Abstract
The Nobel Prize for Medicine in 2017 was awarded to Michael Young, Michael Rosbash and Jeffrey Hall for their discoveries into the molecular mechanisms controlling circadian rhythms (CR). The aims of this paper were to present the mechanisms behind the CRs and discuss the impact this could have on human health. We argued that further research in this field has the potential to revolutionise healthcare through understanding the influence on the pathogenesis of disease, including in cardiovascular, mental and neurological health, as well as influence on cognitive function. The research has shown that intrinsic CRs have physiological and biochemical influences on the body, which may affect the efficiency of drug absorption due to the altered activity of enzymes. There is strong data to suggest CR disturbances, due to either shift work, sleep disorders or frequent travel between time zones, has negative impact on health. This article aims to summarise the extent of this impact and analyse CRs as a potential therapeutic target, as well as describing the pathophysiology and mechanisms driving the course of disease among people with CR disorders. These new discoveries may revolutionise the way in which treatment is provided in the future with more focus on lifestyle changes to provide treatment and more optimal precision medicine. Pharmaceutical companies and healthcare staff must consider the significant message provided from this data and use the information to optimise drug delivery and treatment provision. The facts of CRs role in healthcare can no longer be ignored.
Keywords: Circadian rhythm, Sleep, Cardiovascular health, Neurological health, Cognitive function, Mental health, Drugs
INTRODUCTION
The aetiology of the word “circadian” is derived from Latin where ‘circa’ means ‘around’ and ‘dian’ stands for ‘day’, thereby summating to “around day”. Circadian rhythm (CR) describes the regular, daily physiological changes that occur within the human body. It is important to note that internal CR is able to adapt to external environment, human bodies have adapted to the earth’s 24-hour day-night cycle even though the true internal human clock tends to lie just over 24-hour long.
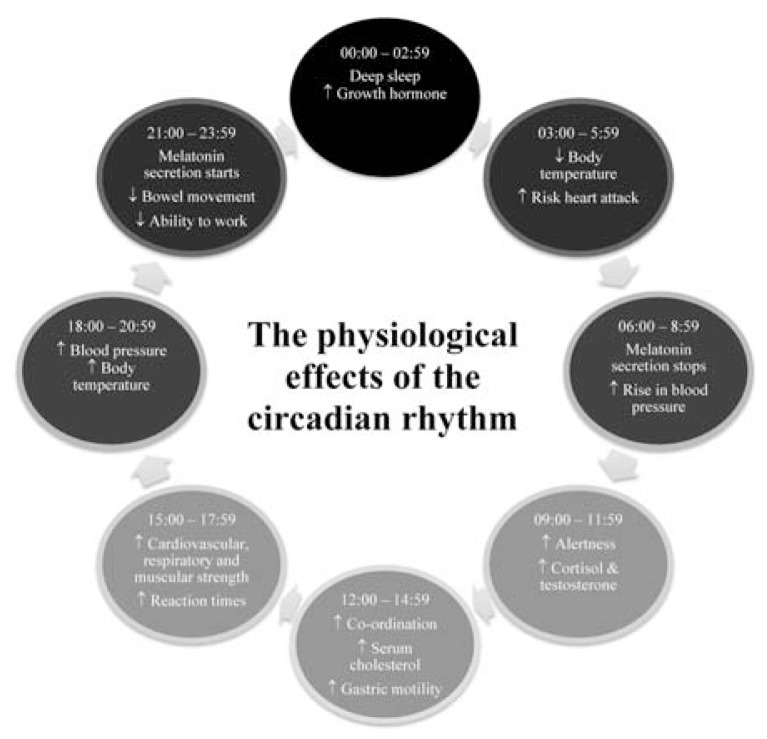
For a while, it was not known whether the circadian rhythm of living organisms existed as a response to the earth’s stimuli or whether there was an endogenous driving factor behind the physiological changes. Research has confirmed that the circadian regulator is endogenous and originates within cells having adapted to external stimuli. The circadian clock is present in every single cell of our bodies, so although the effects of CRs are seen at organ and organ-system level, research has proven that these effects would also be seen on a close-up, cellular level [1]. The physiological changes (Fig. 1) occur as a result of specific genes producing proteins that power the internal biological clock.
Fig. 1.
Diagram showing the process of physiological changes that occur in the body during a 24-hour cycle. The diagram highlights the most prominent physiological changes, such as changes in hormone levels and body temperature, which occur throughout the day and the time at which these changes most often occur in most human bodies.
In 2017, the Nobel Prize in Medicine was awarded to three scientists, Professors: Jeffrey Hall, Michael Rosbash and Michael Young, for their strides in research in the field of CRs and their discovery of molecular mechanisms behind the cycle [2]. Hall, Rosbash and Young were able to decipher the exact genes conducting CRs and pin down the molecular mechanisms as to how biological cycles work. This research continues to better describe the biochemical theories behind CRs and so assist advancements into better understanding how disturbances to internal clocks increase the risk of certain diseases.
There is evidence showing that disturbances to human biological clocks increase the risk of disease. There are many frequent disturbances to the biological clock in the modern world, one example of which includes travelling across time zones, causing misalignment of our internal clock and the earth’s rhythm, a concept described as ‘jet lag’, coming from today’s increased rate of travel. However, the most common disturbance to biological clocks comes from shift work, there are many modern professions whereby working through the night and sleeping through the day is necessary or where night shifts are compulsory and inevitable. It is important to understand what risks these patterns of work carry and how to minimise any detrimental consequences.
