Abstract
The diabetic cornea exhibits pathological alterations, such as delayed epithelial wound healing and nerve regeneration. We investigated the role of semaphorin (SEMA) 3C in corneal wound healing and reinnervation in normal and diabetic B6 mice. Wounding induced the expression of SEMA3A, SEMA3C, and their receptor neuropilin-2 (NRP2), but not NRP1, in normal corneal epithelial cells; this upregulation was suppressed for SEMA3C and NRP2 in diabetic corneas. Injections of Sema3C-specific small interfering RNA and NRP2-neutralizing antibodies in wounded mice resulted in a decrease in the rate of wound healing and regenerating nerve fibers, whereas exogenous SEMA3C had opposing effects in diabetic corneas. NRP1 neutralization, on the other hand, decreased epithelial wound closure but increased sensory nerve regeneration in diabetic corneas, suggesting a detrimental role in nerve regeneration. Taken together, epithelium-expressed SEMA3C plays a role in corneal epithelial wound closure and sensory nerve regeneration. The hyperglycemia-suppressed SEMA3C/NRP2 signaling may contribute to the pathogenesis of diabetic neurotrophic keratopathy, and SEMA3C might be used as an adjunctive therapeutic for treating the disease.
Introduction
Diabetic peripheral neuropathy (DPN) is a common and debilitating complication, with a majority of patients with diabetes developing altered sensation as a result of damage to peripheral sensory nerves, which are preferentially affected in the early stages of diabetes (1–4). DPN is the primary cause of diabetes-related hospital admissions and nontraumatic foot amputations. In the cornea, the major defects are mostly observed in the epithelium, including epithelial edema, fragility, and recurrent erosions, ulcers, superficial punctate keratitis, and delayed and incomplete wound repair (5). In addition to epithelial complications, the decrease in the density of sensory nerves occurs during the early stages of diabetes (5–10), and this nerve damage is known to be directly related to the severity of somatic neuropathy in patients with diabetes (11–13). DPN may result from the slow degeneration of peripheral nerves as well as from defects in their regeneration as a result of environmental challenges, such as wounding and infection (14–16). To date, the underlying basis for nerve damage is not well established. Because the most severe reduction in nerve fiber and branch density occurs in the subbasal nerve plexus close to the corneal epithelium (16), the role of epithelia in the pathogenesis of DPN and diabetic neurotrophic keratopathy has been suggested (5), but the molecules involved have not been fully evaluated.
Our previous studies (8,10) revealed that diabetes causes a significant decrease in epithelium healing rate and delayed epithelium wound closure, hereby termed delayed wound healing (see reviews by Ljubimov [5] and Ljubimov and Saghizadeh [17]). Diabetes also causes defects in sensory nerves, including decreases in the density of nerve endings in the subbasal nerve plexus and in the number of branches that innervate the epithelium (5,8,16). Epithelial debridement wounds result in the loss of nerve endings and retraction of stroma nerve fibers. In normal corneas, sensory nerve regeneration is robust, starting near the limbus with newly formed sensory nerve fibers and endings arranged in parallel and extending radially toward the center of the cornea (16,18,19). Diabetic mouse corneas exhibit changes similar to those observed in diabetic human corneas, with fewer nerve insertion sites near the limbus, and severely delayed regeneration of sensory nerve endings that exhibit a tortuous, fragmented appearance (20). Neurotrophins expressed by the developing cornea, including nerve growth factor, brain-derived neurotrophic factor, ciliary neurotrophic factor, and neurotrophin-3, have been shown to play a critical role in attracting sensory nerves (21). However, these factors function largely as nonspecific chemoattractants, and specific guidance can be realized mainly by guidance molecules (22), of which there are four families: netrins, slits, semaphorins (SEMAs), and ephrins (23–25). Although the reduced number of regenerating sensory nerves in diabetic corneas may be related to the reduction of neurotrophic factors, such as ciliary neurotrophic factor (26) and neurotrophin-3, morphological alteration of the regenerating sensory nerves in diabetic corneas may be due to defects in these guidance molecules.
The SEMAs are a large family of guidance cues that direct neuronal network formation (27). Among this family, the seven members of the class 3 SEMA (SEMA3) subfamily are the only secreted SEMAs (28). SEMA3s require the presence of at least one of the two receptors, neuropilin-1 (NRP1) and NRP2, which form receptor complexes with the plexin (PLXN) receptors A1–A4, or D1, for proper signaling in neurons. SEMA3A binds specifically to NRP1, and SEMA3F and G to NRP2, whereas SEMA3B, SEMA3C, and SEMA3D can bind to both NRPs (29,30). The NRPs also function as coreceptors for several growth factors, such as vascular endothelial growth factor (VEGF), human growth factor, and transforming growth factor-β (31). Although the involvement of SEMA3A and NRP1 in corneal development and wound healing have been characterized (19,32,33), the role of SEMA3C and its preferred receptor NRP2 in the corneas remains elusive.
In our genome-wide cDNA array study, we identified Sema3C as a highly inducible gene in corneal epithelial cells (CECs) responding to wounding in normal and diabetic corneas (34). In this study, we assessed the expression of SEMA3C and its receptors NRP1 and 2 compared with that of SEMA3A. We found that the levels of both SEMA3C and NRP2 were greatly elevated in the healing corneas of normal (NL) but not in diabetic (DM) mice, and SEMA3C and NRP2 play a beneficial role in improving delayed wound healing and sensory nerve regeneration in DM corneas.
Research Design and Methods
Animals and Induction of Diabetes
All investigations conformed to the regulations of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research of the National Institutes of Health. Six-week-old C57BL/6 mice, both males and females, purchased from The Jackson Laboratory, were induced to develop diabetes with streptozocin (STZ) as described previously (9,10). Mice were considered as diabetic with blood glucose levels >350 mg/dL within 8 weeks postinjection and thereafter.
Corneal Epithelial Debridement Wounds
DM and age-matched NL mice were anesthetized by an intraperitoneal injection of xylazine (7 mg/kg) and ketamine (70 mg/kg) plus topical proparacaine, and a 1.5-mm circular wound was first demarcated with a trephine in the central cornea followed by the removal of CECs within the circle with a blunt scalpel blade under a dissecting microscope (Zeiss). Two corneas were pooled in one tube and stored at −80°C. The collected cells were marked as nonwounded (0 h). The progress of wound healing was monitored by fluorescence staining for epithelial defects and photographed with a slit lamp microscope. At the end of healing, the corneas were either snap frozen in optimal cutting temperature (OCT) compound for cryostat sectioning or marked with the same size trephine for CEC collection.
