Abstract
The most common age-related neurodegenerative disease is Alzheimer’s disease (AD) characterized by aggregated amyloid-β (Aβ) peptides in extracellular plaques and aggregated hyperphosphorylated tau protein in intraneuronal neurofibrillary tangles, together with loss of cholinergic neurons, synaptic alterations, and chronic inflammation within the brain. These lead to progressive impairment of cognitive function. There is evidence of innate immune activation in AD with microgliosis. Classically-activated microglia (M1 state) secrete inflammatory and neurotoxic mediators, and peripheral immune cells are recruited to inflammation sites in the brain. The few drugs approved by the US FDA for the treatment of AD improve symptoms but do not change the course of disease progression and may cause some undesirable effects. Translation of active and passive immunotherapy targeting Aβ in AD animal model trials had limited success in clinical trials. Treatment with immunomodulatory/anti-inflammatory agents early in the disease process, while not preventive, is able to inhibit the inflammatory consequences of both Aβ and tau aggregation. The studies described in this review have identified several agents with immunomodulatory properties that alleviated AD pathology and cognitive impairment in animal models of AD. The majority of the animal studies reviewed had used transgenic models of early-onset AD. More effort needs to be given to creat models of late-onset AD. The effects of a combinational therapy involving two or more of the tested pharmaceutical agents, or one of these agents given in conjunction with one of the cell-based therapies, in an aged animal model of AD would warrant investigation.
Keywords: Alzheimer's disease, neuropathology, cognitive deficits, behavioral deficits, immunomodulatory agents, animal models, amyloid deposits, gliosis
Introduction
The most common age-related neurodegenerative disease is Alzheimer’s disease (AD) characterized by aggregated amyloid-β (Aβ) peptides in extracellular plaques and aggregated hyperphosphorylated tau protein in intraneuronal neurofibrillary tangles, together with loss of cholinergic neurons, synaptic alterations, and chronic inflammation within the brain. These lead to progressive impairment of cognitive function and brain tissue destruction (Spires-Jones and Hyman, 2014). Poor facial recognition ability, social withdrawal, increased anxiety, and likelihood of wandering, are other behavioral and cognitive symptoms of AD (Chung and Cummings, 2000).
There is evidence of innate immune activation in AD with microgliosis. Classically-activated microglia (M1 state) secrete inflammatory and neurotoxic mediators, e.g., interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and interferon-γ (Cherry et al., 2014), which increase the activity and expression of γ-secretase, contributing to Aβ deposition and the early pathological changes in AD (Liao et al., 2004; Sastre et al., 2008; Glass et al., 2010; Mandrekar-Colucci and Landreth, 2010). The most pronounced inflammatory reaction occurs around Aβ plaques, which are often completely surrounded by activated microglia in both humans (Sheng et al., 1997) and mouse models of AD (Frautschy et al., 1998). There is also recruitment of peripheral immune cells to inflammation sites in the brain (Heneka et al., 2015). Recent studies in a mouse model of AD have suggested that alternatively-activated microglia (M2 state) might decrease proinflammatory reactions (Cherry et al., 2014), and increase phagocytosis of Aβ plaques, resulting in cognitive improvement (Ohtaki et al., 2008; Lee et al., 2012).
Early-onset (familial, FAD) and late-onset (sporadic) are the two major forms of AD. Early-onset AD is rare accounting for < 5% of cases, whereas late-onset AD accounts for > 95% of cases and occurs later than 65 years of age (Bali et al., 2012). A combination of genetic (70%) and environmental factors (30%) is thought to be important in the etiology of the disease (Dorszewska et al., 2016). The single greatest genetic risk factor for late-onset AD is APOE4 (apollipoprotein E4 gene) (Naj et al., 2011). The few drugs approved by the US FDA for the treatment of AD improve symptoms but do not change the course of disease progression and may cause some undesirable effects (Bassil and Grossberg, 2009; Mimica and Presecki, 2009). AD is a complex multifactorial disease, involving amongst others inflammation, mitochondrial dysfunction, and oxidative stress with formation of reactive oxgen and reactive nitrogen species, and complex network interactions may need to be identified instead of single drug targets. In addition, AD has a long pathophysiological process which begins many years before the symptomatic stage of AD is reached. Therefore, targeting the asymptomatic or preclinical stages may be necessary to successfully treat AD (Riedel, 2014).
Activated microglia drive the inflammatory response induced by extracellular Aβ deposits and later enhanced by aggregates of tau, and which increases with the progression of AD. Translation of active and passive immunotherapy targeting Aβ in many AD animal model trials had limited success in clinical trials. Treatment with immunomodulatory/anti-inflammatory agents early in the disease process, while not preventive, is able to inhibit the inflammatory consequences of both Aβ and tau aggregation (McGeer and McGeer, 2013). A recent review article summarized more than 140 substances, including several anti-inflammatory agents, and treatment modalities in studies of mouse models of AD published in the years 2000–2012 (Li et al., 2013). We have searched the PubMed database for recent studies published in the period January 2012–August 2018 aimed at downregulating immune and inflammatory processes in animal models of AD using immunomodulatory agents and which could be important in slowing disease progression and might be exploited as treatments in human patients with AD. These included pharmacological agents and also cell-based therapies.
Animal Models of Alzheimer’s Disease
Mouse models of AD, while not completely replicating all aspects of the disease, develop specific pathological features which closely mimic aspects of human AD. These animal models can be used to understand some of the pathomechanisms in the progression of AD. Current models use knowledge from FAD, incorporating mutant forms of amyloid precursor protein (APP), presinilin 1 or 2 (PS1 or PS2), tau and other genes (Li et al., 2013). Several APP/PS1 transgenic (Tg) mice have been developed. APP/PS1 are double Tg mice expressing a chimeric mouse/human APP (APP695swe) and a human mutant presinilin 1 (PS1deltaE9). Aβ deposits develop in the brain of Tg mice by 6 to 7 months of age. From 6 to 15 months of age, females develop a 5-fold (Aβ42) and 10-fold (Aβ40) increase in Aβ deposits in the cerebellum by 15 months compared to males (Borchelt, 2018). Mice having three mutant genes beta-APP (betaAPPswe), presenilin-1 (PS1M146V), and tauP301L are known as 3xTg-AD (or 3xFAD) mice. The 3xTg-AD mice progressively develop Aβ and tau pathology that closely resembles that in the human AD brain. Aβ deposits are first apparent in the cortex and progress to the hippocampus with aging, whereas tau pathology is initiated in the hippocampus and then progresses to the cortex. Aβ deposition precedes the tangle pathology (Oddo et al., 2003). Mice having mutant APP (K670N/M671L + I716V + V717I) and PS1 (M146L + L286V) are known as 5xFAD mice (Oakley et al., 2006). They are associated with increasing Aβ accumulation with aging (Holcomb et al., 1998), cognitive defects at an early age and extensive neuronal loss, but not with neurofibrillary tangles (Oakley et al., 2006; Li et al., 2013). APP/PS1 mice carrying mutant APP (K594M/N595L) and PS1 (A246EordE9) have been often used in testing various AD therapies.
While these models have contributed to understanding the biology of key aspects of AD, such as the formation of amyloid plaques and neurofibrillary tangles, they have not proved particularly effective as preclinical models. This may be partly due to the lack of critical hallmarks of AD in the current models, most notably significant neuronal cell loss. Also there may be significant differences between early-onset (familial) and late-onset (sporadic) AD such that treatments tested in existing models may be useful for early-onset AD but not for late-onset AD which is more common in human patients (Onos et al., 2016). In contrast to generating mouse models of early-onset AD, creating models of late-onset AD is more challenging. This is mainly due to the complex genetic and environmental factors that interact to cause late-onset AD, many of which are still unknown or uncertain.
Techniques for genetic manipulation in rats have lagged behind that of mice in the development of AD animal models. Transgenic rat models offer distinctive advantages over mice. The rat is more physiologically, genetically and morphologically closer to humans than is the mouse. In addition, the rat has a well-characterized behavioral pattern. Postnatal brain development in rats would lead to a greater number of synapses and a more complex synaptic organization than in mice. Rat models of AD should enable a more accurate assessment of the impact of AD pathology on cognitive outcomes (Do Carmo and Cuello, 2013). Transgenic rat models of AD have recently been described (Do Carmo and Cuello, 2013; Petrasek et al., 2016; Joo et al., 2017). Non-transgenic rat models that exhibit neurodegeneration and cognitive decline include those injected intracerebroventricularly with streptozotocin (Bassani et al., 2017), and animals that are olfactory bulboectomized (OBX) (Borre et al., 2014; Yehuda and Rabinovitz, 2014).
Immunomodulatory Therapies for Alzheimer’s Disease
Pharmaceutical therapies
The pharmaceutical therapies were with fasudil, LW-AFC, curcumin, TNF inhibitor XPro1595, IL-1 receptor antagonist (IL-1RA), pioglitazone, rosiglitazone, quercetin, IL-1β, cannabidiol, multi-targeted diet (zinc, melatonin, curcumin, piperine, eicosapentaenoic acid, docosahexaenoic acid, uridine, choline), and clioquinol. These have all been shown to have immunomodulatory properties (fasudil: Thorlacius et al., 2006; Song et al., 2013; Liu et al., 2015; LW-AFC: Wang et al., 2016; curcumin: Gaulam et al., 2007; TNF inhibitor XPro1595: Fischer et al., 2015; IL-1RA: Granowitz et al., 1992; Nedumpun et al., 2017; pioglitazone: Singh et al., 2011; El-Sisi et al., 2015; rosiglitazone: Liu et al., 2009; Serghides et al., 2009; quercetin: Li et al., 2016; Casas-Grajales et al., 2017; IL-1β: Chen et al., 2010; cannabidiol: Mecha et al., 2013; Zgair et al., 2017; memantine: Lowinus et al., 2016; Lee et al., 2015; melatonin: Giannoulia-Karantana et al., 2006; Medrano-Campillo et al., 2015; piperine: Sunila and Kuttan, 2004; Rodgers et al., 2009; eicosapentaenoic acid: Iwami et al., 2011; Hirahashi et al., 2014; decosahexaenoic acid: Koch et al., 2006; Hjorth and Freund-Levi, 2012; uridine: Abood et al., 2014; choline: Pavlov et al., 2003; Parrish et al., 2006; Parrish et al., 2008; Rowley et al., 2010; clioquinol: Kidd et al., 2016).
The thirteen animal studies utilizing these pharmaceutical agents are summarized in Table 1. Eleven of these studies had used mouse models, and two a rat model. In the mouse studies, the ages of the animals at which treatment was started ranged from 6 weeks to 21 months and where gender was specified 4 had used males, 3 females, and 2 both males and females. The rat studies had used males, with ages ranging from 8 weeks to 4 months. The treatment period with the pharmacological agent ranged from 30 days to 5 months.
Table 1.
Studies of pharmacological therapies with immunomodulatory properties in animal models of Alzheimer’s disease (AD)
| Study | Details |
|---|---|
| Fasudil | |
| Reference | Yu et al. (2017) |
| Number of animals, gender, ages, and treatment | Adult male amyloid precursor protein (APP)/presinilin 1 (PS1) transgenic (Tg) mice (APPswe/PSEN1deltaE9), 8 months of age, treated with Fasudil intraperitoneally (i.p.) (25 mg/kg per day, n = 8) for 2 months. Behavior was tested by Morris water maze (MWM) test. Animals were anesthetized and brains removed for biochemical analysis and immunohistochemistry (IHC). |
| Comparison | APP/PS1 Tg mice treated with saline i.p. (n = 8) for 2 months. Age-matched male wild type (WT) also served as control. |
| Functional outcomes | In MWM test, APP/PS1 Tg mice exhibited increased latency to target, latency to 1st entrance to southwest zone and mean distance to target compared to WT mice, suggesting that the learning and memory deficits in APP/PS1 Tg mice appeared. Significantly shorter time and distance spent by mice from the starting point on to the platform zone were observed in APP/PS1 + Fasudil mice compared with APP/PS1 + saline mice. APP/PS1 + Fasudil mice spent significantly greater time in southwest zone and distance in southwest zone. Using IHC to determine the amyloid-β (Aβ)42 expression in the hippocampus, the area of immunoreactive deposits of Aβ42 was decreased in APP/PS1 + Fasudil mice compared with APP/PS1 + saline mice. Also by Western blot assay, Fasudil significantly inhibited the levels of Aβ42 in the brains of APP/PS1 + Fasudil mice compared with APP/PS1 + saline mice. By IHC, the number of p-Tau/Ser396-positive cells in the hippocampus was significantly reduced in APP/PS1 + Fasudil mice compared with APP/PS1 + saline mice. Analysis by Western blot showed that expression of p-Tau/Ser396 protein was increased in APP/PS1 + saline mice compared with WT mice and there was a significant decrease in APP/PS1 + Fasudil mice compared with APP/PS1 + saline mice. IHC revealed a marked increase in the number of β-secretase (BACE)-positive cells in the hippocampus of APP/PS1 + saline mice compared with WT mice, and that Fasudil treatment partially attenuated this increase. Western blot assay indicated that expression of BACE protein in APP/PS1 Tg mice was significantly elevated compared with WT mice and was significantly decreased by Fasudil treatment for 8 weeks. Postsynaptic density-95 (PSD-95) is a synaptic protein regulating glutamate receptor anchoring, synaptic stability and certain types of memory regulated by Aβ. By IHC, the number of PSD-95-positive cells in the hippocampus of APP/PS1 + saline mice was decreased compared with WT mice, while treatment of APP/PS1 Tg mice with Fasudil increased the expression. Using Western blot assay, the expression of PSD-95 was significantly higher in APP/PS1 + Fasudil mice compared with APP/PS1 + saline mice. By IHC and Western blot for cortex and hippocampus, p-nuclear factor-kappa B (NF-κB)/p65 expression was increased in APP/PS1 + saline mice compared with WT mice and Fasudil treatment decreased p-NF-κB/p65 expression, which was co-located in CD11b+ microglia. The levels of Rho-associated protein kinase-II (ROCK-II), Toll-like receptor (TLR)-4 and myeloid differentiation primary response 88 (MyD88) proteins were increased in APP/PS1 + saline mice compared with WT mice and Fasudil treatment significantly inhibited the expression of ROCK-II, TLR-4 and MyD88 proteins compared with APP/PS1 + saline mice. However, there was no significant difference in the expression of TLR-2 protein among the three groups. The levels of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α in hippocampus were higher in APP/PS1 + saline mice compared with WT mice. Fasudil treatment significantly decreased the production of IL-1β, IL-6 and TNF-α, and significantly increased the production of IL-10 and interferon (IFN)-γ compared with APP/PS1 + saline mice. |
| Conclusion | Fasudil improved memory, reduced Aβ deposition and tau phosphorylation, decreased BACE, increased PSD-95 and inhibition of TLR-NF-κB-MyD88 inflammation and decreased production of proinflammatory cytokines IL-1β, IL-6 and TNF-α with increased production of anti-inflammatory cytokine IL-10 in APP/PS1 mice. |
| LW-AFC (a formula derived from Liuwei Dihuang Decoction) | |
| Reference | Wang et al. (2017) |
| Number of animals, gender, ages, and treatment | Adult male SAMP8 mice (senescence-accelerated mouse prone 8), 6 months of age, received intragastric administration of LW-AFC (0.1 mL/10 g body weight, 80, 160 and 320 mg/mL in water, n = 9–11) once a day for 150 days. After administration for 3 months, behavioral tests performed. Following the behavioral studies, blood plasma, hypothalamus, pituitary and spleen were collected for analysis. A graded scoring system is used for evaluation of degree of senescence in SAMP8 mice and was designed to assess changes in behavior and appearance of the mice. Grade 0 represented no particular changes and grade 4 represented the most severe changes (Hosokawa et al., 1984). |
| Comparison | SAMP8 mice as model group and SAMR1 mice (senescence-accelerated mouse-resistant 1) (n = 10) as control given an equal volume of water. |
| Functional outcomes | SAMP mice are a model of age-related/late-onset AD (Cheng et al., 2014), while SAMR1 mice represent a normal aging control (Shimada and Hasegawa-Ishii, 2011). The grading score for evaluation of degree of senescence in SAMP8 mice was significantly higher than in SAMR1 mice, and the grading score was significantly reduced after being treated with medium dosage LW-AFC (1.6 g/kg) while there was no significant difference between other treated groups and model group. The total distance of SAMP8 mice in the spontaneous locomotor test was significantly decreased compared with SAMR1 mice. SAMP8 mice administered LW-AFC (0.8, 1.6, 3.2 g/kg) had significantly increased locomotor activity compared to model group. The short (1 hour) and long term (24 hours) object recognition memory were deficit in SAMP8 mice compared to SAMR1 mice, while that of all the treated SAMP8 mice improved and administration with LW-AFC 1.6 g/kg significantly reversed the deficit. In MWM test, SAMP8 mice showed longer escape tendency than SAMR1 mice on the final day, and the latencies of SAMP8 mice treated with LW-AFC (0.8, 1.6 g/kg) were significantly less than the model group, thereby showing that LW-AFC (0.8, 1.6 g/kg) could ameliorate the impairment of spatial learning in SAMP8 mice. Biochemical analysis showed that in hypothalamic-pituitary-adrenal (HPA) axis the concentrations of corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and corticosterone were significantly increased in SAMP8 mice compared to SAMR1 mice, while the level of CRH was significantly decreased by LW-AFC) (1.6, 3.2 g/kg), and ACTH was significantly decreased by LW-AFC (0.8, 1.6 g/kg). In hypothalamic-pituitary-gonadal (HPG) axis, the concentrations of gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were significantly increased and testosterone (T) was significantly decreased in SAMP8 mice compared with SAMR1 mice, while the level of GnRH, FSH and LH was significantly decreased by LW-AFC (0.8, 1.6 g/kg) and T was significantly increased by LW-AFC (0.8, 1.6, 3.2 g/kg). These data show LW-AFC ameliorates imbalance of HPA and HPG axis in SAMP8 mice. Using multiplex bead analysis of blood plasma, IL-1β, IL-2, IL-6, IL-23, granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, chemotactic factor exotoxin, regulated on activation in normal T-cell expressed and secreted (RANTES), TNF-α and TNF-β were significantly increased, while IL-4 and IL-10, chemotactic factor monocyte chemotactic protein 1 (MCP-1) were significantly decreased in SAMP8 mice compared to SAMR1 mice. LW-AFC treatment decreased IL-1β, IL-2, IL-23, GM-CSF, IFN-γ, exotoxin, TNF-α and TNF-β and increased MCP-1 production in 0.8, 1.6, 3.2 g/kg dosage, and decreased IL-6 and RANTES in 0.8, 1.6 g/kg dosage, and meanwhile increased IL-5 production in 1.6 g/kg dosage. These results show LW-AFC administration regulated and restored the aberrant immune function in SAMP8 mice. |
| Conclusion | LW-AFC ameliorated cognitive deterioration and restored the imbalance in HPA and HPG axis, and regulated the abnormal production of cytokines in SAMP8 mice. |
| Reference | Wang et al. (2016) |