This article focuses on four main areas regarding CR disturbances, initially looking at cognitive function in the eyes of a tired healthcare worker and how this may place patients at risk. The article then goes on to focus on the effects of sleep disturbances on the individual’s health, including the risk of cardiovascular injury and the effects on the cardiovascular system, the negative effects on mental health and how poor mental health and mental health diseases arise in those with regular CR disturbances and finally looking at how CR disturbances negatively effect neurological health, seeing it as a risk factor of neurodegenerative diseases. In this article we aim to give an overall picture of what we currently know to be true in the field of circadian rhythms and summarise the main areas of research.
THE MOLECULAR MECHANISMS CONTROLLING THE CR
Some of the first ground breaking research carried out in the field of CRs took place in 1729, by a French astronomer names Jean Jacques d’Ortous de Mairan. His interest in plants and sleep cycles lead him to carrying out an experiment to see whether the daily opening and closing of the leaves of a heliotrope plant occurred only in response to exposure to sunlight and darkness, or if there was an intrinsic component to this action [3]. In this experiment, he placed the heliotrope plant in a dark storage space and observed the movements of the leaves of the plant, noting that without any exposure to sunlight, the leaves still opened up in the day and wrapped up at night. This proved that an element of the plant’s CR was initiated and carried out from within the organism, intrinsically.
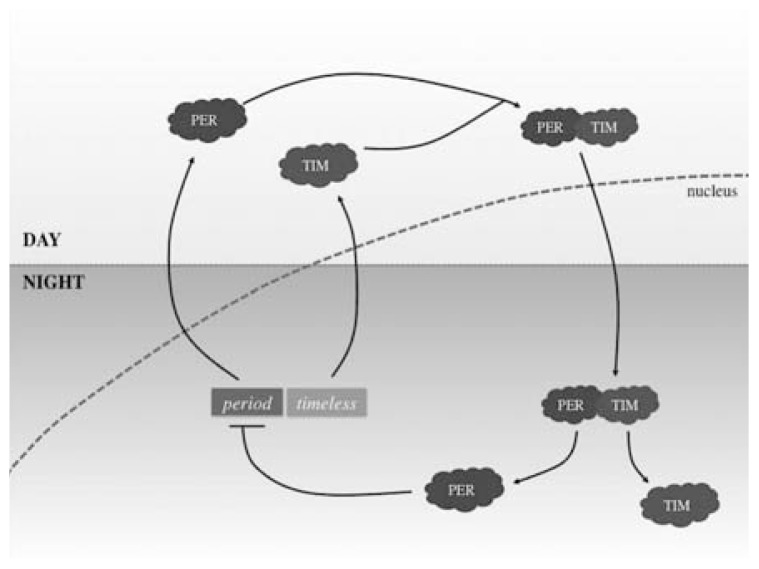
The mechanism behind the perfect synchronisation of CRs relies on genes signalling. Period genes encode for the PER protein, this protein builds up at night and breaks down during the day, representing the rhythm of day to night movement [4]. The period gene was the first clock gene to be discovered, by Jeffrey Hall, Michael Rosbash and Michael Young. The gene is controlled through an inhibitory feedback loop, allowing for its cyclical nature. The PER protein blocks the period gene from producing further PER proteins, hence acting to self-inhibit the cycle. Recent discoveries showed that the timeless gene encodes for the TIM protein, which binds to PER hence allowing it to enter the nucleus of the cell in order to bind to the period gene and thus prevent further PER production (Fig. 2) [5]. Another gene, also recently discovered, is called the double-time gene, which encodes for the DBT protein. The DBT protein prevents the accumulation of the PER protein which describes how the rhythm can be adapted to the earth’s 24-hour cycle [6].
Fig. 2.
Diagram showing one part of the molecular mechanism of CRs. The diagram highlights the mechanism of the period and timeless gene and proteins PER and TIM, showing the way in which they interact in order to differentiate CRs. Day and night is detected by the human body through the build-up of PER protein in the day and an inhibitory feedback loop that halts production of PER at night.
In humans, the circadian master clock resides in the suprachiasmatic nucleus, SCN, of the anterior hypothalamus. Though this master clock has intrinsic rhythmicity, the retinohypothalamic tract light entrains the central oscillation of the SCN that in turn coordinates peripheral oscillators [7]. The oscillations are thought to be regulated by the CLOCK and BMAL genes. CLOCK is at a constant level throughout a 24-hour cycle but BMAL is highest in the morning. When these two genes bind, they activate other genes, such as the period gene, which then take part in the negative feedback cycle described above.
At this point it is important to mention that there are other genes and proteins involved in CR programming are being researched at the moment but in this article only the primary, most researched genes and corresponding proteins were mentioned. Another protein, called CRY, encoded by the cryptochrome gene has also been noted to heterodimerise with PER whilst in the cytosol in order to allow re-entry back into the nucleus where inhibition of CLOCK and BMAL genes prevents further production of CRY and PER [8]. It is important to note that the PER protein is the most prominent and seemingly has the largest effect on control of the circadian rhythm.
WHAT ARE CR DISORDERS AND WHY DO THEY MATTER?
