Abstract
Objective:
To determine if T1ρ magnetic resonance imaging (T1ρ MRI) could assess early articular cartilage changes in knees of asymptomatic female collegiate athletes. It was hypothesized that impact cohort would demonstrate greater changes than nonimpact cohort.
Design:
An institutional review board–approved prospective cohort study. Blinded MRI analyses.
Setting:
Participants from collegiate athletic program. Imaging at university hospital, February 2008 to July 2009.
Participants:
Inclusion criteria were female collegiate athletes in athletic season and asymptomatic. Exclusion criteria were previous/current knee injuries/surgeries. Twenty-one female NCAA Division I athletes, 11 impact (basketball players) and 10 nonimpact (swimmers) participants were consented and imaged with 3.0-T MRI (Siemens) and T1ρ sequence (University of Pennsylvania). One patient was removed (injury diagnosis). Final roster was 10 impact and 10 nonimpact participants. No difference in cohort body mass index, height, or weight.
Main Outcome Measures:
Average T1ρ relaxation times (ART) for patellar and femoral cartilage to analyze defined regions and depth and modified International Cartilage Repair Society classification.
Results:
Statistical analyses showed that ART of radial zone of central third weight-bearing region of cartilage in basketball players was significantly greater (P = 0.041) than swimmers and ART of the superficial zone in basketball players was significantly less (P = 0.003) than that of swimmers. For both groups, the ART of super-ficial zones were significantly greater than that of radial zones (P < 0.001). Four impact athletes showed macroscopic changes (none in nonimpact cohort).
Conclusions:
T1ρ MRI detected early changes in articular cartilage of asymptomatic collegiate female impact athletes, with significant differences between cohorts in radial zone of central third weight-bearing region and superficial zones ART. Both cohorts showed increased ART in superficial zone. Four impact athletes showed macroscopic changes.
Clinical Relevance:
This study demonstrates a quantitative MRI sequence able to detect signal differences in articular cartilage in asymptomatic athletes.
Keywords: T1ρ MRI, NCAA, impact, nonimpact, female athlete, cartilage changes
INTRODUCTION
Conventional magnetic resonance imaging (MRI) is well-suited to identify gross morphologic changes within articular cartilage. The complexity of the interaction between collagen molecules and water protons has led to varying contrast between hyaline cartilage and scar tissue, thus making MRI well-suited to identify gross abnormalities within cartilage. However, the T2 dependence of articular cartilage is highly anisotropic and could potentially be a source of clinical misinterpretation.1–3
Ideally, more advanced MRI techniques could be used to detect early pathologic and/or biochemical changes in cartilage before gross morphologic changes manifest. If these early cartilage changes could be detected at a young age, modification of activities and/or specialized training programs could be instituted to help decrease the risk of further cartilage damage and its potential for progression to degenerative arthritis.
Traditionally, T2-weighted MRI has been routinely used to visualize cartilage degeneration. T2 relaxation reflects water movement in the cartilaginous matrix, and as damage to the cartilage promotes more free movement of water, T2 relaxation time is elevated.4 Although elevated T2 values have been attributed to gross degeneration,5,6 no observable correlation has been found between T2 relaxation time and specific extracellular matrix components, such as proteoglycan (PG).7
Development of MRI sequences and technologies may allow for early detection of pathologic changes within articular cartilage. Recent advances in MRI have increased our ability to analyze the microstructure of human cartilage.8–10 One technique, T1ρ magnetic resonance imaging (T1ρ MRI), assesses low-frequency interactions between hydrogen and structural macromolecules, a process termed spin lattice relaxation in the rotating frame. Early studies with the spin lock (SL) method by Jones,11 Santyr et al,12 and Sepponen et al13 have shown that the proton relaxation takes place along the locking field and it is not influenced by the much higher main magnetic field. The advantage of the SL technique is that the image contrast is characterized by the relaxation processes, which are effective at high field strengths, whereas the high signal-to-noise ratio is maintained by the main magnetic field. This phenomenon enables T1ρ MRI to be selectively sensitive to PG content in articular cartilage, the accurate correlation of which has been shown by several laboratory studies,14–16 animal studies,17 and studies using retrieved clinical specimens.16 Wheaton et al16 showed that T1ρ relaxation times of bovine and clinical osteoarthritic cartilage specimens degraded enzymatically highly correlated to fixed charge density from Na-23 and alcian blue–periodic acid–Schiff staining. Feasibility studies have also been completed to show that T1ρ MRI may be used as a clinical tool to assess cartilage integrity.18–22 Additionally, unlike T2 relaxation time, which varies with collagen content and orientation of fibers in the collagen matrix,3 T1ρ relaxation time is independent of collagen orientation and is sensitive to only PG variations.20
The constituents of articular cartilage, including collagen fibers, water, and PG, serve as the load-bearing material for joints in the human body.13 It has been shown that PG, which provides the compressive stiffness of articular cartilage, is a marker of cartilage health. Because its depletion has been associated with early pathologic changes in articular cartilage and osteoarthritis,23–25 the ability to measure PG content could permit detection of poor cartilage health, early cartilage damage, or early degenerative changes before these changes were allowed to progress to more advanced joint degeneration.
