Abstract
Background:
Lead may be added to the opium by drug smugglers. It can cause elevated blood lead level (BLL) in opium-addicted patients. Erythrocyte pyrimidine 5′-nucleotidase (P5N) activity is susceptible to high BLL. The aim of this study was to find out whether opium-addicted patients show erythropathy and elevated liver enzymes explainable by high BLL and decreased P5N activity.
Methods:
Forty orally opium-addicted subjects and 40 normal healthy volunteers were enrolled in this study. BLL was measured in whole blood specimens using atomic absorption spectrometry instrumentation. Enzymatic activity, protein amount of P5N, and erythrocyte purine/pyrimidine ratio were determined. Blood films were analyzed for the presence of basophilic stippling of red cells and hemolytic anemia. The level of liver function enzymes was measured.
Results:
The mean BLL for opium-addicted patients was significantly higher than control group (P < 0.001). On the contrary, P5N activity showed a valid decrease in opium-addicted patients when compared with control group (P < 0.001). In line with repressed P5N activity, erythrocyte purine/pyrimidine ratio in patients was lower than control group (P < 0.001). A statistically significant reverse correlation was found between BLL and P5N activity (P < 0.05, r = −0.85). The prevalence of both basophilic stippling (P < 0.001, z = 6.62) and hemolytic anemia (P < 0.001, z = 6.52) in study population was significantly associated with elevated BLL. We could not find any significant correlation between serum level of liver enzymes and BLL.
Conclusions:
Opium-addicted patients in Tehran, Iran, are at high risk of lead poisoning which may result in hematologic problems and possibly hepatic damage.
Keywords: Anemia, lead poisoning, liver function tests, opium, pyrimidine 5′-nucleotidase
Introduction
Lead poisoning may arise from different sources. Exposure to automobile exhaust, industrial emissions, and contaminated foods through ingestion, inhalation, and dermal contacts are the known causes of chronic and acute lead poisoning.[1,2] In the majority of cases, lead-exposed individuals are industrial workers dealing with lead-containing materials.[1] Complications resulting from excess lead exposure mainly include hematologic and liver problems. However, lead poisoning could affect multiorgan systems and it usually causes nonspecific clinical presentations such as fatigue, apathy, irritability, and vague gastrointestinal symptoms.[3,4]
Besides environmental and industrial pollutants as the usual sources of lead exposure, some uncommon causes of lead intoxication have been described. According to previous findings, some drug abusers may represent clinical manifestations associated with lead poisoning. In this regard, some cases of plumbism due to abuse of opium have been reported.[5] Seemingly, substance abuse, as a major social and public health problem, is going to cause a new health issue as a source of lead exposure owing to fraud street-level substances sold in black markets especially in middle-East countries.[1,6] As for Iran, Aghaee-Afshar et al.[7] have proven the presence of noticeable amount of lead in illegal opium samples which may be added to the opium by drug smugglers to increase its weight. Akhgari et al.[2] confirmed the presence of lead in opium, heroin, ecstasy tablets, and crystal methamphetamine in Iran. Regarding its geographical position, Iran is a major rout of opium trafficking worldwide and opium is the most common abused substance in the country. Thus, Iranian young population are vulnerable to becoming endangered by many social and health consequences of opium abuse.[8] Pyrimidine 5′-nucleotidase (P5N) is an essential enzyme for erythrocyte integrity and akin described for pathogenesis of hereditary P5N deficiency. Shortened erythrocyte survival is associated with accumulation of pyrimidine nucleotides and decreased P5N activity. P5N induces dephosphorylation of pyrimidine 5′-monophosphate to nucleotides that diffuse out of cells. Lead can inhibit P5N, and hence induces hemolysis and also basophilic stippling (BS) in lead-poisoned cases.[9] In fact, lead intoxication shares some features with hereditary P5N deficiency.[10] As reported by previous studies, elevated blood lead concentration is associated with hematologic findings such as BS of red cells and hemolytic anemia, and it has been proven that decreased P5N activity is partially responsible for these findings.[10,11] Also, high blood lead level (BLL) could damage the majority of soft tissues mainly the liver.[10] The central role of hepatic tissue in detoxification emphasizes the importance of liver function in clearance of xenobiotics especially in drug-addicted individuals. Meanwhile, lead poisoning impairs detoxification of xenobiotics in liver which may result in more complicated problems in drug abusers.[12]
In this study, we hypothesized that opium-addicted patients may exhibit elevated BLL accompanied with hematologic findings and elevated liver function enzymes. We aimed to find out whether these pathological findings are explainable by high BLL and decreased P5N activity.