CR disorders exist when there has been severe disruption to a person’s internal body clock. These disturbances can arise from various sources, some of which include: night shift working, jetlag from long-haul travel, insomnia, advanced sleep phase disorder and narcolepsy. These disturbances are important to identify because they can cause dysfunction in the gastrointestinal and cardiovascular system and are known to be a risk factor for colorectal and breast cancer as well as being a causal factor of many other diseases and illnesses, including stroke [8]. CR disorders not only increase the risk of certain diseases but they also impair cognitive performance and increase the frequency of errors in those suffering from regular sleep disturbance, this includes the cognition of healthcare workers that are providing treatment and therapy to patients in hospital. For this reason, a summary (Table 1) involving both the effect of CR disorders on individuals with regular sleep disturbances and the effect of sleep disturbances on healthcare workers’ cognition needs to be considered.
Table 1.
A summary of the overall effects of regular circadian rhythms disturbances on cognition, the cardiovascular system, mental health and neurological health
| The effects of circadian rhythms disturbances on health | |
|---|---|
| Cognitive function |
|
| Cardiovascular system |
|
| Mental health |
|
| Neurological health |
|
Keys: ↓, decrease; ↑, increase.
COGNITIVE FUNCTION
Working at night is widespread amongst many occupations but particularly prominent in healthcare. Recent studies have shown that impaired quality of sleep has negative effects on the cognitive performance of healthcare workers working overnight. A recent study [9] was carried out involving over 100 nurses in order to test their cognitive performance after a night shift compared to their cognitive function at the end of a day shift. The study showed that sleep deprivation exists amongst over half of the staff nurses, whether it is from night shifts or from extended working hours, 13/14-hour shifts with travel to and from work would also effect sleep patterns and disrupt the CR. The study concluded that sleep deprivation impairs cognitive ability, including general intellect, reaction time, attention, vigilance and memory. This increases the chance of errors occurring in the hospital workplace, hence putting hospital patients at risk.
CR disorders matter because they affect the way in which healthcare workers deliver treatment and therapy to patients in hospitals. Extended working shifts mean that healthcare workers are working long periods of time without many rest breaks or naps and may need to wake up early to get to work or stay awake later as they finish late in the day then journey home. A study [10] looking into the relationship between the hours worked by medical school graduates in hospitals and frequency of medical errors found that fatigue from extended working hours and night shift work resulted in a greater frequency of medical errors. There are limitations to this study as it relies on the honest and unbiased opinion of recent medical school graduates completing a survey, but it begins to uncover the scale of this problem.
The significance of the effect of CR disruptions on health is that healthcare work inevitably involves shift work as medical emergencies and in-ward patients require attention during all hours of the day and night. This is dangerous as it decreases performance levels of healthcare staff and leaves patients vulnerable to medical errors. Although this situation is mostly inevitable, there are measures that can be carried out to ensure safer practice and reduced levels of CR disturbances. Some of these measures include ensuring night shifts are shared out equally amongst all healthcare workers and that breaks for naps are permitted through the night so that no single healthcare worker is working throughout the entire night. With such measures we can aim to decrease the effect on cognitive function brought about by planned CR dysfunction and thus reduce the incidence on medical errors taking place in the hospital due to healthcare workers from untimely shift work.
Studies have shown the administration of melatonin to be the potential treatment for patients suffering from secondary sleep disorders, sleep disruption caused by sleep restriction and not with an organic cause, such as in shift work [11]. Studies report that treating patients with secondary sleep disorder, specifically not primary sleep disorder, with exogenous melatonin can improve sleep quality during the hours which the patient can sleep (non-working hours) without the typical hangover effect of other typical sleep-inducing drugs [12]. This is because melatonin is known to be a part of the CR mechanism and one of the important chemicals that controls sleep-wake cycles [13]. Studies in this area have great potential to help make safer workers required to do shift-work and take on multiple night shifts, in turn improving their cognitive function during the atypical working hours [14]. If this treatment were to be provided to healthcare workers it would mean cognitive functional improvement during shift-work and so working night shifts would be a lesser risk for patients being treated overnight.
CARDIOVASCULAR SYSTEM
CR continuously controls our cardiovascular system through gene and protein manipulation. Human heart rate and blood pressure increase in the day and decreases at night [15]. Oxidative stress is exerted onto the cells of the human body when there is CR disruption and sleep disturbance and this is the cause of the high risk of disease that comes from disturbed sleep cycles. CR effects human body in many ways and hence can affect the heart, both directly and indirectly. Large population studies [16] have shown that CR disruption can cause obesity and it is well known that obesity is a prominent risk factor of cardiovascular disease. Release of reactive oxygen species (ROS) makes the blood vessels vulnerable to oxidative injury, which results in atherosclerosis [17]. This, in turn, puts people with regular CR disruptions at an increased risk of cardiovascular disease.
Experimental studies have been carried out on animals that show that prolonged CR misalignment between the body and the external environment is a risk factor for heart disease, as the heart is no longer protected from injury [18]. In the morning, fibrinolysis decreases and heart rate, blood pressure, platelet reactivity, vasoconstriction and intra-arterial pressures increase, after the night-time dip. This means that in the morning, the cardiovascular system is more vulnerable to acute cardiac events, such as sudden cardiac death, myocardial ischemia (MI), stroke and ventricular arrhythmias, due to the sudden physiological changes that occur [19]. When comparing the size of the acute MI versus the time at which the MI occurred, using magnetic resonance imaging (MRI) [20], research showed that the largest sized MIs occur between the hours of 00:00 and 01:00 whereas the smallest sized MIs occur between 12:00 and 13:00. This outcome proves that CR mechanisms can protect the heart and if disturbed can leave the heart vulnerable to acute injury.
