Summary
WHIM syndrome (warts, hypogammaglobulinemia, infections, and myelokathexis), a primary immunodeficiency disorder involving panleukopenia, is caused by autosomal dominant gain-of-function mutations in CXC chemokine receptor 4 (CXCR4). Myelokathexis is neutropenia caused by neutrophil retention in bone marrow. Patients with WHIM syndrome are often treated with granulocyte colony-stimulating factor (G-CSF), which can increase neutrophil counts but does not affect cytopenias other than neutropenia. In this investigator-initiated, open-label study, three severely affected patients with WHIM syndrome who could not receive G-CSF were treated with low-dose plerixafor, a CXCR4 antagonist, for 19 to 52 months. Myelofibrosis, panleukopenia, anemia, and thrombocytopenia were ameliorated, the wart burden and frequency of infection declined, human papillomavirus–associated oropharyngeal squamous-cell carcinoma stabilized, and quality of life improved markedly. Adverse events were mainly infections attributable to the underlying immunodeficiency. One patient died from complications of elective reconstructive surgery. (Funded by the National Institutes of Health.)
CXC CHEMOKINE RECEPTOR 4 (CXCR4), WHICH BINDS CXC CHEMOKINE ligand 12 (CXCL12), is expressed on most leukocyte subsets and regulates leukocyte development and trafficking, among other activities.1 In WHIM syndrome (warts, hypogammaglobulinemia, infections, and myelokathexis), autosomal dominant gain-of-function CXCR4 mutations impair CXCL12-induced receptor down-regulation, thereby increasing CXCR4 signaling.2,3 Hematologic consequences include myelokathexis and defective early B-cell and T-cell development, which result in panleukopenia, abnormal architecture of secondary lymphoid tissue, and immuno-deficiency.4–8 Patients typically present with recurrent bacterial infections, usually in the otosinopulmonary tract and skin,4,9 and skin or anogenital warts that are refractory to conventional treatments and that may progress to human papilloma-virus (HPV)–associated squamous-cell carcinoma.4,9–13
Treatment includes granulocyte colony-stimulating factor (G-CSF) and immunoglobulin replacement; however, long-term efficacy remains undefined.4,9 Moreover, G-CSF does not correct monocytopenia, lymphopenia, and hypogammaglobulinemia, and disabling bone pain and hematopathologic conditions may occur as a consequence of its use.10 Here, we evaluate the CXCR4 antagonist plerixafor as a mechanism-based treatment in patients with WHIM syndrome who cannot receive G-CSF.
Methods
Medication
Plerixafor (also called AMD3100; brand name, Mozobil) is a parenterally administered small-molecule competitive antagonist of CXCR4 with a half-life of approximately 5 hours.14 Plerixafor increases circulating levels of mature and immature leukocytes10,15,16 and is Food and Drug Administration–approved in combination with G-CSF for hematopoietic stem-cell mobilization for transplantation in patients with multiple myeloma or non-Hodgkin’s lymphoma.
We have previously reported the results of our phase 1 trial of plerixafor (ClinicalTrials.gov number, NCT00967785) involving patients with WHIM syndrome. This investigator-initiated study was approved by the National Institute of Allergy and Infectious Diseases (NIAID) and the NIAID institutional review board and is overseen by the NIAID Division of Clinical Research. Genzyme (and later Sanofi after acquisition of Genzyme) has provided plerixafor for this protocol since 2011 under a Research Support Agreement. In our earlier report, we found that plerixafor durably increased circulating neutrophil, lymphocyte, and monocyte counts for 6 months in three patients with WHIM syndrome; no dose-limiting toxic effects or side effects were noted.16 Accordingly, we designed a randomized, double-blind, phase 3 trial of G-CSF versus plerixafor to assess clinical efficacy and to acquire additional safety information (NCT02231879).