Subconjunctival Injection of Small Interfering RNAs, Neutralizing Antibodies, and Proteins
Subconjunctival injection is a routine procedure used in the ophthalmology clinic to treat ocular diseases because it allows injected materials to slowly diffuse into the cornea with minimal systemic effects. The subconjunctival injection volume for mice was 5 μL per injection. Anesthetized mice were injected with anti-NRP1 or NRP2 (AF566, AF567; R&D Systems, Minneapolis, MN) or control IgG. Recombinant SEMA3C (1728; R&D Systems) with PBS containing 0.1% BSA as the control were injected 4 h before wounding. Mouse Sema3c-specific small interfering RNA (siRNA) (AM16708; Ambion) and control nontargeting siRNAs (AM4611) were injected at concentrations of 20 mmol/L twice (24 and 4 h) before wounding.
RNA Extraction and PCR Analysis
RNA was extracted from the collected CECs using an RNeasy Mini Kit (QIAGEN, Valencia, CA). cDNA was generated with an oligo(dT) primer followed by analysis using RT-PCR or quantitative real-time PCR (qRT-PCR) with SYBR Green (StepOnePlus; Applied Biosystems, Carlsbad, CA), with β-actin expression as an internal control. qRT-PCR results were first normalized with the levels of β-actin and then compared with the levels of NL (value = 1) presented as fold changes. Primer sequences (Table 1) were selected using Primer-BLAST (National Center for Biotechnology Information, Bethesda, MD) and synthesized using oligo(dT).
Table 1.
Primer sets for qRT-PCR analysis
| Gene | Direction | Primer sequence | Base pairs |
|---|---|---|---|
| Sema3A | Forward | GTTGTAGACCGGGTGGATGC | 100 |
| Reverse | TCTCCTTGGGGACTGAAACCA | ||
| Sema3C | Forward | TGCCATAGTCCCCTCTCATC | 143 |
| Reverse | GAGGGCTCTACCCTTCCATC | ||
| Nrp1 | Forward | GGCACAGGTGATGACTTCCA | 194 |
| Reverse | GTCAGCACACTCCACCTGAG | ||
| Nrp2 | Forward | CCCATATCAGCTTTTGCAGATGAA | 90 |
| Reverse | TTTGCCAGATGAGGGGTCAC | ||
| PlxA1 | Forward | CTGTGGACCTGCATGACTGT | 287 |
| Reverse | GGAGTGTGAGGGACCACAAG | ||
| PlxA2 | Forward | CAGACCTTTCCCCATGACACA | 213 |
| Reverse | TGTCCTTGGCTTGCAAAATAGG | ||
| PlxA3 | Forward | AGCAGGCCATTTCGTACCTT | 365 |
| Reverse | TAGTGCTCCTTGCGATGGTG | ||
| PlxA4 | Forward | CTTCGTGGGCACCAAAAGTG | 146 |
| Reverse | AGAGTTGCTCGTGGTCCTTG | ||
| Actin | Forward | CTCTCAGCTGTGGTGGTGAA | 228 |
| Reverse | AGCCATGTACGTAGCCATCC |
Western Blotting
CEC lysates with an equal amount of proteins (20 μg) were separated with 5–15% gradient SDS-PAGE and transferred to nitrocellulose membranes. The membranes were probed with antibodies against SEMA3C (24997; Thermo Fisher Scientific), SEMA3A (19775); NRP1 (D62C6; Cell Signaling), NRP2 (D39A5); and nonmuscle β-actin (A1978; Sigma-Aldrich) as a loading control followed by incubation with horseradish peroxidase–conjugated donkey secondary antibodies (1:4,000) (Jackson ImmunoResearch). The bands were visualized with enhanced SuperSignal chemiluminescence (Thermo Fisher Scientific), and the images were acquired using Kodak Image Station 4000R Pro.
Immunohistochemistry of Mouse Corneas
Mouse eyes were enucleated, embedded in Tissue-Tek OCT compound, and frozen in liquid nitrogen. Six-micrometer–thick sections were cut and mounted to poly-l-lysine–coated glass slides, fixed in 2% paraformaldehyde, blocked with PBS containing 2% BSA for 1 h at room temperature, and incubated with SEMA3A (28867; Santa Cruz Biotechnology) and SEMA3C (AF1728; R&D Systems), NRP1 (AF566), or NRP2 (AF567) antibodies. This was followed by a fluorescein isothiocyanate (FITC)–conjugated anti-goat or rat secondary antibody (1:100) (Jackson ImmunoResearch). Slides were mounted with Vectashield mounting medium containing DAPI. Controls were similarly treated with corresponding IgG from the same animal as the primary antibody. The sections were examined under a Nikon ECLIPSE 90i microscope. The central cornea was photographed, including the leading edge of healing corneas.
Whole-Mount Immunostaining and Quantitation of Innervation of B6 Mouse Corneas
Whole-mount immunostaining and quantitation of innervation of B6 mouse corneas were performed as described in our previous study (16). In brief, the enucleated eyes were fixed, and corneas were then isolated and further fixed for an additional 10 min. The corneas were cut radially into six standardized sections and incubated at 37°C in 20 mmol/L EDTA for 30 min followed by 2-day incubation in 0.025% hyaluronidase and 0.1% EDTA in PBS. The tissues were blocked at room temperature for 2 h in PBS-Triton X-100 containing 2% BSA followed by incubation overnight at 4°C with antibody against β-tubulin III (NL557; R&D Systems). After the addition of secondary antibody, the tissues were mounted and examined under a confocal microscope (TCS SP2; Leica, Heidelberg, Germany). Innervation in a region was calculated as the percent area positive for β-tubulin III staining by ImageJ software.
Statistical Analysis
The statistical analyses were performed using GraphPad Prism 6 software. Data are presented as mean ± SD. Experiments with two treatments and/or conditions were analyzed for statistical significance using a two-tailed Student t test. Experiments with more than two conditions were analyzed using one-way ANOVA, and if more than two groups of mice were used, such as wounded and nonwounded DM and NL mice, a two-way ANOVA was used for analysis to determine overall differences. A Bonferroni posttest was performed to determine statistically significant differences. Significance was accepted at P < 0.05. Experiments were repeated at least twice to ensure reproducibility.
Results
Expression and Distribution of SEMA3A, SEMA3C, and Their Receptors in NL and DM CECs With or Without Wounding
Our cDNA array data identified Sema3C mRNA levels as being significantly increased in healing rat CECs compared with nonwounded epithelium (34). Using an STZ mouse model, we investigated the expression of Sema3C in nonwounded and healing CECs of NL and DM mice. Sema3C transcripts were significantly increased in healing epithelia relative to the control, nonwounded CECs as shown on qRT-PCR (Fig. 1A). Moreover, this wound-induced upregulation in Sema3C transcripts was observed in DM wounded eyes compared with NL wounded eyes. At the protein level, Western blot analysis confirmed the expression pattern determined by qRT-PCR, with an increase of SEMA3C protein expression in wounded NL corneas, which was partially suppressed in wounded DM corneas (Fig. 1A).
Figure 1.
