Abstract
Background:
The integrase inhibitor regimen [elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate (TDF)] demonstrated superior efficacy when compared with a protease inhibitor regimen [ritonavir-boosted atazanavir (ATV + RTV) and FTC/TDF] in 575 treatment-naive women at week 48. We investigated the efficacy, safety, and tolerability of switching to a TAF-based, single-tablet regimen containing elvitegravir, cobicistat, FTC, and tenofovir alafenamide (E/C/F/TAF) versus remaining on ATV + RTV plus FTC/TDF.
Methods:
After completing the initial randomized, blinded phase, virologically suppressed (HIV-1 RNA <50 copies/mL) women on ATV + RTV plus FTC/TDF were rerandomized (3:1) to receive open-label E/C/F/TAF versus remaining on their current regimen. The primary end point was proportion of participants with plasma HIV-1 RNA <50 copies per milliliter at week 48 (U.S. FDA snapshot algorithm), with a prespecified noninferiority margin of 12%. Safety [adverse events (AEs)] and tolerability were also assessed.
Results:
Of 575 women originally randomized and treated in the blinded phase, 159 were rerandomized to switch to E/C/F/TAF and 53 to remain on ATV + RTV plus FTC/TDF. At week 48, virologic suppression was maintained in 150 (94%) of women on E/C/F/TAF and 46 (87%) on ATV + RTV plus FTC/TDF [difference 7.5% (95% confidence interval −1.2% to 19.4%)], demonstrating noninferiority of E/C/F/TAF to ATV + RTV and FTC/TDF. Incidence of AEs was similar between groups; study drug–related AEs were more common with E/C/F/TAF (11% versus 4%).
Conclusions:
Switching to E/C/F/TAF was noninferior to continuing ATV + RTV plus FTC/TDF in maintaining virologic suppression and was well tolerated at 48 weeks.
Keywords: HIV, women, INSTI, tenofovir, TAF
INTRODUCTION
The integrase strand transfer inhibitor (INSTI) elvitegravir coformulated with cobicistat, emtricitabine, and tenofovir alafenamide in a single-tablet regimen (E/C/F/TAF) is a preferred antiretroviral regimen in HIV-infected, treatment-naive individuals.1–3 TAF achieves comparable antiretroviral efficacy at a lower dose compared with tenofovir disoproxil fumarate, resulting in 91% lower plasma tenofovir (TFV) exposures.4 Randomized, phase 3 studies established virologic noninferiority of TAF versus TDF.5–7 In these studies, participants treated with TAF-based regimens consistently showed improvements in markers of renal function and bone mineral density compared with those treated with TDF-based regimens.
We conducted a phase 3, noninferiority study to assess clinical efficacy, safety, and tolerability of switching to the INSTI- and TAF-based, single-tablet regimen of E/C/F/TAF versus remaining on the protease inhibitor –based, multi-tablet regimen of ritonavir-boosted atazanavir (ATV + RTV) plus FTC/TDF in HIV-infected, virologically suppressed women. Herein, we present results 48 weeks after switching.
METHODS
Study Design and Participants
We previously reported inclusion criteria from the randomized, double-blind phase of the Women AntiretroViral Efficacy and Safety study, an international, phase 3 study in treatment-naive women.8 In the double-blind phase, women were randomized 1:1 to receive coformulated elvitegravir, cobicistat, FTC, and TDF (E/C/F/TDF) or ATV 300 mg + RTV 100 mg plus FTC/TDF 200/300 mg. Women virologically suppressed (HIV-1 RNA <50 copies/mL) at week 48 of the double-blind phase were eligible to receive treatment in the open-label extension phase. Women on ATV + RTV plus FTC/TDF were randomized (3:1) to receive E/C/F/TAF (150/150/200/10 mg) or continue ATV + RTV plus FTC/TDF.