The purpose of this study was to determine whether T1ρ MRI could be applied in sports medicine to detect early changes in the articular cartilage of asymptomatic, collegiate-level, female impact compared with nonimpact athletes. We hypothesized that because of differences in impact profiles and training regimens, the impact athletes would exhibit differences in T1ρ relaxation time, signifying early cartilage changes when compared with nonimpact athletes.
METHODS
Study Design and Magnetic Resonance Imaging
Eleven impact (basketball players) and 10 nonimpact (swimmers) female NCAA Division I (collegiate-level) athletes, all training and participating on the active roster and within the season of their respective athletic schedule, were identified for this prospective, institutional review board (IRB)–approved cohort study. The average ages of the impact and nonimpact groups were 20.1 years (range, 18.7–21.8 years) and 20.2 years (range, 18.5–22.8 years), respectively.
Each athlete was questioned about her history of knee injuries or surgeries and if she currently had a knee injury or knee pain. If the athlete denied these 4 questions, they were considered asymptomatic and gave informed consent to participate in the study under an IRB-approved protocol. One impact athlete was removed from the study after an injury diagnosis. Each imaging study was performed less than 24 hours from the most recent athletic practice or refereed match. Magnetic resonance imaging was performed on a 3.0-T scanner (Siemens Medical Solutions, Melvern, Pennsylvania) equipped with a 7-channel knee coil. For imaging, routine 3 plane localizers were obtained to position the 2-dimensional T1ρ imaging plane. In each subject, axial and sagittal planes were identified to capture the patellofemoral joint. The pulse sequence for T1ρ imaging was provided by the University of Pennsylvania (Philadelphia, Pennsylvania).
T1ρ-weighted images were obtained using the sequence previously developed based on SL techniques and B1 inhomogeneities were minimized by using a rotatory echo technique as previously described.26,27 The acquisition parameters of our sequences are summarized in the Table. Spin lock range was limited to 36 ms to minimize imaging time, and to maximize T1ρ-weighted contrast, the SL amplitude coincided with T1ρ dispersion.28
TABLE.
Magnetic Resonance Imaging Parameters for Sagittal and Axial T1ρ
| Parameter | Sagittal T1ρ | Axial T1ρ |
|---|---|---|
| TR, ms | 2000 | 2000 |
| TE, ms | 8.6 | 8.6 |
| TSL, ms | 12, 24, 36 | 12, 24, 36 |
| Field of view, mm | 137 × 140 | 140 × 140 |
| Slice thickness, mm | 3 | 3 |
| Interslice gap, mm | 0.3 | 0.3 |
| Pixel matrix | 256 × 252 | 256 × 256 |
| SL frequency, Hz | 500 | 500 |
| Averages | 2 | 2 |
| Flip angle, degrees | 180 | 180 |
| Pixel bandwidth, Hz/pixel | 260 | 260 |
| Acquisition time, min | 2:22 | 2:22 |
TE, echo time; TR, repetition time; TSL, spin lock time.
The axial T1ρ-weighted images were prescribed on sagittal localizer images, covering regions from the top of the patellar cartilage to the femoral condylar cartilage. Axial cuts from the T1ρ-weighted MRI sequences were used in the data analysis for the patellar cartilage and sagittal cuts were used for the lateral and medial femoral cartilage.
Magnetic Resonance Evaluation
Gross examination of each participant’s magnetic resonance images was also performed by a musculoskeletal fellowship-trained radiologist and orthopaedic surgery resident masked to the athlete groups, to assess any macroscopic abnormalities within the cartilage matrix, according to the modified International Cartilage Repair Society (ICRS) classification system.
Computational Image Processing and Analysis
Absolute pixel-by-pixel T1ρ relaxation times were obtained by using the mathematical relationship between varying SL lengths and the T1ρ image pixel intensity, using the equation:29
| Eq. 1 |
where Sy, S0, and K are the components of transverse magnetization, equilibrium magnetization, and a constant, respectively.14
Using Eq. 1, data from 3 different SL lengths (12, 24, and 36 ms) were linearly fitted to the natural logarithm of signal intensity of corresponding pixels to derive T1ρ relaxation times (MATLAB, Version 7.8; Mathworks, Natick, Massachusetts). The quality of MRI acquisition was con-firmed by analyzing the regression coefficient of fitted data. Mean R2 values of analyzed cartilage exceeded 0.98 to 0.99. Region of interest (ROI) functions were used to select only articular cartilage in both sagittal and axial images. The axial images were used to select all patellar cartilage from the medial to lateral, whereas the sagittal images were used to select articular cartilage from the anterior to posterior (A-P) margins of the medial and lateral femoral condyles. During ROI selection, additional filtering was used to remove noise by removing data below 10 ms and above 100 ms. This filter was found not to exclude any meaningful cartilage pixels but enabled the removal of both noise, as noise pixels exhibited poor curve fits and, thus, either very high or very low T1ρ relaxation times, and pixels from surrounding tissues, such as bone or synovial fluid, exhibiting very low or very high T1ρ relaxation times, that were inadvertently included during ROI selection. All ROIs were selected and analyzed in triplicate and averaged to reduce subjectivity of ROI selection.
