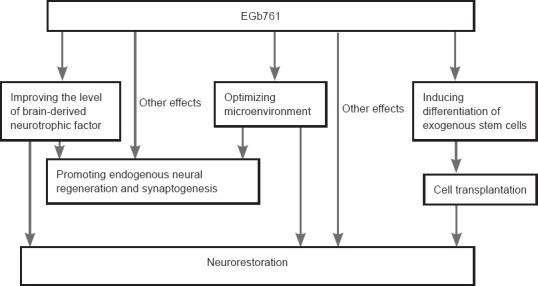
Possible effects of EGb761 on nerve repair.
While it is worthwhile to further study the possibility of the combined use of EGb761 and stem cell transplantation, as well as its specific mechanisms and targets, because the components of the traditional Chinese medicines are complicated, the potential side effects of EGb761 or its impact on other systems should be of concern. For example, it may cause nervous system effects such as dizziness and headache. However, sometimes it might be better to pay attention to promoting clinical transformation (Freedman and Uccelli, 2012). As Sir Martin Evans, the Nobel Prize winner, said in an interview with Life Times, the current focus of stem cell research is to apply experimental results to practical clinical treatment.
China is rich in Ginkgo biloba resources, yielding 70% of the world total of leaves. The use of EGb761 is a merger of eastern and western medicine, but the contribution of the east is often minimized or left out. Ginkgo biloba has been used in Chinese Medicine for centuries, however, its research, development, and utilization in China still falls far behind that of developed countries, despite the raw materials of Ginkgo biloba leaves used for pharmaceuticals being mainly produced in China. The development and utilization of Ginkgo biloba leaves should be accelerated in China, especially when stem cell research is a hot topic, to benefit people worldwide.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was funded by the National Natural Science Foundation of China, No. 81501185 (to CR); the Key Research & Development Project of Shandong Province of China, No. 2017GSF218043 (to CR); the Science and Technology Planning Project of Yantai of China, No. 2016WS017 (to LNG), 2017WS105 (to HL). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was funded by the National Natural Science Foundation of China, No. 81501185 (to CR); the Key Research & Development Project of Shandong Province of China, No. 2017GSF218043 (to CR); the Science and Technology Planning Project of Yantai of China, No. 2016WS017 (to LNG), 2017WS105 (to HL).
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Brooks W, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Chan PC, Xia Q, Fu PP. Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25:211–244. doi: 10.1080/10590500701569414. [DOI] [PubMed] [Google Scholar]
- 2.Chan WH. Ginkgolide B induces apoptosis and developmental injury in mouse embryonic stem cells and blastocysts. Hum Reprod. 2006;21:2985–2995. doi: 10.1093/humrep/del255. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekaran K, Mehrabian ZB, Chinopoulos C, Drieu K, Fiskum G. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry. 2003;36:89–94. doi: 10.1055/s-2003-40447. [DOI] [PubMed] [Google Scholar]
- 4.Chao R, Yin P, Ren N, Zhe W, Wang J, Zhang C, Wei G, Geng D, Wang X. Cerebrospinal fluid-stem cell interactions may pave the path for cell-based therapy in neurological diseases. Stem Cell Res Ther. 2018;9:66–76. doi: 10.1186/s13287-018-0807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C, Wei T, Gao Z, Zhao B, Hou J, Xu H, Xin W, Packer L. Different effects of the constituents of EGb761 on apoptosis in rat cerebellar granule cells induced by hydroxyl radicals. Biochem Mol Biol Int. 1999;47:397–405. doi: 10.1080/15216549900201423. [DOI] [PubMed] [Google Scholar]
- 6.Chi J, Xu L, Ma C, Liu A. Studies on the chemical constituents of the leaves of Ginkgo biloba. Zhongguo Zhongyao Zazhi. 1997;22:106–107. [PubMed] [Google Scholar]