Postbaseline study visits occurred at weeks 4, 12, 24, 36, and 48. Laboratory tests included hematologic analyses, serum chemistry tests, fasting lipid parameters, CD4+ cell counts, measures of renal function {estimated glomerular filtration rate [eGFR] [Cockcroft-Gault], tubular proteinuria [retinol binding protein (RBP) to creatinine (CR) ratio, β2-microglobulin (β2M) to Cr ratio]} (Covance Laboratories, Indianapolis, IN), and measurement of HIV-1 RNA concentration (Roche TaqMan 2.0; Roche Diagnostics, Rotkreuz, Switzerland). Confirmatory plasma samples from participants with confirmed virologic failure at any time during the study (2 consecutive viral load samples >50 copies/mL) and HIV-1 RNA >400 copies per milliliter at the confirmatory visit were sent for resistance analysis using GeneSeq Integrase, PhenoSense GT, and PhenoSense Integrase assays (Monogram Biosciences, South San Francisco, CA). Dual-energy x-ray absorptiometry of the hip and lumbar spine was performed in a subset of women before study drug administration at baseline and weeks 24 and 48 (BioClinica, Newton, PA). Adverse events (AEs) and concomitant drugs were assessed at each visit.
The study was performed in accordance with the Declaration of Helsinki and approved by central or site-specific review boards or ethics committees. Each participant provided a written informed consent. Women who became pregnant during the study had the option to continue treatment after providing additional informed consent. The study was registered with ClinicalTrials.gov (NCT01705574).
Statistical Analyses
Efficacy end points included proportion of participants with plasma HIV-1 RNA <50 copies per milliliter at week 48, as determined using the United States (U.S.) Food and Drug Administration (FDA)-defined snapshot algorithm.9 Other secondary end points included change from baseline in CD4 cell count and safety through week 48. Safety assessments included standard laboratory testing and AEs, coded with Medical Dictionary for Regulatory Activities version 19.
Baseline characteristics were summarized with descriptive statistics. For categorical data, we calculated P values using the Cochran–Mantel–Haenszel test (general association statistic was used for nominal data, and row mean scores differ statistic for ordinal data). For continuous data, we calculated P values using the two-sided Wilcoxon rank-sum test. The proportions of participants with plasma HIV-1 RNA <50 copies per milliliter at week 48 (U.S. FDA snapshot algorithm) for both treatment groups were calculated. The 2-sided 95% confidence interval (CI) of the proportional difference was estimated through an unconditional exact method using 2 inverted 1-sided tests with the standardized statistic. Noninferiority of E/C/F/TAF compared with ATV + RTV and FTC/TDF was concluded if the lower bound of the two-sided 95% CI was greater than −12%. With the assumption of response rates being 82% and 87% in ATV + RTV plus FTC/TDF and E/C/F/TAF groups, respectively, a sample size of 210 subjects and a 3:1 allocated randomization to E/C/F/TAF (n = 157) and ATV + RTV plus FTC/TDF (n = 53) ensure 85% power for the test of noninferiority. We did a prespecified subgroup analysis of treatment differences on the basis of baseline age (<40 years, ≥40 years), race (black, non-black), and study drug adherence (<95%, ≥95%). Adherence to study drug was calculated as the number of pills taken (as measured by pill counts conducted at each study visit) divided by the number of pills prescribed. Changes from baseline in CD4 cell count at week 48 were summarized with descriptive statistics.
RESULTS
Of the 286 women who received ATV + RTV plus FTC/TDF in the double-blind phase, 212 ATV + RTV plus FTC/TDF subsequently switched to E/C/F/TAF (n = 159) or continued on ATV + RTV plus FTC/TDF (n = 53) in the open-label extension (Supplemental Digital Content Fig. S1, http://links.lww.com/QAI/B129). Demographic and baseline clinical characteristics were balanced between groups (Supplemental Digital Content Table S1, http://links.lww.com/QAI/B129). Median age of participants was 36 years, and 101 (48%) were black. Median CD4 count was 610 cells per microliter; 5 (2%) of 211 were positive for hepatitis B surface antigen, and 18 (9%) of 211 were positive for hepatitis C antibody.