Articular cartilage on the medial and lateral femoral condyles was analyzed in 2 ways: (1) all cartilage A-P horns (A-P), and (2) the isolated central third weight-bearing (CWB) region. Patellar cartilage was analyzed in 4 ways: (1) all cartilage from medial to lateral (Full), (2) the medial third (Med), (3) the central third (Ctr), and (4) the lateral third (Lat) (Figure 1).
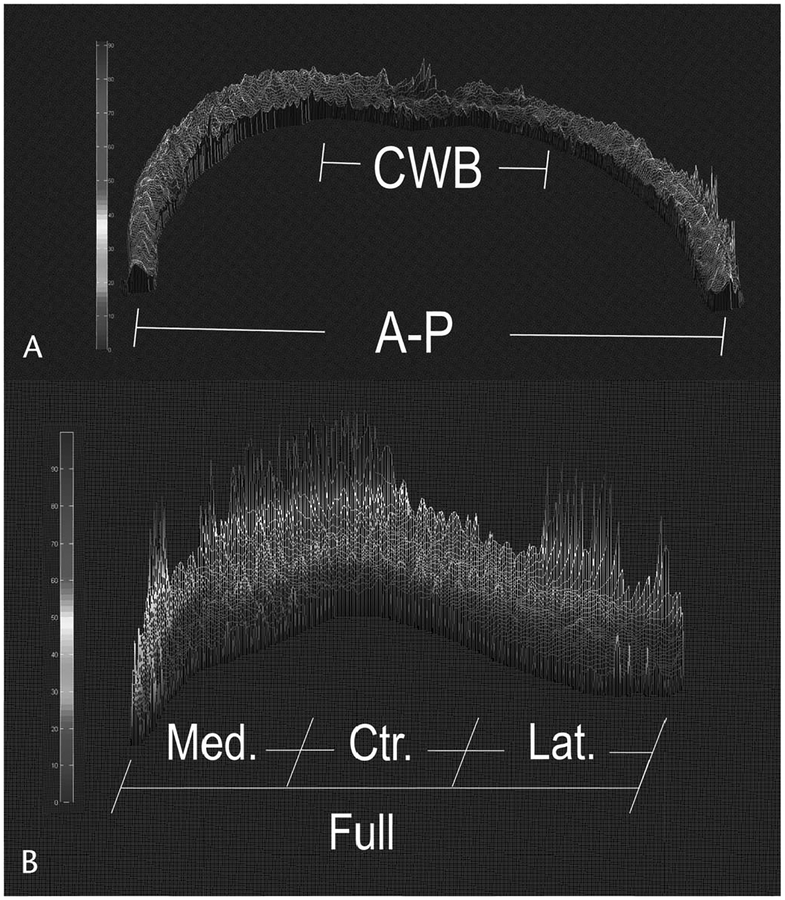
FIGURE 1.
A flow chart of the computational process for postimaging data analysis of medial and lateral femoral cartilage imaged in the sagittal plane (A) and patellar cartilage imaged in the axial plane (B). Color bars represent values from 0 to 100.
To analyze the variations in T1ρ relaxation times due to cartilage depth, quantitative depth analysis was performed by determining the physical height of cartilage in each column of pixels in the obtained parametric maps using the same ROIs as previous analyses outlined above. “Superficial” and “deep” (radial) zone data were collected by dividing the obtained height into 2 halves and collecting T1ρ values for each pixel in each half. This was done to maximize the number of pixels available for analysis. Although zones were not divided by histologic reference, Potter et al10 describe the superficial zone as 10% to 20% of the articular cartilage thickness and the transitional zones as 40% to 60% of the thickness. The deep/radial zone comprises 30% of the articular cartilage thickness.30 Each height of cartilage was divided in half; moving pixel-by-pixel across the width ensured the superficial layer and the superficial part of the transitional zone would be captured in the analyzed superficial layer. All remaining pixels would contain the deep part of the transitional zone and the entire radial zone. As the PG content of the transitional zone increases with increasing depth,10 analyzed cartilage in 2 depth-dependent halves was determined to show significant differences in T1ρ relaxation time between the 2 layers, while maximizing the number of pixels for statistical analysis. Depth analysis was performed in the CWB of medial and lateral femoral condyles and all zones of the patella.
Statistical Analysis
Normalized, average T1ρ relaxation times (ART) were statistically compared between the impact and nonimpact groups. Normalized ART of the superficial and deep layers were compared both within and between each cohort. Additionally, data sets with the ART of the patellar cartilage with macroscopic changes were compared against the ART of the patellar cartilage without macroscopic changes. All statistics used a 1-way analysis of variance and Dunn post hoc test (α = 0.05) (SigmaPlot, Version 11; SYSTAT, Chicago, Illinois).