Sema3C, Sema3A, Nrp1, and Nrp2 transcription and translation in corneal epithelia of NL and DM mice with or without wounding. STZ-induced DM and age-matched NL mice were wounded by epithelium debridement (1.5-mm diameter), and the epithelial cells collected were used as the nonwounded controls (n = 5). At 20 h postwounding, cells that migrated into the original wounds were scraped off the corneas and collected as wounded CECs. CECs collected were subjected to RNA isolation for qRT-PCR and protein extraction for Western blotting. The results of the qRT-PCR analysis of Sema3C (A), Sema3A (B), Nrp1 (C), and Nrp2 (D) in CECs are presented as fold increase (mean ± SD) over the NL nonwounded CECs, which were set as a value of 1 (n = 3). Western blot analyses (two samples, lanes represent each condition), with actin as the internal loading control, are also shown. Three independent experiments were performed for each. *P < 0.05, **P < 0.01, ***P < 0.001 (two-way ANOVA). NLW, NL wounded; DMW, DM wounded.
SEMA3A is the best-studied member of SEMA3s. Consistent with previous findings (19), Sema3A transcripts were dramatically upregulated in both NL and DM wounded CECs (Fig. 1B). At the protein level, SEMA3A was detected only in the healing CECs of both NL and DM mice (Fig. 1B).
Because SEMA3C binds to NRP1 and/or preferably to NRP2 or NRP1/2 (35), we then investigated whether their expression is also altered during induction of diabetes and/or wounding. The expression of Nrp1 was higher in DM than in NL nonwounded CECs, but this elevated expression was not seen in healing NL and DM CECs (Fig. 1C). Western blotting revealed that NRP1 was expressed constitutively in both NL nonwounded and wounded CECs (Fig. 1C). In contrast, Nrp2 transcription was greatly upregulated in wounded NL CECs; like Sema3C, this wound-induced upregulation was suppressed in the DM wounded CECs (Fig. 1D). Protein levels of NRP2 were undetectable in nonwounded NL or DM CECs but were abundant in wounded CECs with a decreased amount in DM wounded CECs relative to NL wounded counterparts (Fig. 1D). Using regular PCR, the mRNA of Plxna1–4 was observed with wounding or diabetes induction in either trigeminal ganglia or CECs (Supplementary Fig. 1).
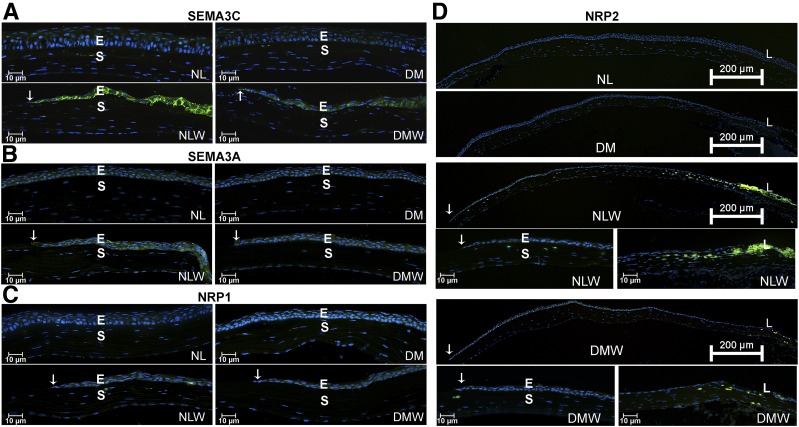
Tissue distribution of SEMA3A and SEMA3C and their two receptors was assessed using immunohistochemistry (Fig. 2). In nonwounded corneas, very faint or no staining can be detected for all four proteins in NL and DM corneas as well as in the limbus (data not shown). Strong staining of SEMA3C was observed mainly in the healing epithelium of NL but not DM corneas (Fig. 2A), whereas SEMA3A staining was observed in both NL and DM healing epithelia (Fig. 2B). NRP1 immunoreactivity was detected in the epithelia in all four groups (Fig. 2C). No changes in the staining pattern of the limbal region were observed for these proteins. NRP2 staining was readily detected in healing epithelia at the junction region between the cornea and the limbus. A large population of NRP2-positive cells was also seen in the corneal stroma near the limbus; the density of NRP2-positive cells gradually decreased toward the central cornea with only a few NRP2-positive cells near the leading edge (arrows). Of note, only a few NRP2-positive cells were seen throughout the stromal region of DM healing corneas (Fig. 2D). Taken together, SEMA3C and NRP2 were upregulated in the healing epithelia, and this upregulation was markedly suppressed in diabetes.
Figure 2.
Localization of SEMA3C, SEMA3A, NRP1, and NRP2 in NL and DM nonwounded and healing corneal epithelia. B6 mice were induced with diabetes and wounded as described in Fig. 1. At 20 h postwounding, wounded corneas (three per group) along with nonwounded controls were excised, snap frozen in OCT compound, and cryostat sectioned. Three sections from each cornea were stained with their respective primary antibodies followed by FITC-conjugated secondary antibodies. The cryostat sections were also counterstained with DAPI, showing nuclei (blue). Two independent experiments were performed for each. For each set of experiments, optimization of the exposure time and other settings was determined using NL healing corneas, and the same settings were kept during the analysis of all samples. Immunofluorescence showing expression (green) of SEMA3C (A), SEMA3A (B), NRP1 (C), and NRP2 (D) in B6 mouse corneas. Arrows mark the leading edge of healing epithelium. The results are representative of two independent experiments, three corneas each. E, epithelium; L, limbus; S, stroma.
NRP1 and NRP2 Expression in Sensory Nerve Fibers
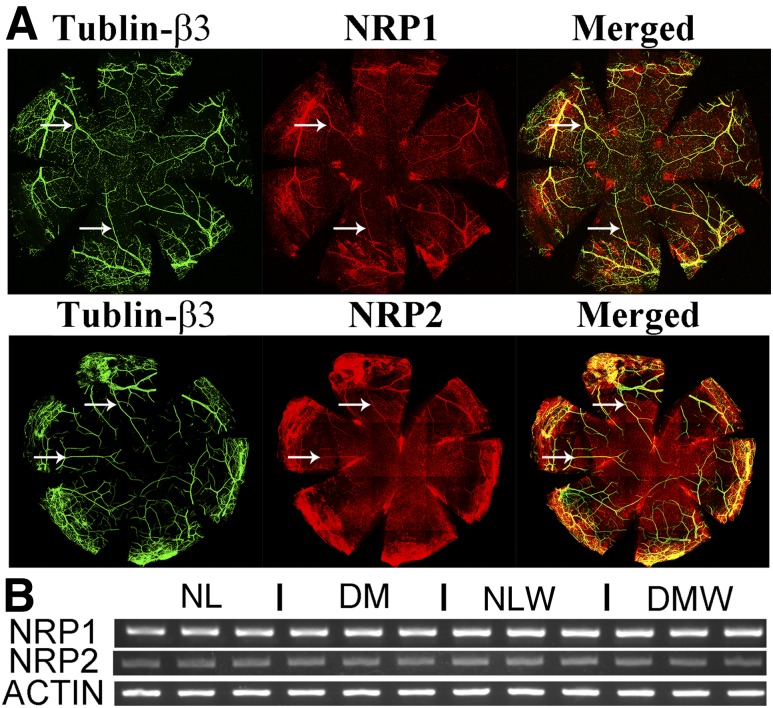
Having shown the expression of SEMA3/NRP/PLXNA both during homeostasis and during wound healing, we next investigated the expression and distribution of NRPs in corneal sensory nerve fibers/endings using whole-mount confocal microscopy. Because a strong background was seen in the cornea for both NRP1 and NRP2, the epithelium had to be removed for visualizing these proteins in the sensory nerves (Fig. 3). Whole-mount confocal microscopy revealed colocalization of both NRP1 and NRP2 (Fig. 3A) with major nerve fibers, but not smaller branches, in the stroma of NL corneas. Regular PCR using cDNA from trigeminal ganglia showed expression of both receptors, suggesting that they may be targeted by epithelium-expressed Sema3s (Fig. 3B).