Methods
Patients and controls’ selection
The study was designed as a cross-sectional study. Forty opium-addicted and 40 normal men were enrolled in this study. Systematic incidental sampling was implemented to select 40 patients among 198 opium-addicted men who referred to Noor Medical Laboratory, Tehran, Iran, over the 1-year study period, 1st of May 2017 to 30th April 2018, to confirm opium abuse for the beginning of opioid replacement therapy. Patients had at least 2 years history of oral opium abuse. Similarly, control healthy men subjects were randomly selected from cases with no history of exposure to lead. Potential confounding factors such as age, body mass index, and smoking habit were tested and adjusted one by one using regression methods. Information on lifestyle and health status of all study participants were collected using a personal data questionnaire. Exclusion criteria were applied to exclude the subjects with a history of possible occupational, environmental, and other sources of lead exposure. Exclusion criteria were daily working with batteries, paints, radiators, tin cans, ceramics, and wires. Also, individuals with any history of pathologic and hematologic disorders were not included in the study. All study participants signed a written informed consent.
Sample collection
Ten milliliters of blood samples was obtained from the study and control subjects by venipuncture. Each sample was divided into 5-mL aliquots in sterile blood-collecting tubes either containing ethylenediaminetetraacetic acid (EDTA) for determination of whole blood lead or without any anticoagulants for biochemical and enzyme assays. After being coded, all blood samples were stored at −20°C. A peripheral blood smear was made for each study participant to evaluate the BS. Urine samples to be used for urine opiates analysis were collected in acid-washed polyethylene flasks.
Urine opiate analysis
To confirm the presence of opium alkaloids in the urine, samples were hydrolyzed using hydrochloric acid and heating for 60 min on 90°C water bath. After alkalinization of the experiment medium with potassium hydroxide 50%, chloroform: isopropanol (80:20, v/v) was used as extractant solvent for efficient extraction of opium alkaloids in pH 8.5–9. Thin-layer chromatography was performed on precoated aluminum sheets (20 × 20), with 0.25-mm silica gel layer thickness and ultraviolet fluorescent indicator ALUGRAM® Xtra SIL G SIL UV254 (Macherey-Nagel Gmbh, Duren, Germany).
Whole blood lead assay
Doubly distilled deionized water 18 MΩ (Millipore Corp., Merk, Germany) was used throughout the analysis procedure for standard, stock, and sample preparation. Nitric acid (65%) was supplied by Sigma-Aldrich, Switzerland (TraceSELECT). To delete contamination, all glass wares and plastic containers were acid-washed by soaking in diluted nitric acid (10%) at least one night prior to each experimental work. All containers were then rinsed three times with deionized water. Different concentrations of standard solutions were prepared by diluting 1000 mg/L pb in nitric acid (Lead Standard for Atomic Absorption, Trace CERT®) stock solution supplied by Sigma-Aldrich. A serial dilution as the calibrator solutions was prepared by the addition of the known amounts of lead to the bovine blood. The blood samples stored at −20°C were thawed using an electric heater. Twenty-five microliters of blood sample was mixed with Triton X-100 buffer and deproteinized by the addition of 25 μL of 0.8 M nitric acid. After an overnight incubation at 4°C, the samples were centrifuged at 11,000 RPM for 5 min. Afterward, the supernatants were analyzed for the lead level by atomic absorption spectroscopy at 283.8 nm (Agilent's 240 AA Graphite Furnace, USA). Triplicate analysis was performed for all samples.
Pyrimidine 5′-nucleotidase assay
Enzyme assay was implemented in both protein level and enzymatic activity of the P5N. Activity of the P5N in blood samples was measured in triplicate using high-performance liquid chromatography (HPLC)-based method first developed by Sakai et al.[13] Briefly, erythrocytes were washed and suspended in 0.9% saline solution. Test solution for each sample was prepared by diluting 50 μL of erythrocyte suspension to 200 μL in distilled water. Tris-buffered UMP (400 μM) was prepared and stored at 4°C as the substrate solution. The total 2 mL of assay mixture contained 200 μL test solution, 200 μL MgCl2 (0.1 M), and 1 mL of substrate solution in deionized water. The assay mixture was incubated at 37°C for 60 min. The reaction was heat-inactivated by placing the tubes at boiling water for 2 min. The mixture was centrifuged at 3000 RPM for 5 min and the supernatant was used for HPLC analysis. A 100-μM uridine solution in deionized water was used as the HPLC standard solution.