| Number of animals, gender, ages, and treatment | Adult male APP/PS1 mice, 9 months of age, received intragastric administration of LW-AFC (0.1 mL/10 g body weight, 160 mg/mL in water, n = 10–11) once a day for 150 days. Behavioral tests were performed starting at 102 days (locomotor activity test) from the beginning of LW-AFC administration and the last one at 131 days. Following the behavioral studies, blood plasma, hypothalamus, pituitary and spleen were collected for analysis. |
| Comparison | APP/PS1 mice as model group and age-matched male WT mice (n = 15) as control given an equal volume of water. |
| Functional outcomes | The locomotor activity test assessed the spontaneous motor activity of APP/PS1 mice, and no significant difference was found between the groups. The novel object recognition test assessed the object recognition memory of mice. LW-AFC treatment in APP/PS1 mice significantly decreased the preferential index, indicating that LW-AFC ameliorated the object recognition memory deficit of APP/PS1 mice. The MWM test assessed the spatial learning and memory of APP/PS1 mice. For the learning task, APP/PS1 mice had longer escape latencies than WT mice on the final test day and the latencies of LW-AFC treated APP/PS1 mice were significantly less than those of the model group. These results showed that LW-AFC ameliorated the spatial learning impairment of APP/PS1 mice. In the probe trial, the escape latency was increased, the number of plate crossings decreased and the time in the target quadrant was decreased, but swimming speed was not significantly different for APP/PS1 mice compared to WT mice. The escape latency decreased, the number of plate crossings increased, and the time in the target quadrant increased in APP/PS1 mice treated with LW-AFC. These findings indicated that LW-AFC significantly improved the spatial learning and memory deficits of APP/PS1 mice. LW-AFC also improved the passive avoidance impairment of APP/PS1 mice. Nissl staining showed typical neuropathological changes in the CA1 and CA3 regions of hippocampus in APP/PS1 mice compared to WT mice, including neuronal loss and nucleus shrinkage or disappearance. Significantly lower Nissl body numbers were seen in the whole brain, hippocampus, and CA1 and CA3 regions of APP/PS1 mice than in WT mice. LW-AFC treatment significantly decreased these neuropathological changes and increased the density of healthy neurons in the hippocampus and CA3 region of APP/PS1 mice. Thus, LW-AFC protected against neuronal loss in the hippocampus of APP/PS1 mice. APP/PS1 mice developed a significant number of Aβ plaques in the brain at 14 months, while Aβ plaques were not found in WT mice. LW-AFC treated APP/PS1 mice had a significantly smaller number of Aβ deposits in the whole brain and hippocampus. Hence, LW-AFC alleviated Aβ deposition in the brain of APP/PS1 mice. The levels of Aβ42 and Aβ40 in the hippocampus and blood plasma of APP/PS1 mice were significantly higher than for WT mice. LW-AFC treatment of APP/PS1 mice led to significantly lower Aβ42 levels in the hippocanpus and in the plasma than in WT mice. Within the HPA axis, the concentration of CRH, ACTH and corticosterone were significantly higher in APP/PS1 mice than in WT mice. LW-AFC significantly decreased the CRH and ACTH level. Within the HPG axis, the concentration of GnRH, FSH and LH were higher in APP/PS1 mice, but T was not significantly different between APP/PS1 and WT mice. LW-AFC significantly decreased the concentration of GnRH, FSH and LH in APP/PS1 mice. Significantly fewer CD8+CD28+ T cells and significantly more CD3+CD2+Foxp3+ T cells were observed in APP/PS1 mice than in WT mice. LW-AFC treatment of APP/PS1 mice increased the expression of CD8+CD28+ T cells and decreased that of CD3+CD2+Foxp3+ T cells. These findings showed that LW-AFC treatment partially restored nomal lymphocyte expression in APP/PS1 mice. By multiplex bead analysis of blood plasma, increases in the levels of IL-1β, IL-2, IL-6, IL-23, GM-CSF, TNF-α, TNF-β, eotaxin, and decreases in the levels of IL-4 and granulocyte-colony stimulating factor (G-CSF) were seen in APP/PS1 mice compared to WT mice. The levels of of IL-1β, IL-2, IL-6, IL-23, GM-CSF, TNF-α, TNF-β, eotaxin were decreased, and IL-4 and G-CSF were increased, after LW-AFC treatment. These results showed that cytokine secretion was abnormal in APP/PS1 mice and that administration of LW-AFC restored this aberrant immune function in APP/PS1 mice. |
| Conclusion | LW-AFC ameliorated behavioral and pathological deterioration, and restored the imbalance in HPA and HPG axis, and the aberrant immune function in APP/PS1 mice. |
| Curcumin | |
| Reference | Sundaram et al. (2017) |
| Number of animals, gender, ages, and treatment | p25 single Tg mice were crossed with Ca2+/calmodulin-dependent protein kinase IIα single Tg mice to generate bi-transgenic offspring (p25Tg mice) that inducibly overexpress the human p25 gene under the control of the Ca2+/calmodulin-dependent protein kinase promoter-regulated tet-off system. p25Tg mice were maintained on deoxycycline (200 µg/mL, in drinking water) from conception to 6 weeks postnatal to avoid any posible developmental consequences from the p25 expression. Hemizygous mice either male or female were used. p25 expresion was induced in 6-week-old mice by removal of deoxycycline in water and concurently treated with an optimized curcumin formulation, Longvida, orally via their feed (4 g/kg, 0.8 g/kg curcumin) of chow for 12 weeks. Behavioral studies were performed for 12-week induced (18-week-old) p25Tg and control mice with and without curcumin treatment (n = 5–6/group). Also biochenical assays were carried out on brain samples from p25Tg and control mice with and without curcumin treatment (n = 3/group). |
| Comparison | WT littermates were used as control. |
| Functional outcomes | Using IHC and Western blot with anti-green fluorescent protein (GFP) antibody, equivalent levels of p25 expression were confirmed in the curcumin-treated as well as non-treated p25Tg mice. There was no obvious change in cyclin-dependent kinase 5 (Cdk5) protein levels between the 12-week induced curcumin-treated and non-treated p25Tg mice. However, p-25-mediated Cdk5 hyperactivity was significantly decreased in curcumin-treated 12-week induced p25Tg mice compared to non-treated p25Tg mice. By IHC, the intensity of glial fibrillary acidic protein (GFAP) staining was reduced in the cortex and hippocampus of curcumin-treated 12-week induced p25Tg mice compared to non-treated p25Tg mice. Western blot analysis of GFAP levels showed there was a 2–3-fold significant decrease in GFAP expression in the forebrain of curcumin-treated p25Tg mice compared to non-treated p25Tg mice. Western blot analysis and cPLA2 activity assay results showed an approximately 3-fold significant decrease in p25-mediated cPLA2 upregulation in curcumin-treated 12-week induced p25Tg mice. Mass spectrometry data indicated that lysophosphatidylcholine (LPC) levels were significantly decreased by curcumin treatment in p25Tg mice. With IHC, altered immunostaining pattern with anti-CD11b (a microglial activation marker) was observed in both cortex and hippocampus of curcumin-treated 12-week induced p25Tg mice compared to non-treated p25Tg mice. Western blot analysis indicated a modest reduction in microglial activation in curcumin-treated 12-week induced p25Tg mice but was not significant with the small group sizes used. By real time polymerase chain reaction, the predominantly anti-inflammatory cytokine transforming growth factor-β levels were unaltered in curcumin-treated p25Tg mice. However, the proinflammatory cytokines macrophage inflammatory protein-1α, TNF-α, IL-1β expression levels in p25TG mice were significantly downregulated by curcumin treatment. Using IHC to study tau hyperphosphorylation levels, ATB (tau) immunostaining levels were decreased in curcumin-treated p25Tg mice compared to non-treated p25Tg mice. Western blot analysis showed a significant 2-fold reduction in ATB expression levels in curcumin-treated p25Tg mice. Histochemical staining assays showed a marked decrease in Aβ42 immunostaining of cortex and hippocampus in curcumin-treated p25Tg mice compared to non-treated p25Tg mice. Forebrain atrophy and neuronal apoptosis were apparent in 12-week induced p25Tg mice. IHC analysis showed cleaved caspase-3 immunostaining was reduced in cortex and hippocampus of 12-week induced p25Tg mice after curcumin treatment, indicating that curcumin confers neuroprotection against p25-induced neuronal death. Spatial memory was assessed using radial arm maze analyses and curcumin-treated p25Tg mice displayed better performance compared to non-treated p25Tg mice. Working memory errors were reduced almost to normal levels and reference memory errors decreased in curcumin-treated p25Tg mice. Hence, curcumin had a neuroprotetive capability to restore p25-inducd cognitive deficits in p25Tg mice. |
| Conclusion | Curcumin ameliorated neuroinflammation, neurodegeneration, and memory deficits in p25Tg mice. |
| Reference | Bassani et al. (2017) |
| Number of animals, gender, ages, and treatment | Adult male Wistar rats, 3-4 months of age, randomly divided into groups: streptozotocin (STZ, n = 7), STZ + curcumin 25 mg/kg (STZ + cu 25, n = 6), STZ + curcumin 50 mg/kg (STZ + cu 50, n = 8), STZ + curcumin 100 mg/kg (STZ + cu 100, n = 7). The animals in STZ groups received a single bilateral intracerebroventricular injection of STZ (3 mg/kg total dose) in saline (4.5 µL/injection site). Treatment with curcumin (25, 50 and 100 mg/kg, per os (p.o.)) or its vehicle (0.5% carboxymethylcellulose in water with 1% Tween 80) was performed over 30 days, once daily in afternoon, and started 1 hour before the beginning of stereotactic surgeries. Behavioral evaluations were started at 3 weeks after surgeries. The animals were assessed in the open field test to evaluate spontaneous locomotor activity and exploratory behavior on day 21 after surgery (day 0 = day of surgery) and in the elevated plus maze (EPM) on day 22 to assess anxiety-like behavior. Cognitive performance was evaluated in the object location test (OLT) on day 28, in the object recognition test on day 29, and in the spatial version of the Y maze on day 30. Immediately after the last behavioral analysis, blood glucose levels were measured using blood samples collected by tail prick. Afterwards animals were anaesthetized and intracardially perfused for IHC evaluation of brains. |
| Comparison | Sham treated animals served as control (n = 7). |
| Functional outcomes | The analysis of spontaneous locomotor activity in the open field test did not reveal any changes in the locomotor parameters analysed in all of the groups compared to the sham group. In the EPM, a decrease in the time spent in closed arms and percentage of time spent in closed arms was seen in the STZ and STZ + cu 100 groups compared to the sham group, which is an indication of decreased anxiety-like behavior in these animals. None of the groups showed more anxiety-like behavior than the sham group in all of the EPM parameters tested. Anxiety-like behavior can reduce the exploration of novelty in the OLT, object recognition test, and Y maze. There were no significant alterations in blood glucose level in all of the groups compared with the sham group. Elevated blood glucose is another factor that could interfere with cognitive performance. The STZ animals showed deficits in short-term spatial memory, reflected in a decrease in the discrimination index in the OLT and a decrease in the time spent on the new arm in the Y maze when compared with the sham group. In the OLT, none of the curcumin-treated groups showed an increased discrimination index compared with the STZ group. In the Y maze, none of the curcumin-treated groups spent more time in the new arm than the STZ group, nor was the time spent on the new arm greater than 33%. Therefore treatment of STZ-lesioned animals with curcumin in different doses did not prevent the impairment in short-term spatial memory. In the object recognition test, the STZ animals exhibited deficits in short-term recognition memory, reflected by a decrease in the discrimination index compared with the sham group. The STZ + cu 50 and STZ + cu 100 groups exhibited an increased discrimination index compared with the STZ group, thereby suggesting a possible beneficial effect of curcumin on short-tem recognition memory at these doses. The STZ animals exhibited decreased newborn neurons in both the subventricular zone of the lateral ventricles (LVs) and subgranular zone of the dentate gyrus (DG) of the hippocampus, reflected by decreased doublecortin (DCX) immunoreactivity (IR) in the subventricular zone of the LVs and decreased DCX-positive cells in the DG of the hippocampus compared with the sham group. Treatment with curcumin in any dose did not restore DCX-IR in the subventricular zone and DCX-positive cells in the DG when compared with the STZ group. At 30 days after surgery, the STZ group showed a marked increase in ionized calcium binding adaptor molecule-1 (Iba-1)-IR in all periventricular areas analysed (i.e. LVs, septum and corpus callosum) and also in the CA1 and CA2 areas of the dorsal hippocampus, but not in the DG, when compared with the sham group. Curcumin treatment at 25 and 50 mg/kg caused an improvement in Iba-1-IR in corpus callosum but not in the LVs and septum, despite an important but not significant reduction in the LVs and septum. In the hippocampus, no improvement in Iba-1-IR was found in the DG, CA1 and CA2 areas for all of the curcumin doses tested. Similarly, the STZ animals showed a marked increase in GFAP-IR in all periventricular areas analysed (i.e., LVs, septum and corpus callosum) and also in the DG, CA1 and CA2 areas of the dorsal hippocampus when compared with the sham group. Curcumin treatment did not improve GFAP-IR in the periventricular areas and the hippocampus with all of the doses tested. |
| Conclusion | Curcumin improved short-term recognition memory and exerted only slight improvement in neuroinflammation, resulting in no improvement in hippocampal and subventricular neurogenesis. |
| Soluble TNF (sTNF) inhibitor XPro1595 | |
| Reference | MacPherson et al. (2017) |
| Number of animals, gender, ages, and treatment | Female 5xFAD mice were used for all flow cytometry, XPro1595, and electrophysiology experiments. Female mice show accelerated AD-like pathology when compared to male 5xFAD mice. For measurement of cytokines in cerebrospinal fluid and inflammatory mRNA analysis, both male and female mice at 2, 4 and 6 months of age were used. To assess the role of soluble TNF in AD-like pathology and immune cell trafficking in the CNS, Tg female mice were treated subcutaneous (s.c.) with XPro1595 (10 mg/kg) twice weekly for 2 months. To assess the role of sTNF on trafficking immune cell population, female mice were treated between 5 and 7 months of age with XPro1595 (n = 10). A second cohort of mice was treated between 2 and 4 months of age with XPro1595 (n = 7). To assess the role of sTNF on AD-like amyloid accumulation and mRNA associated with inflammatory markers, female Tg and non-Tg mice were treated from 5 to 7 months of age with XPro1595 (Tg XPro1595 n = 5, non-Tg XPro1595 n = 6). Brains were removed for IHC and histological analysis. |
| Comparison | Female Tg mice at 2, 4 and 6 months of age were treated s.c. with saline vehicle twice weekly for 2 months as control. Female mice were treated between 5 and 7 months of age with saline as control (n = 9). Other female mice were treated between 2 and 4 months of age with saline (n = 8). Female Tg and non-Tg mice were treated from 5 to 7 months of age with saline (Tg saline n = 3, non-Tg saline n = 8). |
| Functional outcomes | The 5xFAD Tg mouse model of AD shows progressive Aβ plague accumulation before 3 months of age, progressive synaptic protein loss as well as later neuronal degeneration, and cognitive deficits by 5 months of age (Oakley et al., 2006). In this study, male and female Tg mice were not found to express significantly different levels of several cytokines in cerebrospinal fluid at 2, 4 or 6 months of age. Levels of murine KC/chemokine (C-X-C motif) ligand 1 protein were significantly lower in Tg mice at 6 months of age when compared to non-Tg mice. Cerebrospinal fluid levels of IFN-γ, IL-10, IL-1β, IL-6, and IL-12p70 protein levels were undetectable. TNF protein levels were not detectable at 2 months of age and no significant differences were found at 4 and 6 months of age. Within the cortex, there was a significant increase in expression of CD45 mRNA at 6 months in Tg mice compared to non-Tg mice, male and female. Within the midbrain, expression of CD45 and TNF mRNA was significantly increased at 6 months in Tg mice compared to non-Tg mice. Immune cells were isolated from the CNS of Tg and non-Tg mice at 3.5, 5, 7 and 12 months of age. These ages correspond to early stages of amyloid deposition (3.5 months), onset of cognitive impairment (5 months), and aggressive amyloid burden (7 and 12 months) (Oakley et al., 2006). Peripheral macrophages that have trafficked into the brain are CD11b+ and CD45high. The CD11b+CD45high population was not purely peripheral macrophages but also contained activated microglia and dendritic cells, which also express CD11b and CD45 at high level. Within the brain there was no effect of genotype or age on the number of CD11b+CD45high cells, but within this population there was a significant decrease in the frequency of CD11b+-CD45highMHCII (major histocompatibility complex II)+ cells in Tg mice compared to non-Tg mice at 7 months of age. In Tg mice there was a significant increase in the ratio of CD11b+Ly6Chigh:Ly6Chigh at 5 months in the brain compared to non-Tg mice. In the brains of Tg and non-Tg mice, there were no significant differences in the frequency or number of neutrophils (CD3–Ly6G+), B cells (CD3–Ly6G–CD11b–CD19+), or dendritic cells (CD3–Ly6G–CD11b+CD11c+). At 12 months of age, the frequency of CD3+ T cells of all CD45+ brain cells was significantly increased in Tg mice compared to non-Tg mice. The number of CD4+ T cells was not significantly different between Tg and non-Tg mice; however, at 12 months of age there was a significant increase in the number of CD8+ T cells in the brains of Tg mice compared to non-TG mice. Tg mice showed alterations of the CD8+ effector (CD62L–CD44–) T cell population compared to non-Tg mice. At 3.5 months of age, there was a significant decrease in the frequency of CD8+ effectors, while at 5 months of age there was a significant increase in the number of CD8+ effectors in Tg mice compared to non-Tg mice. No significant changes were found in the other subpopulations of CD8+ or CD4+ T cells. Tg mice were treated s.c. with Xpro1595 (10 mg/kg) twice weekly for 2 months starting at 5 months of age or starting at 2 months of age to assess the role of sTNF on AD-like pathology after significant plaque deposition or as plaque deposition began to build up, respectively. Inhibition of sTNF with Xpro1595 between 5 and 7 months of age decreased MHCII+ microglia/macrophages within both populations of activated microglia/peripheral cells (CD11b+CD45high, frequency but not number) and quiescent/homeostatic resident microglia (CD11b+CD45low, frequency and number) immune cells. While these effects on MHCII+ populations were not observed in Tg mice between 2 and 4 months of age, there was an overall decrease in the frequency of CD11b+CD45high populations and an increase in CD11b+CD45low populations. Among other immune call populations in the brain, no changes were detected in frequency or number of B cells or neutrophils when XPro1595 was administered from 5 to 7 months of age in Tg mice. Within the dendritic cell population, there was a significant increase in frequency of CD11c+ cell population in the brain of Tg mice compared to non-Tg mice but there was no significant increase in cell number. When XPro1595 was administered to mice from 2 to 4 months of age, there was no significant increase in number or frequency of CD11c+ cells within the CD11b+ population. Within this population, only when XPro1595 was administered from 2 to 4 months of age but not from 5 to 7 months of age there was a significant decrease in the frequency of a MHCII+ population. Inhibition of sTNF by XPro1595 significantly decreased the density (% of area) of Aβ in the subiculum but not the entire hippocampus of Tg mice. Within the subiculum there was a significant decrease in Aβ density in the dorsal and posterior subiculum but not the ventral subiculum. No significant changes were found in the hippocampus or subiculum in the density of amino cupric silver stain which denotes disintegration of multiple neuronal elements including cell bodies, axons, dendrites and synaptic teminals. When the contralateral hemisphere was used to measure transcriptional changes in gene expression, gene expression associated with an inflammatory response and its resolution was found to be reduced by inhibition of sTNF with XPro1595. Transforming growth factor-β and chemokine (C-C motif) ligand 2 mRNA expression was significantly reduced in Tg mice, but not non-Tg mice, treated with Xpro1595 from between 5 and 7 months of age. Deficits in hippocampal CA1 synaptic function have been reported in several mouse models of AD and are associated with cognitive deficits. To determine if in vivo peripheral administration of XPro1595 modulates synaptic function in Tg mice, brain slices were harvested for analysis of CA3-CA1 synaptic strength curves and long-term potentiation (LTP) levels. At 4 months of age, basal synaptic strength deficits were found to be relatively mild in vehicle-treated Tg mice, characterized by a modest downward shift in the synaptic strength curve and a significant reduction in the maximal excitatory postsynaptic potential/fiber volley ratio, relative to vehicle-treated non-Tg mice. XPro1595 had minimal effect on synaptic strength, though it did slightly reduce and slightly increase the maximal excitatory postsynaptic potential/fiber volley ratio in non-Tg and Tg mice, respectively. Relative to vehicle-treated non-Tg mice, Tg mice showed a significant reduction in LTP levels at 60 minutes post-100 Hz stimulation that was blocked by in vivo treatment with XPro1595. In contrast, XPro1595 had minimal effect on LTP in non-Tg mice. |