RESULTS
Macroscopic Evaluation
Cartilage defects were graded in a blinded fashion according to the “modified ICRS cartilage classification”30 and demonstrated 4 findings in 4 of the participants in the impact athlete (basketball players) cohort, despite all participants presenting as asymptomatic. Specifically, 1 participant had a grade 1 patella cartilage defect in the lateral third (subject 9), 1 participant had diffuse cartilage thinning at the apex of the patella (subject 11), 1 participant had a grade 2 to 3 defect of the lateral third of the patella (subject 12), and 1 participant had a grade 3 defect at the patella apex (subject 19). These defects were individually analyzed and did not exhibit significantly different T1ρ relaxation times compared with the average of other patients. No gross structural defects were appreciated in the nonimpact athlete (swimmers) cohort.
Computational Analysis
Raw MRI data with varying SL time values were successfully converted into a graphical matrix indicating varying T1ρ relaxation times. Regional and depth-dependent differences in T1ρ relaxation times are evident in both 2-dimensional and 3-dimensional maps, which were used to demonstrate regional differences (Figure 2).
FIGURE 2.
Three-dimensional mesh plot of femoral articular cartilage (A) and patellar articular cartilage (B) showing distinct regional and zonal differences of T1ρ relaxation times. Color bar represents T1ρ relaxation times from 0 to 100 ms.
Patellar Cartilage
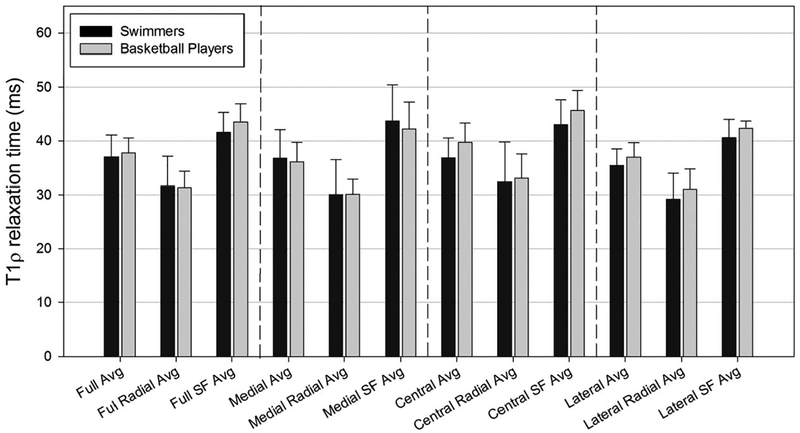
Using axial data acquired from the patellar cartilage parametric maps, ART between the 2 groups of athletes (basketball vs swimmers) were compared for multiple data sets. Statistical analysis indicated no significant differences in T1ρ relaxation time between each participant with a defect classified during macroscopic evaluation and the other participants in the impact cohort. Additionally, no significant differences were found between ART of full patellar or any defined patellar cartilage region (medial, lateral, or central) between cartilage of impact versus nonimpact athletes (Figure 3).
FIGURE 3.
Average T1ρ relaxation times of patellar cartilage zones in impact (basketball players) and nonimpact (swimmers) athletes. Avg, average; SF, superficial.
Lateral Femoral Cartilage
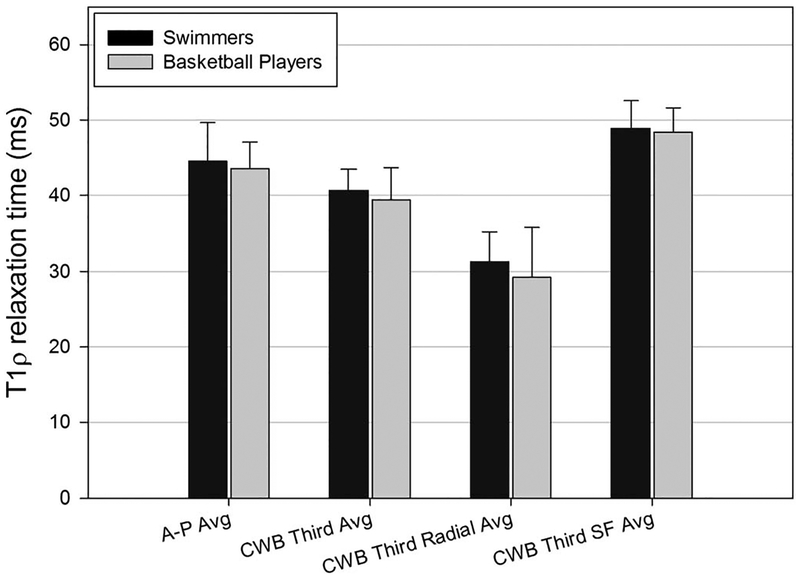
Data for the lateral compartment of the femoral cartilage were captured from the sagittal images and analyzed using a 1-way analysis of variance model. The ART of the A-P and CWB regions showed no significant difference between swimmers and basketball players. When lateral femoral cartilage was separated into superficial and radial zones for depth analysis, there was no significant difference between the 2 groups of athletes (Figure 4). There were, however, significant differences within the cartilage layers of each group. Within the swimmers’ cohort, the superficial layer of the CWB third region had significantly higher T1ρ relaxation time (48.90 ± 3.65 ms) than the radial layer of the CWB third region (31.31 ± 3.96 ms, P < 0.001). Similarly, within the basketball cohort, the superficial layer of the CWB third region had significantly higher T1ρ relaxation time (48.40 ± 3.21 ms) than the radial layer of the CWB third region (29.21 ± 6.63 ms, P < 0.001).