Figure 3.
Expression and localization of NRPs in sensory nerve axons and trigeminal ganglia. A: Whole-mount confocal microscopy of NL nonwounded corneas, with the epithelium removed to reduce background staining. The treated corneas were costained with β-tubulin III, a pan-neuronal marker (green), and NRP1 or NRP2 (red). Arrows mark fibers that costained for both β-tubulin III and NRPs. B: RT-PCR analysis of Nrp1 and Nrp2 of trigeminal ganglia. Three samples from different mice were used, with actin as an internal loading control. Two independent experiments were performed. NLW, NL wounded; DMW, DM wounded.
SEMA3C Promotes Corneal Epithelial Wound Healing and Sensory Nerve Regeneration In Vivo
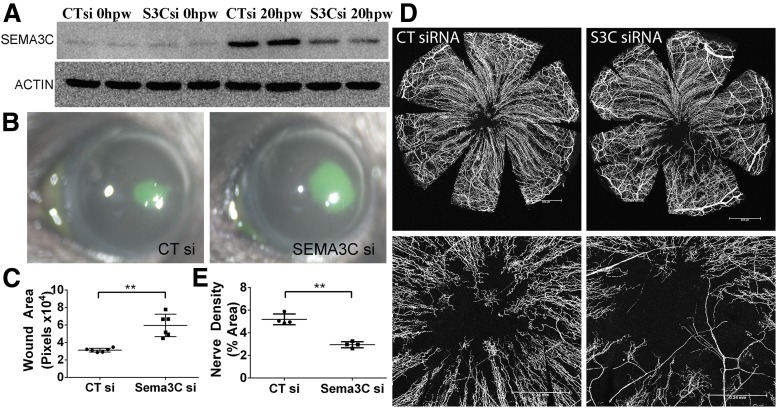
Given the upregulation of SEMA3C in healing NL epithelium, we tested whether SEMA3C played a role in corneal wound healing using two complementary approaches: siRNA downregulation and exogenous SEMA3C administration in wounded CECs. Sema3c-specific siRNA was injected before epithelium debridement; Western blot analysis revealed significant downregulation of SEMA3C at the protein level in healing NL CECs (Fig. 4A). Downregulation of Sema3c resulted in larger wound sizes compared with eyes injected with control, nonspecific siRNA (Fig. 4B and C). Supplementation of recombinant SEMA3C alongside Sema3c-specific siRNA rescued the decreased wound healing, further confirming the specificity of our siRNA (Supplementary Fig. 3). Whole-mount confocal microscopy showed that SEMA3C knockdown resulted in decreased sensory nerve regeneration, as identified using β-tubulin III antibody (Fig. 4D and E). Nerve pixel area in control siRNA-injected eyes was 5.298 ± 0.52% in the central area (bottom panels) compared with 2.95 ± 0.33% in siRNA-injected eyes.
Figure 4.
Suppression of SEMA3C expression decreases epithelial wound healing and corneal nerve regeneration in healing corneas. NL corneas were pretreated with the nonspecific control siRNA (CTsi, right eyes) or Sema3C-specific siRNA (S3Csi, left eyes) 24 and 4 h before epithelium debridement. At 0 h, the corneas were wounded by epithelium debridement (1.5-mm diameter) (n = 5) and allowed to heal for 20 h (A and B) or 3 days (C). A: Epithelial cells collected during wounding (0 hours postwounding [hpw]) and from the original wound beds (20 hpw) were subjected to Western blot analysis to illustrate the downregulation of SEMA3C by siRNA. Two samples for each condition were used. B: At 20 hpw, injured corneas were stained with FITC to show remaining wound and photographed. C: The wound sizes were calculated using Adobe Photoshop software and presented as the average of total pixels (mean ± SD, n = 6). D: Whole-mount confocal microscopy of the corneas pretreated with CTsi or S3Csi and processed at 3 days postwounding. An additional subconjunctival injection of CTsi or S3Csi was performed at 1 day postwounding. Top panels show whole corneal images of sensory nerve fibers/endings. Scale bar = 652 μm. Bottom panels show high-magnification images of the centers of the corneas, with voided areas indicating regenerating nerve endings. Scale bar = 340 μm. E: Nerve densities at the central areas (bottom panels of D) were calculated using ImageJ software from the areas covered with β-tubulin III staining and are presented as percent area (mean ± SD, n = 4). Two independent experiments were performed. **P < 0.01 (Student t test).