During recruitment, we identified three patients with advanced disease who were ineligible for the phase 3 trial because they could not receive G-CSF. We therefore treated these patients with open-label plerixafor according to the phase 1 protocol. All patients provided written informed consent. Sanofi approved all changes to the protocol and consent documents, which mostly involved amendments to extend the duration of treatment, and the company received this article before publication and provided comments. (The original protocol, final protocol, and summary of protocol changes are available with the full text of this article at NEJM.org.) One patient (Patient P1 in our previous report) was enrolled 22 days before registration of the phase 1 trial on ClinicalTrials.gov, in conformance with National Institutes of Health regulations and instruction and as explained in further detail in the Methods section in the Supplementary Appendix (available at NEJM.org). All other patients, including the three patients we report here, were enrolled after trial registration.
Study Assessments
Clinical laboratory assessments were performed in accordance with Clinical Laboratory Improvement Amendments standards. HPVs and polyoma-viruses were identified in skin swabs by rolling-circle amplification and next-generation DNA sequencing, as reported previously.17 Relatedness of adverse events to treatment was determined by the first author, in consultation with three other authors.
Phenotypes of the Patients
Patient 1 was a previously unreported man from Portugal who received a diagnosis of WHIM syndrome at 19 years of age on the basis of myelokathexis, recurrent infections, and a heterozygous frameshift mutation designated CXCR4 p.Ser324Val fsX20.11 At 4 years of age, he underwent splenectomy for presumptive autoimmune neutropenia. This procedure did not modify the severity of neutropenia. Since the patient was 14 years of age, the clinical course had been dominated by chronic, intensely pruritic folliculitis of the lower legs that was complicated by recurrent acute cellulitis and progressive, multifocal, painful, chronic inflammatory lesions that limited ambulation to approximately 2 minutes. He was receiving immunoglobulin replacement and G-CSF (Zarxio, Sandoz) therapy, but myelofibrosis, anemia, and severe thrombocytopenia developed by 20 years of age. After his daily 300-μg dose of G-CSF was reduced to 150 μg every other day given only intermittently during infections, the platelet count normalized (Fig. 1), but severe neutropenia recurred (Fig. S1 in the Supplementary Appendix) and hospitalizations for lower-leg cellulitis became more frequent. Bone marrow transplantation was considered, but a suitable donor was not identified.
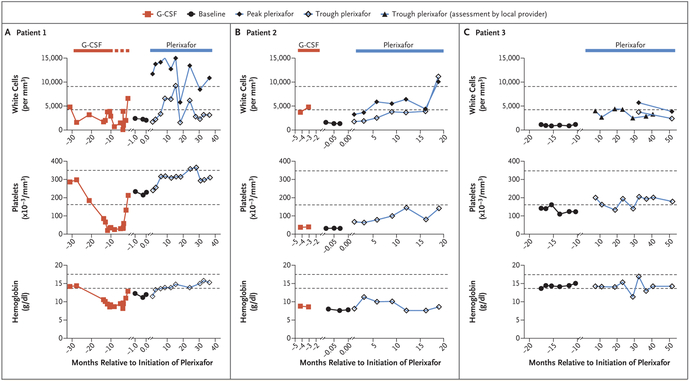
Figure 1. Hematologic Improvement during Long-Term, Low-Dose Plerixafor Treatment.
The periods of treatment with granulocyte colony-stimulating factor (G-CSF) and plerixafor are demarcated by the red and blue bars at the top of each corresponding column of graphs. The dotted portion of the red line for Patient 1 denotes the time when high-dose G-CSF treatment (300 μg subcutaneously every day) was interrupted owing to severe thrombocytopenia and the drug was given at a reduced dose (150 μg subcutaneously every other day) only during episodes of cellulitis. Horizontal dashed black lines in each graph designate the upper and lower limits of the normal range for each variable, as determined by the National Institutes of Health (NIH) Clinical Center Clinical Hematology Laboratory. Baseline values were assessed when neither drug was being taken (solid circles). All values for Patients 1 and 2 at baseline and during plerixafor treatment were determined at the NIH. Peak refers to values obtained approximately 3 hours after plerixafor administration, the time of the peak white-cell count, as defined previously.10 Trough refers to values obtained approximately 12 hours after a dose of plerixafor was administered. Most white-cell values for Patient 3 were determined at trough by the local provider in Germany owing to travel limitations; exceptions are designated by the peak and trough symbols in the figure key, and these values were determined at the NIH. Values during G-CSF treatment were obtained for Patients 1 and 2 at trough (defined as just before a dose was administered) by the local provider and reported to the NIH.