FIGURE 4.
Average T1ρ relaxation times of lateral femoral cartilage zones in impact (basketball players) and nonimpact (swimmers) athletes. Avg, average; SF, superficial.
Medial Femoral Cartilage
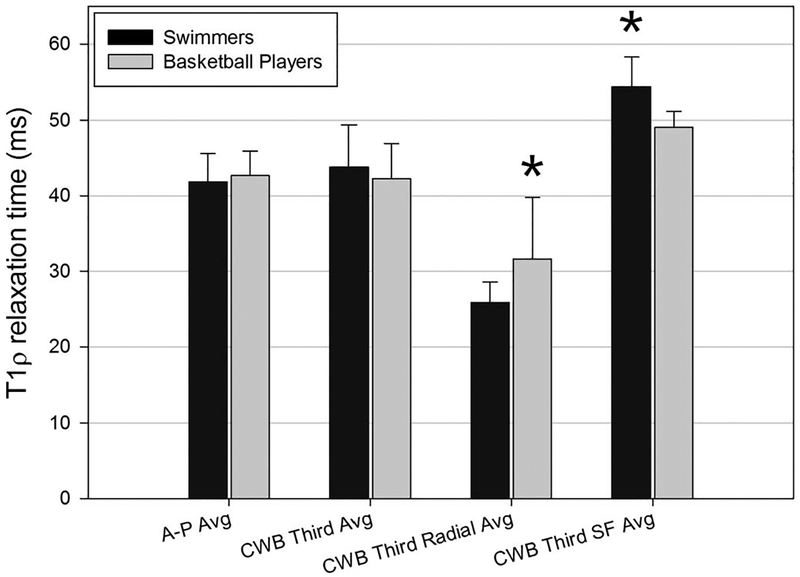
In the medial compartment of femoral cartilage, ART of the A-P and CWB regions also showed no significant difference between the 2 cohorts of athletes. Depth analysis in the medial compartment of the femoral cartilage showed several significant results. The T1ρ relaxation time of the radial zone of the CWB region of cartilage in basketball players (31.63 ± 8.11 ms) was significantly higher (P = 0.041) than the ART of the radial zone of cartilage in swimmers (25.85 ± 2.71 ms), relating to a lower PG content in the radial zone of the basketball players. In addition, the T1ρ average of the superficial zone in basketball players (49.05 ± 2.11 ms) was significantly lower (P = 0.003) than the superficial zone in swimmers (54.35 ± 3.92 ms) (Figure 5), relating to a higher PG content in the basketball players.
FIGURE 5.
Average T1ρ relaxation times of medial femoral cartilage zones in impact (basketball players) and nonimpact (swimmers) athletes. Avg, average; SF, superficial. Asterisk indicates significant difference at P < 0.05.
Within the swimming cohort, the superficial layer (54.35 ± 3.92 ms) was significantly higher (P < 0.001) than the radial layer (25.85 ± 2.71 ms). Similarly, within the basketball cohort, the superficial layer (49.05 ± 2.11 ms) was significantly higher (P < 0.001) than the radial layer (31.63 ± 8.11 ms).
DISCUSSION
Numerous studies have investigated changes in volume and thickness of articular cartilage of the knee to assess loading and overuse of articular cartilage and the likelihood of progressing to degenerative joint disease (DJD).31–33 Kessler et al34 have shown short-term volume changes in the patellar and tibial cartilage of marathon runners and the increased time to completely recover the volume, pointing to the effects of fatigue loading of cartilage during athletic activity, causing repetitive fluid extravasation, which results in inferior biomechanical properties and the potential for degradation because of adverse loading. Still, there is no proven link between physical behavior, the use of the knee cartilage, and DJD. Until recently, traditional imaging modalities, which can evaluate macrostructural cartilage changes, have been unable to identify early asymptomatic changes in articular cartilage. New imaging techniques are sensitive to differences in the distribution and concentration of matrix macromolecules of cartilage, namely collagen and PGs. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) offers quantitative assessment of PG content in cartilage and can be currently considered the standard in quantitative articular cartilage imaging.10 The dGEMRIC relies on an inverse relationship between gadolinium agent uptake and glycosaminoglycan (GAG) concentration in the cartilage extracellular matrix (ECM). Gadolinium inversely affects the T1 component of cartilage, enhancing the contrast in T1-weighted imaging and providing clinically useful information about relative concentrations of GAGs.35 However, dGEMRIC requires intravenous injections of a contrast agent, a period of exercise to facilitate uptake into the cartilage ECM, and a waiting period before imaging. Furthermore, the optimal level of exercise and waiting time have not been elucidated, presenting potential patient-to-patient variability and potentially skewed results. The T1ρ MRI assesses low-frequency interactions between hydrogen and PG in the cartilage, a process called spin lattice relaxation in the rotating frame, and does not rely on contrast agent or any periods of exercise or waiting.10 The T1ρ MRI thus has extensive potential as a clinical tool for assessing relative molecular changes in articular cartilage.