Melatonin is released during sleep and protects the human heart. If there are disturbances to the sleep/wake cycle then there will be reduced release of melatonin and so the heart will be left vulnerable to inflammation and free radical oxidative stress [21]. Melatonin is produced and released from the pineal gland in the brain and has both anti-inflammatory, antihypertensive and antioxidant features. This is why the release of a regular, high volume of melatonin is important in protecting the heart from cardiovascular diseases. Studies recognise that patients suffering from cardiovascular disease also tend to have poor melatonin release [22]. Melatonin has direct free radical scavenging properties, which means it prevents injury that can arise from ROS. CR dysfunction means reduced release of melatonin and hence increased levels ROS, causing cardiovascular injury [23]. Therefore decreased melatonin release, a hormone that protects the heart, is a crucial risk factor of cardiovascular disease and closely related to and regulated by CR cycles. This further strengthens the correlation between poor cardiovascular health and CR dysfunction.
MENTAL HEALTH
Sleep abnormalities are listed as key criteria for current disease classification manuals including the ICD-10 and DSM-IV, with more than 80% of patients suffering from depression or schizophrenia reporting sleep pattern disturbances [24]. Despite this close link between sleep and mental health having long been recognised, the association is typically attributed to medication side effects, abnormal social environment and abnormal light exposure [25]. However, prodromal periods of sleep disturbances are a common feature in many mental health conditions, which suggests that sleep disturbances are intrinsic in the development of mental health disorders and not just a symptom of them. In addition, polymorphisms in clock genes have been associated with the development of multiple mental health disorders [26] providing further evidence that CR disturbance may contribute directly to mental illness.
Evidence of such polymorphisms include the fact that mutations of the clock gene in mice show reduced sleep, increased motor activity, lower anxiety and increased affiliation for cocaine. This is a phenotype comparable to that of the mania state in bipolar [27]. In bipolar disorder, sleep disruption appears to trigger the transition into mania. This insinuates that CRs influence disease progression. Furthermore, in human genetic association studies of sufferers of bipolar disorder, links between polymorphisms in clock genes and frequency of depressive relapses have been demonstrated. These polymorphisms also correlate with responses to both conservative and pharmacologic therapy [24]. Polymorphisms in the melanopisn photopigment gene have also been linked to seasonal affective disorder [28]. Studies of these polymorphisms further provide evidence for the link between mental health and CRs.
A systematic review looking into shift work at night and depression demonstrated, across occupation groups, an increased risk of depression in workers who regularly worked at night [29]. However, it is worth noting that the statistical power of this particular meta-analysis is limited by the small data set and variable measured parameters between studies. It is also confounded by the fact that night shifts typically involve reduced control over an individual’s work and less support by superiors, both independent risk factors for depression. Nevertheless, the evidence discussed above provides support for the theory of circadian clocks involvement in mental health disorders.
A plausible mechanism by which CR disruption could predispose to depression is through reduced sleep time, reduced peripheral cortisol receptor sensitivity, functional hypercortisolaemia and reduced melatonin production. Here, we will discuss evidence supporting the idea that these features occur in mental illness. Reduced sleep time is a common feature in many mental illnesses due to insomnia. However, a more generalised disruption of circadian controlled sleep patterns appears to contribute, as hypersomnia, delayed sleep phase and irregular sleep-wake schedules are also commonly comorbid with many mental illnesses. Furthermore, the HPA axis is regulated by CRs and hypercortisolaemia has been implicated in both the development of bipolar disorder and in the neurocognitive deficits that are a feature of the disease [26]. Likewise, elevated cortisol levels have been reported in patients with schizophrenia [30]. Finally, it is known that endogenous secretion of melatonin is decreased in both depression and anxiety. Collectively this evidence suggests that there is biological plausibility in the theory that mental health disorders and circadian disturbances are interlinked.
Mental health patients often state sleep improvement as their highest priority in what they hope to achieve from treatment [31]. Therefore it should be seen as our responsibility to find treatments that relieve sleep disturbances that result from CR disruption. It has already been established that cognitive behavioural therapy for insomnia (CBT-I) effectively treats insomnia that is comorbid with a wide range of mental illnesses. Medications used to treat depression have known significant sedative effects and thus part of their efficacy may be attributable to the stabilisation of the sleep-wake cycle. Furthermore, drugs which directly target components of the circadian controlled sleep cycle, such as melatonin agonists, have proven to stabilise the quantity and quality of sleep in depression and so offer a strategy to improve affective states [32]. On the other hand, our current best treatments for schizophrenia and bipolar disorder leave a persisting CR disruption [33]. Reducing sleep disruptions through CBT or pharmacological treatment will at least improve symptoms and may even intervene with the underlying pathology of these conditions. Given that mental health disorders are so frequently comorbid, it makes clinical sense to try and target an underlying symptom of all of these conditions to which sleep disturbance presents itself as an attractive target.
NEUROLOGICAL HEALTH
The area of neurological health that appears to be most closely entwined with CR disorders is neurodegenerative disease (NDD). The term NDD refers to a range of disorders stemming from the progressive loss of specific neuronal populations. Underpinning this loss is the aberrant aggregation of normally soluble proteins. For many NDDs, neuronal loss ultimately results in dementia of which around 35 million people worldwide suffer from: a staggering figure that is only set to worsen as the population ages. Therefore understanding the mechanisms that contribute to the pathogenesis of these diseases is paramount if we are to therapeutically intervene to improve the quality of life for the fastest growing subsection of society, the elderly.