| Conclusion | Administration of XPro1595 reduced the age-dependent increase in activated immune cells in Tg mice, while decreasing the overall number of CD4+ T cells. In addition, XPro1595 treatment in vivo rescued LTP measured in brain slices in association with decreased Aβ plaques in the subiculum. |
| IL-1 receptor antagonist (IL-1RA) | |
| Reference | Zou et al. (2016) |
| Number of animals, gender, ages, and treatment | APPswe/PSEN1deltaE9 mice were crossed with GFP-M mice to obtain double Tg offspring which were heterozygous for the corresponding genes (deltaE9+/– × GFP+/–). Adult female Tg mice (4–5 months of age were used. Mice were anesthetized, dexamethasone (6 mg/kg) was injected to prevent development of cerebral edema, and a piece of the skull was removed above the somatosensory cortex. The exposed brain was cleaned with sterile saline and covered with a round glass coverslip (D = 4 mm). The margin between the glass and skull was sealed with dental cement.Post-surgical mice were s.c. injected with carprofen (4 mg/kg) and cefotaxime (250 mg/kg). Lentiviruses encoding IL-1RA were intraparenchymally injected into the cortex before implanting the coverslip. Injection of lentiviruses (200 nL per time at a titre of ~1 × 108 infecting units/mL) was performed at 4 different sites in the exposed area of brain at a depth of 700–800 µm. After 4 weeks recovery period, apical dendrites originating from GFP-labeled layer V pyramidal neurons were imaged in consecutive sessions (once/week). GFP was excited by a femtosecond laser at 880 nm wavelength. To ensure the dendrites were chosen in amyloid plaque-free regions, methoxy-X04 (1 mg/kg) was injected i.p. 24 hours before imaging in the first and last time points (n = 4–6/group). |
| Comparison | GFP positive littermates without APP/PS1 transgenes were used as controls (deltaE9+/– × GFP+/–). |
| Functional outcomes | Replicating the preclinical stages of AD, 4–5 months old APPswe/PSEN1deltaE9 mice develop Aβdeposits without cognitive decline. In a previous study normal spine density and dynamics were observed far away from Aβ deposits in deltaE9 at this age (Zou et al., 2015). To assess if activity-induced structural spine plasticity on these dendrites was disturbed in preclinical AD, APP/PS1deltaE9 mice at 4–5 months of age were housed under enriched environment (EE) over 5 weeks and the apical tufts of layer V pyramidal neurons in the somatosensory cortex monitored. EE, which provides a spectrum of synaptic inputs and which leads to adaptive synaptic alterations within the adult brain, induced a steady increase of spine density in the control group. In contrast, EE failed to increase spine density in deltaE9 mice. Unlike control mice in which there was a gradual decline in dendritic spine elimination upon EE, the rate of spine elimination in deltaE9 mice remained unchanged. EE did not alter the rate of spine formation in both groups. IL-1β is a key mediator of the inflammatory response in AD and is known to have deleterious effects on synaptic plasticity (Tong et al., 2012). The expression of IL-1β was significantly increased in cortical tissue of deltaE9 mice. To diminish IL-1β activity, lentiviruses expressing IL-1RA was injected into the somatosensory cortex. IL-1RA rectified the adaptive gain of spines upon EE in deltaE9 mice, accompanied with the gradual decline in spine elimination instead of rising spine formation. |
| Conclusion | Anti-inflammatory treatment with IL-1RA in deltaE9 mice successfully rescued the impairment in increasing spine density during EE. |
| Pioglitazone | |
| Reference | Zou et al. (2016) |
| Number of animals, gender, ages, and treatment | APPswe/PSEN1deltaE9 mice were crossed with GFP-M mice to obtain double Tg offspring which were heterozygous for the corresponding genes (deltaE9+/– × GFP+/–). Adult female Tg mice 4–5 months of age were used. Pioglitazone (350 ppm) was supplemented into rodent chow. Cranial window implantation and in vivo imaging as described above in the study with IL-1RA (n = 4–6/group). |
| Comparison | GFP positive littermates without APP/PS1 transgenes were used as controls (deltaE9+/– × GFP+/–). |
| Functional outcomes | It is known that amyloid plaques are surrounded by activated glial cells that release proinflammatory cytokines (Watkins et al., 2001). To investigate if these cytokines caused the impaired adaptive plasticity, deltaE9 mice were treated with pioglitazone, a peroxisome proliferators-activated receptor-γ agonist which inhibits the production of proinflammatory cytokines without affecting synaptic plasticity (Jiang et al., 1998; Chen et al., 2015). Pioglitazone treatment p.o. over 6 weeks period rehabilitated the steady increase of spine density in deltaE9 mice during exposure to EE. As in control mice, the EE-induced spine density increase resulted from the gradual decline in spine elimination, while the rate of spine formation was unchanged. |
| Conclusion | Anti-inflammatory treatment with pioglitazone in deltaE9 mice successfully rescued the impairment in increasing spine density during EE. |
| Reference | Yu et al. (2016) |
| Number of animals, gender, ages, and treatment | Homozygous 3xTg AD mice with PS1M146V, APPSwe and tauP301L transgenes were used. Female 3xTg mice at 10 months of age were subjected to behavioral tests and then treated with an experimental diet containing pioglitazone hydrochloride for 4 months. The experimental diet contained 200 mg pioglitazone/kg of rodent chow, and unmodified chow was used as the control diet. The body weight of mice and food consumption were measured every week. After the drug treatment (at age of 14 months) the mice were subjected to behavioral tests again. Then the mice were euthanized and the brains removed and hippocampi and cerebral cortices dissected and frozen for biochemical analyses. Behavioral tests (n = 11–12/group) and biochemical and IHC analyses (n = 4–8/group) were performed. |
| Comparison | WT mice served as control. |
| Functional outcomes | The 3xTg AD mice had slightly smaller body weight than WT control mice. Treatment with pioglitazone started to show a slight reduction in body weight after treatment for 13 weeks, and this decrease became statistically significant after treatment for 16 weeks. This decrease in body weight was not seen in WT mice after treatment with the same dose of the drug for the same period of time. During the same period of time, the food consumption among the groups or treatments was not different. The 3xTg AD mice explored for a longer distance in the open field and showed a higher fall latency in the rotarod test than WT mice. These findings indicated that the 3xTg AD mice have a more active spontaneous exploratory activity and perform better in motor coordination and balance. Treatment of the 3xTg AD mice and WT control mice with pioglitazone for 4 months did not significantly alter the spontaneous exploratory activity or locomotion. At 10 months of age, the 3xTg AD mice spent significantly less time in the open arm of the elevated plus maze than did the WT mice, but they spent approximately double the time and traveled double the distance than the WT mice in the center of the open field. These observations suggest a decreased anxiety level of the 3xTg AD mice at 10 months of age. Treatment of 3xTg AD mice and WT mice with pioglitazone did not significantly alter the time spent in the open arm of the EPM or the time spent and distance traveled in the center of the open field. These results suggest that pioglitazone treatment did not affect the anxiety level of the mice. At the age of 10 months before the treatment, the 3xTg AD mice took a longer time and swam a longer distance than WT mice to find the escape platform during the training phase, although improvement from day to day was seen in the 3xTg AD mice. This suggested a learning impairment and it appeared more severe when the mice reached 14 months of age. Treatment of 3xTg AD mice with pioglitazone for 4 months improved the learning of the mice as evidenced by significantly shorter escape latency and swimming distance on day 4 to reach the escape platform than the untreated 3xTg AD mice. These results suggested that pioglitazone improved learning of the 3xTg AD mice. Probe trials were performed 1 day after the last training in the MWM to assess the spatial reference memory of the mice. An impairment of the spatial reference memory in the 3xTg AD mice was observed at both 10 and 14 months of age, as evidenced by less time spent and shorter distance swam within the target quadrant by the 3xTg AD mice than WT mice during the probe test. Pioglitazone treatment did not significantly affect the performance in the probe test, suggesting that treatment with pioglitazone for 4 months did not improve spatial reference memory. The total tau level in the hippocampus was several fold higher, but in the cerebral cortex only slightly higher, in the 3xTg AD mice than in WT mice. Treatment of the mice with pioglitazone for 4 months did not significantly alter the total tau level. Treatment with pioglitazone reduced tau phosphorylation at several AD-related sites in the hippocampus of both 3xTg AD and WT mice. This reduction was only seen at a few phosphorylation sites in the cerebral cortex. Treatment of both 3xTg AD and WT mice with pioglitazone for 4 months increased phosphorylation of AKT at Ser473 and of GSK3β at Ser9 in the hippocampus. These findings suggested that pioglitazone treatment promoted insulin-AKT signaling that in turn led to inhibition of GSK3β. No significant promotion of AKT and inhibition of GSK3β were found in the cerebral cortex. A significant increase of GFAP staining (a marker of astrocytes and neuroinflammation) was observed in the brains of the 3xTg AD mice compared with WT mice. Treatment with pioglitazone for 4 months reduced GFAP staining in the brains of both 3xTg AD and WT mice, suggesting that pioglitazone treatment may attenuate neuroinflammation. |
| Conclusion | Pioglitazone treatment improved spatial learning, enhanced AKT signaling, and decreased tau hyperphosphorylation and neuroinflammation in 3xTg AD mice. |
| Rosiglitazone | |
| Reference | Yu et al. (2015) |
| Number of animals, gender, ages, and treatment | Homozygous 3xTg AD mice with PS1M146V, APPSwe and tauP301L transgenes were used. Female 3xTg mice at 10 months of age were subjected to behavioral tests and then treated with an experimental diet containing rosiglitazone maleate for 4 months. The experimental diet contained 50 mg rosiglitazone/kg of rodent chow. After the drug treatment (at age of 14 months) the mice were subjected to behavioral tests again. Then the mice were euthanized and the brains removed and hippocampi and cerebral cortices dissected and frozen for biochemical analyses. Behavioral tests (n = 11–12/group) and biochemical and IHC analyses (n = 4–8/group) were performed. |
| Comparison | WT mice served as control. |
| Functional outcomes | Treatment with rosiglitazone started to show a slight reduction in body weight after treatment for 13 weeks, and this decrease became statistically significant after treatment for 16 weeks. This decrease in body weight was not seen in WT mice after treatment with the same dose of the drug for the same period of time. During the same period of time, the food consumption among the groups or treatments was not different. Treatment of the 3xTg AD mice and WT control mice with rosiglitazone for 4 months did not significantly alter the spontaneous exploratory activity or locomotion. Treatment of 3xTg AD mice and WT mice with rosiglitazone did not significantly alter the time spent in the open arm of the elevated plus maze or the time spent and distance traveled in the center of the open field. These results suggest that rosiglitazone treatment did not affect the anxiety level of the mice. Treatment of 3xTg AD mice with rosiglitazone for 4 months did not improve the learning of the mice or the spatial reference memory. Treatment of the mice with rosiglitazone for 4 months did not significantly alter the total tau level. Treatment of both 3xTg AD and WT mice with rosiglitazone for 4 months increased phosphorylation of AKT at Ser473 and of GSK3β at Ser9 in the hippocampus. These findings suggested that rosiglitazone treatment promoted insulin-AKT signaling that in turn led to inhibition of GSK3β. No significant promotion of AKT and inhibition of GSK3β were found in the cerebral cortex. A significant increase of GFAP staining (a marker of astrocytes and neuroinflammation) was observed in the brains of the 3xTg AD mice compared with WT mice. Treatment with rosiglitazone for 4 months reduced GFAP staining in the brains of both 3xTg AD and WT mice, suggesting that rosiglitazone treatment may attenuate neuroinflammation. |
| Conclusion | Rosiglitazone treatment enhanced AKT signaling, and decreased tau hyperphosphorylation and neuroinflammation in 3xTg AD mice. |
| Quercetin | |
| Reference | Sabogal-Guaqueta et al. (2015) |
| Number of animals, gender, ages, and treatment | Homozygous 3xTg AD and non-Tg mice, 18–21 months of age, were treated i.p. with quercetin 25 mg/kg in 0.1% dimethyl sulfoxide every 48 hours for 3 months. At 48 hours after the final treatment, behavioral tests (8–16 mice for each test) were performed. At 24 hours after the final behavioral test, the animals were anesthetized, perfused with saline and 4% paraformaldehyde, and the brains removed for IHC and histology (3–5 mice for each study) and biochemical analyses (4–6 mice for each analysis). |
| Comparison | 3xTg AD and non-Tg mice received i.p. injections of 0.1% dimethyl sulfoxide vehicle. |
| Functional outcomes | The subiculum showed a decrease of cell density in the vehicle treated 3xTg AD mice, and quercetin treatment increased the cell density in the subiculum to a level similar to that in non-Tg mice treated with vehicle or quercetin. No changes in cell density were observed in the other structures evaluated. These findings were supported by a significant loss of NeuN immunoreactivity in the subiculum of 3xTg AD mice treated with vehicle, which was prevented by quercetin treatment. NeuN immunoreactivity in the subiculum of the quercetin-treated 3xTg AD mice was similar to that in the non-Tg mice. The CA1 area of hippocampus, the entorhinal area and the amygdala did not show any alteration in NeuN immunoreactivity. The 3xTg AD mice showed strong Aβ immunoreactivity when compared with non-Tg mice 21–24 months of age. Quercetin treatment resulted in a significant decrease in the amount of extracellular Aβ deposition in all of the cerebral regions of the brain examined compared to vehicle treatment. The vehicle- or quercetin-treated non-Tg mice did not show Aβ immunoreactivity. A significant decrease was found in the levels of C-terminal APP fragments (β) in the quercetin-treated 3xTg AD mice, which were significantly lower than those in the vehicle-treated 3xTg AD mice. Also there was a significant reduction in Aβ40 and Aβ42 levels in the hippocamus of the quercetin-treated 3xTg AD mice compared to the vehicle-treated 3xTg AD mice. The vehicle-treated 3xTg AD mice had abundant ATB immunoreactivity whereas the quercetin-treated 3xTg AD mice displayed a significant decrease in these neurofibrillary tangles in the CA1 area, the subiculum and the amygdala but not in the entorhinal area. These findings were confirmed by a significant reduction in paired helical filament-1 and ATB protein levels in hippocampal and amygdalar lysates from quercetin-treated 3xTg AD mice compared to vehicle-treated 3xTg AD mice. The total tau level was not altered by any treatment. The 3xTg AD mice had a significant increase in GFAP immunoreactivity compared to non-Tg mice. However, quercetin treatment significantly reduced GFAP immunoreactivity in the CA1 area, the entorhinal area and the amygdala compared to vehicle treatment, but no changes were seen in the subiculum. Quercetin treatment did not affect the non-Tg mice. The quercetin-treated 3xTg AD mice had significantly reduced microglial activation in the CA1 area, the subiculum and the amygdala compared to vehicle-treated 3xTg AD mice, and was similar to that in the non-Tg mice. There were no changes in the entorhinal area. Quercetin treatment for 3 months of 18–21-month-old 3xTg AD mice significantly reduced the latency to locate the platform in the MWM test and improvement occurred in the spatial learning tasks after 8–10 trials. AD mice exhibit synaptic dysfunction and LTP deficits. In the EPM test, the non-Tg mice treated with quercetin or vehicle rarely visited the open arm, very similar to the 3xTg AD mice treated with vehicle, whereas the 3xTg AD mice treated with quercetin spent more time in the open arm. The time spent rearing was significantly lower in the quercetin-treated 3xTg AD mice than in the other mice. |
| Conclusion | Quercetin treatment decreased extracellular β-amylodosis, taupathy, astrogliosis and microgliosis in hippocampus and amygdala of 3xTg AD mice. In addition, quercetin improved performance on learning and spatial memory tasks and greater risk assessment behavior of the 3xTg AD mice in the EPM test. |
| IL-1β | |
| Reference | Rivera-Escalera et al. (2014) |
