In the cerebral cortex, local inhibition is achieved by different types of gamma-aminobutyric (GABA)-expressing interneurons, representing essential key players of cortical information processing (Marin, 2012). Their development has to be highly orchestrated to ensure proper numbers and subtype composition in the adult cortex and defects in interneuron development contribute to the pathophysiology of psychiatric diseases (Marin, 2012). Different from cortical projection neurons, inhibitory interneurons have their origin in proliferative zones in the basal telencephalon from where they have to migrate over long distances up to the cortex (Zimmer-Bensch, 2018). During this migratory process, post-mitotic interneurons have to continuously adopt their morphology and directionality following guidance cues that are expressed in spatiotemporal-specific manner (Zimmer-Bensch, 2018). Detection and integration of environmental signals are achieved by receptor and intracellular signaling proteins that are expressed in a subset-specific manner. This mediates the differential responses of interneuron subtypes to certain guidance factors and provides an explanation for the different migratory streams that appear to correlate with interneuron identity (Zimmer-Bensch, 2018).
During the extended period of post-mitotic development, the regulation of cell survival and subtype-specific differentiation is further of great relevance for proper network formation in the adult cortex (Zimmer-Bensch, 2018). All these processes are coordinated by the specific expression of regulatory proteins, like particular transcription factors, driving downstream gene activation or repression, thereby contributing to the morphological and electrophysiological diverse GABAergic interneuron subtypes. Hence, uncovering regulatory key players of particular genetic networks helps to shed light on subtype-specific interneuron development and could reveal potential causes for associated diseases.
LHX1 regulates the migration of preoptic area (POA)-derived cortical interneurons: Diverse members of the LIM-containing homeodomain transcription factor family have fundamental roles in brain und neuron development regulating cell morphology, differentiation and survival (Hunter and Rhodes, 2005). Some of them are specifically known to participate in the regulation of interneuron development destined for the cortex, spinal cord and cerebellum (Hunter and Rhodes, 2005). Among them, LHX1 was shown to control motor neuron migration (Palmesino et al., 2010) and the maintenance of the inhibitory-neurotransmitter status in Nestin-expressing murine spinal cord interneurons by regulating PAX2 expression, a key regulator for their GABAergic identity (Pillai et al., 2007). Hence, LHX1 seems to be crucial for the migration and differentiation of GABAergic neurons.
In the telencephalon, LHX1 is very locally expressed in the POA, a domain in the basal telencephalon that gives rise to diverse types of GABAergic interneurons targeting many different brain regions including cortical interneurons (Gelman et al., 2009; Pensold et al., 2017). Thereby, LHX1 partially overlaps with HMX3, expressed in early post-mitotic POA cells. We investigated the role of LHX1 in the development of HMX3-expressing POA-derived cortical interneurons (Symmank et al., 2018b).
For this, we established a conditional knockout mouse model in which Lhx1 was deleted in Hmx3-Cre expressing post-mitotic cells (Hmx3-Cre/tdTomato/Lhx1 loxP2; Lhx1 KO). As controls we used Hmx3-Cre/tdTomato (Lhx1 WT) mice in which only Cre-mediated expression of the fluorescent reporter protein tdTomato is induced. Due to the described relevance of LHX1 in regulating migratory aspects in non-cortical interneurons (Palmesino et al., 2010), we performed a detailed analysis of the migration pattern of Hmx3-deriving Lhx1 knockout and wild-type POA cells at embryonic day 16.5, at the peak of migration of HMX3-positive interneurons (Symmank et al., 2018a). Many POA-derived interneurons were shown to preferentially use the superficial migratory stream through the basal telencephalon entering the developing cortical compartment through the piriform cortex (Zimmer et al., 2011; Pensold et al., 2017; Symmank et al., 2018b). In contrast to wild-type cells, Lhx1 knockout embryos displayed an increased proportion of POA-derived cells migrating along the deep route through the subventricular zone of the medial and lateral ganglionic eminence (Figure 1A).
Figure 1.