Each NDD presents differently as a unique protein or group of proteins misfolding and aggregating. The proteins in question are amyloid β in Alzheimer disease (AD), α-synuclein in Parkinson disease (PD) and the protein Huntingtin in Huntington’s disease (HD). There are certain symptoms that affect people irrespective of which NDD they suffer, such as disruption of the sleep-wake cycle. This disruption also occurs as a part of non-pathological ageing, however, the degree of disruption is far greater in NDD and takes affect at a younger age. For example, over 80% of people suffering from REM behaviour disorder will develop PD or another condition caused by the build-up of α-synuclein [34].
A reason for all NDDs accentuating age related decline in circadian modulated sleep patterns is that even in healthy ageing, it is thought that communication between central and peripheral clocks deteriorate before the breakdown of any individual molecular components of a central or peripheral clock [35]. Thus, neurodegeneration in any area of the brain could disrupt the complete synchrony between the various clocks of the brain needed for effective, restorative sleep [36]. A study [36] of post-mortem human brain tissue demonstrated that individuals who suffered from AD exhibited CLOCK gene expression alteration in the pineal gland (which receives central CLOCK inputs) to be comparable to gene expression seen in rats in which the SCN-pineal projections had been experimentally lesioned. This indicates that indeed the central inputs from circadian neurons appear to be lacking in those suffering with AD and that this has implications for gene expression and thus function. The pineal gland itself is responsible for secreting melatonin and so is fundamental for regulating sleep and melatonin has known effects on the neurological system [37]. The consequent sleep disturbances and cognitive implication of this, discussed above, could enhance not only the subjective experience of NDD but also increase the susceptibility of vulnerable subpopulations of neurons to further degeneration. This presents the idea that there is a cycle of dysregulation and destruction involving circadian clocks in NDD.
A viable mechanism by which CR could contribute to NDD pathogenesis is by the loss of the tight maintenance of proteostasis. This would allow aberrant proteins to build up in cells that would otherwise be cleared. Indeed, sleep deprivation has been shown to increase CSF markers of neuronal injury in mice [38], suggesting that disrupted sleep can predispose to NDD by stimulating inflammation and thus synaptic damage thereby making neurons more vulnerable to cell death. It is also thought that CR disruption can contribute directly to the build-up of aggregated proteins. Transgenic mice models of AD have demonstrated an increased amyloid plaque deposition in sleep deprived states [38], a mechanism for this could be that more of the proteins known to misfold in NDDs are produced if more time is spent in an awake state. This is thought to be conserved in humans, as shown by epidemiological data indicating deficient sleep in cognitively normal people as an independent risk factor for the development of clinical AD [39]. Another potential mechanism comes from the association of slow-wave sleep with increased glymphatic flow [40], the process whereby astrocytes clear proteins from the brain. Therefore, CR disruption can affect proteostasis in multiple ways, leading to a predisposition for protein aggregation.
The importance of CR on neurological health is apparent even before birth. Melatonin is produced mainly by the pineal gland under direct influence of the SCN [41]. It shows distinct diurnal variation [42] and so appears to be irrevocably linked to the central clock. Via the placenta, maternal melatonin reaches the fetus, which provides neuroprotection [43] while additionally entraining fetal CR. As the pineal gland does not mature until after birth, the fetus is dependent on maternal melatonin (and thus maternal CR) for entraining its own CR. This can be deduced from the fact that melatonin freely crosses the placental barrier [44] and is detectable in the fetal brain long before maturation of the pineal gland. In addition, melatonin receptors have been identified in the fetal SCN [45] suggesting that maternal melatonin is fundamental to programming fetal CR and therefore laying the foundations for a healthy sleep wake cycle which itself is pivotal to neurodevelopment. Neurodevelopment occurs largely during REM sleep of which new-borns spend 50% of their 16 to 18 hours of sleep in [46].
Furthermore, activation of fetal melatonin receptors protect against oxidative stress leading to an increase in neuronal differentiation and survival [47]. Abnormal maternal melatonin has been suggested to cause excessive oxidative stress as well as increase neonatal inflammation. This has been implicated later in childhood as the development of autism spectrum disorder [48]. In addition, melatonin modulates neural plasticity [49]. It follows therefore that a healthy CR is important throughout life to facilitate learning.
Disruption of CRs may promote NDD through free radical mediated injury which neurons are highly sensitive to. Cellular concentrations of ROS as well as the antioxidant glutathione have clear CR oscillations in drosophila brain and in cultured human fibroblasts [50]. In mice it has further been demonstrated that deletion of BMAL1 increases oxidative stress in the brain. Therefore, showing possible correlation between CR effect on ROS and NDD.
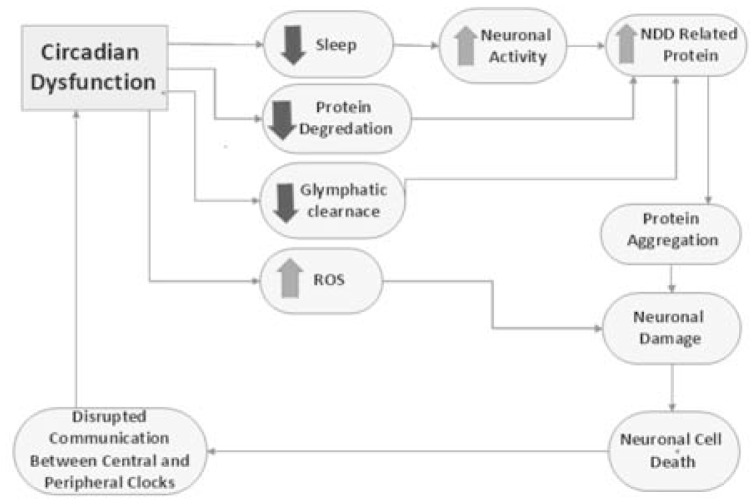
We have so far discussed the ways in which CR dysfunction and neuronal cell death interact (Fig. 3). Targeting the sleep-wake cycle therapeutically has shown some promising early results where both pharmacological and behavioural manipulation of daily activity to promote a more stable sleep cycle lead to improved cognitive and metabolic functions in mouse models of HD [51]. However, the degree to which this will translate to human successes is contentious as melatonin supplementation has been explored as a therapeutic for AD with only modest effect [52]. In addition, light therapy has shown disappointing results, although, it is worth noting that this targets SCN directly and as discussed, it is more the communication between the clocks that becomes dysfunctional with age and in NDDs.