| Number of animals, gender, ages, and treatment | APP/PS1 mice, 7 months of age, were used. APP/PS1 mice were crossed to C-C chemokine receptor type 2 (CCR2)–/– mice, and then backcrossed to CCR2–/– mice to generate APP/PS1/CCR2–/– mice. APP/PS1 mice crossed to IL-1βXAT mice to produce APP/PS1-IL-1βXAT mice were used as recipients in a bonw marrow chimeric experiment. Mice were anesthetized, secured in a stereotactic apparatus and a 0.5 mm burr hole drilled in the skull and a 33 gauge needle pre-loaded with feline immunodeficiency virus (FIV)-Cre was lowered 1.8 mm from the brain surface over 2 minutes. A microsyringe pump controller was used to inject 1.5 µL of virus (~1.5 × 104 IVP) at a constant rate over 10 minutes. Following a 5 minutes delay to allow viral diffusion, the needle was raised slowly over 2 minutes, the burr hole sealed with bone wax and soft tissues sutured. APP/PS1 and APP/PS1-IL-1βXAT bone marrow chimeric animals received unilateral FIV-Cre injections at 7 months of age and were euthanized at 8 months of age for biochemical analysis of brain tissue. For experiments using APP/PS1 and APP/PS1/CCR2–/– mice, two 0.5 mm burr holes were drilled, one on each side, and a 33 gauge needle was lowered 1.5 mm over 2 minutes and 5 µL injected of recombinant adeno-associated virus vector 2 expressing IL-1β (rAAV2-IL-1β) or recombinant adeno-associated virus vector 2 (rAAV2) resulting in delivery of approximately 1.5 × 108 infectious particles/mL into each hippocampus. Following AAV2 delivery, 2 minutes was allowed for diffusion of viral particles. The needle was raised over 2 minutes and the burr hole sealed with bone wax. The procedure was then repeated to deliver the same viral vector on the opposite side. APP/PS1/CCR2–/– bone marrow chimeric animals received rAAV2-IL-1β into one hippocampus and rAAv2-Phe (a control viral vector) into the contralateral hippocampus at 9 months of age. All animals were euthanized 4 weeks post-viral transduction for brain tissue analysis. Group sizes were 4–12/group. For construction of bone marrow chimeras 8 to 12 week-old APP/PS1, APP/PS1-IL-1βXAT, or APP/PS1/CCR2–/– mice received two doses of 6 Gy total body irradiation separated by 4 hours. The head was shielded during the irradiation procedure to avoid confounding effects of cell recruitment and radiation-induced brain inflammation. Immediately after the second total body irradiation dose, APP/PS1, APP/PS1-IL-1βXAT, or APP/PS1/CCR2–/– mice were reconstituted with bone marrow derived from tibias and femurs of either GFP or GFP-CCR2–/– donor mice. Each bone marrow recipient received 200 µL of suspension for a total of 3 × 106 cells via tail vein injection. After a 6-week reconstitution period, APP/PS1 and APP/PS1-IL-1βXAT mice were subjected to tail bleeds for analysis of GFP positive leukocytes by flow cytometry. For APP/PS1/CCR2–/– mice, peripheral blood was collected at the time of euthanasia to determine bone marrow constitution and levels of monocyte subsets in the circulation. |
| Comparison | – |
| Functional outcomes | APP/PS1 or APP/PS1-IL-1βXAT mice received either WT-GFP or CCR2–/–-GFP bone marrow at 2–3 months of age. At 7 months of age all groups received unilateral hippocampal FIV-Cre injections and were euthanized at 8 months of age for analysis of GFP positive cells around amyloid plaques as well as Congo Red and 6E10 plaque indices. No GFP positive cells surrounded plaques without IL-1β overexpression. Significantly fewer GFP positive cells surrounded plaques in APP/PS1-IL-1βXAT mice that received CCR2–/–-GFP bone marrow. There was an overall effect of IL-1β on 6E10 labeled plaques and significant reductions in 6E10 plaques for both WT bone marrow recipients and CCR2–/– recipients following 1 month of IL-1β overexpression. There was a significant reduction in Congo Red plaques for both WT bone marrow recipients and CCR2–/– recipients. These findings suggested that peripheral mononuclear cells are recruited following IL-1β overexpression, but are not necessary for IL-1β-mediated amyloid plaque clearance. The 7-month-old APP/PS1 mice transduced intrahippocampally with rAAV2-IL-1β exhibited robust microglial activation 4 weeks following transduction compared to APP/PS1 mice transduced with rAAV2-Phe, a control vital vector. In addition to microglial activation, APP/PS1 mice transduced with rAAV2-IL-1β had increased levels of murine IL-1β and chemokine (C-C motif) ligand 2 detected in hippocampal tissue compared to APP/PS1 mice transduced with rAAV2-Phe. Transduction with rAAV2-IL-1β significantly reduced 6E10 and Congo Red staining of amyloid plaques in the hippocampus of APP/PS1 mice. Measurements of hippocampal Aβ peptide levels showed APP/PS1 mice transduced with rAAV2-IL-1β had significant decreases in levels of insoluble and soluble Aβ42 compared to APP/PS1 mice transduced with rAAV2-Phe. The neuroinflammation mediated by rAAV2-IL-1β in APP/PS1 mice dd not alter the levels of APP or its processing as the activity of BACE and its β-carboxy terminal fragment cleavage products remained unchanged. CCR2 signaling is important for the recruitment of CCR2+ monocytes to the brain (Saederup et al., 2010). APP/PS1 mice lacking CCR2 were generated as a way of ablating CCR2+ monocyte recrutment to the inflammed hippocampus. APP/PS1/CCR2–/– mice transduced with rAAV2-IL-1β had an increased inflammatory response 4 weeks following transduction as indicated by microglial activation and increased production of murine IL-1β and chemokine (C-C motif) ligand 2 when compared to APP/PS1/CCR2–/– mice transduced with control viral vector. Even in the absence of CCR2, rAAV2-IL-1β decreased 6E10 and Congo Red staining of amyloid plaques. Mesurements of hippocampal Aβ peptide levels showed APP/PS1/CCR2–/– mice transduced with rAAV2-IL-1β had significant decreases in insoluble Aβ42 and insoluble Aβ40 compared to control APP/PS1/CCR2–/– mice transduced with rAAV2-Phe. However, the levels of soluble Aβ42 and soluble Aβ40 were not significantly different from APP/PS1/CCR2–/– mice transduced with rAAV2-Phe. These findings confirmed that CCR2+ mononuclear cells are not necessary for IL-1β-mediated amyloid plaque clearanmce. |
| Conclusion | IL-1β overexpression in APP/PS1 mice ameliorated amyloid pathology, increased plaque-associated microglia, and induced recruitment of peripheral immune cells to the brain parenchyma. The IL-1β-mediated amyloid plaque clearance was independent of CCR2 signaling in the APP/PS1 mouse model of AD. |
| Cannabidiol | |
| Reference | Cheng et al. (2014) |
| Number of animals, gender, ages, and treatment | Adult male APPswe/PSEN1deltaE9 mice, 3 months of age, and their non-Tg WT littermates were treated daily late in the afternoon with a gel pellet containing cannabidiol (CBD) which they consumed within 2–5 minutes. CBD was used at a dose of 20 mg/kg body weight. CBD treatment was carried out for 5 months. Behavioral tests were performed starting at 10 months of age (n = 8–14/group). Mice were anesthetized, blood collected and centrifuged for plasma. Euthanized mice were perfused with phosphate buffer saline, and brains removed for biochemical analyses (n = 8–10). |
| Comparison | APPswe/PSEN1deltaE9 mice and WT littermates were given a vehicle gel pellet daily. |
| Functional outcomes | CBD treatment increased the time that APP/PS1 mice spent with the novel mouse in the social preference test, while no such effect was observed in WT mice, indicating that CBD had a beneficial effect on social recognition memory. In the EPM test, CBD treatment for 5 months had no effect on anxiety behaviors. In the associative learning test, APP/PS1 mice showed increased amounts of freezing at baseline regardless of treatment. CBD treatment had no effect on soluble and insoluble Aβ40 or Aβ42 in the cortex of APP/PS1 mice. Similarly, Aβ levels in the hippocampus were unchanged after CBD treatment. Total F2-isoprostanes (free and esterified corrected for arachidonic acid) were not significantly altered in APP/PS1 mice when compared to WT littermates. For enzymatically oxidised sterols, APP/PS1 mice exhibited significantly decreased overall levels of 24-hydroxycholesterol compared to WT littermates. No differences were found across all four groups for 27-hydroxycholesterol and the reactive species oxidised sterols, 7β-hydroxycholesterol and 7-ketocholesterol. Cholesterol was increased in cortical tissues of APP/PS1 mice compared to WT mice and CBD treatment increased cholesterol levels. There were no significant differences in the levels of mRNA for two inflammayory cytokine markers, IL-1β and TNF-α. No significant effect of CBD treatment on these cytokines was observed. All mice treated with CBD had significantly increased levels of CBD in blood plasma. |
| Conclusion | APP/PS1 mice developed a social recognition deficit that was ameliorated by CBD treatment. CBD treatment had no effect on anxiety or associative learning. The beneficial effect of CBD on social recognition memory was not associated with any changes in amyloid load or oxidative damage. |
| Memantine | |
| Reference | Borre et al. (2014) |
| Number of animals, gender, ages, and treatment | Adult male Sprague-Dawley rats, 240–270 g, 8–10 weeks of age, were anesthetized and two burr holes drilled and the olfactory bulbs aspirated through a blunt hypodermic needle (OBX animals). Group 3: OBX + vehicle (OBX-Veh) (n = 10); Group 4: OBX + memantine (OBX-Mem) (n = 10). Memantine (20 mg/2 mL/kg) or water (vehicle) was administered p.o. via gavage daily starting 2 days prior to OBX surgery and continued to 28 days after surgery. Behavioral tests were performed 7 to 18 days after OBX, with olfactory test at 29 days. Animals were euthanized at 30 days, and brains and spleens removed for analysis. |
| Comparison | Animals underwent sham surgery but without removal of olfactory bulbs. Group 1: sham operated + vehicle (Sham-Veh) (n = 10); Group 2: sham operated + memantine (Sham-Mem) (n = 10) |
| Functional outcomes | The observed weight loss in OBX animals was not due to decreased food consumption. OBX resulted in anosmia (complete loss of smell). The OBX-induced spatial memory deficit was reversed in T-maze by memantine treatment. Also the OBX-induced fear memory loss in the passive avoidance retention task was partly rescued by memantine treatment. Treatment with memantine attenuated the OBX-induced hyperactivity. The hippocampi of OBX animals fed the control diet weighed significantly less compared to sham controls, and memantine treatment attenuated the OBX-induced hippocampal atrophy. OBX resulted in a significantly lower cell count in the CA3, CA1 and DG areas of the dorsal hippocampus. Memantine treatment attenuated OBX-induced cell loss in the DG area but failed to rescue cell loss in the CA3 and CA1 areas. In the ventral hippocampus, OBX resulted in a significantly lower cell count in the CA3, CA1 and DG areas. Treatment with memantine rescued cell loss in the CA3, CA1 and DG areas. The presence of increased numbers of T cells in lymphoid organs signifies the induction of an immune response. OBX induces systemic inflammation (Song et al., 2009) and OBX increased the numbers of T cells in the spleen, suggesting the presence of systemic immune activation and possibly active inflammation. Treatment with memantine significantly decreased splenic T cells in OBX animals. |
| Conclusion | Memamtine treatment of OBX rats prevented/impeded the development of a neurodegenerative and depressive disorder and the concomitant cognitive deficits. |
| Multi-targeted diet | |
| Reference | Borre et al. (2014) |
| Number of animals, gender, ages, and treatment | Adult male Sprague-Dawley rats, 240–270 g, 8–10 weeks of age, were anesthetized and two burr holes drilled and the olfactory bulbs aspirated through a blunt hypodermic needle (OBX animals). Group 3: OBX + control diet (OBX-C) (n = 12); Group 4: OBX + Exp diet (OBX-Exp) (n = 12). Both diets were fed to the animals for 6 weeks (2 weeks prior to surgery and 4 weeks thereafter). The experimental diet was a control diet with supplements in mg/kg of food of zinc 1.63, curcumin 0.25, piperine 0.06, melatonin 0.03, choline 9.5, uridine 15.48, 3% soya + 4% tuna oil (25% docosahexaenoic acid/6% eicosapentaenoic acid). The relative calorific content of the two diets was approximately 4000 kcal/kg. |
| Behavioral tests were performed 7 to 22 days after OBX, with olfactory test at 29 days. Animals were euthanized at 30 days, and brains and spleens removed for analysis. | |
| Comparison | Animals underwent sham surgery but without removal of olfactory bulbs. Group 1: sham operated + control diet (Sham-C) (n = 12); Group 2: sham operated + experimental diet (Sham-Exp) (n = 12) |
| Functional outcomes | The observed weight loss in OBX animals was not due to decreased food consumption. OBX resulted in anosmia (complete loss of smell). The OBX-induced spatial memory deficit was reversed in T-maze, but not in the holeboard test, by the experimental diet. Also the OBX-induced fear memory loss in the passive avoidance retention task was partly rescued by the experimental diet. The experimental diet attenuated the OBX-induced hyperactivity. The hippocampi of OBX animals fed the control diet weighed significantly less compared to sham controls, and the experimental diet attenuated the OBX-induced hippocampal atrophy. OBX resulted in a significantly lower cell count in the CA3, CA1 and DG areas of the dorsal hippocampus. The experimental diet attenuated OBX-induced cell loss in the DG area but failed to rescue cell loss in the CA3 and CA1 areas. In the ventral hippocampus, OBX resulted in a significantly lower cell count in the CA3, CA1 and DG areas. The experimental diet rescued cell loss in the CA3, CA1 and DG areas. The presence of increased numbers of T cells in lymphoid organs signifies the induction of an immune response. OBX induces systemic inflammation (Song et al., 2009) and OBX increased the numbers of T cells in the spleen, suggesting the presence of systemic immune activation and possibly active inflammation. The experimental diet significantly decreased splenic T cells in OBX animals. |
| Conclusion | A diet targeting multiple disease etiologies can prevent/impede the development of a neurodegenerative and depressive disorder and the concomitant cognitive deficits. |
| Clioquinol | |
| Reference | Zhang et al. (2013) |
| Number of animals, gender, ages, and treatment | Adult male and female APPswe/PSEN1deltaE9 mice, 5 months of age, were assigned to 3 groups (n = 8–12/group). WT mice 5 months of age were also used. APP/PS1 mice were treated with clioquinol (CQ) 6 mg/kg p.o. once daily for 5 months. At 11 months of age, mice were anesthetized and blood collected. Euthanized mice were perfused with phosphate buffer saline followed by formalin, and the brains removed and post-fixed in formalin for histology and IHC. |
| Comparison | APPswe/PSEN1deltaE9 mice were dosed p.o with distilled water replacing the CQ stock solution. |
| Functional outcomes | Compared to untreated APP/PS1 mice, treatment with CQ significantly decreased the area fraction and the number of amyloid plaques, with the region of interest including the neocortex and hippocampus. When compared by gender, the decreases in both area fraction and plaque number in males were significantly greater than in females. In CQ treated APP/PS1 mice, conspicuous myelinopathies independent of amyloid plaques were seen in the dorsal lateral geniculate nucleus (DLG) and which consisted of numerous edematous swellings and fragmented fibers (in 11 out of 12 CQ treated mice, 92%). No myelin pathology was seen in the DLG of APP/PS1 control mice. In WT mice, relatively milder myelinopathies were seen in DLG of all of the CQ treated mice (n = 6). No myelin pathology was seen in the DLG of untreated WT mice (n = 5). Plasma levels of Aβ42 in the CQ treated APP/PS1 mice were significantly higher than those in the untreated APP/PS1 mice. Activated astrocytes were located primarily surrounding the amyloid plaques in the untreated and CQ treated APP/PS1 mice. The activated astrocytes exhibited hypertrophic processes and cell bodies and increased expression of GFAP compared with resting astrocytes. The total number of plaque-associated astrocytes was decreased in CQ treated APP/PS1 mice compared to untreated APP/PS1 mice, presumably because the area and number of amyloid plaques were reduced in CQ treated APP/PS1 mice. Activated microglia, identified by labeling with CD68, were found primarily near or surrounding amyloid plaques. There was no significant difference in the morphology of activated or resting microglia in any brain region examined between the different groups. The total number of CD68-labeled (activated) microglia was decreased in CQ treated APP/PS1 mice compared to untreated APP/PS1 in parallel with the decrease of amyloid plaques. |
Animal Alzheimer’s disease or cognitive impaired studies
Fasudil
Fasudil treatment intraperitoneally (i.p.) for 2 months of male APP/PS1 Tg mice (APPswe/PSEN1deltaE9), 8 months of age, ameliorated memory and learning deficits, reduced Aβ42 deposition and decreased the number of β-secretase-positive cells in the hippocampus. Fasudil inhibited Toll-like receptor-nuclear factor-kappa B-myeloid differentiation primary response 88 inflammation, decreased the production of IL-1β, IL-6 and TNF-α (all proinflammatory cytokines), and increased the production of IL-10 (an anti-inflammatory cytokine) in the hippocampus of APP/PS1 Tg mice (Yu et al., 2017).
LW-AFC (a formula derived from Liuwei Dihuang Decoction)
Three kinds of main active components have been extracted from Liuwei Dihuang decoction (LW), which is a traditional Chinese medicinal prescription, based on immunological and endocrinological activity assessment. These included a polysaccharide fraction, a glycoside fraction, and an oligosaccharide fraction, which together formed LW-AFC. Intragastric administration of LW-AFC for 5 months improved the spatial learning and memory deficits of male APP/PS1 mice, 9 months of age. It also improved the passive avoidance impairment of the APP/PS1 mice. LW-AFC protected against neuronal loss in the hippocampus, and alleviated Aβ deposition in the brain of APP/PS1 mice with lower levels of Aβ42 in the hippocampus. LW-AFC restored the imbalance of corticotropin-releasing hormone and adrenocorticotropic hormone within the hypothalamic-pituitary-adrenal axis, and also of gonadotropin-releasing hormone, follicle-stimulating hormone and luteinizing hormone within the hypothalamic-pituitary-gonadal axis of APP/PS1 mice. Treatment of APP/PS1 mice with LW-AFC increased the expression of CD8+ CD28+ T cells and decreased that of CD3+ CD2+ Foxp3+ T cells, showing that LW-AFC treatment partially restored nomal lymphocyte expression in APP/PS1 mice. In addition, the aberrant cytokine secretion in APP/PS1 mice shown by analysis of blood plasma was restored by LW-AFC administration (Wang et al., 2016).
Using male SAMP8 (senescence-accelerated mouse prone 8 strain) mice, 6 months of age, as a model of age-related/late-onset AD, intragastric administration of LW-AFC (0.8, 1.6 g/kg) for 5 months lessened the impairment of spatial learning in these mice. Also LW-AFC reversed the levels of corticotropin-releasing hormone and adrenocorticotropic hormone in the hypothalamic-pituitary-adrenal axis and of gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone and testosterone in the hypothalamic-pituitary-gonadal axis of SAMP8 mice. The aberrant cytokine production in SAMP8 mice was restored by LW-AFC treatment (Wang et al., 2017).
Curcumin
p25 single Tg mice were crossbred with Ca2+/calmodulin-dependent protein kinase single Tg mice to generate bi-transgenic offspring (p25Tg mice) that inducibly overexpress the human p25 gene under the control of the Ca2+/calmodulin-dependent protein kinase promoter-regulated tet-off system. p25Tg mice were maintained on deoxycycline in the drinking water from conception to 6 weeks postnatal to avoid any posible developmental consequences from the p25 expression. Hemizygous mice either male or female were used. p25 expresion was induced in 6 week-old mice by removal of deoxycycline in water and concurently treated with an optimized curcumin formulation, Longvida, orally via their feed of chow for 12 weeks. Deficits in spatial memory, working memory and reference memory were reduced by treatment of p25Tg mice with curcumin, showing that curcumin had a neuroprotective capability to restore p25-induced cognitive deficits in p25Tg mice. Curcumin treatment reduced the immunostaining intensity of glial fibrillary acidic protein (GFAP, an astrocyte activation marker) in the cortex and hippocampus as well as reduced the levels of GFAP expression in the forebrain of p25Tg mice. An altered immunostaining pattern with anti-CD11b (a microglial activation marker) occurred in the cortex and hippocampus of curcumin-treated p25Tg mice. Curcumin treatment downregulated the expression levels of macrophage inflammatory protein-1α, TNF-α and IL-1β (all proinflammatory cytokines) in p25Tg mice. Tau hyperrphosphorylation levels and Aβ42 deposition were decreased in curcumin-treated p25Tg mice. In addition, curcumin treatment reduced cleaved caspase-3 immunostaining in the cortex and hippocampus, indicating that curcumin confers neuroprotection against p25-induced neuronal death (Sundaram et al., 2017).