The protein level of P5N in samples was measured through a two-site sandwich immunoassay method using Human 5-Prime-Nucleotidase (NT5C2) ELISA Kit, Bioassay Technology Laboratory, Shanghai Korain Biotech Co., Ltd., China according to the protocol provided by the manufacturer.
Liver function tests’ assay
Hepatic function was evaluated through determination of the liver enzymes in serum of the study participants. Blood samples for this purpose were collected in clot-activator containers, and the serum was stored at − 20°C. Liver function enzymes including alkaline phosphatase (ALP), aspartate transaminase (AST), and alanine transaminase (ALT) were measured in serum of the patients and controls using a “BiOLiS24i Premium” Automated Clinical Analyzer (Tokyo Boeki Machinery Ltd, Tokyo, Japan).
Reticulocyte count
Reticulocyte count was determined in whole blood specimens containing EDTA as anticoagulant according to the protocols published previously.[14] One drop of New Methylene Blue stain (NMB) was added to 50 μL of blood samples. Following the incubation at 37°C for 30 min, thin blood films were prepared from the specimens. Cell count was performed using a light microscope. In this method, reticulocytes are distinguished from red blood cells (RBCs) by NMB-stained ribosomal RNAs which are not present in the mature RBCs. Reticulocyte percentage was calculated as below:

Determination of purine/pyrimidine distribution in erythrocytes
To evaluate the distribution of purine and pyrimidine nucleotides, the ratio of the purine/pyrimidine content of the RBCs was determined. Plasma, leukocytes, and platelets were separated from the whole blood samples by centrifugation, and the red blood cells were washed twice and suspended in 0.9% saline solution. An aliquot of 200 μL of the suspension was transferred to ice, followed by addition of 800 μL of 4% perchloric acid, and the supernatant was separated as the nucleotide solution. Assay mixture contained 200 μL of nucleotide solution, 300 μL of glycine buffer, and 500 μL of deionized water. Purine/pyrimidine ratio was analyzed in assay mixture by measuring the optical density at 260 and 280 nm wavelengths using LKB Novaspec® (Pharmacia LKB Biotechnology, Uppsala, Sweden) 4049 Spectrophotometer.
Statistical analysis
Outliers and extreme values were detected and excluded from data analysis using quartile method. All parametric datasets were analyzed in terms of normal distribution and linearity using Shapiro–Wilk test (P > 0.05 was considered as normal distribution). Unpaired t-test was used to compare means between two groups for those datasets with normal distribution (P < 0.05 was considered as statistically significant). Mann–Whitney nonparametric test was used to analyze non-normal distributed and nonparametric datasets (P < 0.05 was considered as statistically significant). Data analysis was performed using MS Excel, SPSS® and GraphPad Prism v4.3® (GraphPad software, San Diego, USA). The results were reported as mean ± standard deviation and median (interquartile range) for normally and non-normally distributed datasets, respectively.
Results
Urine opiate analysis results
The results of the urine analysis for the detection of opium alkaloids showed that all opium-addicted cases were positive for opium alkaloids (morphine, codeine, thebaine, papaverine, and noscapine) in urine samples. Whereas opium alkaloids were not detected in urine samples obtained from control cases.
Blood lead level in opium-addicted patients
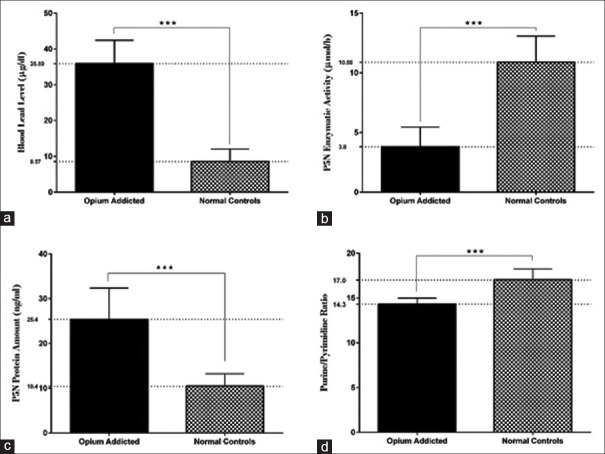
The results of this study concluded that opium-addicted patients showed toxic BLL. The patients group showed an average BLL of 35.89 ± 6.5 μg/dL ranging from 26 to 48.4 μg/dL. As for the control group, the mean BLL was 8.57 ± 3.4 μg/dL ranging from 3.2 to 15.7 μg/dL [Figure 1a]. As analyzed with the independent sample t-test, the mean difference between the two groups was statistically significant [Table 1]. Centers for Disease Control and Prevention (Atlanta, GA, USA) have set elevated BLL to be 10 μg/dL for adults and 5 μg/dL for children.[15] All the opium-addicted subjects in our study were intoxicated with lead regarding the cut-off value of BLL.