Fig. 3.
Circadian rhythm dysfunction has a wide array of effects from those that are behavioural, decreased sleep, to those that are biochemical, increased ROS production. The consequences of these changes ultimately induce neuronal cell death which disrupts communication between central and peripheral clocks thus further disrupting circadian function.
On the other hand, even before we develop therapeutics to target circadian dysfunction at a molecular level, conservative therapy may have a role to play in symptomatic improvements of NDDs. Morning light exposure, day time activity and consistent bed times are known to improve sleep patterns which is important regardless of the impact on disease progression as disordered sleep patterns of carers has been quoted as the primary reason to transfer relatives suffering from NDD into care institutions [53]. Therefore targeting the sleep/wake cycle as a means to slow the cycle of impaired CR function and neurodegeneration could have a huge impact on the life course of those suffering from NDDs.
CONCLUSION AND FUTURE DIRECTION
The recent discoveries in the field of CRs carry important implications for human health and wellbeing and will change the way in which diseases are diagnosed and the method of treatment provided to patients when prescribing drugs, or other treatment modalities provided to treat disease. Much has been discovered about the mechanism driving the CR and the ways by which cells within a single organism signal to each other and communicate their current position within the cycle. This discovery has embarked on huge research for the further understanding of CR mechanism as well as its application in the treatment of patients. Funding in this field has been significantly increased and provided for by grant bodies, such as the NIH and EU, as well as by pharmaceutical industries.
Understanding the biological clock is important because of the physical effects on the human body, through varying the levels of circulating hormone, rate of body metabolism, human body temperatures and blood pressure as well as effecting social behaviour. Understanding the mechanism behind the effects on the human body is vital because of the way in which it can influence precision medicine and the provision of drugs. Taking certain medications at various times throughout the day can result in better or worse absorption or varying intensities in side effects depending on body temperature and the levels of hormones circulating the system at that time. It is vital to carry out further research about all the physical effects of the biological clock and the consequential effects on drug absorption and metabolism.
Understanding more about the process behind CRs carries a lot of clinical importance. An example of this is research [54] suggesting that the rate of wound healing is dependent on CRs with evidence suggesting that patients with CR disruption will inevitably suffer from delayed wound healing. The difference in time taken for wound healing to occur has been explained through CR, cells produce different proteins at varying rates depending on the time of day. This has mass potential for CR theories to be translated and used in clinical application with treatment provision. As mentioned before, certain proteins, including PER, TIM and DBT, are produced more at night whereas others are more vastly produced in the daytime. The way in which these proteins are produced allow human bodies to live by a regular rhythm, often involving sleeping at night-time when it is dark and working during the day under sunlight conditions, described as diurnal rhythm.
With the understanding that sleep deprivation negatively impairs cognitive behaviour, it should be an imperative aim to reduce the effects of shift work and extended working hours on healthcare workers in order to ensure the safe management of patients in hospital. Some ways to minimise sleep disturbance and CR disorders are through allowing nap-breaks during night shifts and ensuring no single healthcare worker is burdened with more nightshifts than necessary, by equally sharing the sum of nightshifts for each division amongst all healthcare workers working in that division. There needs to be an increase in the number of rest breaks that doctors and nurses are allowed to take during their shifts in order to ensure their bodies are not being overworked at the wrong times.
The pharmaceutical industry is spending billions of dollars in research on therapeutic strategies being developed and implemented in order to treat sleep and metabolic disorders; optimising the precise timings of drug delivery, in order to maximise uptake; and gaining control in various cellular pathways. The budget exists for this research to be carried further and the future implications involve greater global standards of treatment for all varieties of diseases as covered in this article. With further research, society becomes one step closer in understanding the ingrained body clocks thus allowing for the ability to manipulate the body clock in order to best benefit human bodies and prevent and treat disease.
ACKNOWLEDGEMENTS
We acknowledge the comments and help received from Professor Alexander Seifalian on the manuscript.
Footnotes
CONFLICT OF INTERESTS
None to declare.