Usng male rats 3 to 4 months of age which received an intracerebroventricular (i.c.v.) injection of streptozotocin that induced cognitive impairment, treatment with curcumin p.o. for 30 days did not prevent the impairment in short-term spatial memory but there was a possible beneficial effect on short-term recognition memory when administered at 50 and 100 mg/kg per day. Curcumin treatment at 25 and 50 mg/kg per day caused an improvement in immunoreactivity of ionized calcium binding adaptor molecule 1 (Iba-1) in the corpus callosum but not in the lateral ventricles and septum. For all of the curcumin doses tested there was no improvement in Iba-1-immunoreactivity in the DG, CA1 and CA2 areas of the hippocampus. Also, curcumin treatment at all of the doses tested did not improve GFAP-immunoreactivity in the periventricular areas and the hippocampus, and did not cause an improvement in hippocampal and subventricular neurogenesis (Bassani et al., 2017).
Soluble tumor necrosis factor inhibitor XPro1595
Treatment with XPro1595 s.c. for 2 months of 5xFAD Tg mice, 2 to 4 months and 5 to 7 months of age, reduced the age-dependent increase in activated immune cells and decreased the overall number of CD4+ T cells. XPro1595 treatment decreased the density (% of area) of Aβ plaques in the subiculum but not the entire hippocampus of Tg mice. Aβ density was decreased in the dorsal and posterior subiculum but not the ventral subiculum. Treatment with XPro1595 rescued long-term potentiation measured in brain slices, indicating that XPro1595 modulated synaptic function in Tg mice (MacPherson et al., 2017).
Interleukin-1 receptor antagonist
Injection of lentivirus (LV) expressing IL-1RA into the somatosensory cortex of female APP/PS1deltaE9 mice, 4 to 5 months of age, rescued the impairment in increasing spine density under an enriched environment over 5 weeks, and this resulted from a gradual decline in spine elimination instead of rising spine formation (Zou et al., 2016).
Pioglitazone
Pioglitazone treatment p.o. (by supplementing the rodent chow) over 6 weeks regained the steady increase in spine density of female APPswe/PSEN1deltaE9 mice, 4 to 5 months of age, under enriched environment and resulted from the gradual decline in spine elimination, with the rate of spine formation unchanged (Zou et al., 2016).
Treatment of female 3xTg (PS1M146V, APPSwe and tauP301L) mice, 10 months of age, with pioglitazone p.o. (by supplementing the rodent chow) over 4 months did not affect the spontaneous exploratory activity, locomotion, or anxiety level of the mice. However, pioglitazone improved learning of the 3xTg mice as evidenced by shorter escape latency and swimming distance on day 4 of the elevated plus maze test. Pioglitazone treatment did not affect the performance in the probe test, suggesting that treatment with pioglitazone for 4 months did not improve spatial reference memory. Total tau level of the mice was not altered by pioglitazone treatment for 4 months. GFAP staining in the brains of 3xTg mice was reduced by pioglitazone treatment for 4 months, suggesting that pioglitazone may attenuate neuroinflammation (Yu et al., 2016).
Rosiglitazone
Treatment of female 3xTg (PS1M146V, APPSwe and tauP301L) mice, 10 months of age, with rosiglitazone p.o. (by supplementing the rodent chow) for 4 months did not affect the spontaneous exploratory activity, locomotion, or anxiety level of the mice. Moreover, rosiglitazone treatment did not improve the learning of the mice or the spatial reference memory. Total tau level of the mice was not altered by rosiglitazone treatment for 4 months. Treatment with rosiglitazone reduced GFAP staining in the brains of 3xTg mice, suggesting that rosiglitazone treatment may decrease neuroinflammation (Yu et al., 2016).
Quercetin
Quecetin treatment i.p. of 3xTg AD mice, 18 to 21 months of age, with treatment given every 48 hours for 3 months, increased cell density in the subiculum. There was a decrease in the amount of extracellular Aβ deposition in all of the cerebral regions of the brain examined in the quercetin-treated 3xTg AD mice, with a reduction in Aβ40 and Aβ42 levels in the hippocampus. Neurofibrillary tangles were decreased in the CA1 area, the subiculum and the amygdala, but not in the entorhinal area, of the quercetin-treated 3xTg AD mice. Total tau level was not altered by quercetin treatment. GFAP immunoreactivity was reduced in the CA1 area, the entorhinal area and the amygdala, but no changes were seen in the subiculum, on quercetin treatment. The quercetin-treated 3xTg AD mice had decreased microglial activation in the CA1 area, the subiculum and the amygdala, but no changes in the entorhinal area. Performance on learning and spatial memory tasks and greater risk assessment behavior of the 3xTg AD mice in the elevated plus maze test was improved by quercetin treatment (Sabogal-Guaqueta et al., 2015).
Interleukin-1β
Treatment of APP/PS1 mice, 7 months of age, with recombinant adeno-associated virus vector 2 expressing IL-1β (rAAV2-IL-1β) injected into each hippocampus resulted in marked microglial activation 4 weeks following transduction. The levels of IL-1β and chemokine (C-C motif) ligand 2 were increased, and there was a reduction in 6E10 and Congo Red staining of amyloid plaques, in the hippocampus of APP/PS1 mice transduced with rAAV2-IL-1β. The levels of insoluble and soluble Aβ42 in the hippocampus were decreased but there was no alteration in the levels of APP or the activity of β-secretase in APP/PS1 mice transduced with rAAV2-IL-1β. Transduction with rAAV2-IL-1β suggested that peripheral mononuclear cells were recruited to the brain parenchyma following IL-1β overexpression, but were not necessary for IL-1β-mediated amyloid plaque clearance in APP/PS1 mice (Rivera-Escalera et al., 2014).
Cannabidiol
Cannabidiol treatment p.o. (via a gel pellet) daily for 5 months of male APP/PS1 (APPswe/PSEN1deltaE9) mice, 3 months of age, ameliorated the social recognition deficit but had no effect on anxiety behaviors, with behavioral testing started at 10 months of age. Levels of soluble and insoluble Aβ40 or Aβ42 in the cortex of APP/PS1 mice were unchanged by cannabidiol treatment, and similarly Aβ levels in the hippocampus were unchanged after cannabidiol treatment. No significant effect of cannabidiol treatment on the levels of mRNA for IL-1β and TNF-α was observed (Cheng et al., 2014).
Memantine
Memantine treatment of olfactory bulbectomized (OBX) male Sprague-Dawley rats, 8 to 10 weeks of age, p.o. via gavage daily, starting 2 days prior to surgery and continued to 28 days after surgery, reversed the short-term spatial memory deficit in T maze behavioral test, but is without effect on reference memory in the holeboard (Borre et al., 2012), and partly rescued OBX-induced fear memory loss in the passive avoidance retention task. Also treatment with memantine attenuated OBX-induced hyperactivity. Memantine treatment attenuated OBX-induced cell loss in the dentate gyrus (DG) area but failed to rescue cell loss in the CA3 and CA1 areas of the dorsal hippocampus, and attenuated OBX-induced cell loss in the CA3, CA1 and DG areas of the ventral hippocampus. Treatment with memantine decreased splenic T cells in OBX animals (Borre et al., 2014).
Multi-targeted diet
The multi-targeted diet (which included curcumin, piperine, melatonin, choline, uridine, decosahexaenoic acid, eicosapentaenoic acid) to OBX male Sprague-Dawley rats, 8 to 10 weeks of age, for 2 weeks prior to surgery and 4 weeks thereafter reversed the short-term spatial memory deficit in T maze behavioral test, but was without effect on reference memory in the holeboard, and partly rescued OBX-induced fear memory loss in the passive avoidance retention task. The OBX-induced hyperactivity was reduced by the experimental diet. The experimental diet attenuated OBX-induced cell loss in the DG area but did not rescue cell loss in the CA3 and CA1 areas in the dorsal hippocampus. In the ventral hippocampus, the experimental diet rescued OBX-induced cell loss in the CA3, CA1 and DG areas. The experimental diet significantly decreased splenic T cells in OBX animals (Borre et al., 2014).
Clioquinol
Treatment of male and female APP/PS1 (APPswe/PSEN1deltaE9) mice, 5 months of age, with clioquiniol p.o. daily for 5 months decreased the area fraction and the number of amyloid deposits in the neocorex and hippocampus in animals at 11 months of age. Activated astrocytes were located primarily surrounding the amyloid plaques, and the total number of plaque-associated astrocytes was decreased in clioquinol-treated APP/PS1 mice, presumably because of the decrease in the area and number of amyloid plaques in these animals. Activated microglia (CD68-labeled) were found primariy near or surrounding amyloid plaques, and the number of CD68-labeled microglia was reduced in clioquinol-treated APP/PS1 mice in parallel with the decrease of amyloid plaques (Zhang et al., 2013).
Cell-based therapies
The cell-based therapies were with M2 macrophages, CD4+ CD25+ Foxp3+ Treg cells, Th1 T cells stimulated with anti-CD3, APOE3 (apolipoprotein E3) bone marrow cells, and amniotic stem cells. They have all been associated with immunomodulatory properties (M2 macrophages: Parsa et al., 2012; Loi et al., 2016; CD4+ CD25+ Foxp3+ Treg cells: Shi et al., 2011; Luz-Crawford et al, 2013; Th1 T cells stimulated with anti-CD3: Sinha and Bagchi, 2004; Abraham et al., 2008; APOE3 bone marrow cells: Yang et al., 2013; amniotic stem cells: Insausti et al., 2014; Magatti et al., 2018).
The five animal studies utilizing these cell-based therapies are summarized in Table 2. Four of these studies had used mouse models, and one a rat model. In the mouse studies, the ages of the animals at which treatment was started ranged from 4 to 16 months and where gender was specified 1 had used males, and 1 both males and females. The rat study had used males 9 to 10 weeks of age. The treatment period with the cell-based therapy ranged from 7 days to 8 months.
Table 2.
Studies of cell-based therapies with immunomodulatory properties in animal models of Alzheimer’s disease (AD)
| Study | Details |
|---|---|
| M2 macrophages | |
| Reference | Zhu et al. (2016) |
| Number of animals, gender, ages, and treatment | Adult male F344 rats, 8–9 weeks of age, were randomly divided into groups: model group (intracerebroventricular injection (i.c.v.) amyloid-β (Aβ42) + intravenous injection (i.v.) phosphate buffer saline (PBS), n = 15) and M2-transplantation group (i.c.v. Aβ42 + i.v. M2 macrophages, n = 15). Aβ42 peptide was dissolved in sterile water at a concentration of 6 mg/mL and diluted to 2 mg/mL with 0.01 M PBS. The peptide was incubated at 37°C for 4 days to aggregate it before injection. Rats were anesthetized, placed in the stereotaxic apparatus, and a hole driled in the skull. Then 5 µL of aggregated Aβ42 suspension was administered by i.c.v. injection. Bone marrow cells were collected from the tibial and femoral shafts of male F344 rats. Macrophages were isolated from the bone marrow cell suspensions, cultured and differentiated in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum and 10 ng/mL recombinant macrophage colony-stimulating factor for 6 days. On 7th day, macrophages were skewed to M2 phenotype by adding 15 ng/mL recombinant interleukin (IL)-4 for 48 hours. On 9th day, M2 macrophages were labeled with DiI and then detached with 0.25% trypsin for 10 minutes. At 5 days after stereotactic surgery, 1 × 106 M2 macrophages/rat were injected via tail vein. Morris water maze test was carried out from day 12 to day 14 after Aβ42 injection. Spontaneous locomotor activity was tested by Y maze on day 16. For immunohistochemistry, rats in each group (n = 5) were anesthetized, and perfused with 0.1 M PBS followed by 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde. The remaining rats were euthanized and the cortex isolated and homogenized for analysis of cytokine levels. |
| Comparison | Rats received i.c.v. PBS + i.v. PBS as control sham group (n = 15) |
| Functional outcomes | M2 macrophages were labeled with DiI to distinguish transplanted cells from endogenous M2 macrophages/microglia. In Morris water maze, escape latency was prolonged in the model group compared with the sham group, indicating spatial memory deficits. In the Y maze, the proportion of AD model rats that entered three consecutive different arms was significantly lower than that of the sham control rats. Compared to model group rats, M2-transplantation rats showed an increase in both tests. Immunohistochemistry analysis of the density of total neurons (NeuN+ cells) in cortex and cholinergic neurons (ChAT+ cells) in nucleus basalis of Maynert were significantly decreased in the model group compared to sham group. In the M2-transplantation group, the densities were restored to the levels of the sham group. These findings demonstrated that M2 macrophage transplantation significantly improved learning and memory in the AD model rats and exerted neuroprotective effects. Immunohistochemistry analysis showed that the densities of inducible nitric oxide synthase-positive cells (M1 macrophages/microglia) and CD206+ cells in the cortex of rats in the model group were significantly increased and decreased, respectively, compared to sham group. In the M2-transplantation group, these changes were reversed. immunohistochemistry showed no DiI+ cells in the brain. These findings demonstrated that transplanted M2 macrophages were not trafficked to the brain, but were able to induce an increase in the numbers of endogenous M2 microglia. Expression levels of IL-4, IL-5 and β-nerve growth factor in the cortex of rats in the model group were decreased compared to the sham group, indicating that administration of Aβ42 led to the supression of anti-inflammatory factors and triggered an inflammatory response and neuroinflammatory injury. All of these changes were reversed by M2 macrophage transplantation. Western blots showed interferon regulatory factor (IRF)5 levels in cortex were significantly raised, while levels of IRF4 were significantly lowered, in model rats compared to sham rats. M2 macrophage transplantion rats had significantly lower levels of IRF5 and significantly higher levels of IRF4 compared to model rats. These results indicated that transplantation promoted M2 phenotype polarization. In vitro studies with primary microglia isolated from cortex of neonatal rats at postnatal day 1 suggested that Aβ42 induced a neuroinflammatory response that stimulated microglia to polarize into the M1 phenotype. In contrast, nerve growth factor induced polarizartion of microglia toward M2 and rebalanced the ratio of M1 and M2 phenotype cells. |
| Conclusion | M2 macrophage transplantation attenuated neuroinflammation, reversed Aβ42-induced changes in IRF4 and IRF5, drove endogenous microglial polarization toward M2 phenotype, and ameliorated cognitive impairment. |
| CD4+CD25+Foxp3+ Treg cells | |
| Reference | Baek et al. (2016) |
| Number of animals, gender, ages, and treatment | Adult male 3xFAD Tg AD mice with PS1M146V, APPKM670/671NL, tauP301L transgenes, 4 months of age, were used. CD4+CD25+ T cells and CD4+CD25– T cells were isolated from the spleens obtained from male 6-week-old C57BL6 mice. Either 1 × 106 Treg or Teff cells were adoptively transferred by i.v. injection (tail vein) of 3xTg AD mice. Spatial learning and memory in mice was tested using Morris water maze. After the behavioral test, mice were transcardially perfused with saline containing 0.5% sodium nitrate and heparin (10 U/mL) and then fixed with 4% paraformaldehyde in 0.1 M PBS. Each brain was removed and post-fixed overnight at 4°C, and stored in 30% sucrose for sectioning and immunohistochemistry. Single-cell suspension of splenocytes was cultured in RPMI-1640 with 10% fetal bovine serum and antibiotics. Cultures were activated in the presence of plate-bound anti-CD3 and soluble anti-CD28 antibody. Cytokines were assayed in the supernatants. |
| Comparison | Male 3xTg AD mice as control. Also wild type (WT) mice. |
| Functional outcomes | Treg treatment of 3xTg AD mice improved cognitive impairments as indicated by a decreased escape latency response and increased platform entries in the spatial probe test compared with the 3xTg group. The expression of IL-2, IL-6, interferon-γ and IL-17A in supernatants of splenocyte cultures was significantly increased for 3xTg AD mice compared with WT group. Administration of CD4+CD25+ T cells significantly decreased these cytokine levels compared with the 3xTg group. Administration of CD4+CD25– T cells significantly reduced interferon-γ levels but was without effect on the other cytokines compared to 3xTg group. In addition, IL-10 levels were significantly increased in Treg transferred to 3xTg AD mice compared with the 3xTg group, but not with Teff transfer. Treatment with CD4+CD25+ T cells of 3xTg AD mice significantly decreased Aβ burdens in both cortex and hippocampus compared with the 3xTg group. In contrast to treatment with CD4+CD25+ T cells, treatment with CD4+CD25– T cells had no effect on Aβ in hippocampus or cortex. Treg treatment reduced the number of Iba-1+ microglia in hippocampal CA1 region of 3xTg AD mice, whereas Teff treatment had no effect on Iba-1 expression, compared with the 3xTg group. |
| Conclusion | Transplantation of purified CD4+CD25+ T cells into 3xTg AD mice improved cognitive functions and reduced Aβ deposition. |
| Th1 T cells stimulated with anti-CD3 | |
| Reference | Fisher et al. (2014) |
| Number of animals, gender, ages, and treatment | Adult amyloid precursor protein (APP)/presinilin 1 (PS1) transgenic (Tg) mice, 5 months and 12–15 months of age, were used. Aβ-specific T cell line was generated by immunizing mice 2 months of age by footpad injection of Aβ42 (100 µg) emulsified in complete Freund’s adjuvant H37Ra. At 10 days later, popliteal, ingunal, and iliac lymph nodes were extracted and cells seeded (5 × 106 cells/mL) in Biotarget medium supplemented with 10 µg/mL Aβ42. Every other day thereafter, human recombinant IL-2 (rIL-2) (10 U/mL) in complete Dulbecco’s modified Eagle medium was added. Following 1 week and every 2 weeks later, the T cell cultures were restimulated with irradiated (6000 rad) splenocytes and reseeded (2 × 105 T cells/mL, 5 × 106 irradiated splenocytes/mL) CD4+ T cells were restimulated with 1 µg/mL anti-CD3 for 48 hours. Cells were then harvested and resuspended in PBS at a concentration of 5 × 104 cells/µL. For Th1 cell subpopulation generation, anti-IL-4 (20 µg/mL) and mouse IL-12 (20 ng/mL) were supplemented in the first three stimulations during seeding and then 2 days later. OVA-specific T cell line was also generated from spleens from OT-II OVA (ovalbumin) TCR Tg mice cultured with OVA 323–339 peptide (OVA323–339) (10 µg/mL). Cells (2.5 × 105) were slowly injected over 5 minutes into each of the lateral ventricles of the brain of APP/PS1 Tg mice, 5 months of age, with a stereotactic device (n = 3–5). |
| Comparison | WT mice were i.c.v. injected with Aβ-specific Th1 T cells (n = 3–5) |