Our study sought to investigate whether specific biochemical differences in cartilage of 2 distinct groups of athletes could be discerned using T1ρ MRI, which has been shown to correlate with PG content in cartilage.14 Several of our findings can be compared with differences in PG content in articular cartilage between the 2 types of athletes. The radial zone of the CWB of medial femoral cartilage in basketball players exhibited a higher T1ρ relaxation time than the same zone in swimmers, indicating a lower PG content in the radial zone of basketball players. A similar effect was illustrated in the superficial zone, where swimmers exhibited a higher signal and, hence, a lower PG content in this zone. As such, our study shows that T1ρ MRI is effective in distinguishing differences in articular cartilage that cannot be seen using conventional MRI sequences. Whether these significant differences in signal can be correlated to sport-specific, early degenerative conditions have yet to be determined and warrant further investigation. However, these results demonstrate that the impact cohort had potentially degenerative changes originating from the radial zone and the nonimpact cohort illustrated degenerative changes deriving from the superficial zone of the CWB of the medial fem-oral cartilage. Differences in tibiofemoral and patellofemoral loading between knee joints of basketball players, who experience uniaxial loading of high magnitude during a predominately running exercise, and swimmers, who experience little high-magnitude loading but high-frequency tibiofemoral flexion/extension causing shear, is a possible explanation for our results. This implies the possibility of detecting early degenerative changes in articular cartilage before clinical diagnosis of DJD using conventional imaging techniques and also suggests differences in the pattern of progression of DJD as a function of sport. Therefore, our hypothesis was proven in the context of significant changes in the radial zone (basketball players), but disproven in the superficial zone, as the swimming cohort showed significant increases in T1ρ signal.
Four of the 10 impact athletes exhibited patellar articular cartilage defects on conventional MRI. To analyze whether these defects increased or decreased T1ρ signal, we analyzed whether the zones in which the defect was found were outliers compared with nondefect individuals, which was not the case in any of the 4 athletes. These defects did not significantly influence T1ρ relaxation time and therefore did not significantly affect the impact cohorts’ mean relaxation time. Although the precise nature of these defects is unknown, we conclude that T1ρ signal in the specific zone containing the defect was unaffected because the defect, while diagnosable on a single image, was not severe enough to adequately increase/decrease the mean T1ρ signal given the resolution of the sequence; the defect did not directly affect components of cartilage to which T1ρ imaging is sensitive (ie, water, PGs); or the change in T1ρ signal was so small in a few pixels that it did not statistically affect the mean T1ρ relaxation time of the entire zone.
Previous studies have used T1ρ to quantify changes in articular cartilage of athletes to detect degenerative conditions. Luke et al36 used 3-T T1ρ MRI to image the knee cartilage of asymptomatic athletes before and after running a marathon. Although gross changes could not be detected using conventional MRI, significant increases in T1ρ signal were measured for up to 3 months. The study also found most significant results in the medial compartment of the knee, a result consistent with significant findings in the medial compartment from our study. Several studies have shown that the tibiofemoral forces through the joint are primarily transferred through the medial compartment, with the medial compartment experiencing approximately 75% of the load and the remainder through the lateral compartment.37 A larger medial condyle and a medial shift in the mechanical axis when weight bearing in a single-leg position, especially during dynamic activity, have been suggested as possible explanations for this differential force distribution.38 Stehling et al39 have shown signal changes in the meniscus of athletes after the completion of a marathon, a further indication that T1ρ MRI is an effective tool for assessing orthopaedic soft tissues. Literature has shown that high-impact exercise may be beneficial in one knee compartment but detrimental in another. Vogelsong et al40 imaged 21 Division I basketball players using T1ρ, T2, and Na+ maps before and after the sport season and found that tibiofemoral cartilage exhibited a decrease in T1ρ relaxation time, potentially indicating PG deposition/increase, whereas Na+ imaging demonstrated a decrease in the patellofemoral joint, indicating a potential loss of PG content.