Figure 1.
Blood lead concentration and P5N-related analysis. (a) An approximate 4-fold increased BLL was seen in opium-addicted patients compared with control group. (b) Enzymatic activity of P5N in erythrocytes of opium-addicted patients was significantly lower than control group. (c) In contrast to enzymatic activity, protein amount of P5N in erythrocytes of patients was significantly higher than controls. (d) In line with decreased P5N activity, purine/pyrimidine ratio of the nucleotides in erythrocytes of the opium-addicted patients was lower than control subjects. ***P< 0.001
Table 1.
Statistical findings of BLL and P5N-related variables for patients and controls analyzed by unpaired t-test
| Variable | Study subjects | Mean | Range | SD | Mean difference | t | P | |
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | |||||||
| BLL (μg/dL) | Patients | 35.89 | 26 | 48.4 | 6.41 | 27.32 | 23.16 | <0.001 |
| Controls | 8.57 | 3.2 | 15.7 | 3.42 | ||||
| P5N enzymatic activity (μmol uridine/h) | Patients | 3.8 | 1.7 | 5.0 | 1.0 | 7.2 | 18.7 | <0.001 |
| Controls | 10.8 | 7.0 | 14.0 | 2.1 | ||||
| P5N protein amount | Patients | 25.4 | 16.0 | 45.0 | 7.0 | 14.9 | 11.7 | <0.001 |
| Controls | 10.4 | 6.0 | 17.0 | 2.7 | ||||
| Purine/pyrimidine ratio in red cells | Patients | 14.3 | 13.0 | 15.3 | 0.6 | 2.7 | 9.2 | <0.001 |
| Controls | 17.0 | 15.0 | 20.0 | 1.6 | ||||
BLL: blood lead level; P5N: pyrimidine 5′-nucleotidase; SD: standard deviation, t: the size of the difference relative to the variation of samples
Enzymatic activity of pyrimidine 5′-nucleotidase
The results of this study demonstrated that enzymatic activity of the P5N is negatively correlated with BLL. Enzymatic activity of P5N in opium-addicted subjects was dramatically decreased when compared with the control group (P < 0.001) [Figure 1b]. The mean P5N activity for opium-addicted and control subjects is presented in Table 1. The difference between means was statistically significant as analyzed with unpaired t-test (P < 0.001). Also, the purine/pyrimidine ratio in erythrocytes of opium-addicted patients was significantly lower than control group [Figure 1d]. The mean purine/pyrimidine ratio for patients and controls is demonstrated in Table 1. Enzymatic activity of P5N was negatively correlated with BLL [Figure 2a]. Pearson's correlation coefficient (r) for the analysis was − 0.85 with a two-tailed P value of lower than 0.001. The mean protein amount of P5N in patients group was significantly higher than that of control group (25.4 ± 7.0 vs 10.4 ± 2.7 ng/mL) [Figure 1c], and it was reversely correlated with the enzymatic activity of P5N. When analyzed against BLL, a strong correlation was found between P5N protein amount and BLL (r = 0.85) [Figure 2b].
Figure 2.
Blood lead concentration and its correlation with erythrocyte P5N. (a) Enzymatic activity of P5N shows a valid correlation with blood lead concentration (P < 0.001, Pearson's r = −0.85). (b) Protein expression of P5N in erythrocytes is significantly correlated with blood lead concentration (P < 0.001, Pearson's r = 0.85)
Liver function enzymes’ activity
Liver function enzymes in serum of opium-addicted patients showed a significant elevation when compared with the control group [Table 2]. Ten patients showed an ALT level of higher than upper reference limit (URL: 40 U/L). The difference between mean ALT activity in two groups was statistically significant (Mann–Whitney: P < 0.001, z = 6.18). In line with ALT results, a significantly increased AST was observed in serum of opium-addicted subjects when compared with that of control group (Mann–Whitney: P < 0.001, z = 6.39). Serum AST values for nine patients exceeded the URL, whereas all controls showed a normal AST level regarding the reference values (URL: 40 U/L). The difference between two groups for serum ALP was found to be statistically significant when analyzed with Mann–Whitney test (P = 0.001, z = 3.28).
Table 2.