Switching to E/C/F/TAF was noninferior to continuing ATV + RTV plus FTC/TDF for the outcome of HIV–1 RNA <50 copies per milliliter at week 48 (U.S. FDA snapshot algorithm); virologic suppression was maintained in 150 women (94%) on E/C/F/TAF and 46 (87%) on ATV + RTV plus FTC/TDF (difference 7.5%, 95% CI −1.2% to 19.4%; Table 1). Response rates were generally consistent across prespecified subgroups (age, race, and study drug adherence); non-black women and those with adherence <95% had response rates that favored E/C/F/TAF (Supplemental Digital Content Fig. S2, http://links.lww.com/QAI/B129). Virologic suppression with HIV-1 RNA cutoff at <20 copies per milliliter was noted in 135 participants (85%) on E/C/F/TAF and 38 (72%) on ATV + RTV plus FTC/TDF (difference 13.2%, 95% CI: −0.0% to 27.5%, P = 0.041). Mean (SD) changes from baseline in CD4 cell counts were similar between groups: 35 (137.5) cells per microliter for E/C/F/TAF and 49 (204.8) cells per microliter for ATV + RTV and FTC/TDF.
TABLE 1.
Virologic Outcomes at Week 48
| E/C/F/TAF vs ATV + RTV plus FTC/TDF |
||||
|---|---|---|---|---|
| E/C/F/TAF (n = 159) | ATV+RTV plus FTC/TDF (n = 53) | P | Difference in Percentages (95% CI)† | |
| HIV-1 RNA <50 copies/mL† | 150 (94%) | 46 (87%) | 0.13 | 7.5% (21.2% to 19.4%) |
| HIV-1 RNA ≥50 copies/mL | 3 (2%) | 2 (4%) | ||
| HIV-1 RNA ≥50 copies/mL | 2 (1%) | 2 (4%) | ||
| Discontinued because of the lack of efficacy | 0 | 0 | ||
| Discontinued because of other reasons and the last available HIV-1 RNA ≥50 copies/ mL* | 1 (1%) | 0 | ||
| Added new ARV | 0 | 0 | ||
| No virologic data | 6 (4%) | 5 (9%) | ||
| Discontinued because of AE/death | 1 (1%) | 1 (2%) | ||
| Discontinued because of other reasons* and the last available HIV-1 RNA <50 copies/ mL | 4 (3%) | 3 (6%) | ||
| Missing data but on study drug | 1 (1%) | 1 (2%) | ||
| HIV-1 RNA <50 copies/mL by missing = failure‡ | 150 (94%) | 47 (89%) | 0.21 | 5.7% (22.5% to 17.2%) |
| HIV-1 RNA <50 copies/mL by missing = excluded‡ | 150/153 (98%) | 47/49 (96%) | 0.60 | 2.1% (22.9% to 11.4%) |
Data are n (%).
Other reasons include subjects who discontinued study drug because of investigator’s discretion, withdrawal of consent, lost to follow-up, noncompliance with study drug, protocol violation, and pregnancy.
P values were from the Fisher exact test. Difference in percentages of virologic success and its 95% CI were calculated based on exact method. The exact CI was estimated based on an unconditional exact method using 2 inverted 1-sided tests with the standardized statistic.
P value, difference in percentages, and 95% CI were based on a dichotomized response: success (HIV-1 RNA <50 copies/mL) or failure (HIV-1 RNA ≥50 copies/mL or missing) for missing = failure approach or missing = excluded approach. Difference in percentage of virologic success and its 95% CI were calculated based on an unconditional exact method using 2 inverted one-sided tests with the standardized statistic.
Resistance analysis was performed in 5 women (3.1%) in the E/C/F/TAF group and 2 (3.8%) in the ATV + RTV plus FTC/TDF group. None had emergent genotypic or phenotypic resistance to study drugs.
Both treatments were well tolerated; most AEs were reported as mild or moderate in severity. Types of AEs were similar between groups (Supplemental Digital Content Table S2, http://links.lww.com/QAI/B129). AEs leading to study drug discontinuation were uncommon, occurring in 1 participant (1%) on E/C/F/TAF (confusional state secondary to lymphoma) and 1 (2%) on ATV + RTV plus FTC/TDF (hepatitis); the hepatitis AE was considered treatment related by the investigator. Overall, treatment-related AEs were reported for 18 participants (11%) on E/C/F/TAF and 2 (4%) on ATV + RTV plus FTC/TDF. No women died during the study. Grade 3 or 4 laboratory abnormalities were reported for 41 of 158 women (26%) on E/C/F/TAF and 21 of 53 (40%) on ATV + RTV plus FTC/TDF. The difference between groups was driven by higher incidence of grade 3 or 4 hyperbilirubinemia with ATV + RTV plus FTC/TDF compared with E/C/F/TAF [28% (15/53) versus 0].