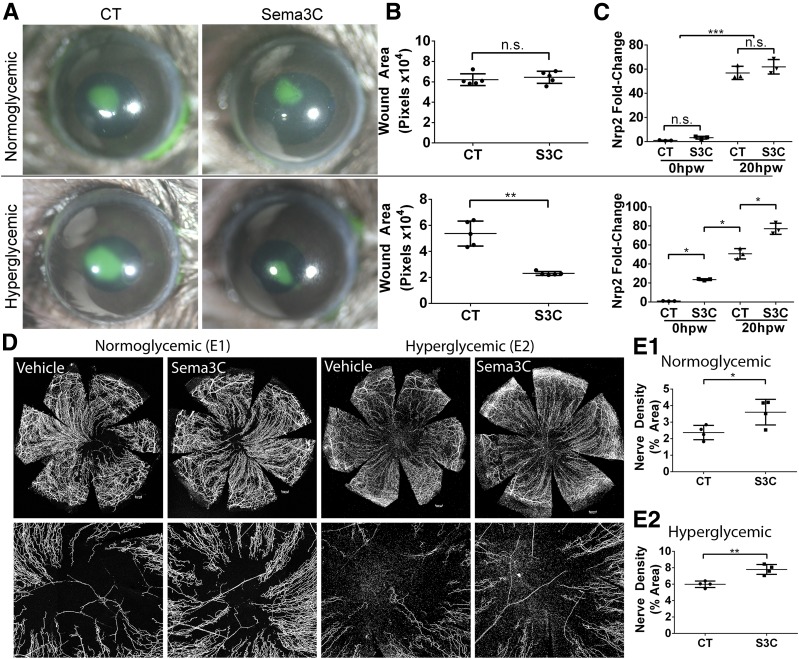
We next administered exogenous bioactive recombinant SEMA3C before epithelium wounding. The presence of SEMA3C did not appear to influence epithelial wound closure in NL corneas but significantly ameliorated the delayed healing phenotype seen in DM corneas (Fig. 5A and B). SEMA3C supplementation also significantly increased nerve regeneration in both NL and DM corneas, which were collected at 3 vs. 4 days postwounding, respectively, to have similar coverage of regenerating nerves (Fig. 5D and E). To understand whether compensatory changes occurred in the expression of other SEMA3s or NRPs in response to SEMA3C treatment, epithelia from both NL and DM eyes were analyzed with qRT-PCR. We observed that only the expression of NRP2 was significantly upregulated by SEMA3C treatment in both wounded and nonwounded states in DM, but not NL, corneas (Fig. 5C).
Figure 5.
Exogenous SEMA3C (S3C) increases DM wound healing and corneal nerve regeneration in healing corneas. NL and DM corneas pretreated with BSA as the control (CT, right eyes) or recombinant S3C (50 ng/μL, left eyes) 4 h before epithelium debridement. At 0 h, the corneas were wounded by epithelium debridement (1.5-mm diameter) and allowed to heal for 20 h (A–C) or 3 days (D and E). A: At 20 hours postwounding (hpw) in NL mice and 24 hpw in DM mice, injured corneas were stained with FITC to photograph the remaining wound area. B: The wound sizes were calculated using Adobe Photoshop software and presented as the average of total pixels (mean ± SD, n = 5). **P < 0.01 (Student t test). C: CECs collected during wounding (20 hpw) and from original wound beds (0 hpw) were subjected to RNA qRT-PCR analysis of Nrp2 expression. The results are presented as fold increase (mean ± SD) over the NL nonwounded CECs, which were set as a value of 1 (n = 3). *P < 0.05, ***P < 0.001 (two-way ANOVA). D: Representative corneas harvested at 3 days postwounding (dpw) for NL and 4 dpw for DM, with an additional subconjunctival injection of BSA or recombinant S3C at 1 dpw. Staining for β-tubulin III was performed, and images of the entire cornea were captured (top panels), with high-magnification images shown in bottom panels. Scale bars: 176 μm (E1), 202 μm (E2). E1 and E2: Nerve densities at the central areas (bottom panels) were calculated using ImageJ software from the areas covered with β-tubulin III staining and presented as percent area (mean ± SD, n = 4). Two independent experiments were performed. *P < 0.05, **P < 0.01 (Student t test). n.s., not significant.
NRP1 and NRP2 Play Opposing Roles in Sensory Nerve Regeneration
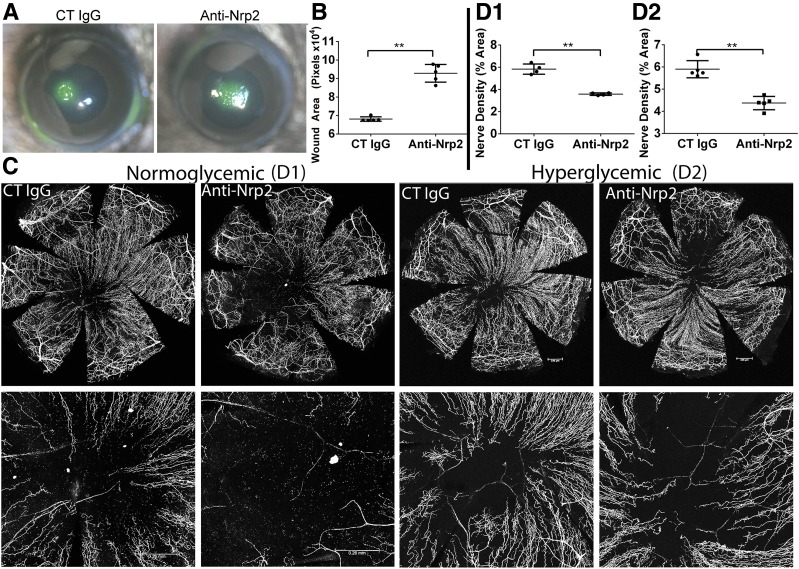
Having identified a parallel pattern of expression of SEMA3C and NRP2 in wounded NL and DM corneas, we next investigated the role of NRP2 signaling in the healing cornea. NRP2-neutralizing antibody treatment resulted in significant increases in the wound sizes of NL (Fig. 6A and B), but not DM (data not shown), corneas. For sensory nerve regeneration, NRP2 blockade in wounded DM corneas resulted in a decrease of regenerating nerve fibers in wounded NL corneas and in an exacerbation of the delayed nerve regeneration in DM wounded corneas (Fig. 6C and D).
Figure 6.
NRP2 blockade decreases epithelial wound healing and corneal nerve regeneration in healing corneas. NL corneas pretreated with control (CT) IgG (10 μg/mL, right eyes) and NRP2-neutralizing antibodies (10 μg/mL, left eyes) 4 h before epithelium debridement. A: At 20 hours postwounding (hpw), injured corneas were stained with FITC to show remaining wound and photographed. B: The wound sizes were calculated using Adobe Photoshop software and presented as the average of total pixels (mean ± SD, n = 5). C: Representative corneas harvested at 3 days postwounding (dpw) for NL and 4 dpw for DM, with an additional subconjunctival injection of nonspecific CT IgG or NRP2-neutralizing antibodies at 1 dpw. Staining for β-tubulin III was performed, and images of the entire cornea were captured (top panels), with high-magnification images shown in the bottom panels. Scale bars: 260 μm (D1), 336 μm (D2). D1 and D2: Quantification of β-tubulin III staining pixels of central cornea from C (bottom panels) were calculated using ImageJ software and presented as percent area (mean ± SD, n = 4). Two independent experiments were performed. **P < 0.01 (Student t test).