Patient 2 was a 47-year-old man from Chicago and a member of a previously reported three-generation family with WHIM syndrome.2 The clinical diagnosis was made at 30 years of age on the basis of warts, hypogammaglobulinemia, myelokathexis, and recurrent infections since childhood, and a CXCR4R334X mutation was identified at 35 years of age. Past medical conditions included recurrent otosinopulmonary infections (complicated by hearing loss), skin abscesses, severe periodontitis resulting in complete tooth loss, and a malar Epstein–Barr virus–associated B-cell lymphoma at 30 years of age in complete remission after chemotherapy.18 Warts appeared on the patient’s hands and feet in childhood and progressed at these locations.
When Patient 2 was 35 years of age, HPV type 16 (HPV-16)–associated head and neck squamous-cell carcinoma was diagnosed, and over a period of 10 years he underwent disfiguring surgery and received radiation therapy and chemotherapy.19 Complications included dysphagia (resulting in gastric-tube feeding) and right mandibular osteoradionecrosis and osteomyelitis (the latter was suppressed with long-term ciprofloxacin and amoxicillin–clavulanate). A tracheostomy was performed. Head and neck cancer recurred at 45 years of age. He was receiving G-CSF at a dose of 300 μg daily, which maintained the absolute neutrophil count at more than 500 cells per cubic millimeter, but anemia and severe thrombocytopenia developed. He was not a candidate for bone marrow transplantation because of a poor prognosis.
Patient 3 was a 52-year-old man from Germany who received a diagnosis of WHIM syndrome at 51 years of age on the basis of myelokathexis, hypogammaglobulinemia, recurrent infections, persistent warts since 29 years of age involving mainly the hands and forearms, condyloma acuminatum, anal Bowen’s disease (squamous-cell carcinoma in situ), and a CXCR4R334X mutation. Frequent otosinopulmonary infections resulted in partial hearing loss and bronchiectasis. He received G-CSF at 25 years of age but declined it thereafter because he had severe bone pain. He was hospitalized frequently for pneumonia despite receiving monthly immunoglobulin and prophylactic trimethoprim–sulfamethoxazole.
Results
Hematologic and Immunologic Variables
G-CSF was discontinued by Patients 1 and 2 and was not being taken by Patient 3. After the establishment of baseline complete blood counts, plerixafor was injected subcutaneously at a dose of 0.01 to 0.02 mg per kilogram of body weight twice daily by Patients 1, 2, and 3 for 36, 19, and 52 months, respectively. Results of the first 6 months have been reported previously for Patient 3 (designated Patient P3 in that trial).16 Approximately 1.5 years later, he resumed plerixafor treatment at the same dose.
Within 6 weeks, anemia and severe thrombocytopenia were ameliorated in Patient 1 after daily G-CSF was discontinued, and levels of red cells and platelets stabilized in the normal range during plerixafor treatment. Similarly, the severity of thrombocytopenia in Patient 2 decreased markedly after G-CSF was discontinued and plerixafor was initiated 3 days later; however, early increases in levels of red cells were not sustained. Patient 3 had mild thrombocytopenia, and higher platelet counts were sustained during plerixafor treatment (Fig. 1).
Over the course of treatment, the peak total white-cell count after plerixafor administration exceeded both the trough and baseline values in all three patients (Fig. 1), mainly owing to increased levels of lymphocytes in Patients 1 and 2 and of neutrophils in Patient 3 (Fig. S1 in the Supplementary Appendix). The oscillation between peak and trough values is a biomarker indicating sustained plerixafor bioavailability and bioactivity. The absolute neutrophil count showed little if any oscillation as the drug was cleared between doses, a finding consistent with our previous report.16 Circulating total and memory B-cell and T-cell subsets were increased by plerixafor treatment in all patients (Fig. S2A through S2D in the Supplementary Appendix), whereas natural killer and natural killer T cells were less responsive (Fig. S2C in the Supplementary Appendix).