- 7.Defeudis FV, Drieu K. Ginkgo biloba extract (EGb 761) and CNS functions: basic studies and clinical applications. Curr Drug Targets. 2000;1:25–58. doi: 10.2174/1389450003349380. [DOI] [PubMed] [Google Scholar]
- 8.Ding Y, Zeng Y, Zhang W, Chen S. The effects of ginkgolid B of various consistency on the differentiation of neural stem cells. Jiepou Xuebao. 2004;35:484–488. [Google Scholar]
- 9.Dong XX, Hui ZJ, Xiang WX, Rong ZF, Jian S, Zhu CJ. Ginkgo biloba extract reduces endothelial progenitor-cell senescence through augmentation of telomerase activity. J Cardiovasc Pharmacol. 2007;49:111–115. doi: 10.1097/FJC.0b013e31802ef519. [DOI] [PubMed] [Google Scholar]
- 10.Freedman MS, Uccelli A. Neurorepair with mesenchymal stem cells: hope or hype? Lancet Neurol. 2012;11:123–125. doi: 10.1016/S1474-4422(12)70001-7. [DOI] [PubMed] [Google Scholar]
- 11.Gesine K, Sandra S, A AJ, Thorsten T, Markus M, Niklas F, Stefanie L, V SR, Johannes F, Claudia R. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Q, Chen C, Zhang Z, Wu Z, Fan X, Di W, Shi L. Ginkgo biloba extract promotes osteogenic differentiation of human bone marrow mesenchymal stem cells in a pathway involving Wnt/β-catenin signaling. Pharmacol Res. 2015;97:70–78. doi: 10.1016/j.phrs.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Hao F, Li A, Yu H, Liu M, Wang Y, Liu J, Liang Z. Enhanced neuroprotective effects of combination therapy with bone marrow-derived mesenchymal stem cells and ginkgo biloba extract (EGb761) in a rat model of experimental autoimmune encephalomyelitis. Neuroimmunomodulation. 2016;23:41–57. doi: 10.1159/000437429. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Jin GH, Zhang XH. The inducing effects of ginkgolide b on neural stem cells differentiating into neurons. Jiepou Xuebao. 2003;34:367–371. [Google Scholar]
- 15.Kang XQ, Zang WJ, Bao LJ, Li DL, Xu XL, Yu XJ. Differentiating characterization of human umbilical cord blood-derived mesenchymal stem cells in vitro. Cell Biol Int. 2006;30:569–575. doi: 10.1016/j.cellbi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Kleijnen J, Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136–1139. doi: 10.1016/0140-6736(92)93158-j. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Kumar P. Multi-component botanical drugs for degenerative diseases. Clin Trials Degener Dis. 2018;3:115–118. [Google Scholar]
- 18.Lang F, Hoerr R, Noeldner M, Koch E. Ginkgo biloba extract EGb 761®: from an ancient asian plant to a modern european herbal medicinal product. In: Evidence and Rational Based Research on Chinese Drugs. In: Wagner H, Ulrich-Merzenich G, editors. Vienna: Springer; 2013. [Google Scholar]
- 19.Li S, Tang D, Xue Z, Zhang Z, Sun X, Liu Y, Dong H, Yin X. Biphasic effect of EGb761 on simulated ischemia-induced rat BMSC survival in vitro and in vivo. Life Sci. 2011;88:853–863. doi: 10.1016/j.lfs.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Liang Z, Liu J. The effects of extract of Ginkgo biloba on the neuron differentiation of human adipose-derived stem cells. Shenjing Jiepouxue Zazhi. 2013;29:543–551. [Google Scholar]
- 21.Li X, Lu L, Li Y. The effects of Ginkgo biloba extract on differentiation of neural stem cells into neuron like cells in rats. Zhongguo Laonianxue Zazhi. 2010;30:2002–2003. [Google Scholar]
- 22.Li XT, Song YJ, Li YJ. Role of EGB in differentiation of Wistar rat’s NSC_S into astrocyte-like cell. Heilongjiang Yiyao Kexue. 2006;29:4–5. [Google Scholar]
- 23.Logani S, Chen MC, Tran T, Le T, Raffa RB. Actions of Ginkgo biloba related to potential utility for the treatment of conditions involving cerebral hypoxia. Life Sci. 2000;67:1389–1396. doi: 10.1016/s0024-3205(00)00741-4. [DOI] [PubMed] [Google Scholar]