| Functional outcomes | Significantly more CD4 T cells were observed at 28 days post-injection in the hippocampus, thalamus and cortex of 5 month-old APP/PS1 Tg mice injected i.c.v. with Aβ-specific Th1 T cells (Aβ -> AD mice) as compared with WT mice injected i.c.v. with Aβ-specific Th1 T cells (Aβ -> WT mice) and with APP/PS1 Tg mice injected i.c.v. with OVA323–339-specific T cells (OVA-> AD mice). Immunohistochemistry of the hippocampus showed that whereas the cells were randomly distributed in Aβ -> WT mice, they were clustered around Aβ plaques in Aβ -> AD mice at 28 days. In Aβ -> AD mice compared to OVA-> AD mice, major histocompatibility complex II was increased and was colocalized with T cells at the sites of Aβ plaques. These findings suggested that the accumulation of Aβ in the brain of APP/PS1 Tg mice promoted the targeting of T cells specifically to their Aβ antigens and thereby increasing major histocompatibility complex II expression, which presumably facilitated longer retention of these cells in the brain. Aβ-specific Th1 cells or PBS were injected i.c.v. to APP/PS1deltaE9 Tg mice (Aβ -> AD and PBS ->AD, respectively) 12–15 months of age. Brain sections were immunolabeled with anti-Aβ at 28 days post-injection and the areas occupied by Aβ plaques were analyzed. Compared with PBS ->AD mice, a 56% and 30% reduction in plaque burden was found in the hippocampus and cortex, respectively, of Aβ-> AD mice. By real time polymerase chain reaction analysis, major histocompatibility complex II, interferon-γ and tumor necrosis factor (TNF)-α in the brains of Aβ -> AD mice remained significantly upregulated as compared with PBS ->AD mice at 28 days post-injection (~3-fold), but to a markedly lower extent than at 5 days post-injection. Similarly, signal regulatory protein-1β, which was shown to increase Aβ uptake by microglia, was induced by Th1 cells (but not Th2 or Th17 cells) at 5 days post-injection and to a lesser extent at 28 days post-injection. Moreover, of all of the chemokines induced at 5 days post-injection, only chemokine (C-X-C motif) ligand 9 remained significantly upregulated. These results suggested a very low grade inflammatory reaction induced by Aβ-plaque-associated Th1 T cells sufficient to enhance the clearance of Aβ by microglia. Using the terminal dexynucleotidyl transferase(TdT)-mediated dUTP nick end labeling staining, the number of apoptotic cells was very low and similar in the brain of Aβ -> AD mice and of PBS ->AD mice, whereas the positive control (sections from a mouse brain following vascular injury) showed markedly increased apoptosis. The amount of proliferating and differentiating neuronal progenitors in the hippocampus was measured in brain sections from 6-month-old WT mice and from 10- to 12-month-old APP/PS1deltaE9 Tg mice, each left untreated or i.c.v. injected with Aβ-specific Th1 T cells or with PBS. Sections were immunolabeled with anti-doublecortin, a microtubule-associated protein expressed by neuronal precursor cells. The total number of doublecortin-positive cells was similar in all groups. However, while the number of neuronal progenitors in subgranular layer of dentate gyrus was also similar in all 6-month-old WT groups, it was significantly increased in 10- to 12-month-old APP/PS1deltaE9 Tg mice i.c.v. injected with Aβ-specific Th1 T cells. These findings suggested that i.c.v. injected Aβ-specific Th1 T cells induced neither neuronal loss nor chronic neuroinflammation, and that in APP/PS1deltaE9 Tg mice they induced a temporary increase in dentate gyrus proliferating neuronal progenitors. |
| Conclusion | Aβ-specific Th1 T cells when injected into APP/PS1 Tg mice target Aβ plaques, increase Aβ uptake, and promote neurogenesis with no evidence of neuronal loss. |
| Apolipoprotein (APOE) 3 bone marrow cells (BMCs), APOE4 BMCs | |
| Reference | Yang et al. (2013) |
| Number of animals, gender, ages, and treatment | Adult APPswe/PS1deltaE9 mice, 5 months of age, received total body 10.5 Gy single dose irradiation at approximately 2 Gy/min from a cesium-137 source. BMCs were isolated from 8-week-old male APOE3/3;GFP (green fluorescent protein) or APOE4/4;GFP transgenic mice by flushing femurs and tibias with RPMI media with 10% fetal bovine serum. The samples were combined, passed through a 25-G needle filtered through a 70-µm nylon mesh and centrifuged. Erythrocytes were lysed in ammonium chloride potassium buffer, and the remaining leukocytes resuspended in sterile PBS at a concentration of approximately 5 × 106 viable nucleated cells/200 µL. Irradiated APPswe/PS1deltaE9 mice received APOE3/3;GFP (n = 11) or APOE4/4;GFP (n = 8) BMCs via retro-orbital venous plexus injections 1 day after total body irradiation. Chimeric mice underwent behavioral testing at 8 months after transplantation and were then euthanized for tissue analysis. Blood was collected by cardiac puncture for complete blood counts and flow cytometry and mice were transcardially perfused with ice-cold PBS. Brains were removed for analysis. |
| Comparison | – |
| Functional outcomes | White bood cell, red blood cell, and platelet counts did not differ between groups. Multilineage differentiation of hematopoietc stem cells was within the normal range, with no significant differences between groups. There was no differential influence of APOE on the proportions of T and B lymphocytes and neutrophils. Although differential blood counts showed no differences in total monocytes, flow cytometry of peripheral blood indicated APOE4/4 BMC transplantation (BMT) gave rise to fewer CD11b+ monocytes/macrophages than did APOE3/3 BMT, suggesting effects of APOE on monocyte molecular phenotype in the periphery. Mononuclear cells were isolated for flow cytometry from cerebral cortex and were then probed for microglia, which unlike peripheral monocytes are CD11b+ and CD45low cells. Although almost half of the CD11b+CD45low cells were BMT derived (GFP+) in APOE3/3 recipients, less than a third of microglia in APOE4/4 recipients were derived from BMT. Using immunofluorescence histology, hippocampus and cerebral cortex from the contralateral hemisphere were analysed to further quantitate APOE genotype effects on BMT-derived monocyte/microglia engulfment. BMT-derived cells were identified by strong GFP autofluorescence in both groups, and on the basis of Iba-1 immunopositivity were almost uniformly microglia. Stereological analysis showed significantly increased donor-derived microglia in APOE3/3 compared to APOE4/4 recipients in cerebral cortex and in hippocampus. Overall, cerebral cortical and hippocampal microglia densities were not significantly different between the two groups, and there was no significant APOE effect on total microglia density between BMC recipients. APOE3/3 transplantation resulted in 45% and 40% greater APOE protein levels in the cerebral cortex and hippocampus, respectively, than did APOE4/4 transplantation. In the behavior tests, APOE3/3 mice displayed habitation to a novel environment as shown by a progressive significant decrease in total distance traveled over successive days, whereas APOE4/4 mice showed no significant decrease in distance traveled over successive days. There was no significant difference in baseline locomotor function between the two groups, nor any significant differences in the acquisition phase of the Barnes maze test; however, reversal learning was significantly preserved in APOE3/3 recipients compared with APOE4/4 recipients. APOE3/3 mice exhibited decreased distance traveled, shorter escape latency, and fewer errors than APOE4/4 mice. The APOE4/4 recipients only used a spatial or serial search strategy 16% of the time, whereas APOE3/3 recipients used one of these strategies 50% of the time. These findings demonstrated better spatial working memory in APPswe/PS1deltaE9 recipients of APOE3/3 compared to APOE4/4 BMT. Using immunohistochemistry of sections stained with a pan-Aβ antibody, total area occupied by Aβ plaques and plaque frequency were significantly decreased in the hippocampus of APOE3/3 mice compared to APOE4/4 mice. APOE genotype effects were less apparent in the cerebral cortex, where plaque frequency was lower in APOE3/3 recipients, but there was no significant effect of donor APOE genotype on total area occupied by plaques. Average plaque size was not affected by donor genotype in either cortex or hippocampus. Qualitatively more bone marrow-derived cells were found in association with Aβ plaques in the hippocampi of APOE3/3 recipients compared with APOE4/4 recipients. In both groups, GFP+ cells around plaques had a less ramified morphology, with blunted processes extending around and into the immunopositive amyloid core. There were no significant differences in Tris-HCl buffer-soluble Aβ40 or Aβ42 between the two groups in cerebral cortex or hippocampus. However, APOE3/3 recipients had less guanidine-soluble Aβ40 in cerebral cortex and hippocampus compared to APOE4/4 recipients. There was no significant effect of donor APOE genotype on levels of guanidine-soluble Aβ42 in cerebral cortex or hippocampus. Using real time polymerase chain reaction, the levels of TNF-α and macrophage migration inhibitory factor in cerebral cortex were significantly increased in APOE4/4 compared to APOE3/3 recipients, and levels of IL-10 were significantly lower in the APOE4/4 compared to APOE3/3 recipients. Donor APOE genotype did not promote differences in cerebral cortex expression levels of IL-6, IL-4, chemokine (C-C motif) ligand 2, chemokine (C-X3-C motif) ligand 1, and chemokine (C-C motif) ligand 8. Also there was no effect of donor APOE genotype on major histocompatibility complex II microglia expression. Overall, these findings indicated that APOE4/4 transplantation resulted in a more proinflammatory state in cerebral cortex and hippocampus than did APOE3/3 transplantation. |
| Conclusion | Bone marrow-derived APOE3 expressing cells were superior to those expressing APOE4 in their ability to mitigate the behavioral and neuropathological changes in APP/PS1 mouse model of AD. |
| Amniotic stem cells (AMSCs) | |
| Reference | Kim et al. (2013) |
| Number of animals, gender, ages, and treatment | Tg2576 mice were used to evaluate the effect of AMSC transplantation. Adult female APPswe mice. 15–16 months of age, were used for the behavioral studies and for pathological analysis at 12 weeks after transplantation (n = 8/group). Also adult male APPswe mice. 12–13 months of age, were used for additional pathological analysis at 1 week after transplantation (n = 8/group). To evaluate the immunomodulatory effects of AMSCs on AD pathology, 3xTg AD mice, 6–7 months of age, were used (3 female mice at each time point). Normal human placentae (≥ 37 gestational weeks) were obtained after Cesarean section. Each placenta was carefully dissected and the tissue washed in PBS and then mechanically minced and digested with 0.5% collagenase IV for 30 minutes at 37°C. Harvested cells were cultured in α-MEM with 10% fetal bovine serum, antibiotics, 25 ng/mL fibroblast growth factor 4 and 1 µg/mL heparin. Cells were cultured in complete medium containing 25 ng/mL fibroblast growth factor 4 and 1 µg/mL heparin for 6 days at 37°C. For i.v., 200 µL of cell suspension (approximately 2 × 106 cells) was injected into the tail vein (AMSC-injected group). All behavioral tests were performed 6 weeks after AMSC transplantation. For immunohistological analysis, mice were euthanized at 1 week (n = 8/group) and 12 weeks (n = 8/group) after injection. |
| Comparison | WT littermates (n = 10) were used as normal control group. Also female 3xTg AD mice, 6–7 months of age, injected i.v. with PBS (PBS-injected group) |
| Functional outcomes | The water maze test (WMT) was performed 6 weeks after transplantation. In trial block 1, the normal control mice tended to find the hidden platform more quickly than the PBS- and AMSC-injected groups, while in trial block 2 the mean escape latencies of the 3 groups were not significantly different. The latency in the normal control group was significantly faster in trial block 3 than the PBS- and AMSC-injected groups. From trial block 3 to trial block 5, the AMSC-injected group showed a marked change in mean escape latency, indicating that impaired memory function was reversed by stem cell injection. Although there was no significant difference between the AMSC-injected group and the normal control group in trial blocks 4 and 5, there was a significant difference between the AMSC-injected group and the PBS-injected group for trial blocks 4 and 5. A probe test was performed on day 5, 24 hours after the last training trial. This involved removing the platform and recording the length of time each nouse spent within the zone previously occupied by the platform over a period of 60 seconds. The time spent in the zone was significantly less for the PBS-injected group than for the normal group and the AMSC-injected group. There was no difference between the normal group and the AMSC-injected group in terms of swimming velocity throughout the first four trial blocks, but there was a significant difference between the normal group and the PBS-injected group. There was no difference between the WT mice (normal control group), APPswe mice injected with PBS (PBS-injected group) or APPswe mice injected with AMSCs (AMSC-injected group) in terms of locomotor tests, the elevated plus maze test, light/dark transition tests, or open field test tests. Analysis of sections stained eith cresyl violet and thioflavin S showed that the number of Aβ plaques in the cortex and hippocampus was significantly lower in the AMSC-injected group (treated for 12 weeks with AMSCs) than in the PBS-injected group. The numbers of small (< 50 µm in diameter) and intermediate (50–100 µm in diameter) plaques were significantly lower in AMSC-injected group than in the PBS-injected group, whereas the number of large plaques (> 100 µm in diameter) was not different between the groups. The AMSC-injected group tended to show a positive correlation between mean escape latency in the WMT and the number of amyloid plaques (P < 0.06), but no such correlation was found in the PBS-injected group. At 1 weeks post-injection, the number of Iba-1+ microglia was significantly greater in the AMSC-injected group compared with the PBS-injected group, whereas the number of Iba-1+ microglia was not different beween the two groups at 12 weeks post-injection. The area of Iba-1+ microglia was significantly higher in the AMSC-injected group than in the PBS-injected group at 1 week post-injection, but significantly lower at 12 weeks post-injection. These findings suggested that AMSCs were able to recruit microglial cells during the initial acute stage after transplantation in the AD mouse model, and after the initial stage AMSCs maintained a lower number of resident microglial cells despite the proinflammatory environment. This supports an immunosuppresive role for AMSCs. At 1 week post-injection, the levels of mRNA expression of the proinflammatory cytokines IL-1 and TNF-α in the brain were significantly lower in the AMSC-injected group than in the PBS-injected group, whereas the levels for the anti-inflamatory cytokines IL-10 and transforming growth factor-β were significantly higher in the AMSC-injected group than in the PBS-injected group. At 12 weeks post-injection, the levels of mRNA expression of IL-1 and TNF-α in the brain were not significantly different between the two groups, whereas the levels for IL-10 and transforming growth factor-β were significantly higher in the AMSC-injected group than in the PBS-injected group. At 1 week post-injection, the levels of Aβ-degrading enzymes, including insulin-degrading enzyme and matrix metalloprotein-9, were significantly higher in the AMSC-injected group than in the PBS-injected group. |
| Conclusion | At 6 weeks after treatment with AMSCs, AD mice showed improved spatial learning which was significantly correlated with fewer Aβ plaques in the brain. The level of proinflammatory cytokines IL-1 and TNF-α was lower and that of anti-inflammatory cytokines IL-10 and transforming growth factor-β was higher in AMSC-injected mice than in PBS-injected mice. |
Animal Alzheimer’s disease or cognitive impaired studies
M2 macrophages
Intravenous injection (i.v.) of M2 macrophages at 5 days after male F344 rats, 8 to 9 weeks of age, had been administered intracerebroventricular injection (i.c.v.) aggregated Aβ42 suspension caused an improvement in learning and menory deficits in MWM and Y maze test performed from day 12 to day 14 after Aβ42 administration. M2-transplantation restored the density of total neurons (NeuN+ cells) in the cortex and cholinergic neurons (ChAT+ cells) in the nucleus basalis of Maynert of the AD model rats. In the M2-transplantation group, the densities of inducible nitric oxide synthase-positive cells (M1 macrophages/microglia) were reduced and CD206+ cells (M2 macrophages/microglia) were increased in the cortex, but there were no M2 transplanted cells in the brain. These findings showed that transplanted M2 cells were not trafficked to the brain, but were able to induce an increase in the numbers of endogenous M2 macrophages/microglia. M2 macrophage transplantation reversed the decrease in expression levels of IL-4, IL-6 and β-nerve growth factor in the cortex of AD rats brought about by administration of Aβ42. M2 macrophage transplantation rats had lower levels of interferon regulatory factor 5 and higher levels of interferon regulatory factor 4 compared to untreated AD rats, indicating that transplantation promoted M2 phenotype polarization (Zhu et al., 2016).
CD4+CD25+Foxp3+ Treg cells
CD4+ CD25+ Treg cells adoptively transfered i.v. to male 3xTg AD mice (PS1M146V, APPKM670/671NL and tauP301L transgenes), 4 months of age, improved cognitive impairments in spatial probe test after 2 months of cell transfer. Administration of Treg cells increased IL-10 levels in splenocytes from 3xTg AD mice. Aβ deposition in the cortex and hippocampus was decreased by treatment with Treg cells. The number of Iba-1+ microglia in hippocampal CA1 region of 3xTg AD mice was reduced by Treg treatment (Baek et al., 2016).
Th1 T cells
The number of CD4 T cells was increased at 28 days post-injection in the hippocampus, thalamus and cortex of APP/PS1 (APPswe/PSEN1deltaE9) Tg mice, 5 months of age, injected i.c.v. with Aβ-specific T cells compared with APP/PS1 Tg mice injected i.c.v. with ovalbumin (OVA)-specific T cells. The cells were clustered around Aβ plaques in the hippocampus of the APP/PS1 Tg mice administered Aβ-specific T cells. Major histocompatibility complex II (MHCII) was increased and colocalized with T cells at the sites of Aβ plaques. It was suggested that the accumulation of Aβ in the brain of APP/PS1 Tg mice promoted the targeting of T cells specifically to their Aβ antigens and thereby increased MHCII expression, which presumably facilitated longer retention of these cells in the brain. On injecting i.c.v. Aβ-specific T cells to APP/PS1 Tg mice at 12 to 15 months of age, a decrease in Aβ plaque deposition occurred in the hippocampus and cortex at 28 days post-injection. MHCII, interferon-γ and TNF-α were upregulated in the brains of APP/PS1 Tg mice administered i.c.v. Aβ-specific T cells, but to a lower extent than at 5 days post-injection. Signal regulatory protein-1β, shown to increase Aβ uptake by microglia, was induced by Th1 cells at 5 days post-injection and to a lesser extent at 28 days post-injection. Of all of the chemokines induced at 5 days post-injection, only chemokine (C-X-C motif) ligand 9 remained upregulated. These findings suggested a very low grade inflammatory reaction induced by Aβ-plaque-associated Th1 T cells that was sufficient to increase the clearance of Aβ by microglia. The number of apoptotic cells in the brains of APP/PS1 Tg mice injected with Aβ-specific T cells was low. Aβ-specific Th1 T cells did not induce neuronal loss or chronic neuroinflammation, and in APP/PS1 Tg mice they induced a temporary increase in proliferating neuronal progenitors in the dentate gyrus (Fisher et al., 2014).