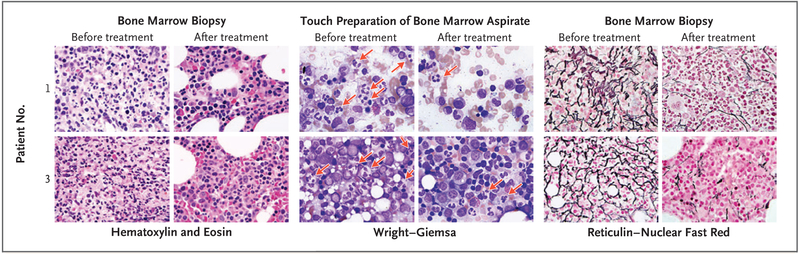
Sequential bone marrow–biopsy specimens that were obtained from Patients 1 and 3 revealed severe pretreatment myelofibrosis and myelokathexis that decreased markedly during plerixafor treatment (Fig. 2). Immunoglobulin replacement was discontinued by Patients 1 and 3 before they started plerixafor. The IgG level of Patient 1 remained stable, and levels of IgA and IgM increased; in Patient 3, immunoglobulins declined to stable lower levels. Patient 2 had never received immunoglobulin therapy despite a low IgG level, which remained low during plerixafor treatment (Fig. S3 in the Supplementary Appendix).
Figure 2. Amelioration of Myelokathexis and Myelofibrosis during Long-Term, Low-Dose Plerixafor Treatment.
Core bone marrow–biopsy samples were obtained from Patients 1 and 3 approximately 3 days before starting plerixafor (before treatment) and 24 and 52 months after starting plerixafor (after treatment) for Patients 1 and 3, respectively. Pretreatment hematoxylin and eosin–stained biopsy samples from both patients show markedly hypercellular marrow with granulocytic hyperplasia, right-shifted myelopoiesis, an elevated myeloid-to-erythroid ratio of approximately 5:1, and abundant neutrophils consistent with myelokathexis. This pattern was found in approximately 90% of the pretreatment marrow but in only 40 to 50% of the post-treatment marrow. The post-treatment images depict areas of normocellular marrow with normal myelopoiesis and a normal myeloid-to-erythroid ratio of 2:1. The pretreatment touch preparations of bone marrow aspirate (Wright–Giemsa stain) show frequent atypical neutrophils with pyknotic nuclear segments connected by thin, wispy strands of chromatin that are characteristic of myelokathexis (red arrows). These neutrophils can still be seen in the post-treatment samples but are less frequent. Both patients had severe myelofibrosis as defined by pretreatment dense reticulin staining of bone marrow; myelofibrosis was ameliorated after plerixafor treatment. All images are at 1000× magnification.
HPV-Associated Disease
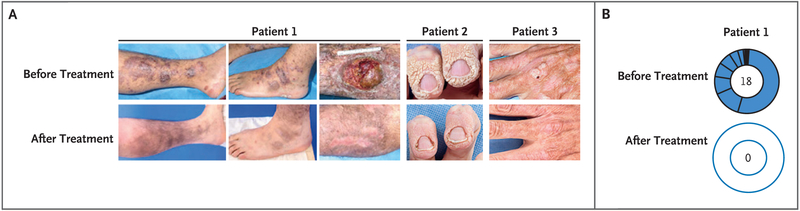
In addition to plerixafor, Patients 2 and 3 received imiquimod for 12 and 6 weeks, respectively. Patient 2 also received both the HPV quadrivalent and HPV 9-valent vaccines during plerixafor treatment. The wart burden of Patient 2 decreased markedly on the hands (Fig. 3A, and Fig. S4 in the Supplementary Appendix) and feet. In addition, two HPV-16–associated head and neck cancer lesions in the anterior maxilla resolved. A remaining posterior pharynx tumor enlarged slowly but was controlled by periodic debulking. The genital warts of Patient 3 completely resolved within 6 months, as reported previously16; by 12 months, the cutaneous wart burden was reduced markedly on the dorsum of the hands but not the palms (Fig. 3A, and Fig. S4 in the Supplementary Appendix). The Bowen’s disease lesion was treated surgically.