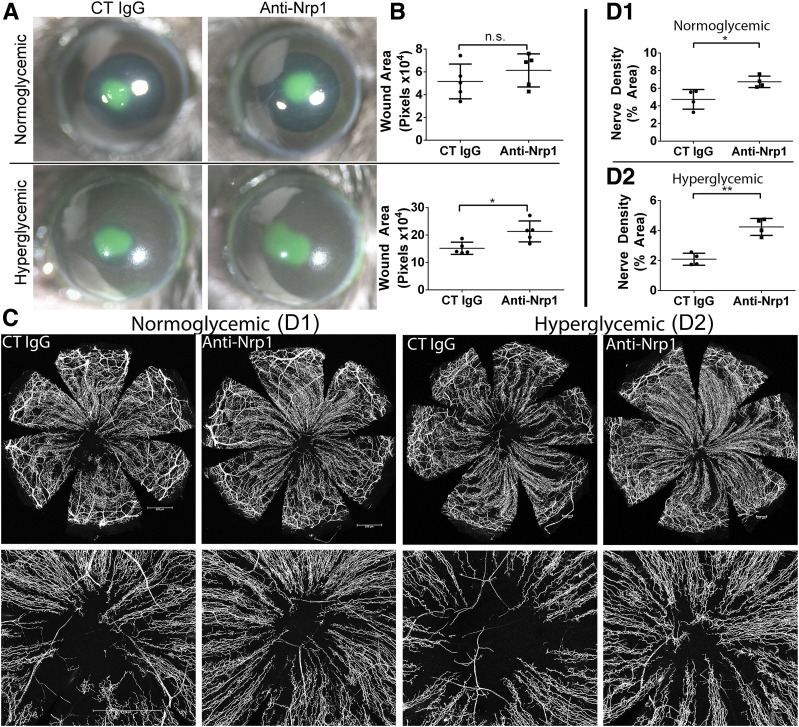
The role of NRP1 in the healing cornea was also assessed using neutralizing antibodies. NRP1 blockade in normal mice had no significant effect on wound healing rates as assessed by FITC staining (Fig. 7A and B), whereas NRP1 blockade resulted in a mild, but significant further increase in wound size in DM mice, suggesting a beneficial role for NRP1 in the process. In contrast to the results with epithelial wound closure, we found that NRP1 blockade significantly improved corneal sensory nerve regeneration in both NL and DM mice (Fig. 7C and D), suggesting a detrimental role for this process.
Figure 7.
NRP1 blockade decreases DM epithelial wound healing but increases corneal nerve regeneration in healing corneas. NL or DM corneas were pretreated with control (CT) IgG (10 μg/mL, right eyes) and NRP1-neutralizing antibodies (10 μg/mL, left eyes) 4 h before epithelium debridement. A: At 20 hours postwounding (hpw), injured corneas were stained with FITC to show remaining wound and photographed. B: The wound sizes were calculated using Adobe Photoshop software and presented as the average of total pixels (mean ± SD, n = 5). C: Representative corneas harvested at 3 days postwounding (dpw) for NL and 4 dpw for DM, with an additional subconjunctival injection of nonspecific IgG or NRP1-neutralizing antibodies at 1 dpw. Staining for β-tubulin III was performed, and images of the entire cornea were captured (top panels), with high-magnification images shown in bottom panels. Scale bars: 529 μm (D1), 217 μm (D2). D1 and D2: Nerve densities at the central areas (bottom panels) were calculated using ImageJ software from the areas covered with β-tubulin III staining and presented as percent area (mean ± SD, n = 4). Two independent experiments were performed. *P < 0.05 and **P < 0.01 (Student t test). n.s., not significant.
Discussion
In this study, we investigated the role of SEMA3C and its receptors NRP1 and NRP2 in corneal epithelial wound healing. We identified that SEMA3C, SEMA3A, and NRP2 are upregulated in CECs postwounding and that the upregulations of SEMA3C, NRP2, but not SEMA3A were suppressed in the diabetic state. Expression of both NRPs was detected in corneal sensory nerve fibers. Functionally, suppression of SEMA3C expression or blockade of NRP2 resulted in both delayed corneal wound healing and sensory nerve regeneration in NL corneas, whereas the addition of exogenous SEMA3C ameliorated delayed DM corneal wound healing as well as increased sensory nerve regeneration in both DM and NL corneas. In contrast, blockade of NRP1 resulted in improved sensory nerve regeneration in both DM and NL corneas but exacerbated DM corneal epithelial wound closure, whereas downregulation of SEMA3A yielded nonconclusive results (data not shown). Taken together, these results suggest that SEMA3C-NRP2 signaling plays a role in both corneal epithelial wound healing and sensory nerve regeneration, whereas NRP1 signaling appears to have negative effects on the sensory nerve regeneration in both NL and DM corneas.
In the current study, we focused on the epithelial effects on wound healing and on sensory nerve innervation and regeneration by focusing on a factor highly induced in response to wounding in CECs, SEMA3C. Our study reveals that SEMA3C is expressed in the corneal epithelium and that wounding induced a significant upregulation in its expression, whereas diabetes resulted in suppression of this upregulation. Similarly, for the first time to our knowledge, we show that NRP2 is not expressed in nonwounded corneas but is induced upon wounding. Like SEMA3C, the wound-induced NRP2 upregulation is repressed in the DM cornea. Unlike NRP2, NRP1 is constitutively expressed in NL corneas but at higher levels in DM nonwounded CECs, whereas SEMA3A was only found in healing CECs of DM and NL mice. Hence, in nonwounded corneas, SEMA3C may have minimal effects on CECs. In response to wounding in NL corneas, CEC expression of SEMA3C, 3A, and NRP2 was either enhanced or induced, whereas NRP1 remained unchanged. This would result in an increase in SEMA3C-NRP2 or SEMA3C-NRP1/2 signaling that benefits epithelial wound repair, as shown in alveolar epithelial cells (36). Using two complementary approaches, Sema3c-specific siRNA and NRP2-neutralizing antibodies, we show that both treatments resulted in decreased epithelial wound closure in NL corneas, supporting the idea that SEMA3C, through NRP2, plays an important role in epithelial wound healing. One limitation is that we cannot determine whether the wound difference is due to a delay in the start of healing, an overall decrease in the rate of wound healing, or both.
Compared with NL corneas, in DM wounded corneas, the expression of both SEMA3C and NRP2 in CECs was repressed at both mRNA and protein levels, unlike SEMA3A and NRP1. Consistent with delayed wound closure in DM corneas, diabetes induction results in decreases in both the ligand (SEMA3C) and its preferred receptor (NRP2), suggesting that the defects in SEMA3C-NRP2 and/or SEMA3C-NRP1/2 signaling contribute to the defects in the epithelial wound closure observed in DM corneas. This report is the first to our knowledge to link SEMA3C-NRP2 expression and signaling to diabetic keratopathy. Of note, although NRP1 levels in these corneas were either unchanged with wounding in NL corneas or decreasing with wounding in DM corneas, neutralizing NRP1 also resulted in larger wound sizes in DM corneas, suggesting a supportive role of NRP1 in mediating CEC migration. Considering that the level of SEMA3A was elevated in DM wounded CECs compared with DM nonwounded CECs, we postulate that the formation of NRP1/2 dimers, which are lacking in diabetic and/or NRP1-neutralized CECs, is most effective for transducing SEMA3C signals into healing corneal epithelia. This is supported by findings that SEMA3C has maximal affinity for NRP1/2 heterodimers (35) and that NRP2 neutralization only reduced 50% of SEMA3C binding to the surface of dendritic cells, which express both NRP isoforms during differentiation (37). Hence, we propose that defects in SEMA3C-NRP1/2 signaling is partially responsible for decreased reepithelialization in DM corneas.