Apolipoprotein E3- and apolipoprotein E4-bone marrow cells
Injection of APOE3- or APOE4-bone marrow cells via retro-orbital venous plexus at 1 day after total body irradiation of APP/PS1 (APPswe/PSEN1deltaE9) mice, 5 months of age, had no differential influence of APOE on the proportions of T and B lymphocytes and neutrophils. While differential blood counts showed no differences in total monocytes, flow cytometry of peripheral blood indicated that APOE4 bone marrow cell transplantation (BMT) resulted in fewer CD11b+ monocyte/macrophages than did APOE3 BMT. Almost half of the microglia were BMT derived in APOE3 recipients, while only a third of the microglia were derived from BMT in APOE4 recipients. APOE3 transplantation resulted in higher APOE protein levels in the cortex and hippocampus than did APOE4 transplantation. APOE3 transplantation was superior to APOE4 transplantation in mitigating the cognitive deficit in APP/PS1 mice. Total area of Aβ plaques and plaque frequency were significantly reduced in the hippocampus of APOE3 mice compared to APOE4 mice, and a greater number of bone marrow-derived cells were associated with Aβ plaques in the hippocampus of APOE3 recipients compared to APOE4 recipients. The levels of TNF-α and macrophage migration inhibitory factor in the cortex were greater in APOE4 compared to APOE3 recipients, and levels of IL-10 were lower in the APOE4 compared to APOE3 recipients. Donor APOE genotype did not promote differences in cortex expression levels of IL-6, IL-4, chemokine (C-C motif) ligand 2, chemokine (C-X3-C motif) ligand 1, and chemokine (C-C motif) ligand 8. Also there was no effect of donor APOE genotype on MHCII microglia expression. These findings indicated that APOE4 transplantation resulted in a greater inflammatory state in the cortex and hippocampus than did APOE3 transplantation (Yang et al., 2013).
Amniotic stem cells
Injection i.v. of amniotic stem cells in female APPswe mice, 15 to 16 months of age, resulted in a reversal of impaired memory function assessed at 6 weeks after transplantation. At 12 weeks after amniotic stem cell (AMSC) transplantation, the number of Aβ plaques was decreased compared to PBS-injected mice, with lower numbers of small and intermediate plaques in the AMSC-injected group than in the PBS-injected group, while there was no difference in the number of large plaques between the two groups. At 1 week post-injection of AMSC in male APPswe mice, 12 to 13 months of age, the number of Iba-1+ microglia was greater in the AMSC-injected group compared with the PBS-injected group, whereas at 12 weeks post-injection the number of Iba-1+ microglia did not dffer beween the two groups. The area of Iba-1+ microglia was higher in the AMSC-injected group than in the PBS-injected group at 1 week post-injection, but lower at 12 weeks post-injection. These findings suggested that AMSCs were able to recruit microglial cells during the initial acute stage after transplantation in the AD mouse model, and after this stage AMSCs maintained a lower number of resident microglial cells despite the proinflammatory environment. At 1 week post-injection, the levels of mRNA expression of IL-1 and TNF-α in the brain were lower in the AMSC-injected group than in the PBS-injected group, whereas the levels of IL-10 and transforming growth factor-β were higher in the AMSC-injected group than in the PBS-injected group. At 12 weeks post-injection, the levels of mRNA expression of IL-1 and TNF-α in the brain did not differ between the two groups, whereas the levels for IL-10 and transforming growth factor-β were higher in the AMSC-injected group than in the PBS-injected group. At 1 week post-injection, the levels of Aβ-degrading enzymes, which included insulin-degrading enzyme and matrix metalloprotein-9, were higher in the AMSC-injected group than in the PBS-injected group (Kim et al., 2013).
Ameliorating Effects of Immunomodulatory Agents in Alzheimer’s Disease
The amyloid cascade hypothesis proposed that abnormal production of Aβ is the cause of AD and that the neurotoxicity is due to Aβ itself or its oligomeric forms (Selkoe, 1991; Hardy and Higgins, 1992). However it has been suggested that this, in itself, canot be the cause of AD because micromolar concentrations of these Aβ forms would be required for such toxicity, and their levels in the brain are much lower being in the picomolar range (McGeer and McGeer, 2013). AD most likely results from the inflammatory response induced by extracellular Aβ deposits, which later becomes enhanced by aggregates of tau. Activated microglia drive the inflammatory response which increases over time as the disease progresses. Pharmacological and cell-based therapies that target inflammation are promising approaches for the treatment of Alzheimer’s disease (Figure 1).
Figure 1.
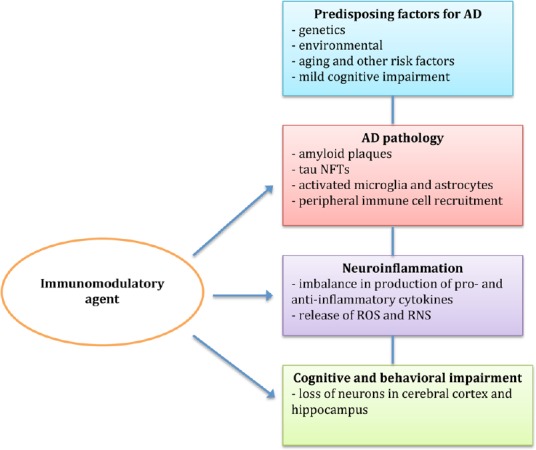
Targeting pathophysiological parameters of Alzheimer’s disease by immunomodulatory agents.
AD: Alzheimer’s disease; NFTs: neurofibrillary tangles; ROS: reactive oxygen species; RNS: reactive nitrogen species.
The pharmaceutical studies described in this review have identified several agents with immunomodulatory properties that alleviated AD pathology and cognitive impairment in animal models of AD. The agents found to ameliorate memory and learning deficits were fasudil, LW-AFC, curcumin, pioglitazone, quercetin, memantine, and multi-targeted diet, while cannabidiol improved the social recognition deficit. Aβ deposition was reduced by fasudil, LW-AFC, curcumin, XPro1595, quercetin, IL-1β, and clioquinol, whereas tau hyperphosphorylation levels and neurofibrillary tangles were lowered by curcumin. The agents that protected against neuronal cell loss were LW-AFC, curcumin, quercetin, memantine, and multi-targeted diet. Attenuation of neuroinflammation by immunomodulatory agents was suggested by several findings. Astrocyte activation in the brain was decreased by curcumin, pioglitazone, rosiglitazone, quercetin, and clioquinol, while curcumin, quercetin, and clioquinol also reduced microglial activation in the brain. The levels of proinflammatory cytokines were decreased by fasudil and curcumin, while that of anti-inflammatory cytokines was increased by fasudil. LW-AFC also restored aberrant cytokine secretion. In addition, LW-AFC restored the imbalance in the hypothalamic-pituitary-adrenal axis and the hypothalamic-pituitary-gonadal axis, and partially restored normal lymphocyte expression. However, it should be noted that while these immunomodulatory compounds alleviated the inflammatory response in AD, they may also affect other systems that may be important in AD pathogenesis e.g., clioquinol is a metal chelator (Rodriquez-Santiago et al., 2015), quercetin is an antioxidant (Zheng et al., 2017), memantine targets glutamatergic signaling (Damasceno et al., 2014; Carvajal et al., 2016). So while these compounds may influence immune function, it is not clear whether this is their primary mechanism of action or a secondary consequence of actions on some other target.
The cell-based therapies reviewed have been shown to improve functional and neurological outcomes in animal models of AD. Learning and memory deficits were ameliorated by M2 macrophages, CD4+ CD25+ Treg cells, APOE3 BMCs, and AMSCs. In addition, CD4+ CD25+ Treg cells, Aβ-specific Th1 T cells, APOE3 BMCs, and AMSCs reduced Aβ deposition. M2 macrophages restored the density of total neurons, and AMSCs increased the levels of IL-10 and transforming growth factor-β, both of which are anti-inflammatory cytokines.
Perspectives
Recent studies in animal models of AD have mainly investigated immunotherapy against Aβ or use of immunomodulatory agents as intervention strategies. Active and passive immunotherapy targeting Aβ was found to be successful in reducing Aβ deposition and preventing cognitive decline in many AD animal model trials, but failed to translate to effective treatment of AD in clinical trials (Rosset et al., 2015). Encephalitis occurred in a minority of patients treated with active immunization and vasogenic edema or amyloid-related imaging abnormalities was a complication in some passive immunization trials (Wisniewski and Goni, 2014). The amyloid-related imaging abnormalities were detected by magnetic resonance imaging and considered to represent transient accumulation of fluid/edema or microhemorrhages, and it was proposed that anti-Aβ immunotherapy may affect blood-brain barrier integrity (Blockx et al., 2016).
One important difference betwen animal models of AD and the human disease is the age of the subject. AD occurs mainly in humans aged 65 years and older (Burns and Iliffe, 2009; Bali et al., 2012), whereas most mouse models use juvenile or young adult animals. In spite of greater amyloid clearance in middle-aged APP/PS1 mice than aged mice, microhemorrhages only developed in old animals treated with anti-Aβ antibody (Li et al., 2012). Aging in the immune system results in a tendency to proinflammatory responses. T-cell proliferation and proinflammatory cytokines were found in mice which received Aβ immunization and contraindicated the use of such interventions in elderly AD patients (Lambracht-Washington and Rosenberg, 2015). In view of the above and with aging being the greatest risk factor for Alzheimer’s disease (Guerreiro and Bras, 2015), immunomodulatory agents may hold the most potential for the development of novel AD therapeutics (Fernandez et al., 2013). Many of the immunomodulatory compounds tested are phytochemicals found in plants and foods e.g., quercetin, curcumin, cannabidiol (Libro et al., 2016). Several studies have shown that increased consumption of fruits and vegetables can reduce the risk of developing AD and retard its progression (Hughes et al., 2010; Hu et al., 2013; Hartman and Ross, 2018). However, epidemiological evidence that individual phytochemicals can affect AD is lacking.
The majority of the animal studies reviewed had employed Tg models of early-onset or FAD, with the animals being young adult mice 2 to 10 months of age. Only one study had used Tg AD mice 18 to 21 months of age (Sabogal-Guaqueta et al., 2015). All of the immunomodulatory pharmaceutical agents and cell-based therapies tested had a beneficial effect on the AD parameter(s) targeted (Figure 1). In a survey of studies between 2000–2012 by Li et al. (2013), clioquinol, memantine and rosiglitazone were the only immunomodulatory pharmaceutical agents reported on and had similar effects to those desribed in the present review. The only model of age-related/late-onset AD was SAMP8 mice 6 months of age (Wang et al., 2017). More effort needs to be given to creating new models of late-onset AD and also rat Tg models of AD. A greater number of synapses and a more complex synaptic organization occurs in post-natal brain development in rats than in mice, and should allow a more accurate assessment of the impact of AD pathology on cognitive outcomes (Do Carmo and Cuello, 2013). Rat models used in the studies reviewed had involved i.c.v. injection of streptozotocin or removal of the olfactory bulbs. The latter represents a putative model for AD with the animals exhibiting depression and an increase in hyperphosphorylation of brain tau protein and in the number of tangles (Yehuda and Rabinovitz, 2014). Current animal models of AD have severe limitations in their capacity to facilitate immunological investigations. The animals are usually studied after life-long housing in the absence of natural microbial pathogens, possibly resulting in reduced host fitness and poor disease resistance (Rosshart et al., 2017; Cao and Zheng, 2018). This has important implications for trying to develop animal models of late-onset AD, as etiology of AD involves a combination of genetic (70%) and environmental factors (30%) (Dorszewska et al., 2016).
Finally, just as the multi-targeted diet had beneficial effects in OBX rats, consideration should be given to investigating the effects of a combinational therapy involving two or more of the tested pharmaceutical agents together, or one of these agents given in conjunction with one of the cell-based therapies, in an aged animal model of AD.
Additional file: Open peer review report 1 (97.8KB, pdf) .
Footnotes
Conflicts of interest: There are no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Hans-Gert Bernstein, Otto-von-Guericke University Magdeburg, Germany.
P-Reviewer: Bernstein HG; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Abood WN, Fahmi I, Abdulla MA, Ismail S. Immunomodulatory effect of an isolated fraction from Tinospora crispa on intracellular expression of INF-γ, IL-6 and IL-8. BMC Complement Altern Med. 2014;14:205. doi: 10.1186/1472-6882-14-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham M, Karni A, Dembinsky A, Miller A, Gandhi R, Anderson D, Weiner HL. In vitro induction of regulatory T cells by anti-CD3 antibody in humans. J Autoimmun. 2008;30:21–28. doi: 10.1016/j.jaut.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baek H, Ye M, Kang GH, Lee C, Lee G, Choi DB, Jung J, Kim H, Lee S, Kim JS, Lee HJ, Shim I, Lee JH, Bae H. Neuroprotective effects of CD4+ CD25+ Foxp3+ regulatory T cells in a 3xTg-AD Alzheimer’s disease model. Oncotarget. 2016;7:69347–69357. doi: 10.18632/oncotarget.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bali J, Gheinani HA, Zurbriggen S, Rajendran L. Role of genes linked to sporadic Alzheimer’s disease risk in the production of β-amyloid peptides. Proc Nat Acad Sci U S A. 2012;109:15307–15311. doi: 10.1073/pnas.1201632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassani TB, Turnes JM, Moura EL, Bonato JM, Cóppola-Segovia V, Zanata SM, Oliveira RM, Vital MA. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced model of dementia of Alzheimer’s type. Behav Brain Res. 2017;335:41–54. doi: 10.1016/j.bbr.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Bassil N, Grossberg GT. Novel regimens and delivery systems in the pharmacological treatnent of Alzheimer’s disease. Drugs. 2009;23:293–307. doi: 10.2165/00023210-200923040-00003. [DOI] [PubMed] [Google Scholar]
- 7.Blockx I, Einstein S, Guns PJ, Van Audekerke J, Guglielmetti C, Zago W, Roose D, Verhoye M, Van der Linden A, Bard F. Monitoring blood-brain barrier integrity following amyloid-β immunotherapy using gadolinium-enhanced MRI in a PDAPP mouse model. J Alzheimers Dis. 2016;54:723–735. doi: 10.3233/JAD-160023. [DOI] [PubMed] [Google Scholar]
- 8.Borchelt DR. B6.Cg-Tg(APP695)3Dbo Tg(PSEN1dE9)S9Dbo/Mmjax. 2018. [Accessed August 5, 2018]. https://www.jax.org/strain/005866 .