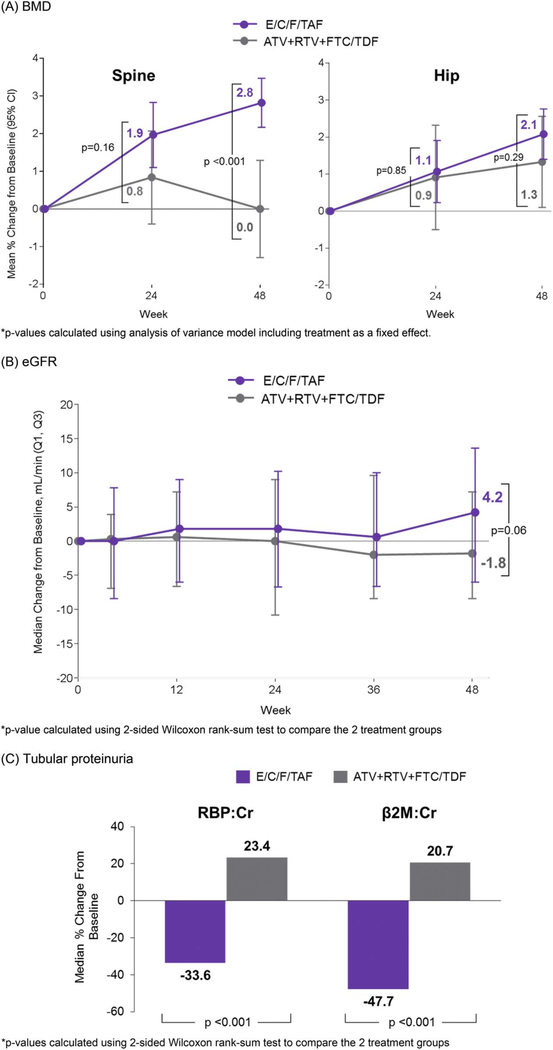
Mean spine BMD increased 2.8% from baseline to week 48 with E/C/F/TAF, whereas minimal changes were seen with ATV + RTV plus FTC/TDF (P < 0.001 for difference in changes between groups) (Fig. 1A). More participants on E/C/F/TAF had increases in spine BMD that exceeded a 3% minimum threshold,15 whereas fewer had decreases of >3% compared with ATV + RTV plus FTC/TDF (>3% increases: 47% versus 10%; >3% decreases: 3% versus 7%; P < 0.001 for difference in distribution between groups). Changes in hip BMD at weeks 24 and 48 were not different between groups (P ≥ 0.29). Two women, 1 in each group, had a fracture; both resulted from trauma and were considered unrelated to treatment.
FIGURE 1.
Key bone and renal end points through week 48. A, BMD: *P values calculated using analysis of variance model including treatment as a fixed effect. B, eGFR: *P value calculated using the 2-sided Wilcoxon rank-sum test to compare the 2 treatment groups. C, Tubular proteinuria: *P values calculated using the 2-sided Wilcoxon rank-sum test to compare the 2 treatment groups. ATV, atazanavir; β2M, β2-microglobulin; BMD, bone mineral density; Cr, creatinine; E/C/F/TAF, coformulated elvitegravir, cobicistat, emtricitabine, tenofovir alafenamide; Q, quartile; RBP, retinol binding protein; RTV, ritonavir.
No cases of proximal tubulopathy were reported by the investigator in either group, and no participant discontinued because of a study drug–related renal AE. In both groups, we observed no notable changes from baseline in median eGFR through week 48 (Fig. 1B), although a trend toward increased eGFR was observed with E/C/F/TAF. At week 48, tubular proteinuria (RBP:Cr, β2-microglobulin:Cr) decreased with E/C/F/TAF while increasing with ATV + RTV plus FTC/TDF (P < 0.001 for both, Fig. 1C).