Our immunohistochemistry study revealed an intriguing distribution pattern of NRP2 in healing corneas. Although both NRP1 and NRP2 can be detected in the entire healing epithelial layer with a well-defined leading edge, strong staining of NRP2 was observed in the boundary region between the cornea and the limbus. This strong staining was found not only in the epithelial layer but also in the stromal layer with individually labeled, infiltrating cells. Because the limbal region contains epithelial, stromal stem, and progenitor cells in injured corneas (38–42), it is tempting to suggest that this may be the identity of these NRP2-high cells. These stem cells possess a certain proliferative capacity and are critical for wound closure in our debridement wounding model (39). NRP2 is also expressed in periodontal ligament, a source of mesenchymal stem cells (41). Unfortunately, limbal stem-cell markers are not well established, and a combination must be used, including ΔNp63α, C/EBPδ, Bmi-1, ABCG2, and Notch-1 for limbal stem cells (reviewed in Joe and Yeung [43]). Alternatively, the stromal NRP2-expressing cells may be infiltrating maturating dendritic cells (37), which express NRP1 and 2 (Supplementary Fig. 2). The number of high NRP2-expressing cells was dramatically reduced in the DM healing corneas, further suggesting that the defects in SEMA3C-NRP2 signaling might be responsible in part for decreased epithelial wound healing in diabetes.
Unlike our results regarding epithelial wound closure, our functional study revealed that NRP1 neutralization improves sensory nerve regeneration in wounded NL and DM corneas (44). The role of VEGF/NRP interaction in sensory nerve de-/regeneration in the corneas may not be ruled out by the current study. In the retina, NPR1-expressing mononuclear phagocytes are selectively recruited to sites of pathological neovascularization in response to locally produced SEMA3A as well as VEGF (45), whereas NRP2 colocalized with a vascular marker and was suggested to facilitate VEGF-induced retinal neovascularization. Of note, NRP1 was reported to be upregulated in patients with diabetes with proliferative retinopathy and to mediate SEMA3A-induced vascular permeability (44), whereas NRP1 was found to inhibit pathological angiogenesis in the presence of SEMA3C (46). In the skin, elevated NRP1 was linked to epidermal nerve fiber loss and/or defective regeneration (47). Consistent with the contradicting role of NRP1, the levels of NRP1 remained the same in healing corneas of NL and DM mice, which express different levels of SEMA3A and SEMA3C. High SEMA3A levels and decreased SEMA3C levels in diabetic healing corneas would result in a decrease in SEMA3C/NRP complexes. The increase in SEMA3A/NRP1 and/or decrease in SEMA3C/NRP may be a major cause of severely delayed sensory nerve regeneration in diabetic corneas (34). This was supported by the fact that exogenous SEMA3C enhanced nerve regeneration in both NL and DM eyes and ameliorated the decreased epithelial wound healing observed in DM corneas. Moreover, exogenous SEMA3C increased hyperglycemia-suppressed Nrp2 in DM wounds, suggesting a positive feedback loop in SEMA3C/NRP2 signaling that further reduces detrimental effects of SEMA3A/NRP1. Consistent with this, blockade of NRP2 resulted in a similar phenotype to that observed with suppression of SEMA3C expression, with decreased wound healing and nerve regeneration. Hence, we suggest that NRP1-caused defects in sensory nerve regeneration are related to the levels of SEMA3A and SEMA3C in the cornea and that NRP2 expressed in sensory nerves may influence nerve regeneration directly through epithelium-produced SEMA3C and indirectly through an increase in the proliferation of corneal stem and progenitor cells, which express high levels of NRP2 because these are necessary for ocular surface innervation (48).
Taken together, our study reveals that although both NRP1 and NRP2 ameliorated delayed epithelial wound healing, they have opposing effects in sensory nerve regeneration, with NRP2 enhancing sensory nerve regeneration while NRP1 attenuates the process. Moreover, we suggest that exogenous supplementation of SEMA3C, which may tip the balance in favor for the formation of SEMA3C-NRP1/2 over SEMA3A-NRP1 complexes, may serve as a new strategy for restoring impaired nerve regeneration in patients with diabetes.
Supplementary Material
Article Information
Funding. We acknowledge support from National Eye Institute grants R01-EY-010869, F30-EY-025923 (to P.S.-Y.L.), R01-EY-017960 (to F.-S.X.Y.), and P30-EY-04068 (National Eye Institute core grant to Wayne State University) and from Research to Prevent Blindness (to Kresge Eye Institute).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.S.-Y.L. and N.G. performed laboratory testing and edited and checked the accuracy of the manuscript. M.D., O.S., and R.M. performed laboratory testing. Y.Z. contributed to the sample collection and data analysis. F.-S.X.Y. was responsible for study design and recruitment, contributed to the sample collection and data analysis, and reviewed and edited the manuscript. F.-S.X.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the Association for Research in Vision and Ophthalmology 2018 Annual Meeting, Honolulu, HI, 29 April–2 May 2018.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-1172/-/DC1.