- 9.Borre Y, Bosman E, Lemstra S, Westphal KG, Olivier B, Oosting RS. Memantine partly rescues behavioral and cognitive deficits in an animal model of neurodegeneration. Neuropharmacology. 2012;62:2010–2017. doi: 10.1016/j.neuropharm.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Borre YE, Panagaki T, Koelink PJ, Morgan ME, Hendriksen H, Garssen J, Kraneveld AD, Olivier B, Oosting RS. Neuroprotective and cognitive enhancing effects of a multi-targeted food intervention in an animal model of neurodegeneration and depression. Neuropharmacology. 2014;79:738–749. doi: 10.1016/j.neuropharm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Burns A, Iliffe S. Alzheimer’s disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 12.Cao W, Zheng H. Peripheral immune system in aging and Alzheimer’s disease. Mol Neurodegener. 2018;13:51. doi: 10.1186/s13024-018-0284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvajal FJ, Mattison HA, Cerpa W. Role of NMDA-mediated glutamatergic signaling in chronic and acute neuropathologies. Neural Plasticity. 2016;2016:2701526. doi: 10.1155/2016/2701526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casas-Grajales S, Vázquez-Flores LF, Ramos-Tovar E, Hernández-Aquino E, Flores-Beltrán RE, Cerda-García-Rojas CM, Camacho J, Shibayama M, Tsutsumi V, Muriel P. Quercetin reverses experimental cirrhosis by immunomodulation of the proinflammatory and profibrotic processes. Fundam Clin Pharmacol. 2017;31:610–624. doi: 10.1111/fcp.12315. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Li S, Sun W, Li J. Anti-diabetes drug pioglitazone ameliorates synaptic defects in AD transgenic mice by inhibiting cyclin-dependent kinase5 activity. PLoS One. 2015;10:e0123864. doi: 10.1371/journal.pone.0123864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YT, Nikulina K, Lazarev S, Bahrami AF, Noble LB, Gallup M, McNamara NA. Interleukin-1 as a phenotypic immunomodulator in keratinizing squamous metaplasia of the ocular surface in Sjogren’s syndrome. Am J Pathol. 2010;177:1333–1343. doi: 10.2353/ajpath.2010.100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng D, Spiro AS, Jenner AM, Garner B, Karl T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J Alzheimers Dis. 2014;42:1383–1396. doi: 10.3233/JAD-140921. [DOI] [PubMed] [Google Scholar]
- 18.Cheng XR, Zhou WX, Zhang YX. The behavioral, pathological and therapeutic features of the senescence-accelerated mouse prone 8 strain as an Alzheimer’s disease animal model. Ageing Res Rev. 2014;13:13–37. doi: 10.1016/j.arr.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung JA, Cummings JL. Neurobehavioral and neuropsychiatric symptoms in Alzheimer’s disease: characteristics and treatment. Neurol Clin. 2000;18:829–846. doi: 10.1016/s0733-8619(05)70228-0. [DOI] [PubMed] [Google Scholar]
- 21.Damasceno FS, Barison MJ, Pral EM, Paes LS, Silber AM. Memantine, an antagonist of the NMDA glutamate receptor, affects cell proliferation, differentiation and the intracellular cycle and induces apoptosis in Trypanosoma cruzi. PLoS Negl Trop Dis. 2014;8:e2717. doi: 10.1371/journal.pntd.0002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Do Carmo S, Cuello AC. Modeling Alzheimer’s disease in transgenic rats. Mol Neurodegener. 2013;8:37. doi: 10.1186/1750-1326-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorszewska J, Prendecki M, Oczkowska A, Dezor M, Kozubski W. Molecular basis of familial and sporadic Alzheimer’s disease. Curr Alzheimer Res. 2016;13:952–963. doi: 10.2174/1567205013666160314150501. [DOI] [PubMed] [Google Scholar]
- 24.El-Sisi AE, Sokar SS, Salem TA, Abu Risha SE. PPARγ-dependent anti-tumor and immunomodulatory sctions of pioglitazone. J Immunotoxicol. 2015;12:308–316. doi: 10.3109/1547691X.2014.978055. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez PL, Britton GB, Rao KS. Potential immunotargets for Alzheimer’s disease treatment strategies. J Alzheimers Dis. 2013;33:297–312. doi: 10.3233/JAD-2012-121222. [DOI] [PubMed] [Google Scholar]
- 26.Fischer R, Kontermann RE, Maier O. Targeting sTNF/TNFR1 signaling as a new therapeutic strategy. Antibodies. 2015;4:48–70. [Google Scholar]
- 27.Fisher Y, Strominger I, Biton S, Nemirovsky A, Baron R, Monsonego A. Th1 polarization of T cells injected into the cerebrospinal fluid induces brain immunosurveillance. J Immunol. 2014;192:92–102. doi: 10.4049/jimmunol.1301707. [DOI] [PubMed] [Google Scholar]
- 28.Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPSW transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- 29.Giannoulia-Karantana A, Vlachou A, Polychronopoulou S, Papassotiriou I, Chrousos GP. Melatonin and immunomodulation: connections and potential clinical applications. Neuroimmunomodulation. 2006;13:133–144. doi: 10.1159/000097258. [DOI] [PubMed] [Google Scholar]
- 30.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granowitz EV, Porat R, Mier JW, Pribble JP, Stiles DM, Bloedow DC, Catalano MA, Wolff SM, Dinarello CA. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992;4:353–360. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- 32.Guerreiro R, Bras J. The age factor in Alzheimer’s disease. Genome Med. 2015;7:106. doi: 10.1186/s13073-015-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 34.Hartman RE, Ross DM. Effects and mechanisms of actions of phytochemicals on Alzheimer’s disease neuropathology. Front Biosci (Elite Ed) 2018;10:300–333. doi: 10.2741/e824. [DOI] [PubMed] [Google Scholar]
- 35.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirahashi J, Kawahata K, Arita M, Iwamoto R, Hishikawa K, Honda M, Hamasaki Y, Tanaka M, Okubo K, Kurosawa M, Takase O, Nakakuki M, Saiga K, Suzuki K, Kawachi S, Tojo A, Seki G, Marumo T, Hayashi M, Fujita T. Immunomodulation with eicosapentaenoic acid supports the treatment of autoimmune small-vessel vasculitis. Sci Rep. 2014;4:6406. doi: 10.1038/srep06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hjorth E, Freund-Levi Y. Immunomodulation of microglia by docosahexaenoic acid and eicosapentaenoic acid. Curr Opin Clin Nutr Metab Care. 2012;15:134–143. doi: 10.1097/MCO.0b013e32835017cc. [DOI] [PubMed] [Google Scholar]
- 38.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 39.Hosokawa M, Kasai R, Higuchi K, Takeshita S, Shimizu K, Hamamoto H, Honma A, Irino M, Toda K, Matsumura A, et al. Grading score system: a method for evaluation of the degree of senescence in senescence accelerated mouse (SAM) Mech Ageing Dev. 1984;26:91–102. doi: 10.1016/0047-6374(84)90168-4. [DOI] [PubMed] [Google Scholar]
- 40.Hu N, Yu JT, Tan L, Wang YL, Sun L, Tan L. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes TF, Andel R, Small BJ, Borenstein AR, Mortimer JA, Wolk A, Johansson B, Fratiglioni L, Pedersen NL, Gatz M. Midlife fruit and vegetable consumption and risk of dementia in later life in Swedish twins. Am J Geriat Psychiatry. 2010;18:413–420. doi: 10.1097/JGP.0b013e3181c65250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Insausti CL, Blanquer M, García-Hernández AM, Castellanos G, Moraleda JM. Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 2014;7:53–63. doi: 10.2147/SCCAA.S58696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwami D, Nonomura K, Shirasugi N, Niimi M. Immunomodulatory effects of eicosapentaenoic acid through induction of regulatory T cells. Int Immunopharmacol. 2011;11:384–389. doi: 10.1016/j.intimp.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 44.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 45.Joo IL, Lai AY, Bazzigaluppi P, Koletar MM, Dorr A, Brown ME, Thomason LA, Sled JG, McLaurin J, Stefanovic B. Early neurovascular dysfunction in a transgenic rat model of Alzheimer’s disease. Sci Rep. 2017;7:46427. doi: 10.1038/srep46427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kidd BA, Wroblewska A, Boland MR, Agudo J, Merad M, Tatonetti NP, Brown BD, Dudley JT. Mapping the effects of drugs on the immune system. Nat Biotechnol. 2016;34:47–54. doi: 10.1038/nbt.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim KS, Kim HS, Park JM, Kim HW, Park MK, Lee HS, Lim DS, Lee TH, Chopp M, Moon J. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol Aging. 2013;34:2408–2420. doi: 10.1016/j.neurobiolaging.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 48.Koch C, Brovkina L, Ruehl R, Worm M. Immunomodulation by dietary n3-polyunsaturated acids (PUFA) in vitro. J Allergy Clin Immunol. 2006;117:S147. [Google Scholar]
- 49.Lambracht-Washington D, Rosenberg RN. A noninflammatory immune response in aged DNA Aβ42-immunized mice supports its safety for possible immunotherapy in AD patients. Neurobiol Aging. 2015;36:1274–1281. doi: 10.1016/j.neurobiolaging.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, Yang YS, Suh JG, Lee BH, Jin HK, Bae JS. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588–602. doi: 10.1016/j.neurobiolaging.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 51.Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, Tzeng NS, Wang LJ, Lee IH, Wang TY, Chen KC, Yang YK, Hong JS, Lu RB. Low-dose memantine attenuated methadone dose in opioid-dependent patients: a 12-week double-blind randomized controlled trial. Sci Rep. 2015;5:10140. doi: 10.1038/srep10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C, Ebrahimi A, Schluesener H. Drug pipeline in neurodegeneration based on transgenic mice models of Alzheimer’s disease. Ageing Res Rev. 2013;12:116–140. doi: 10.1016/j.arr.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Li Q, Lebson L, Lee DC, Nash K, Grimm J, Rosenthal A, Selenica ML, Morgan D, Gordon MN. Chronological age impacts immunotherapy and monocyte uptake independent of amyloid load. J Neuroimmune Pharmacol. 2012;7:202–214. doi: 10.1007/s11481-011-9329-9. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. Quercetin inflammation and immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein though a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–49532. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- 56.Libro R, Giacoppo S, Soundara Rajan T, Bramanti P, Mazzon E. Natural phytochemicals in the treatment and prevention of dementia: An oveview. Molecules. 2016;21:518. doi: 10.3390/molecules21040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu CY, Guo SD, Yu JZ, Li YH, Zhang H, Feng L, Chai Z, Yuan HJ, Yang WF, Feng QJ, Xiao BG, Ma CG. Fasudil mediates cell therapy of EAE by immunomodulating encephalomyelitic T cells and macrophages. Eur J Immunol. 2015;45:142–152. doi: 10.1002/eji.201344429. [DOI] [PubMed] [Google Scholar]
- 58.Liu DS, Liu WJ, Chen L, Ou XM, Wang T, Feng YL, Zhang SF, Xu D, Chen YJ, Wen FQ. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates acrolein-induced airway mucus hypersecretion in rats. Toxicology. 2009;260:112–119. doi: 10.1016/j.tox.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Loi F, Cordova LA, Zhang R, Pajarinen J, Lin TH, Goodman SB, Yao Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016;7:15. doi: 10.1186/s13287-016-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lowinus T, Bose T, Busse S, Busse M, Reinhold D, Schraven B, Bommhardt UH. Immunomodulation by memantine in therapy of Alzheimer’s disease is mediated through inhibition of Kv1. 3 channels and T cell responsiveness. Oncotarget. 2016;7:53797–53807. doi: 10.18632/oncotarget.10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, Noël D, Jorgensen C, Figueroa F, Djouad F, Carrión F. Mesenchymal stem cells generate a CD4+ CD25+ Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacPherson KP, Sompol P, Kannarkat GT, Chang J, Sniffen L, Wildner ME, Norris CM, Tansey MG. Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiol Dis. 2017;102:81–95. doi: 10.1016/j.nbd.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magatti M, Vertua E, Cagnoni A, Silini A, Parolini O. The immunomodulatory properties of amniotic cells: The two sides of the coin. Cell Transplant. 2018;27:31–44. doi: 10.1177/0963689717742819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandrekar-Colucci S, Landreth GE. Microglia and inflammation in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2010;9:156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126:479–497. doi: 10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- 66.Mecha M, Feliú A, Iñigo PM, Mestre L, Carrillo-Salinas FJ, Guaza C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis. 2013;59:141–150. doi: 10.1016/j.nbd.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Medrano-Campillo P, Sarmiento-Soto H, Álvarez-Sánchez N, Álvarez-Ríos AI, Guerrero JM, Rodríguez-Prieto I, Castillo-Palma MJ, Lardone PJ, Carrillo-Vico A. Evaluation of the immunomodulatory effect of melatonin on the T-cell response in peripheral blood from systemic lupus erythematosus patients. J Pineal Res. 2015;58:219–226. doi: 10.1111/jpi.12208. [DOI] [PubMed] [Google Scholar]
- 68.Mimica N, Presecki P. Side effects of approved antidementives. Psychiatr Danub. 2009;21:108–113. [PubMed] [Google Scholar]
- 69.Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer‘s disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nedumpun T, Wongyanin P, Sirisereewan C, Ritprajak P, Palaga T, Thanawongnuwech R, Suradhat S. Interleukin-1 receptor antagonist: an early immunomodulatory cytokine induced by porcine reproductive and respiratory syndrome virus. J Gen Virol. 2017;98:77–88. doi: 10.1099/jgv.0.000665. [DOI] [PubMed] [Google Scholar]
- 71.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 73.Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Onos KD, Sukoff Rizzo SJ, Howell GR, Sasner M. Toward more predictive genetic mouse models of Alzheimer’s disease. Brain Res Bull. 2016;122:1–11. doi: 10.1016/j.brainresbull.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parsa R, Andresen P, Gillett A, Mia S, Zhang XM, Mayans S, Holmberg D, Harris RA. Adoptive transfer of imunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 2012;61:2881–2892. doi: 10.2337/db11-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parrish WR, Gallowitsch Puerta M, Ochani M, Ochani K, Moskovic D, Lin X, Czura CJ, Miller EJ, Al-Abed Y, Tracey KJ, Pavlov VA. Choline suppresses inflammatory responses. Shock. 2006;25:45. [Google Scholar]
- 77.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, Chavan S, Goldstein RS, Czura CJ, Miller EJ, Al-Abed Y, Tracey KJ, Pavlov VA. Modulation of TNF release requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 79.Petrasek T, Skurlova M, Maleninska K, Vojtechova I, Kristofikova Z, Matuskova H, Sirova J, Vales K, Ripova D, Stuchlik A. A rat model of Alzheimer’s disease based on Abeta42 and pro-oxidative substances exhibits cognitive deficit and alterations in glutamatergic and cholinergic neurotransmitter systems. Front Aging Neurosci. 2016;8:83. doi: 10.3389/fnagi.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riedel WJ. Preventing cognitive decline in preclinical Alzheimer’s disease. Curr Opin Pharmacol. 2014;14:18–22. doi: 10.1016/j.coph.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 81.Rivera-Escalera F, Matousek SB, Ghosh S, Olschowka JA, O’Banion MK. Inteleukin-1β mediated amyloid plaque clearance is independent of CCR2 signaling in the APP/PS1 mouse model of Alzheimer’s disease. Neurobiol Dis. 2014;69:124–133. doi: 10.1016/j.nbd.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodgers G, Doucette CD, Spurrell DR, Hoskin DW, West KA, Liwski RS. Immunomodulatory effect of piperine on dendritic cell function (50. 36) J Immunol. 2009;182:5036. [Google Scholar]
- 83.Rodriquez-Santiago L, Alí-Torres J, Vidossich P, Sodupe M. Coordination properties of a metal chelator clioquinol to Zn(2+) studied by static DFT and ab initio molecular dynamics. Phys Chem Chem Phys. 2015;17:13582–13589. doi: 10.1039/c5cp01615k. [DOI] [PubMed] [Google Scholar]
- 84.Rosset MB, Lui G, Dansokho C, Chaigneau T, Dorothée G. Vaccine-induced Aβ-specific CD8+ T cells do not trigger autoimune neuroinflammation in a murine model of Alzheimer’s disease. J Neuroinflammation. 2015;12:95. doi: 10.1186/s12974-015-0317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, Hickman HD, McCulloch JA, Badger JH, Ajami NJ, Trinchieri G, Pardo-Manuel de Villena F, Yewdell JW, Rehermann B. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell. 2017;171:1015–1028. doi: 10.1016/j.cell.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth. 2010;105:201–207. doi: 10.1093/bja/aeq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sabogal-Guaqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sastre M, Walter J, Gentleman SM. Interactions between APP secretases and inflammatory mediators. J Neuroinflammation. 2008;5:25. doi: 10.1186/1742-2094-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 91.Serghides L, Patel SN, Ayi K, Lu Z, Gowda DC, Liles WC, Kain KC. Rosiglitazone modulates the innate immune response to Plasmodium falciparum infection and improves outcome in experimental cerebral malaria. J Infect Dis. 2009;199:1536–1545. doi: 10.1086/598222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheng JG, Mrak RE, Griffin WS. Neuritic plaque evolution in Alzheimer’s disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol. 1997;94:1–5. doi: 10.1007/s004010050664. [DOI] [PubMed] [Google Scholar]
- 93.Shi M, Liu ZW, Wang FS. Inmmunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimada A, Hasegawa-Ishii S. Senescence-accelerated Mice (SAMs) as a Model for Brain Aging and Immunosenescence. Aging Dis. 2011;2:414–435. [PMC free article] [PubMed] [Google Scholar]
- 95.Singh S, Loke YK, Furberg CD. Long-term use of thiazolidenediones and the associated risk of pneumonia or lower respiratory tract infection: systematic review and meta-analysis. Thorax. 2011;66:383–388. doi: 10.1136/thx.2010.152777. [DOI] [PubMed] [Google Scholar]
- 96.Sinha AK, Bagchi AK. Role of anti-CD3 in modulation of Th1-type immune response in Shigella dysenteriae infection. J Med Microbiol. 2004;53:1075–1081. doi: 10.1099/jmm.0.05420-0. [DOI] [PubMed] [Google Scholar]
- 97.Song C, Zhang XY, Manku M. Increased phospholipase A2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: effects of chronic ethyl-eicosapentaenoate treatment. J Neurosci. 2009;29:14–22. doi: 10.1523/JNEUROSCI.3569-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song Y, Chen X, Wang LY, Gao W, Zhu MJ. Rho kinase inhibitor fasudil protects against β-amyloid-induced hippocampal neurodegeneration in rats. CNS Neurosci Ther. 2013;19:603–610. doi: 10.1111/cns.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau synapses in Alzheimer’s disease. Neuron. 2014;82:756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sundaram JR, Poore CP, Sulaimee NHB, Pareek T, Cheong WF, Wenk MR, Pant HC, Frautschy SA, Low CM, Kesavapany S. Curcumin ameliorates neuroinflammation, neurodegeneration, and memory deficits in p25 transgenic mousae model that bears hallmarks of Alzheimer’s disease. J Alzheimers Dis. 2017;60:1429–1442. doi: 10.3233/JAD-170093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sunila ES, Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J Ethnopharmacol. 2004;90:339–346. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 102.Thorlacius K, Slotta JE, Laschke MW, Wang Y, Menger MD, Jeppsson B, Thorlacius H. Protective effect of fasudil, a Rho-kinase inhibitor, on chemokine expression, leukocyte recruitment, and hepatocellular apoptosis in septic liver injury. J Leukoc Biol. 2006;79:923–931. doi: 10.1189/jlb.0705406. [DOI] [PubMed] [Google Scholar]
- 103.Tong L, Prieto GA, Kramár EA, Smith ED, Cribbs DH, Lynch G, Cotman CW. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. J Neurosci. 2012;32:17714–17724. doi: 10.1523/JNEUROSCI.1253-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J, Lei X, Cheng XR, Zhang XR, Liu G, Cheng JP, Xu YR, Zeng J, Zhou WX, Zhang YX. LW-AFC, a new formula derived from Liuwei Dihuang decoction, ameliorates behavioral and pathological deterioration via modulating the neuroendocrine-immune system in PrP-hAβPPswe/PS1ΔE9 transgenic mice. Alzheimer's Res Ther. 2016;8:57. doi: 10.1186/s13195-016-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J, Zhang X, Cheng X, Cheng J, Liu F, Xu Y, Zeng J, Qiao S, Zhou W, Zhang Y. LW-AFC, a new formula derived from Liuwei Dihuang decoction, ameliorates cognitive deterioration and modulates neuroendocrine-immune system in SAMP8 mouse. Curr Alzheimer Res. 2017;14:221–238. doi: 10.2174/1567205013666160603001637. [DOI] [PubMed] [Google Scholar]
- 106.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 107.Wisniewski T, Goni F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88:499–507. doi: 10.1016/j.bcp.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang Y, Cudaback E, Jorstad NL, Hemingway JF, Hagan CE, Melief EJ, Li X, Yoo T, Khademi SB, Montine KS, Montine TJ, Keene CD. APOE3, but not APOE4, bone marrow transplantation mitigates behavioral and pathological changes in a mouse model of Alzheimer disease. Am J Pathol. 2013;183:905–917. doi: 10.1016/j.ajpath.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yehuda S, Rabinovitz S. Olfactory bulbectomy as a putative model for Alzheimer’: the protective role of essential fatty acids. PharmaNutrition. 2014;2:12–18. [Google Scholar]
- 110.Yu JZ, Li YH, Liu CY, Wang Q, Gu QF, Wang HQ, Zhang GX, Xiao BG, Ma CG. Multitarget therapeutic effect of fasudil in APP/PS1 transgenic mice. CNS Neurological Disorders-Drug Targets. 2017;16:199–209. doi: 10.2174/1871527315666160711104719. [DOI] [PubMed] [Google Scholar]
- 111.Yu Y, Li X, Blanchard J, Li Y, Iqbal K, Liu F, Gong CX. Insulin sensitizers improve learning and attenuate tau hyperphosphorylation and neuroinflammation in 3xTg-AD mice. J Neural Transm. 2016;122:593–606. doi: 10.1007/s00702-014-1294-z. [DOI] [PubMed] [Google Scholar]
- 112.Zgair A, Lee JB, Wong JCM, Taha DA, Aram J, Di Virgilio D, McArthur JW, Cheng YK, Hennig IM, Barrett DA, Fischer PM, Constantinescu CS, Gershkovich P. Oral administration of cannabis with lipids leads to high levels of cannabinoids in the intestinal lymphatic system and prominent immunomodulation. Sci Rep. 2017;7:14542. doi: 10.1038/s41598-017-15026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang YH, Raymick J, Sarkar S, Lahiri DK, Ray B, Holtzman D, Dumas M, Schmued LC. Efficacy and toxicity of clioquinol treatment and A-beta42 inoculation in the APP/PS1 mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2013;10:494–506. doi: 10.2174/1567205011310050005. [DOI] [PubMed] [Google Scholar]
- 114.Zheng YZ, Deng G, Liang Q, Chen DF, Guo R, Lai RC. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci Rep. 2017;7:7543. doi: 10.1038/s41598-017-08024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu D, Yang N, Liu YY, Zheng J, Ji C, Zuo PP. M2 macrophage transplantation ameliorates cognitive dysfunction in amyloid-β-treated rats through regulation of microglial polarization. J Alzheimers Dis. 2016;52:483–495. doi: 10.3233/JAD-151090. [DOI] [PubMed] [Google Scholar]
- 116.Zou C, Montagna E, Shi Y, Peters F, Blazquez-Llorca L, Shi S, Filser S, Dorostkar MM, Herms J. Intraneuronal APP and extracellular Aβ independently cause dendritic spine pathology in transgenic mouse models of Alzheimer’s disease. Acta Neuropathol. 2015;129:909–920. doi: 10.1007/s00401-015-1421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zou C, Shi Y, Ohli J, Schüller U, Dorostkar MM, Herms J. Neuroinflammation impairs adaptive structural plasticity of dendritic spines in a preclinical model of Alzheimer’s disease. Acta Neuropathol. 2016;131:235–246. doi: 10.1007/s00401-015-1527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