Fasting total cholesterol, LDL, HDL, and triglycerides increased with E/C/F/TAF at week 48 and remained stable with ATV + RTV plus FTC/TDF (Supplemental Digital Content Table S4, http://links.lww.com/QAI/B129). However, we noted no difference in change in total cholesterol to HDL ratio between groups (median change 0.1 versus 0, P = 0.075). Two (1%) of 159 participants on E/C/F/TAF began lipid-lowering drugs compared with none on ATV + RTV plus FTC/TDF.
Women enrolled were required to use 2 forms of birth control, with pregnancy testing at every study visit. Nineteen women became pregnant (20 pregnancies): 13 on E/C/F/TAF and 6 on ATV + RTV plus FTC/TDF. Of these 19 women, 17 elected to continue study drugs (11 on E/C/F/TAF and 6 on ATV + RTV plus FTC/TDF). Uncomplicated term delivery was confirmed for 7 pregnancies (4 on E/C/F/TAF and 3 on ATV + RTV plus FTC/TDF) with no congenital malformations reported. Virologic suppression was confirmed in all of these women at week 48. Of the remaining pregnancies, 5 ended in elective abortions (n = 4 E/C/F/TAF, n = 1 ATV + RTV plus FTC/TDF), 6 (2 pregnancies in 1 woman) ended in spontaneous abortions (n = 4 and n = 1, respectively), and outcome is unknown for 2 (n = 1 and n = 1, respectively).
DISCUSSION
This study demonstrated efficacy and safety in women who switched to an INSTI- and TAF-based, single-tablet regimen (E/C/F/TAF). A high rate of virologic suppression was maintained in women on E/C/F/TAF, with no evidence of treatment-emergent resistance after 48 weeks.
The safety profiles were comparable between treatment groups through 48 weeks. More treatment-related AEs were reported for participants on E/C/F/TAF, which was not unexpected for individuals initiating a novel therapy with 3 new antiretroviral agents versus those continuing their well-tolerated baseline regimen in an open-label study.
Most remarkable in this study is demonstration of improved bone and kidney safety among women switched to E/C/F/TAF. Significant improvements at week 48 in spine bone mineral density were observed among women switched to E/C/F/TAF compared with those continuing ATV + RTV plus FTC/TDF. Although there were no statistical significant differences between groups demonstrated for hip BMD, absolute increases were greater with E/C/F/TAF. Our results are consistent with previous findings of improved BMD when switching from TDF- to TAF-containing regimens; this switch strategy may be particularly beneficial for women, given the anticipated decreases in BMD occurring at the time of menopause.6,7,10,11
Although no women developed tubulopathy in this study, long-term TDF has been associated with proximal tubular function.12–14 Sensitive laboratory markers of tubular function (ie, RBP:CR and β2M:Cr) were used to assess for evidence of TDF-associated nephrotoxicity. These markers are specific for proximal renal tubular dysfunction and recommended for monitoring TFV nephrotoxicity in current Infectious Diseases Society of America Guidelines.15 In our study, women who had mild to no renal impairment at baseline (ClCr ≥50 mL/min) had improvements in tubular proteinuria after switching to E/C/F/TAF, suggesting a decreased risk of nephrotoxicity compared with remaining on a TDF regimen. These findings are consistent with renal marker results of other large TDF-to-TAF switch studies.6,7,10 Long-term renal and bone benefits of E/C/F/TAF will require further follow-up. Nonetheless, our study reinforces findings of improved clinical bone and renal parameters with TAF-versus TDF-containing regimens.