References
- 1.Premkumar LS, Pabbidi RM. Diabetic peripheral neuropathy: role of reactive oxygen and nitrogen species. Cell Biochem Biophys 2013;67:373–383 [DOI] [PubMed] [Google Scholar]
- 2.Jack M, Wright D. Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl Res 2012;159:355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobrowsky RT, Rouen S, Yu C. Altered neurotrophism in diabetic neuropathy: spelunking the caves of peripheral nerve. J Pharmacol Exp Ther 2005;313:485–491 [DOI] [PubMed] [Google Scholar]
- 4.Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 2011;7:573–583 [DOI] [PubMed] [Google Scholar]
- 5.Ljubimov AV. Diabetic complications in the cornea. Vision Res 2017;139:138–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson EP, Coppey LJ, Holmes A, Yorek MA. Changes in corneal innervation and sensitivity and acetylcholine-mediated vascular relaxation of the posterior ciliary artery in a type 2 diabetic rat. Invest Ophthalmol Vis Sci 2012;53:1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson EP, Coppey LJ, Yorek MA. Early loss of innervation of cornea epithelium in streptozotocin-induced type 1 diabetic rats: improvement with ilepatril treatment. Invest Ophthalmol Vis Sci 2012;53:8067–8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F, Gao N, Yin J, Yu FS. Reduced innervation and delayed re-innervation after epithelial wounding in type 2 diabetic Goto-Kakizaki rats. Am J Pathol 2012;181:2058–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu K, Yu FS. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest Ophthalmol Vis Sci 2011;52:3301–3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin J, Huang J, Chen C, Gao N, Wang F, Yu FS. Corneal complications in streptozocin-induced type I diabetic rats. Invest Ophthalmol Vis Sci 2011;52:6589–6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavakoli M, Petropoulos IN, Malik RA. Assessing corneal nerve structure and function in diabetic neuropathy. Clin Exp Optom 2012;95:338–347 [DOI] [PubMed] [Google Scholar]
- 12.Tavakoli M, Mitu-Pretorian M, Petropoulos IN, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes 2013;62:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efron N. The Glenn A. Fry award lecture 2010: ophthalmic markers of diabetic neuropathy. Optom Vis Sci 2011;88:661–683 [DOI] [PubMed] [Google Scholar]
- 14.Pop-Busui R, Stevens MJ, Raffel DM, et al. Effects of triple antioxidant therapy on measures of cardiovascular autonomic neuropathy and on myocardial blood flow in type 1 diabetes: a randomised controlled trial. Diabetologia 2013;56:1835–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry JR, Bril V. Complications of sural nerve biopsy in diabetic versus non-diabetic patients. Can J Neurol Sci 1994;21:34–37 [DOI] [PubMed] [Google Scholar]
- 16.Gao N, Yan C, Lee P, Sun H, Yu FS. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. J Clin Invest 2016;126:1998–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res 2015;49:17–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu FS, Yin J, Lee P, Hwang FS, McDermott M. Sensory nerve regeneration after epithelium wounding in normal and diabetic cornea. Expert Rev Ophthalmol 2015;10:383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Zhou Q, Luo Y, Nguyen T, Rosenblatt MI, Guaiquil VH. Semaphorin3A induces nerve regeneration in the adult cornea-a switch from its repulsive role in development. PLoS One 2018;13:e0191962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagali N, Poletti E, Patel DV, et al. Focused tortuosity definitions based on expert clinical assessment of corneal subbasal nerves. Invest Ophthalmol Vis Sci 2015;56:5102–5109 [DOI] [PubMed] [Google Scholar]
- 21.Conrad AH, Strafuss JM, Wittman MD, Conway S, Conrad GW. Thyroxine increases the rate but does not alter the pattern of innervation during embryonic chick corneal development. Invest Ophthalmol Vis Sci 2008;49:139–153 [DOI] [PubMed] [Google Scholar]
- 22.Dickson BJ. Molecular mechanisms of axon guidance. Science 2002;298:1959–1964 [DOI] [PubMed] [Google Scholar]
- 23.Bolsover S, Fabes J, Anderson PN. Axonal guidance molecules and the failure of axonal regeneration in the adult mammalian spinal cord. Restor Neurol Neurosci 2008;26:117–130 [PubMed] [Google Scholar]
- 24.Chen SY, Cheng HJ. Functions of axon guidance molecules in synapse formation. Curr Opin Neurobiol 2009;19:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giger RJ, Hollis ER II, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol 2010;2:a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Q, Chen P, Di G, et al. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem Cells 2015;33:1566–1576 [DOI] [PubMed] [Google Scholar]
- 27.Koropouli E, Kolodkin AL. Semaphorins and the dynamic regulation of synapse assembly, refinement, and function. Curr Opin Neurobiol 2014;27:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Winter F, Holtmaat AJ, Verhaagen J. Neuropilin and class 3 semaphorins in nervous system regeneration. Adv Exp Med Biol 2002;515:115–139 [DOI] [PubMed] [Google Scholar]
- 29.Neufeld G, Mumblat Y, Smolkin T, et al. The role of the semaphorins in cancer. Cell Adhes Migr 2016;10:652–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma A, Verhaagen J, Harvey AR. Receptor complexes for each of the class 3 semaphorins. Front Cell Neurosci 2012;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer 2008;8:632–645 [DOI] [PubMed] [Google Scholar]
- 32.McKenna CC, Munjaal RP, Lwigale PY. Distinct roles for neuropilin1 and neuropilin2 during mouse corneal innervation. PLoS One 2012;7:e37175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko JA, Mizuno Y, Yanai R, Chikama T, Sonoda KH. Expression of semaphorin 3A and its receptors during mouse corneal development. Biochem Biophys Res Commun 2010;403:305–309 [DOI] [PubMed] [Google Scholar]
- 34.Bettahi I, Sun H, Gao N, et al. Genome-wide transcriptional analysis of differentially expressed genes in diabetic, healing corneal epithelial cells: hyperglycemia-suppressed TGFβ3 expression contributes to the delay of epithelial wound healing in diabetic corneas. Diabetes 2014;63:715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruediger T, Zimmer G, Barchmann S, Castellani V, Bagnard D, Bolz J. Integration of opposing semaphorin guidance cues in cortical axons. Cereb Cortex 2013;23:604–614 [DOI] [PubMed] [Google Scholar]
- 36.Vadivel A, Alphonse RS, Collins JJ, et al. The axonal guidance cue semaphorin 3C contributes to alveolar growth and repair. PLoS One 2013;8:e67225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curreli S, Wong BS, Latinovic O, Konstantopoulos K, Stamatos NM. Class 3 semaphorins induce F-actin reorganization in human dendritic cells: role in cell migration. J Leukoc Biol 2016;100:1323–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun TT, Tseng SC, Lavker RM. Location of corneal epithelial stem cells. Nature 2010;463:E10–E11;discussion E11 [DOI] [PubMed]
- 39.Amitai-Lange A, Altshuler A, Bubley J, Dbayat N, Tiosano B, Shalom-Feuerstein R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells 2015;33:230–239 [DOI] [PubMed] [Google Scholar]
- 40.Ksander BR, Kolovou PE, Wilson BJ, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014;511:353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li GG, Zhu YT, Xie HT, Chen SY, Tseng SC. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci 2012;53:5686–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells 2005;23:1266–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joe AW, Yeung SN. Concise review: identifying limbal stem cells: classical concepts and new challenges. Stem Cells Transl Med 2014;3:318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerani A, Tetreault N, Menard C, et al. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab 2013;18:505–518 [DOI] [PubMed] [Google Scholar]
- 45.Dejda A, Mawambo G, Cerani A, et al. Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk. J Clin Invest 2014;124:4807–4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang WJ, Hu J, Uemura A, Tetzlaff F, Augustin HG, Fischer A. Semaphorin-3C signals through neuropilin-1 and plexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol Med 2015;7:1267–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Acker N, Ragé M, Vermeirsch H, et al. NRP-1 receptor expression mismatch in skin of subjects with experimental and diabetic small fiber neuropathy. PLoS One 2016;11:e0161441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueno H, Ferrari G, Hattori T, et al. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci 2012;53:867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.