- 24.Low CB, Liou YC, Tang BL. Neural differentiation and potential use of stem cells from the human umbilical cord for central nervous system transplantation therapy. J Neurosci Res. 2010;86:1670–1679. doi: 10.1002/jnr.21624. [DOI] [PubMed] [Google Scholar]
- 25.Maclennan KM, Darlington CL, Smith PF. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog Neurobiol. 2002;67:235–257. doi: 10.1016/s0301-0082(02)00015-1. [DOI] [PubMed] [Google Scholar]
- 26.Nathan P. Can the cognitive enhancing effects of ginkgo biloba be explained by its pharmacology? Med Hypotheses. 2000;55:491–493. doi: 10.1054/mehy.2000.1099. [DOI] [PubMed] [Google Scholar]
- 27.Ni Y, Zhao B, Hou J, Xin W. Preventive effect of Ginkgo biloba extract on apoptosis in rat cerebellar neuronal cells induced by hydroxyl radicals. Neurosci Lett. 1996;214:115–118. doi: 10.1016/0304-3940(96)12897-4. [DOI] [PubMed] [Google Scholar]
- 28.Niu G, Wang J, Shang F. Effects of Ginkgolide B on endogenous neural stem cells of newborn rats with hypoxic- ischemic brain damage. Zhongguo Kangfu Yixue Zahzi. 2014;29:415–420. [Google Scholar]
- 29.Osman NMS, Amer AS, Abdelwahab S. Effects of Ginko biloba leaf extract on the neurogenesis of the hippocampal dentate gyrus in the elderly mice. Anat Sci Int. 2016;91:280–289. doi: 10.1007/s12565-015-0297-7. [DOI] [PubMed] [Google Scholar]
- 30.Pan HP. Progress in studies of pharmacological activities and clinical applications of preparations of dried leaf of Ginkgo biloba. Zhongguo Zhong Yao Za Zhi. 2005;30:93. [PubMed] [Google Scholar]
- 31.Pardon MC, Hanoun N, Perezdiaz F, Joubert C, Launay JM, Christen Y, Hamon M, Cohensalmon C. Long-term treatment with the antioxidant drug EGb 761 at senescence restored some neurobehavioral effects of chronic ultramild stress exposure seen in young mice. Neurobiol Aging. 2004;25:1067–1083. doi: 10.1016/j.neurobiolaging.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Ponto LL, Schultz SK. Ginkgo biloba extract: review of CNS effects. Ann Clin Psychiatry. 2003;15:109–119. doi: 10.1023/a:1024688326023. [DOI] [PubMed] [Google Scholar]
- 33.Ren C, Geng RL, Ge W, Liu XY, Chen H, Wan MR, Geng DQ. An observational study of autologous bone marrow-derived stem cells transplantation in seven patients with nervous system diseases: a 2-year follow-up. Cell Biochem Biophys. 2014;69:179–187. doi: 10.1007/s12013-013-9756-8. [DOI] [PubMed] [Google Scholar]
- 34.Schneider LS. Ginkgo biloba extract and preventing Alzheimer disease. JAMA. 2008;300:2306–2308. doi: 10.1001/jama.2008.675. [DOI] [PubMed] [Google Scholar]
- 35.Shi C. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against β-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem Biol Interact. 2009;181:115–123. doi: 10.1016/j.cbi.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Shi C, Wu F, Xu J. H2O2 and PAF mediate Abeta1-42-induced Ca2+ dyshomeostasis that is blocked by EGb761. Neurochem Int. 2010;56:893–905. doi: 10.1016/j.neuint.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Si YC, Li Q, Xie CE, Niu X, Xia XH, Yu CY. Chinese herbs and their active ingredients for activating xue (blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells. Chin Med. 2014;9:13–20. doi: 10.1186/1749-8546-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smriga M, Saito H, Nishiyama N. Ginkgo biloba facilitates synaptic plasticity in the rat perforant path: dentate gyrus projections in-vivo. Pharm Sci. 1997;3:521–523. [Google Scholar]
- 39.Su P, Huang J, Luo X. Effect of ginkgolide B on differentiation of bone marrow mesenchymal stem cells into neuron like cells. Guangdong Yixue. 2007;28:33–35. [Google Scholar]