Figure 3. Amelioration of Skin Pathologic Conditions during Long-Term, Low-Dose Plerixafor Treatment.
Panel A shows the left medial lower leg, left foot, and right medial lower leg of Patient 1 both before starting plerixafor and after 36, 36, and 28 months of plerixafor therapy for the left, middle, and right post-treatment images, respectively. The fingers of Patient 2 are shown before plerixafor was started and after 9 months of treatment, and the hand of Patient 3 is shown before plerixafor was started and after32 months of treatment. Patient 1 had chronic eczematoid dermatitis associated with recurrent cellulitis for 6 years. The left and middle post-treatment images of this patient show resolution of inflammation, with residual areas of hyperpigmentation probably representing uncleared hemosiderin; recurrent cellulitis ceased. The right pretreatment image of this patient shows a chronic inflammatory mass centered at a site of recurrent cellulitis and saphenous-vein insufficiency that was removed surgically 6 months after plerixafor was started. The surgical wound (16×10×2 cm) healed completely (right post-treatment image). Patients 2 and 3 had a reduced cutaneous wart burden after plerixafor therapy. Both patients also received topical imiquimod, and Patient 2 received human papillomavirus (HPV) vaccination during treatment. (For photographs of the hands of Patients 2 and 3 before and after plerixafor treatment, see Fig. S4 in the Supplementary Appendix.) Panel B shows clearance of 17 HPVs and Trichodysplasia spinulosa polyomavirus in Patient 1 after 18 months of plerixafor therapy. The relative abundance of each of the viruses detected is conveyed by the arc length on the donut plot. The number in the center is the number of different HPV types plus polyomavirus species detected (see Table S1 in the Supplementary Appendix for quantitative details and virus names). Patient 1 was not given imiquimod or other treatments for HPV. He had received HPV quadrivalent vaccination before starting plerixafor and HPV 9-valent vaccination 2 years after starting plerixafor. Those vaccines do not include any HPV types recovered from the patient.
Susceptibility to Infection
Patient 1 had a mixture of inflammatory bilateral lower-leg skin lesions, including pruritic folliculitis, and tender violaceous raised and ulcerated chronic inflammatory lesions (Fig. 3A). Histo-pathological findings were most consistent with a chronic eczematoid and inflammatory process (data not shown). Warts were absent, yet molecular analysis of lesion swabs before plerixafor therapy revealed 17 HPVs in variable amounts (6 β types and 11 γ types, including the known HPV types 36, 107, 115, 122, 150, and 156; 1 β type and 10 γ types were novel)20 (Fig. 3B, and Table S1 in the Supplementary Appendix). Two years previously he had received the HPV quadrivalent vaccine, which does not include any of these types. Trichodysplasia spinulosa polyomavirus (TSPyV), a rare polyomavirus associated with folliculitis,21 was also detected with an average read depth of 50. The half maximal effective concentration for TSPyV neutralization in serum occurred at a dilution of 1:85,000. During plerixafor treatment, most lower-leg lesions and associated cellulitis resolved within several months, pruritus decreased markedly, and the 17 HPVs and TSPyV became undetectable (Fig. 3B, and Table S1 in the Supplementary Appendix).
At 6 months after plerixafor was started, a large inflammatory and vascular pretibial mass persisted, which was associated with right great-saphenous-vein insufficiency and recurrent cellulitis at this site. The vein and mass were removed surgically, with complete healing of the surgical wound (16×10×2 cm) over a period of 18 months (Fig. 3A), and there was only one subsequent episode of cellulitis, at the groin venectomy site. Pain on ambulation resolved, and the patient was able to engage in high-intensity activities (soccer and hiking). One episode of candidal intertrigo and several episodes of otitis externa were treated topically. He continued to receive plerixafor therapy.