Study Limitations
Because analysis is dependent on the resolution of the raw magnetic resonance data, despite all imaging being performed on 3-T MRI, the inherent dimensions of cartilage limit the number of pixels available for analysis. During the depth analysis portion of the study, the zones were not obtained according to histologic references, but instead according to the accepted conventions of articular cartilage zones and thickness that may introduce a dilutive effect by, for example, inadvertently including pixels from the superficial layer in the data set of the deep layer, thereby skewing the results slightly. However, the accuracy of the depth analysis may be reaffirmed by the significant differences found between the T1ρ relaxation time of superficial and radial zones. The radial zone, with known higher PG content than superficial zones,41 consistently exhibited a lower signal (ie, higher PG) than the superficial zone. In addition, our pilot study was a small cohort of participants (n = 20) participating in only 2 sports. An expanded study investigating a spectrum of collegiate-level athletics and collection of supplementary data (ie, comparative fitness/training grading, lower extremity alignment analysis, ethnicity, functional measurements) would be useful in understanding the variables leading to early cartilage changes and possibly DJD.
CONCLUSIONS
T1ρ magnetic resonance imaging detected changes indicative of degeneration of the articular cartilage of asymptomatic collegiate female athletes, as assessed by T1ρ relaxation time. Differences were most apparent in the medial femoral condyles, where the impact athletes exhibited greater signal in the radial zone, whereas the nonimpact athletes exhibited greater signal in the superficial zone. Within each cohort, the participants demonstrated significantly greater relaxation times in the superficial zone compared with the radial zone.
This study builds on the body of literature evaluating T1ρ MRI in clinically applicable environments. Establishing the capabilities and validity of T1ρ MRI presents an opportunity to understand the life cycle of cartilage degeneration, from early changes to eventual destruction.
Clinically, it has been demonstrated that enrolling athletes with preexisting musculoskeletal issues into plyometric and intervention programs may provide techniques to slow or reduce early cartilage degeneration.42 Ultimately, asymptomatic young impact athletes, who have been identified as having early cartilage damage through the use of advanced MRI techniques, could be enrolled in exercise intervention programs composed of lower impact training segments to complement the required in-season practice regimens.
ACKNOWLEDGMENTS
The authors thank Walter Witschey, PhD, of the University of Pennsylvania for providing the T1ρ imaging technique; Oakland University (OU) and William Beaumont Hospital (WBH) for an OU-WBH Collaborative Research Grant. Y. Xia thanks National Institutes of Health for R01 Grants (AR 045172 and AR 052353).
Supported by National Institutes of Health for R01 Grants (AR 045172 and AR 052353).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Xia Y Averaged and depth-dependent anisotropy of articular cartilage by microscopic imaging. Semin Arthritis Rheum. 2008;37:317–327. [DOI] [PubMed] [Google Scholar]
- 2.Xia Y Resolution ‘scaling law’ in MRI of articular cartilage. Osteoarthritis Cartilage. 2007;15:363–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia Y Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol. 2000;35:602–621. [DOI] [PubMed] [Google Scholar]
- 4.Alhadlaq HA, Xia Y, Moody JB, et al. Detecting structural changes in early experimental osteoarthritis of tibial cartilage by microscopic magnetic resonance imaging and polarised light microscopy. Ann Rheum Dis. 2004;63:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology. 2000;214:259–266. [DOI] [PubMed] [Google Scholar]
- 6.Dunn TC, Lu Y, Jin H, et al. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieminen MT, Toyras J, Rieppo J, et al. Quantitative MR microscopy of enzymatically degraded articular cartilage. Magn Reson Med. 2000;43: 676–681. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe A, Boesch C, Obata T, et al. Effect of multislice acquisition on T1 and T2 measurements of articular cartilage at 3T. J Magn Reson Imaging. 2007;26:109–117. [DOI] [PubMed] [Google Scholar]
- 9.Shindle MK, Foo LF, Kelly BT, et al. Magnetic resonance imaging of cartilage in the athlete: current techniques and spectrum of disease. J Bone Joint Surg Am. 2006;88(suppl 4):27–46. [DOI] [PubMed] [Google Scholar]
- 10.Potter H, Black B, Chong L. New techniques in articular cartilage imaging. Clin Sports Med. 2009;28:77–94. [DOI] [PubMed] [Google Scholar]
- 11.Jones G Spin-lattice relaxation in the rotating frame: weak-collision case. Phys Rev. 1966;148:332–335. [Google Scholar]
- 12.Santyr G, Henkelman R, Bronskill M, et al. Spin locking for magnetic resonance imaging with application to human breast. Magn Reson Med. 1989;12:25–37. [DOI] [PubMed] [Google Scholar]