- 40.Tan X, Sun B, Zhu Q, Zhou H, Wang M, Xu Z, Jin G, Anatomy DO. Differentiation from neural stem cells into dopaminergic neurons induced by ginkgolide B. Zhongguo Jiepouxue Zazhi. 2016;39:10–13. [Google Scholar]
- 41.Tchantchou F, Pncao L. Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. J Alzheimers Dis. 2009;18:787–798. doi: 10.3233/JAD-2009-1189. [DOI] [PubMed] [Google Scholar]
- 42.Tchantchou F, Xu Y, Wu Y, Christen Y, Luo Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007;21:2400–2408. doi: 10.1096/fj.06-7649com. [DOI] [PubMed] [Google Scholar]
- 43.Tendi EA, Bosetti F, Dasgupta SF, Stella AM, Drieu K, Rapoport SI. Ginkgo biloba extracts EGb 761 and bilobalide increase NADH dehydrogenase mRNA level and mitochondrial respiratory control ratio in PC12 cells. Neurochem Res. 2002;27:319–323. doi: 10.1023/a:1014963313559. [DOI] [PubMed] [Google Scholar]
- 44.Türeyen K, Vemuganti R, Bowen KK, Sailor KA, Dempsey RJ. EGF and FGF-2 infusion increases post-ischemic neural progenitor cell proliferation in the adult rat brain. Neurosurgery. 2005;57:1254–1263. doi: 10.1227/01.neu.0000186040.96929.8a. [DOI] [PubMed] [Google Scholar]
- 45.Wang A, Yang Q, Li Q, Wang X, Hao S, Wang J, Ren M. Ginkgo Biloba. L., extract reduces H2O2-induced bone marrow mesenchymal stem cells cytotoxicity by regulating mitogen-activated protein kinase (MAPK) signaling pathways and oxidative stress. Med Sci Monit. 2018;24:3159–3167. doi: 10.12659/MSM.910718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Han Z. Ginkgo biloba extract enhances differentiation and performance of neural stem cells in mouse cochlea. Cell Mol Neurobiol. 2015;35:861–869. doi: 10.1007/s10571-015-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang C, Wang B. Ginkgo biloba extract attenuates oxidative stress and apoptosis in mouse cochlear neural stem cells. Phytother Res. 2016;30:774–780. doi: 10.1002/ptr.5572. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Chen W, Wang Y. A ginkgo biloba extract promotes proliferation of endogenous neural stem cells in vascular dementia rats. Neural Regen Res. 2013;8:1655–1662. doi: 10.3969/j.issn.1673-5374.2013.18.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Zhang DX, Wang BZ. Effect of ginkgolide B on Foxg1 mRNA expression and endogenous neural stem cell proliferation of cerebral tissue of rats with hypoxic-ischemic brain damage. Zhongguo Fuyou Baojian. 2014;29:2233–2237. [Google Scholar]
- 50.Wang SJ, Chen HH. Ginkgolide B, a constituent of Ginkgo biloba, facilitates glutamate exocytosis from rat hippocampal nerve terminals. Eur J Pharmacol. 2005;514:141–149. doi: 10.1016/j.ejphar.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 51.Wang YH, Liu HL, Shi YJ. Ginkgolide B promoting neuritc outgrouth of differentiated in vitro. Guoji Naoxueguang Bing Zazhi. 2007a;15:739–743. [Google Scholar]
- 52.Wang YH, Luo X, Shi YJ. Mechanisms and effect of Ginkgolide B on the differentiation of neuron stem cells. Zhongguo Kangfu Lilun yu Shijian. 2007b;13:701–703. [Google Scholar]
- 53.Wei G, Ren C, Duan X, Geng D, Zhang C, Liu X, Chen H, Wan M, Geng R. Differentiation of mesenchymal stem cells into neural stem cells using cerebrospinal fluid. Cell Biochem Biophys. 2015;71:1–7. doi: 10.1007/s12013-014-0222-z. [DOI] [PubMed] [Google Scholar]
- 54.Wei Q, Chen S, Yang S, Xu Q, Xu C, Cai J. The effect of traditional Chinese medicine on neural stem cell proliferation and differentiation. Aging Dis. 2017;8:792–811. doi: 10.14336/AD.2017.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White HL, Scates PW, Cooper BR. Extracts of Ginkgo biloba leaves inhibit monoamine oxidase. Life Sci. 1996;58:1315–1321. doi: 10.1016/0024-3205(96)00097-5. [DOI] [PubMed] [Google Scholar]