Patient 2 had one episode of otitis externa during plerixafor treatment and had markedly improved quality of life. However, mandibular osteomyelitis persisted with pain and fistula development. Because his condition had otherwise improved, a 9-hour operation was performed for débridement and facial reconstruction. Neutropenia did not occur, but he had postoperative bone graft and skin flap failure, ventilator-associated pneumonia with multidrug-resistant Pseudomonas aeruginosa, and sepsis, and he died 19 months after starting plerixafor, 6 weeks after the surgery.
Patient 3 had eight infections over a period of 52 months while receiving plerixafor: two upper-airway and four lower-airway infections, one episode of conjunctivitis, and one episode of cervical lymphadenitis, none of which resulted in hospitalization. This represents an 85% lower frequency of infection than his pretreatment norm.16 He reported a markedly improved quality of life, was able to work and enjoy outdoor exercise, and was no longer fearful of recurrent infection and hospitalization. He continued to receive plerixafor.
Safety and Adverse Events
Most adverse events were infections attributable to the underlying immunodeficiency, but with reduced frequency and severity (Table S2 in the Supplementary Appendix). Renal and hepatic toxic effects were not observed (Fig. S5 in the Supplementary Appendix), and bone pain was not reported.
Discussion
We describe the CXCR4 antagonist plerixafor as a mechanism-based therapy for three patients with WHIM syndrome who could not receive G-CSF. There was a reduction in the frequency of infection in all three patients; resolution of chronic, progressive, multifocal eczematoid and follicular lesions in Patient 1, associated with clearance of TSPyV and 17 HPV types; a reduction in the wart burden in Patients 2 and 3; and a partial response of head and neck squamous-cell carcinoma in Patient 2. All three patients reported improved quality of life.
Because Cxcr4 deficiency is lethal in mice22 and because plerixafor is approved only for mobilizing hematopoietic stem cells, our goal was to normalize excessive CXCR4 signaling with the lowest dose that sustained hematologic improvement and a trough absolute neutrophil count of approximately 500 cells per cubic millimeter.16 The required dose was approximately 10% of the stem-cell mobilization dose and was not associated with bone pain or other adverse events attributable to the drug. The long treatment duration in these three patients exceeds the duration of plerixafor treatment in other experimental settings.16
In the three patients in our study, there was not a marked loss of apparent benefit; however, responsiveness varied somewhat among leukocyte subsets and among patients. Lymphocytes were most responsive in Patients 1 and 2, findings that are consistent with previously published results,10,15,16 but neutrophils were most responsive in Patient 3. The responding lymphocytes included naive and memory B-cell and T-cell subsets, whereas natural killer cells and natural killer T cells responded less well. Cell-count oscillation over time after each dose was prominent for lymphocytes and monocytes but not for neutrophils. Explanations for these differences are lacking but could involve patient-specific and cell-type–specific variation in CXCR4 expression and function. Mechanisms that have been proposed for plerixafor-induced leukocyte mobilization are controversial and include blockade of three CXCR4-dependent factors: leukocyte retention in bone marrow and other immune organs, neutrophil retention in the lungs, and the homing of circulating leukocytes to bone marrow.23–25 Plerixafor-induced hematopoietic stem-cell mobilization and seeding of extramedullary sites might also contribute, although the low dose makes this unlikely. Circulating CD34+ cells were not measured.
Unlike panleukopenia, anemia and severe thrombocytopenia are not features of WHIM syndrome, and they were most likely caused in Patients 1 and 2 by high-dose G-CSF treatment analogous to granulocyte–macrophage colony-stimulating factor, which has been reported to cause reversible myelofibrosis in one patient.26 Mild thrombocytopenia has been reported in patients with WHIM syndrome and was observed in Patient 3, who had not received G-CSF within 25 years before the present study yet also presented with myelofibrosis.6 The marked benefit with respect to both myelokathexis and myelofibrosis after plerixafor treatment in Patients 1 and 3 (Patient 2 was not evaluated) suggests a pathogenetic relationship.
Further controlled assessment of the safety and efficacy of plerixafor in patients with WHIM syndrome is a challenge because the disease is extremely rare.4 Nevertheless, our phase 3 trial of G-CSF versus plerixafor in WHIM syndrome is designed to permit this assessment.