Statistical findings of liver function tests for patients and controls analyzed by nonparametric Mann–Whitney test
| Variable | Study subjects | Median | Range | IQR | z | P | |
|---|---|---|---|---|---|---|---|
| Min. | Max. | ||||||
| ALT (U/L) | Patients | 24.0 | 12.0 | 98.0 | 20.0 | 6.18 | <0.001 |
| Controls | 12.0 | 8.0 | 35.0 | 4.1 | |||
| AST (U/L) | Patients | 30.0 | 13.0 | 75.0 | 20.0 | 6.39 | <0.001 |
| Controls | 13.0 | 7.0 | 25.0 | 5.3 | |||
| ALP (U/L) | Patients | 190.0 | 113.0 | 418.0 | 81.0 | 3.28 | <0.01 |
| Controls | 152.0 | 78.0 | 300.0 | 56.0 | |||
IQR: interquartile range; ALT: alanine transaminase; AST: aspartate transaminase; ALP: alkaline phosphatase
Hematologic findings in opium-addicted patients
Elevated BLL is associated with hematologic findings in opium-addicted patients. BS was observed in 22 out of 40 peripheral blood smears taken from opium-addicted subjects (N = 22, 55%). For control group, no blood cell abnormalities were seen in any of the stained blood films. The mean BLL for BS positive and BS negative subjects was 43.72 [standard error (SE = 1.8)] and 15.61 (SE = 1.48), respectively. As analyzed with Mann–Whitney nonparametric test, the BLL of BS-positive subjects was significantly higher than BS-negative subjects (P < 0.001, z = 6.62). Furthermore, P5N activity in BS-positive subjects was significantly decreased (P < 0.001, z = −4.87).
Half of opium-addicted patients were suffering from hemolytic anemia (N = 20, 50%), and all the study controls showed normal results for hematologic tests. When analyzed using Mann–Whitney, a significant association between BLL and hemolytic anemia was found (P = 0.001, z = 6.52). The mean BLL for the anemic and nonanemic subjects was 44.9 (SE = 1.99) and 16.16 (SE = 1.47), respectively. Also, anemic subjects showed significantly lower P5N activity when compared with nonanemic subjects (P < 0.001, z = −4.79). The mean reticulocyte count for patients and controls was 0.99 ± 0.30 and 1.0 ± 0.29, respectively, and the difference between two groups was not statistically significant (P = 0.95). All the study subjects showed normal values for reticulocyte count.
Analysis of stained blood films showed a mean reticulocyte count of 1.0 ± 0.29% (0.55%–1.73%) for study subjects. Reticulocyte count for all control subjects fell within the normal range (normal range: 0.5%–2.5% in adults). As for opium-addicted patients, the mean reticulocyte count was calculated to be 0.99 ± 0.3% (0.5%–1.5%). Also, three patients (with 4.5%, 5%, and 6.5%) who also were positive for hemolytic anemia showed a reticulocyte count higher than URL. However, no significant difference was found in reticulocyte count of two groups when analyzed with nonparametric Mann–Whitney test (P = 0.954, z = 0.057).
Ethics approval and consent to participate
The study protocol was in conformity with the ethical guidelines of the 1975 Declaration of Helsinki, as revised in 1983. Private information, name, surname, and medical information were removed from datasheets to comply with ethical concern. According to Legal Medicine Research Center, Legal Medicine Organization, Iran Ethics Committee, the information about human subjects was fully confidential.