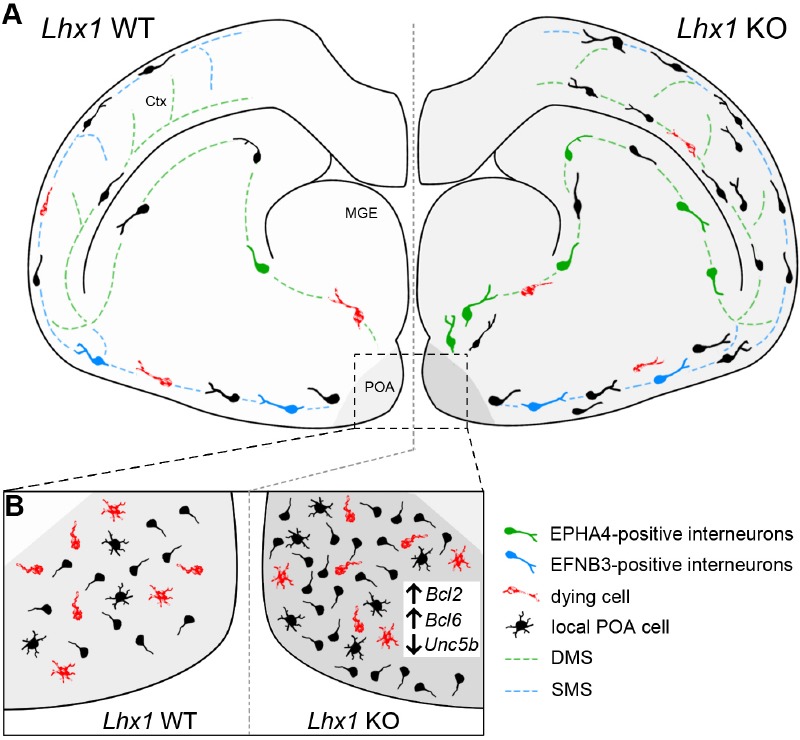
LHX1 regulates the migration and survival of immature preoptic area (POA)-derived interneurons destined for the cerebral cortex.
(A) The population of Hmx3-expressing cells in the POA gives rise to interneurons destined for the cerebral cortex and other brain regions as well as to local cells remaining on site. POA-derived interneurons preferentially are using the superficial migratory stream (SMS, blue line) to migrate through the basal telencephalon into the cortex (Lhx1 WT). A small proportion is also using the deep migratory stream (DMS, green line) through the MGE. Bidirectional EPHA4-EFNB3 signaling contributes to the separation and channeling of immature interneurons into superficial migrating EFNB3-positive cells (blue cells) and deep migrating EPHA4-positive cells (green cells). Post-mitotic deletion of Lhx1 in Hmx3-expressing cells (Lhx1 KO) lead to an enhanced overall cell number and an increased proportion of POA-derived interneurons using the DMS. As possible reason, Lhx1 deficiency promotes higher levels of EPHA4 expression and reduced EFNB3 levels in embryonic POA cells. (B) Prior to migration LHX1 restrict cell survival (indicated by red cells) possibly by repressing pro-survival genes like Bcl2 and Bcl6 and promoting pro-apoptotic Unc5b expression which is misregulated in Lhx1 KO.
Migrating interneurons are guided by diverse attractive and repulsive membrane-bound and secreted cues (Zimmer-Bensch, 2018) and bidirectional repulsive EFNB3/EPHA4 signaling was reported to be involved in the segregation of POA and MGE-derived interneurons into superficial and deep migratory stream (Zimmer et al., 2011). While EPHA4 expressed by MGE-derived interneurons elicits repulsive responses in EFNB3-positive interneurons of the POA through reverse signaling, EFNB3 in turn acts as repellent for MGE-interneurons through EPH4 forward signaling (Zimmer et al., 2011). As a LHX1-dependent transcriptional control of EphA4 was already reported for motorneurons (Kania and Jessell, 2003) and as the preference of POA-derived cells for the superficial migratory stream was reduced in Lhx1 KO, we asked whether LHX1 modulates EFNB3/EPHA4 signaling in immature POA-derived interneurons destined for the cerebral cortex through transcriptional regulation. Indeed, siRNA-mediated depletion of Lhx1 in dissociated embryonic POA cells revealed alterations in both, EPHA4 and EFNB3 expression, which could cause the altered migration pattern (Symmank et al., 2018b). Together, our data suggest that LHX1 is crucial for channeling the migration of POA-derived interneurons by repressing EPHA4 and promoting EFNB3 expression (Figure 1A). This is in agreement with recent studies confirming the relevance of LHX1 for regulating the expression of members of the EPH-receptor/EFN-ligand family during head development (Sibbritt et al., 2018). In contrast to the previous study showing correlated levels of EPHA4 and LHX1 in motor neurons (Kania and Jessell, 2003), we identified a negative correlation between EPHA4 and LHX1 expression, (Symmank et al., 2018b), which could be explained by cell-type specific binding partners, coactivators or repressors.