REFERENCES
- 1.Hoyle NP, Seinkmane E, Putker M, Feeney KA, Krogager TP, Chesham JE, Bray LK, Thomas JM, Dunn K, Blaikley J, O’Neill JS. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci Transl Med. 2017;9:eaal2774. doi: 10.1126/scitranslmed.aal2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burki T. Nobel Prize awarded for discoveries in circadian rhythm. Lancet. 2017;390:e25. doi: 10.1016/S0140-6736(17)32661-2. [DOI] [PubMed] [Google Scholar]
- 3.De Mairan JJ. Observation botanique. Hist l’Academie. R Des Sci. 1729:35. [Google Scholar]
- 4.Reddy P, Zehring WA, Wheeler DA, Pirrotta V, Hadfield C, Hall JC, Rosbash M. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell. 1984;38:701–10. doi: 10.1016/0092-8674(84)90265-4. [DOI] [PubMed] [Google Scholar]
- 5.Noreen S, Pegoraro M, Nouroz F, Tauber E, Kyriacou CP. Interspecific studies of circadian genes period and timeless in Drosophila. Gene. 2018;648:106–14. doi: 10.1016/j.gene.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotwica J, Larson MK, Bebas P, Giebultowicz JM. Developmental profiles of PERIOD and DOUBLETIME in Drosophila melanogaster ovary. J Insect Physiol. 2009;55:419–25. doi: 10.1016/j.jinsphys.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Parekh PK, McClung CA. Circadian mechanisms underlying reward-related neurophysiology and synaptic plasticity. Front Psychiatry. 2016;6:187. doi: 10.3389/fpsyt.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Y, Hur T, Hong Y. Circadian rhythm disruption and subsequent neurological disorders in night-shift workers. J Lifestyle Med. 2017;7:45–50. doi: 10.15280/jlm.2017.7.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaliyaperumal D, Elango Y, Alagesan M, Santhanakrishanan I. Effects of sleep deprivation on the cognitive performance of nurses working in shift. J Clin Diagn Res. 2017;11:CC01–03. doi: 10.7860/JCDR/2017/26029.10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barger LK, Ayas NT, Cade BE, Cronin JW, Rosner B, Speizer FE, Czeisler CA. Impact of extended-duration shifts on medical errors, adverse events, and attentional failures. PLoS Med. 2006;3:e487. doi: 10.1371/journal.pmed.0030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaumont M, Batéjat D, Piérard C, Van Beers P, Denis JB, Coste O, Doireau P, Chauffard F, French J, Lagarde D. Caffeine or melatonin effects on sleep and sleepiness after rapid eastward transmeridian travel. J Appl Physiol. 2004;96:50–8. doi: 10.1152/japplphysiol.00940.2002. [DOI] [PubMed] [Google Scholar]
- 12.Li T, Jiang S, Han M, Yang Z, Lv J, Deng C, Reiter RJ, Yang Y. Exogenous melatonin as a treatment for secondary sleep disorders: A systematic review and meta-analysis. Front Neuroendocrinol. 2018;52:22–8. doi: 10.1016/j.yfrne.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, Ben-Shushan A, Ford I. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Folkard S, Arendt J, Clark M. Can Melatonin improve shift workers’ tolerance of the night shift? Some preliminary findings. Chronobiol Int. 1993;10:315–20. doi: 10.3109/07420529309064485. [DOI] [PubMed] [Google Scholar]
- 15.West AC, Smith L, Ray DW, Loudon ASI, Brown TM, Bechtold DA. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat Commun. 2017;8:417. doi: 10.1038/s41467-017-00462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko SB. Night shift work, sleep quality, and obesity. J Lifestyle Med. 2013;3:110–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Haghikia A, Landmesser U. Lipoproteins and cardiovascular redox signaling: Role in atherosclerosis and coronary disease. Antioxid Redox Signal. 2018;29:337–52. doi: 10.1089/ars.2017.7052. [DOI] [PubMed] [Google Scholar]
- 18.Havakuk O, Zukerman N, Flint N, Sadeh B, Margolis G, Konigstein M, Keren G, Aviram G, Shmilovich H. Shift work and the risk of coronary artery disease: A cardiac computed tomography angiography study. Cardiology. 2018;139:11–6. doi: 10.1159/000481088. [DOI] [PubMed] [Google Scholar]
- 19.Manfredini R, Boari B, Salmi R, Fabbian F, Pala M, Tiseo R, Portaluppi F. Twenty-four-hour patterns in occurrence and pathophysiology of acute cardiovascular events and ischemic heart disease. Chronobiol Int. 2012;30:6–16. doi: 10.3109/07420528.2012.715843. [DOI] [PubMed] [Google Scholar]
- 20.Bulluck H, Nicholas J, Crimi G, White SK, Ludman AJ, Pica S, Raineri C, Cabrera-Fuentes HA, Yellon D, Rodriguez-Palomares J, Garcia-Dorado D, Hausenloy DJ. Circadian variation in acute myocardial infarct size assessed by cardiovascular magnetic resonance in re-perfused STEMI patients. Int J Cardiol. 2017;230:149–54. doi: 10.1016/j.ijcard.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roohbakhsh A, Shamsizadeh A, Hayes AW, Reiter RJ, Karimi G. Melatonin as an endogenous regulator of diseases: The role of autophagy. Pharmacol Res. 2018;133:265–76. doi: 10.1016/j.phrs.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Tengattini S, Reiter RJ, Tan D-X, Terron MP, Rodella LF, Rezzani R. Cardiovascular diseases: Protective effects of melatonin. J Pineal Res. 2008;44:16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC, Reiter RJ. Melatonin and circadian biology in human cardiovascular disease. J Pineal Res. 2010;49:14–22. doi: 10.1111/j.1600-079X.2010.00773.x. [DOI] [PubMed] [Google Scholar]