We observed increases in fasting total cholesterol, HDL, LDL, and triglycerides with E/C/F/TAF, consistent with previous findings in other TDF-to-TAF switch studies.6,7 Treatment with TDF has been associated with lower lipids compared with non-TDF regimens, and this lipid effect is considered to be a function of circulating levels of TFV.16–18 In the current study, increases in fasting total cholesterol, HDL, LDL, and triglycerides in the E/C/F/TAF group after switching from a TDF-containing regimen were observed and were consistent with previous findings in other TDF-to-TAF switch studies.6,7 However, no treatment differences were observed in total cholesterol to HDL ratio, a predictor of cardiovascular risk.19
In conclusion, virologically suppressed, HIV-infected women who switched to E/C/F/TAF ATV + RTV plus FTC/TDF remained virologically suppressed after 48 weeks, with no evidence of antiretroviral resistance. More importantly, significant improvements in measures of bone and renal safety were observed after switching. These results provide important clinical data on TAF-based regimens as a safe and effective treatment option for women.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients who participated in this trial and their families, the principal investigators and their staff, and the Gilead study staff.
S.H. has participated in the advisory boards of Gilead Sciences, Bristol-Myers Squibb, and Janssen and has received personal fees from Gilead Sciences, ViiV, Bristol-Myers Squibb, and Janssen. K.S. has participated in the advisory boards of Gilead Sciences. The remaining authors have no funding or conflicts of interest to disclose.
A.K., S.J., R.K., A.C., and H.C. are employees of the funder Gilead Sciences and shareholders of Gilead stock.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
REFERENCES
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in HIV-1-Infectedadults and Adolescents. Department of Health and Human Services; 2016. Available at: http://www.aidsinfo.nih.gov/ContentFiles/Adul-tandAdolescentGL.pdf. Accessed October 11, 2016. [Google Scholar]
- 2.Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society–USA panel. JAMA. 2016; 316:191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European AIDS Clinical Society (EACS). European Guidelines for Treatment of HIV-positive Adults in Europe, Version 8.2; 2017. Available at: http://www.eacsociety.org/files/guidelines_8.2-english.pdf. Accessed May 12, 2016.
- 4.Ruane PJ, Dejesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63:449–455. [DOI] [PubMed] [Google Scholar]
- 5.Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015; 385:2606–2615. [DOI] [PubMed] [Google Scholar]
- 6.Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, noninferiority study. Lancet Infect Dis. 2016;16:43–52. [DOI] [PubMed] [Google Scholar]
- 7.Gallant JE, Daar ES, Raffi F, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV. 2016;3:e158–65. [DOI] [PubMed] [Google Scholar]
- 8.Squires K, Kityo C, Hodder S, et al. Integrase inhibitor versus protease inhibitor based regimen for HIV-1 infected women (WAVES): a randomised, controlled, double-blind, phase 3 study. Lancet HIV. 2016;3: e410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith F, Hammerstorm T, Soon G, et al. A meta-analysis to assess the FDA DAVP’s TLOVR algorithm in HIV submissions. Drug Inf J. 2011; 45:291–300. [Google Scholar]
- 10.Pozniak A, Arribas JR, Gathe J, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48 week results from a single-arm, multi-center, open-label, Phase 3 study. J Acquir Immune Defic Syndr. 2016;71:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall AM, Hendry BM, Nitsch D, et al. Tenofovir-associated kidney toxicity in HIV-infected patients. Am J Kidney Dis. 2011;57:773–780. [DOI] [PubMed] [Google Scholar]
- 13.Nisjijima T, Kawasaki Y, Tanaka N, et al. Long-term exposure to tenofovir continuously decrease renal function in HIV-1-infected patients with low body weight: results from 10 years of follow-up. AIDS. 2014; 28:1903–1910. [DOI] [PubMed] [Google Scholar]
- 14.Kinai E, Hanabusa H. Progressive renal tubular dysfunction associated with long-term use of tenofovir DF. AIDS Res Hum Retroviruses. 2009; 25:387–394. [DOI] [PubMed] [Google Scholar]
- 15.Lucas GM, Ross MJ, Stock PG, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014; 59:e96–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos JR, Saumoy M, Curran A, et al. The lipid-lowering effect of tenofovir/emtricitabine: a randomized, cross-over, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:403–408. [DOI] [PubMed] [Google Scholar]
- 17.Tungsiripat M, Kitch D, Glesby MJ, et al. A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS. 2010;24:1781–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane HM, Grunfeld C, Willig JH, et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS. 2011;25:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millán J, Pintó X, Muñoz A, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.