In addition to changes in interneuron migration, we observed alterations in cortical positioning in the embryonic cortex as well as in the adult cortex of Lhx1-deficient mice (Figure 1A), suggesting that migratory routes and their environmental stimuli influence or predispose for final positioning (Symmank et al., 2018b). There are several studies, emphasizing the relevance of external stimuli migrating interneurons are confronted with for subtype-specific maturation programs (Zimmer-Bensch, 2018). Hence, although LHX1 is only expressed at early post-mitotic stages, the LHX1-dependent transcriptional networks reach out to later stages of interneuron development like cortical distribution and morphological maturation, presumably due to the changed environmental signals integrated during migration. To what extent the altered distribution of Lhx1-deficient POA-derived interneurons affects neuronal connectivity and network activity, is not investigated so far and will be addressed in future studies.
LHX1 regulates the survival of POA-derived cortical interneurons: Besides correct layer distribution, the final number of interneurons in the cortex is of great importance for inhibitory circuitries. Alongside with our studies on cell migration, we also detected alterations in the total number of Hmx3-deriving cells in Lhx1 knockout mice already at embryonic stages that persisted into adulthood (Symmank et al., 2018b). To this end, we analyzed tdTomato-positive wild-type und Lhx1-deficient cells in the embryonic POA, basal telencephalon and cortex as well as in the adult POA, septum and the visual, somatosensory and motor cortex. We assumed that the increased cell numbers, found in the embryonic POA, basal telencephalon and cortex of Lhx1 knockout mice, were based on diminished apoptotic events normally reducing neuronal numbers at embryonic and postnatal stages. Using the TdT-mediated dUTP-biotin nick end labeling method and quantitative expression analysis of Lhx1-deficient POA cells as well as Neuroblastoma (N2a) cells as cell culture model, we found strong evidence that LHX1 negatively acts on cell survival through transcriptional repression of pro-survival genes like Bcl2 and Bcl6, and positively regulates the expression of the pro-apoptotic gene Unc5b (Figure 1B). Overexpression studies in mouse embryos recently confirmed the transcriptional control of apoptosis-regulating genes of the BCL2-family by LHX1 (Sibbritt et al., 2018).
Although Lhx1-mediated regulation of cell death is known for mitotic cells, our study highlights the role of LHX1 in the post-mitotic regulation of cell death. Even prior to migratory events, LHX1 seems to control cellular survival, as the enhanced cell number was already observed within the embryonic POA in Lhx1-deficient mice. As the HMX3-lineage also generates glial cells for the cerebral cortex, as well as interneurons for other brain regions and non-migrating cells remaining in the POA, one could speculate that LHX1 has an early role in balancing the number of these different cell types. This would fit to the typical functions of LIM-containing homeodomain transcription factors controlling cell fate and thereby also survival (Hunter and Rhodes, 2005). However, in adult mice we found increased numbers of both, cortical neurons and glial cells, which rather suggest that LHX1 regulates cell survival somehow cell type-independently.
Unlike LHX1, which seems to promote cell death in Hmx3-derived interneurons, we recently uncovered the important role of DNMT1 as positive regulator of post-mitotic POA cell survival and modulator of their migratory characteristics (Pensold et al., 2017). By repressing pro-apoptotic genes like Pak6, DNMT1 function is crucial for immature interneuron viability and morphological changes during their post-mitotic migration to cortical target regions (Pensold et al., 2017). Unpublished data of our group indicate that DNMT1 represses Lhx1 thereby acting on neuronal migration and survival as well (data not shown). Similar to the DNMT1-mediated Pak6 repression (Symmank et al., 2018b), Lhx1 expression could be also regulated by a complex interplay of DNMT1 with histone-modifying enzymes.