Discussion
As a toxic trace element, lead has found its way to enter the human body throughout the health history. Occupational exposure is one of the most important causes of lead poisoning. For example, results of a study conducted in Tehran indicated that 35.5% of bus drivers had a BLL higher than 50 μg/dL.[16] Different normal BLL ranges have been established for various populations. As for Iranian population, a mean BLL of 12.37 μg/dL is reported by Farzin et al.[17] A decrease in the incidence of occupational lead poisoning is reported in past decades[18] and it has occurred presumably owing to expended safety awareness. However, another source of lead exposure is emerging to intoxicate a social layer of people who are opium-addicted patients. This unusual lead source is the adulterated opium, containing a large amount of lead which is added to the substance by drug smugglers or petty dealers to increase its specific weight and get more profit.[19]
According to a systematic review, 322 Iranian cases of plumbism due to opium consumption have been reported in the literature during 2004–2016.[20] In line with these reports, we showed that the mean BLL for opium-addicted patients referred to our laboratory was significantly higher than control group. Also, all opium-addicted patients participated in this study showed elevated BLL more than normal value (normal ≤ 10 μg/dL).[21] Our results are in agreement with the reports of the study conducted in Rafsanjan, Iran.[22] The mean values for BLL in this study were higher than their report. This difference might be due to diversity in the sources of opium sold in Rafsanjan and Tehran. Toxic BLL for opium-addicted patients has most likely arisen from orally opium ingestion, since our study groups were matched with regard to age, sex, and other lifestyle factors so that the only different variable between two groups was opium ingestion. These findings suggest that the majority of the opium samples sold in Iran are contaminated with lead. Domeneh et al.,[23] in their study on BLL in opium-dependent cases, indicated that BLL is significantly higher in oral opium-dependent persons. Previous studies confirmed the presence of lead in opium samples in Tehran, Iran.[2]
In various plumbism cases reported in the literature, the patients have shown hemolytic anemia accompanied with BS.[11,20,24] Although BS is a characteristic feature in lead poisoning, it is not a universal finding.[9] Abnormal hematologic findings observable in these patients may partially arise from inhibition of P5N activity. Erythrocyte P5N activity was first introduced as an indicator of lead exposure.[25] Because of its critical role in erythrocyte metabolism, decreased P5N activity could lead to various hematologic deficiencies. Lead causes erythrocyte hemolysis and also inhibits hemoglobin formation. It also inhibits P5N activity in mature erythrocytes, causing reduction in erythrocytes lifespan and an increase in erythrocyte susceptibility resulting in the reduction of erythrocyte count.[26] In this study, we observed a remarkable decrease in P5N activity in opium-addicted patients compared with the control subjects. Also, low erythrocyte purine/pyrimidine ratio in patients group represented accumulation of pyrimidine nucleotides following suppressed P5N activity which eventually leads to hemolytic anemia.[27] Some studies indicated that this complication generally appears in BLL higher than 50 μg/dL.[28] Lead-induced hemolytic anemia may be a result of lipid peroxidation and reactive oxygen species production.[28] Seeking for blood cell disorders, we found considerable BS in 55% of peripheral blood smears obtained from opium-addicted patients. Also, 50% of the patients represented hemolytic anemia. The prevalence of hemolytic anemia and BS in our study population showed a valid correlation with both P5N activity and BLL. Highlighting the importance of P5N activity in erythrocyte metabolism, these findings emphasize the potential risk of hematologic disorders caused by suppression of P5N activity in opium-addicted patients. The results of the study conducted by Kim et al.[24] suggest that in lead-exposed individuals, insufficient P5N activity may affect heme biosynthetic pathways and hemoglobin level in subjects with toxic BLL who are negative for hemolytic anemia. Indeed, decreased P5N activity results in destruction of erythrocytes leading to hemolytic anemia which is not the only type of anemia seen in lead-exposed patients.[24] However, the metabolites of hemoglobin biosynthetic pathway were not evaluated in this study. We found a reverse correlation between P5N activity and BLL. Interestingly, the protein amount of P5N enzyme in patients group was significantly higher than control group. These results indicate that as a consequence of lead exposure, P5N activity is suppressed in opium-addicted patients, and increased protein amount of the enzyme could be indicative of a natural compensatory response to insufficient enzymatic activity.
As indicated in previous studies, high BLL could affect the majority of organs impairing their normal functions. Liver shows notable susceptibility to high BLL among the soft tissues. According to the results of a study conducted previously, exposure to low dose of lead in rats is associated with liver morphologic lesions.[29] In another study, it was concluded that there was a significant correlation between BLL and elevated liver function tests in car paint workers.[30] As the indicators of hepatic damage, we measured liver function enzymes including, ALT, AST, and ALP in serum of the study subjects. Opium is one of the prevalent drugs used by Iranian abusers, and its abuse is one of the challenging public health issues in Iran and other countries.[31,32] Opium-addicted patients showed higher values for all three enzymes. However, we could not find any correlation between BLL and the level of liver function enzymes because of high variation and very low linearity of the datasets. Kshirsagar et al.[12] have found that serum ALT is increased, but the AST level does not exhibit any significant alterations in lead-exposed individuals compared with normal subjects.[12] Mami et al.[33] have shown that consumption of pure opium for 60 days results in elevated liver enzymes in addicted rabbits. Regarding these reports and our results, opium addiction itself might be involved in hepatic damage. Although it is difficult to consider lead exposure as the only cause of elevated liver enzymes in opium-addicted patients.