- 13.Sepponen R, Pohjonen J, Sipponen J, et al. A method for T1 rho imaging. J Comput Assist Tomogr. 1985;9:1007–1011. [DOI] [PubMed] [Google Scholar]
- 14.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–423. [DOI] [PubMed] [Google Scholar]
- 15.Duvvuri U, Reddy R, Patel S, et al. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997; 38:863–867. [DOI] [PubMed] [Google Scholar]
- 16.Wheaton AJ, Casey FL, Gougoutas AJ, et al. Correlation of T1rho with fixed charge density in cartilage. J Magn Reson Imaging. 2004; 20:519–525. [DOI] [PubMed] [Google Scholar]
- 17.Wheaton AJ, Dodge GR, Borthakur A, et al. Detection of changes in articular cartilage proteoglycan by T(1rho) magnetic resonance imaging. J Orthop Res. 2005;23:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Han ET, Ma CB, et al. In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005;54:929–936. [DOI] [PubMed] [Google Scholar]
- 19.Regatte RR, Akella SV, Lonner JH, et al. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23:547–553. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regatte RR, Akella SV, Wheaton AJ, et al. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11:741–749. [DOI] [PubMed] [Google Scholar]
- 22.Regatte RR, Akella SV, Borthakur A, et al. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388–1394. [DOI] [PubMed] [Google Scholar]
- 23.Grushko G, Schneiderman R, Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tissue Res. 1989;19:149–176. [DOI] [PubMed] [Google Scholar]
- 24.Mankin HJ, Johnson ME, Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. III. Distribution and metabolism of amino sugar-containing macromolecules. J Bone Joint Surg Am. 1981;63:131–139. [PubMed] [Google Scholar]
- 25.van de Loo AA, Arntz OJ, Otterness IG, et al. Proteoglycan loss and subsequent replenishment in articular cartilage after a mild arthritic insult by IL-1 in mice: impaired proteoglycan turnover in the recovery phase. Agents Actions. 1994;41:200–208. [DOI] [PubMed] [Google Scholar]
- 26.Borthakur A, Wheaton A, Charagundla SR, et al. Three-dimensional T1ρ‐ weighted MRI at 1.5 Tesla. J Magn Reson Imaging. 2003;17:730–736. [DOI] [PubMed] [Google Scholar]
- 27.Charagundla SR, Borthakur A, Leigh JS, et al. Artifacts in T1ρ-weighted imaging: correction with a self-compensating spin-locking pulse. J Magn Reson. 2003;162:113–121. [DOI] [PubMed] [Google Scholar]
- 28.Witschey WR, Borthakur A, Elliott MA, et al. Artifacts in T1ρ-weighted imaging: compensation for B1and B0 field imperfections. J Magn Reson. 2007;186:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rommel E, Kimmich R. Volume-selective determination of the spin-lattice relaxation time in the rotating frame T1 rho, and T1 rho imaging. Magn Reson Med. 1989;12:209–218. [DOI] [PubMed] [Google Scholar]
- 30.Potter H, Foo L. Magnetic resonance imaging of articular cartilage: trauma, degeneration and repair. Am J Sports Med. 2006;34:661–677. [DOI] [PubMed] [Google Scholar]
- 31.Cicuttini FM, Wluka AE, Wang Y, et al. Compartment differences in knee cartilage volume in healthy adults. J Rheumatol. 2002;29:554–556. [PubMed] [Google Scholar]
- 32.Gandy S, Dieppe P, Keen M, et al. No loss of cartilage volume over three years in patients with knee osteoarthritis as assessed by magnetic resonance imaging. Osteoarthritis Cartilage. 2002;10:929–937. [DOI] [PubMed] [Google Scholar]
- 33.Karvonen R, Negendank W, Teitge R, et al. Factors affecting articular cartilage thickness in osteoarthritis and aging. J Rheumatol. 1994;21: 1310–1318. [PubMed] [Google Scholar]
- 34.Kessler M, Glaser C, Tittel S, et al. Recovery of the menisci and articular cartilage of runners after cessation of exercise. Am J Sports Med. 2008; 36:966–970. [DOI] [PubMed] [Google Scholar]
- 35.Williams A, Gillis A, McKenzie C, et al. Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): potential clinical applications. AJR Am J Roentgenology. 2004;182:167–172. [DOI] [PubMed] [Google Scholar]
- 36.Luke AC, Stehling C, Stahl R, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: does long-distance running lead to cartilage damage? Am J Sports Med. 2010;38:2273–2280. [DOI] [PubMed] [Google Scholar]
- 37.Hsu RW, Himeno S, Coventry MB, et al. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop Relat Res. 1990;215–227. [PubMed] [Google Scholar]
- 38.Schipplein O, Andriacchi T. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–119. [DOI] [PubMed] [Google Scholar]
- 39.Stehling C, Luke A, Stahl R, et al. Meniscal T1rho and T2 measured with 3.0T MRI increases directly after running a marathon. Skeletal Radiol. 2011;40:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogelsong M, Pappas G, Staroswiecki E, et al. Are sports good for your knees? An MRI evaluation of the effects of basketball on knee health in Division I collegiate athletes. Proc Intl Soc Mag Reson Med. 2011;19: 507. [Google Scholar]
- 41.Bullough P, Goodfellow J. The significance of the fine structure of articular cartilage. J Bone Joint Surg Br. 1968;50:852–857. [PubMed] [Google Scholar]
- 42.Hewett T, Stroupe A, Nance T, et al. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24:765–773. [DOI] [PubMed] [Google Scholar]