Taken together, identifying LHX1 as another important regulator of the migration and survival of POA-derived cortical interneurons our study adds an additional key player to the regulatory network of gene expression driving post-mitotic interneuron development.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Sanusi Mohammad Bello, King Saud University, Saudi Arabia; Viviane Rostirola Elsner, Centro Universitário Metodista-IPA, Brazil.
P-Reviewers: Bello SM, Elsner VR; C-Editors: Zhao M, Li JY; T-Editor: Liu XL
References
- 1.Gelman DM, Martini FJ, Nobrega-Pereira S, Pierani A, Kessaris N, Marin O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter CS, Rhodes SJ. LIM-homeodomain genes in mammalian development and human disease. Mol Biol Rep. 2005;32:67–77. doi: 10.1007/s11033-004-7657-z. [DOI] [PubMed] [Google Scholar]
- 3.Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions. Neuron. 2003;38:581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 4.Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 5.Palmesino E, Rousso DL, Kao TJ, Klar A, Laufer E, Uemura O, Okamoto H, Novitch BG, Kania A. Foxp1 and lhx1 coordinate motor neuron migration with axon trajectory choice by gating Reelin signalling. PLoS Biol. 2010;8:e1000446. doi: 10.1371/journal.pbio.1000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pensold D, Symmank J, Hahn A, Lingner T, Salinas-Riester G, Downie BR, Ludewig F, Rotzsch A, Haag N, Andreas N, Schubert K, Hubner CA, Pieler T, Zimmer G. The DNA Methyltransferase 1 (DNMT1) Controls the shape and dynamics of migrating poa-derived interneurons fated for the murine cerebral cortex. Cereb Cortex. 2017;27:5696–5714. doi: 10.1093/cercor/bhw341. [DOI] [PubMed] [Google Scholar]
- 7.Pillai A, Mansouri A, Behringer R, Westphal H, Goulding M. Lhx1 and Lhx5 maintain the inhibitory-neurotransmitter status of interneurons in the dorsal spinal cord. Development. 2007;134:357–366. doi: 10.1242/dev.02717. [DOI] [PubMed] [Google Scholar]
- 8.Sibbritt T, Ip CK, Khoo PL, Wilkie E, Jones V, Sun JQJ, Shen JX, Peng G, Han JJ, Jing N, Osteil P, Ramialison M, Tam PPL, Fossat N. A gene regulatory network anchored by LIM homeobox 1 for embryonic head development. Genesis. 2018;56:e23246. doi: 10.1002/dvg.23246. [DOI] [PubMed] [Google Scholar]
- 9.Symmank J, Bayer C, Schmidt C, Hahn A, Pensold D, Zimmer-Bensch G. DNMT1 modulates interneuron morphology by regulating Pak6 expression through crosstalk with histone modifications. Epigenetics. 2018a;13:536–556. doi: 10.1080/15592294.2018.1475980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Symmank J, Golling V, Gerstmann K, Zimmer G. The Transcription Factor LHX1 Regulates the Survival and Directed Migration of POA-derived Cortical Interneurons. Cereb Cortex. 2018b doi: 10.1093/cercor/bhy063. doi: 10.1093/cercor/bhy063. [DOI] [PubMed] [Google Scholar]
- 11.Zimmer-Bensch G. Diverse facets of cortical interneuron migration regulation-implications of neuronal activity and epigenetics. Brain Res. 2018;1700:160–169. doi: 10.1016/j.brainres.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Zimmer G, Rudolph J, Landmann J, Gerstmann K, Steinecke A, Gampe C, Bolz J. Bidirectional ephrinB3/EphA4 signaling mediates the segregation of medial ganglionic eminence-and preoptic area-derived interneurons in the deep and superficial migratory stream. J Neurosci. 2011;31:18364–18380. doi: 10.1523/JNEUROSCI.4690-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