Conclusions
In conclusion, our results indicate that Iranian opium abusers are at high risk of lead poisoning. In addition, we showed that lead intoxication due to opium ingestion is associated with hematologic findings arising partially from suppressed erythrocyte P5N activity. Finally, our findings highlight the importance of screening for BLL in Iranian opium-addicted patients to prevent complications related to lead exposure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mohajer R, Salehi MH, Mohammadi J, Emami MH, Azarm T. The status of lead and cadmium in soils of high prevalent gastrointestinal cancer region of Isfahan. J Res Med Sci. 2013;18:210–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Akhgari M, Moradi F, Ziarati P. The texture of psychoactive illicit drugs in Iran: Adulteration with lead and other active pharmaceutical ingredients. J Psychoactive Drugs. 2018;50:451–9. doi: 10.1080/02791072.2018.1508791. [DOI] [PubMed] [Google Scholar]
- 3.Sadeghniiat-Haghighi K, Saraie M, Ghasemi M, Izadi N, Chavoshi F, Khajehmehrizi A. Assessment of peripheral neuropathy in male hospitalized patients with lead toxicity in Iran. J Res Med Sci. 2013;18:6–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Mărginean CO, Meliţ LE, Moldovan H, Lupu VV, Mărginean MO. Leadpoisoning in a 16-year-oldgirl: A casereport and a review of the literature (CAREcompliant) Medicine (Baltimore) 2016;95:e4916. doi: 10.1097/MD.0000000000004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afshari R, Emadzadeh A. Shortcommunication: Casereport on adulteratedopium-inducedsevereleadtoxicity. Drug ChemToxicol. 2010;33:48–9. [Google Scholar]
- 6.Akhgari M, Etemadi-Aleagha A, Jokar F. VR, itor Neuropathology of Drug Addictions and Substance Misuse Volume 1: Foundations of Understanding, Tobacco, Alcohol, Cannabinoids and Opioids. UK: Academic Press; 2016. Street level heroin, an overview on its components and adulterants; pp. 867–77. [Google Scholar]
- 7.Aghaee-Afshar M, Khazaeli P, Behnam B, Rezazadehkermani M, Ashraf-Ganjooei N. Presence of lead in opium. Arch Iran Med. 2008;11:553–4. [PubMed] [Google Scholar]
- 8.Kordrostami R, Akhgari M, Ameri M, Ghadipasha M, Aghakhani K. Forensic toxicology analysis of self-poisoning suicidal deaths in Tehran, Iran; trends between 2011-2015. Daru. 2017;25:15. doi: 10.1186/s40199-017-0181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogbenna AA, Ayandokun OA, Roberts AA, Adewoyin AS, Famuyiwa CO. Hematologic profile of battery repair workers occupationally exposed to lead in Lagos. Nigeria. Ann Trop Pathol. 2017;8:68. [Google Scholar]
- 10.Tian W, Wang D, Fan H, Yang L, Ma G. A plasmabiochemicalanalysis of acuteleadpoisoning in a ratmodel by chemometrics-basedfouriertransforminfraredspectroscopy: An exploratorystudy. Front Chem. 2018;6:261. [Google Scholar]
- 11.Alinejad S, Aaseth J, Abdollahi M, Hassanian-Moghaddam H, Mehrpour O. Clinicalaspects of opiumadulterated with leadin Iran: A review. Basic ClinPharmacolToxicol. 2018;122:56–64. doi: 10.1111/bcpt.12855. [DOI] [PubMed] [Google Scholar]
- 12.Kshirsagar M, Patil J, Patil A, Ghanwat G, Sontakke A, Ayachit RK. Biochemical effects of lead exposure and toxicity on battery manufacturing workers of Western Maharashtra (India): With respect to liver and kidney function tests. Al Ameen J Med Sci. 2015;8:107–14. [Google Scholar]
- 13.Sakai T, Yanagihara S, Ushio K. Determination of 5′-nucleotidase activity in human erythrocytes and plasma using high-performance liquid chromatography. J Chromatogr. 1982;239:717–21. doi: 10.1016/s0021-9673(00)82031-2. [DOI] [PubMed] [Google Scholar]
- 14.Adu P, Pobee R, Awuah A, Asiamah PB, Amoani F, Gyabaa S. Reducedhaematopoieticoutput in automobilemechanics and sprayers with chronicexposure to petrochemicals: A case-controlstudy in CapeCoast, Ghana. J Environ Public Health. 2018 doi: 10.1155/2018/9563989. doi: 10.1155/2018/9563989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wani AL, Ara A, Usmani JA. Lead toxicity: A review. InterdiscipToxicol. 2015;8:55–64. doi: 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdollahi M, Nikfar Sh, Jalili N. Monitoring of lead poisoning in bus drivers of Tehran. Iranian J Med Sci. 1995;20:29–33. [Google Scholar]