- 24.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–99. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 25.Wirz-Justice A, Haug HJ, Cajochen C. Disturbed circadian rest-activity cycles in schizophrenia patients: An effect of drugs? Schizophr Bull. 2001;27:497–502. doi: 10.1093/oxfordjournals.schbul.a006890. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci. 2007;104:6406–11. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hankins MW, Peirson SN, Foster RG. Melanopsin: An exciting photopigment. Trends Neurosci. 2008;31:27–36. doi: 10.1016/j.tins.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Angerer P, Schmook R, Elfantel I, Li J. Night work and the risk of depression. Dtsch Arztebl Int. 2017;114:404–11. doi: 10.3238/arztebl.2017.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman SA, Marcu S, Kayumov L, Shapiro CM. Altered sleep architecture and higher incidence of subsyndromal depression in low endogenous melatonin secretors. Eur Arch Psychiatry Clin Neurosci. 2010;260:327–35. doi: 10.1007/s00406-009-0080-7. [DOI] [PubMed] [Google Scholar]
- 31.Auslander LA, Jeste DV. Perceptions of problems and needs for service among middle-aged and elderly outpatients with schizophrenia and related psychotic disorders. Community Ment Health J. 2002;38:391–402. doi: 10.1023/A:1019808412017. [DOI] [PubMed] [Google Scholar]
- 32.De Bodinat C, Guardiola-Lemaitre B, Mocaër E, Renard P, Muñoz C, Millan MJ. Agomelatine, the first melatonergic antidepressant: Discovery, characterization and development. Nat Rev Drug Discov. 2010;9:628–42. doi: 10.1038/nrd3140. [DOI] [PubMed] [Google Scholar]
- 33.Salvatore P, Ghidini S, Zita G, De Panfilis C, Lambertino S, Maggini C, Baldessarini RJ. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008;10:256–65. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 34.Schenck CH, Trenkwalder C. Rapid eye movement sleep behavior disorder: current knowledge and future directions. Sleep Med. 2018;14:699–702. doi: 10.1016/j.sleep.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Chauhan R, Chen K-F, Kent BA, Crowther DC. Central and peripheral circadian clocks and their role in Alzheimer’s disease. Dis Model Mech. 2017;10:1187–99. doi: 10.1242/dmm.030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, Liu RY, Zhou JN, Swaab DF. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J. 2006;20:1874–6. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- 37.Park S, Park K, Lee Y, Chang KT, Hong Y. New Prophylactic and therapeutic strategies for spinal cord injury. J Lifestyle Med. 2013;3:34–40. [PMC free article] [PubMed] [Google Scholar]
- 38.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sterniczuk R, Theou O, Rusak B, Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10:767–75. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- 40.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubocovich ML. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007;8:34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Tortonese DJ, Preedy DF, Hesketh SA, Webb HN, Wilkinson ES, Allen WR, Fuller CJ, Townsend J, Short RV. Experimental jetlag disrupts circadian clock genes but improves performance in racehorses after light-dependent rapid resetting of neuroendocrine systems and the rest-activity cycle. J Neuroendocrinol. 2011;23:1263–72. doi: 10.1111/j.1365-2826.2011.02222.x. [DOI] [PubMed] [Google Scholar]
- 43.Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan D-X. Melatonin: An established antioxidant worthy of use in clinical trials. Mol Med. 2009;15:43–50. doi: 10.2119/molmed.2008.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biran V, Phan Duy A, Decobert F, Bednarek N, Alberti C, Baud O. Is melatonin ready to be used in preterm infants as a neuroprotectant? Dev Med Child Neurol. 2014;56:717–23. doi: 10.1111/dmcn.12415. [DOI] [PubMed] [Google Scholar]
- 45.Voiculescu SE, Zygouropoulos N, Zahiu CD, Zagrean AM. Role of melatonin in embryo fetal development. J Med Life. 2014;7:488–92. [PMC free article] [PubMed] [Google Scholar]
- 46.Mirmiran M, Maas YGH, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003;7:321–34. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- 47.Kaneko Y, Hayashi T, Yu S, Tajiri N, Bae EC, Solomita MA, Chheda SH, Weinbren NL, Parolini O, Borlongan CV. Human amniotic epithelial cells express melatonin receptor MT1, but not melatonin receptor MT2: A new perspective to neuroprotection. J Pineal Res. 2011;50:272–80. doi: 10.1111/j.1600-079X.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 48.Jin Y, Choi J, Won J, Hong Y. The Relationship between autism spectrum disorder and melatonin during fetal development. Molecules. 2018;23:198. doi: 10.3390/molecules23010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serón-Ferré M, Torres-Farfán C, Forcelledo ML, Valenzuela GJ. The development of circadian rhythms in the fetus and neonate. Semin Perinatol. 2001;25:363–70. doi: 10.1053/sper.2001.29037. [DOI] [PubMed] [Google Scholar]
- 50.Khapre RV, Kondratova AA, Susova O, Kondratov RV. Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle. 2011;10:4162–9. doi: 10.4161/cc.10.23.18381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pallier PN, Maywood ES, Zheng Z, Chesham JE, Inyushkin AN, Dyball R, Hastings MH, Morton AJ. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of huntington’s disease. J Neurosci. 2007;27:7869–78. doi: 10.1523/JNEUROSCI.0649-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJG, van Someren EJW. Effect of bright light and melatonin on cognitive and non-cognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA. 2008;299:2642–55. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 53.Simpson C, Carter PA. Pilot study of a brief behavioral sleep intervention for caregivers of individuals with dementia. Res Gerontol Nurs. 2010;3:19–29. doi: 10.3928/19404921-20090731-02. [DOI] [PubMed] [Google Scholar]
- 54.Cable EJ, Onishi KG, Prendergast BJ. Circadian rhythms accelerate wound healing in female Siberian hamsters. Physiol Behav. 2017;171:165–74. doi: 10.1016/j.physbeh.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]