- 56.Woodbury D, Schwarz EJ, Prockop DJ. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2001;27:632–636. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y, Sun J, George J, Ye H, Cui Z, Li Z, Liu Q, Zhang Y, Ge D, Liu Y. Study of neuroprotective function of Ginkgo biloba extract (EGb761) derived-flavonoid monomers using a three-dimensional stem cell-derived neural model. Biotechnol Prog. 2016a;32:735–744. doi: 10.1002/btpr.2255. [DOI] [PubMed] [Google Scholar]
- 58.Wu Z, Zhang J, Gu X, Zhang X, Shi S, Liu C. Effects of the extract of Ginkgo biloba on the differentiation of bone marrow mesenchymal stem cells in vitro. Am J Transl Res. 2016b;8:3032–3040. [PMC free article] [PubMed] [Google Scholar]
- 59.Xin W, Wei T, Chen C, Ni Y, Zhao B, Hou J. Mechanisms of apoptosis in rat cerebellar granule cells induced by hydroxyl radicals and the effects of EGb761 and its constituents. Toxicology. 2000;148:103–110. doi: 10.1016/s0300-483x(00)00200-6. [DOI] [PubMed] [Google Scholar]
- 60.Xu SL, Zhu KY, Bi CW, Yan L, Men SW, Dong TT, Tsim KW. Flavonoids, derived from traditional Chinese medicines, show roles in the differentiation of neurons: possible targets in developing health food products. B Birth Defects Res C Embryo Today. 2013;99:292–299. doi: 10.1002/bdrc.21054. [DOI] [PubMed] [Google Scholar]
- 61.Yan LU, Liu S, Liu D, Liu X, Liu DQ, Liu YH. Electrophysiology study on differentiation of rat bone marrow stromal stem cells into neurom-like cells by Ginkgolide B. Xiandaishengwu Yixue Jinzhan. 2012;28:2170–2176. [Google Scholar]
- 62.Yander H, Tzongfu K, Kunhsiung L, Liu YC, Huang YT, Lai CY, Chan WH. Ginkgolide B induces apoptosis via activation of JNK and p21-activated protein kinase 2 in mouse embryonic stem cells. Ann N Y Acad Sci. 2010;1171:501–508. doi: 10.1111/j.1749-6632.2009.04691.x. [DOI] [PubMed] [Google Scholar]
- 63.Yuan F, Yu R, Yin Y, Shen J, Dong Q, Zhong L, Song L. Structure characterization and antioxidant activity of a novel polysaccharide isolated from Ginkgo biloba. Int J Biol Macromol. 2010;46:436–439. doi: 10.1016/j.ijbiomac.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Zhang C, Ren C, Chen H, Geng R, Fan H, Zhao H, Guo K, Geng D. The analog of ginkgo biloba extract 761 is a protective factor of cognitive impairment induced by chronic fluorosis. Biol Trace Elem Res. 2013;153:229–236. doi: 10.1007/s12011-013-9645-4. [DOI] [PubMed] [Google Scholar]
- 65.Zhang CY, Chen R, Wang F, Ren C, Zhang P, Li Q, Li HH, Guo KT, Geng DQ, Liu CF. EGb-761 Attenuates the anti-proliferative activity of fluoride via DDK1 in PC-12 Cells. Neurochem Res. 2017a;42:606–614. doi: 10.1007/s11064-016-2115-6. [DOI] [PubMed] [Google Scholar]
- 66.Zhang DL, Li D, Wang MJ. Mechanism and application of stem cell transplantation in the treatment of ischemic stroke. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:5393–5398. [Google Scholar]
- 67.Zhang M, Cai J. Extract of Ginkgo biloba leaves reverses yohimbine-induced spatial working memory deficit in rats. Behav Pharmacol. 2005;16:651–656. doi: 10.1097/00008877-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Zhang P, Liao L, Deng Z, Tan Y. Research progress of pharmacological effects and clinical application of ginkgo biloba extract. Jinzhou Yike Daxue Xuebao. 2017b;2017:426–429. [Google Scholar]
- 69.Zheng PD, Mungur R, Zhou HJ, Hassan M, Jiang SN, Zheng JS. Ginkgolide B promotes the proliferation and differentiation of neural stem cells following cerebral ischemia/reperfusion injury, both in vivo and in vitro. Neural Regen Res. 2018;13:1204–1211. doi: 10.4103/1673-5374.232476. [DOI] [PMC free article] [PubMed] [Google Scholar]