- 17.Farzin L, Amiri M, Shams H, AhmadiFaghih MA, Moassesi ME. Bloodlevels of lead, cadmium, and mercury in residents of Tehran. Biol Trace Elem Res. 2008;123:14–26. doi: 10.1007/s12011-008-8106-y. [DOI] [PubMed] [Google Scholar]
- 18.Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, et al. The decline in blood lead levels in the United States: The National Health and Nutrition Examination Surveys (NHANES) JAMA. 1994;272:284–91. [PubMed] [Google Scholar]
- 19.Radfar SR, Nematollahi P, Farhoudian A, Noroozi A. Leadpoisoning among opiumusers in Iran: A possiblenewemergingepidemic in the region. Iran J Public Health. 2017;46:1152–3. [PMC free article] [PubMed] [Google Scholar]
- 20.Soltaninejad K, Shadnia S. Leadpoisoning in opiumabuser in Iran: A systematicreview. Int J Prev Med. 2018;9:3. doi: 10.4103/ijpvm.IJPVM_22_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamani N, Hassanian-Moghaddam H. Notes from the field: Lead contamination of opium- Iran, 2016. MMWR Morb Mortal Wkly Rep. 2018;66:1408–9. doi: 10.15585/mmwr.mm665152a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salehi H, Sayadi AR, Tashakori M, Yazdandoost R, Soltanpoor N, Sadeghi H, et al. Comparison of serumleadlevel in oralopiumaddicts with healthycontrolgroup. Arch Iran Med. 2009;12:555–8. [PubMed] [Google Scholar]
- 23.Domeneh BH, Tavakoli N, Jafari N. Blood lead level in opium dependents and its association with anemia: A cross-sectional study from the capital of Iran. J Res Med Sci. 2014;19:939–43. [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HC, Jang TW, Chae HJ, Choi WJ, Ha MN, Ye BJ, et al. Evaluation and management of lead exposure. Ann Occup Environ Med. 2015;27:30. doi: 10.1186/s40557-015-0085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valentine WN, Paglia D, Fink K, Madokoro G. Lead poisoning: Association with hemolytic anemia, basophilic stippling, erythrocyte pyrimidine 5’-nucleotidase deficiency, and intraerythrocytic accumulation of pyrimidines. J Clin Invest. 1976;58:926–32. doi: 10.1172/JCI108545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oginawati K, Dwilestari H, Junianto N. Hematology analysis of lead exposure on painting workers (Case study: Informal automobile painting industries in Karasak, Bandung) KnE Life Sci. 2018;4:674–86. [Google Scholar]
- 27.Huisjes R, Bogdanova A, van Solinge WW, Schiffelers RM, Kaestner L, van Wijk R. Squeezing for life- Properties of redbloodcelldeformability. Front Physiol. 2018;9:656. doi: 10.3389/fphys.2018.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agyemang V. Blood lead levels and selected haematological parameters among at risk occupational groups and blood donors in Kenyasi, BrongAhafo Region. Doctoral dissertation, University Of Ghana. 2018 [Google Scholar]
- 29.da Silva de Assis HC, Sánchez-Chardi A, Dos Reis RC, Nicaretta L, Mencinauski C, Jakobi SC, et al. Subchronictoxiceffects of tributyltin (TBT) andinorganiclead (PbII) in rats. Environ ToxicolPharmacol. 2005;19:113–20. doi: 10.1016/j.etap.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Tutuarima J. The correlation between lead level and hemoglobin, hematocrit, cystatinC serum, SGOT and SGPT levels of car paint workers. Health Notions. 2018;2:387–97. [Google Scholar]
- 31.Najafipour H, Masoomi M, Shahesmaeili A, Haghdoost AA, Afshari M, Nasri HR, et al. Effects of opiumconsumption on coronaryarterydiseaseriskfactors and oralhealth: Results of KermanCoronaryArteryDiseaseRiskfactorsStudy a population- basedsurvey on 5900subjectsaged15-75 years. Int J Prev Med. 2015;6:42. doi: 10.4103/2008-7802.157470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazemi T, Qasemi M, Hajihosseini M. Opium, an important risk factor for deep vein thrombosis patients. J Res Med Sci. 2017;22:112. doi: 10.4103/jrms.JRMS_454_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mami S, Eghbali M, Cheraghi J, Mami F, Borujeni MP, Salati AP. Effect of opium addiction on some serum parameters in rabbit. Glob Vet. 2011;7:310–4. [Google Scholar]