Supplementary Material
Acknowledgments
Supported by the Divisions of Intramural Research of the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (NIH), and the Clinical Center of the NIH.
We thank the patients and their families for participating in this research, Harry L. Malech, M.D., for patient referrals and helpful advice, and Sergio D. Rosenzweig, M.D., Ph.D., for help with leukocyte flow cytometry.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
References
- 1.Murphy PM, Heusinkveld L. Multisystem multitasking by CXCL12 and its receptors CXCR4 and ACKR3. Cytokine 2018; 109: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez PA, Gorlin RJ, Lukens JN, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet 2003; 34: 70–4. [DOI] [PubMed] [Google Scholar]
- 3.McDermott DH, Lopez J, Deng F, et al. AMD3100 is a potent antagonist at CXCR4(R334X), a hyperfunctional mutant chemokine receptor and cause of WHIM syndrome. J Cell Mol Med 2011; 15: 2071–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai T, Malech HL. WHIM syndrome: congenital immune deficiency disease. Curr Opin Hematol 2009; 16: 20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tassone L, Notarangelo LD, Bonomi V, et al. Clinical and genetic diagnosis of warts, hypogammaglobulinemia, infections, and myelokathexis syndrome in 10 patients. J Allergy Clin Immunol 2009; 123: 1170–3. [DOI] [PubMed] [Google Scholar]
- 6.McDermott DH. Warts, hypogammaglobulinemia, infections, and myelokathexis syndrome In: Sullivan KE, Stiehm ER, eds. Stiehm’s immune deficiencies. London: Elsevier/Academic Press, 2014: 711–9. [Google Scholar]
- 7.Balabanian K, Brotin E, Biajoux V, et al. Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood 2012; 119: 5722–30. [DOI] [PubMed] [Google Scholar]
- 8.Janas ML, Turner M. Stromal cell-derived factor 1α and CXCR4: newly defined requirements for efficient thymic β-selection. Trends Immunol 2010; 31: 370–6. [DOI] [PubMed] [Google Scholar]
- 9.Heusinkveld LE, Yim E, Yang A, et al. Pathogenesis, diagnosis and therapeutic strategies in WHIM syndrome immuno-deficiency. Expert Opin Orphan Drugs 2017; 5: 813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott DH, Liu Q, Ulrick J, et al. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood 2011; 118: 4957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaussant Cohen S, Fenneteau O, Plouvier E, et al. Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet J Rare Dis 2012; 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, Fenyk JR Jr. WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet 2000; 91: 368–76. [PubMed] [Google Scholar]
- 13.Wetzler M, Talpaz M, Kleinerman ES, et al. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med 1990; 89: 663–72. [DOI] [PubMed] [Google Scholar]
- 14.De Clercq E The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil). Biochem Pharmacol 2009; 77: 1655–64. [DOI] [PubMed] [Google Scholar]
- 15.Dale DC, Bolyard AA, Kelley ML, et al. The CXCR4 antagonist plerixafor is a po tential therapy for myelokathexis, WHIM syndrome. Blood 2011; 118: 4963–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood 2014; 123: 2308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peretti A, FitzGerald PC, Bliskovsky V, Buck CB, Pastrana DV. Hamburger polyomaviruses. J Gen Virol 2015; 96: 833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chae KM, Ertle JO, Tharp MD. B-cell lymphoma in a patient with WHIM syndrome. J Am Acad Dermatol 2001; 44: 124–8. [DOI] [PubMed] [Google Scholar]
- 19.Cipriani NA, Blair E, Taxy JB. WHIM syndrome and oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: 105–8. [DOI] [PubMed] [Google Scholar]
- 20.Pastrana DV, Peretti A, Welch NL, et al. Metagenomic discovery of 83 new human papillomavirus types in patients with immunodeficiency. mSphere 2018; 3(6): e00645–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa — a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc 1999; 4: 268–71. [DOI] [PubMed] [Google Scholar]
- 22.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998; 393: 595–9. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Li Z, Gao JL, et al. CXCR4 antagonist AMD3100 redistributes leukocytes from primary immune organs to secondary immune organs, lung, and blood in mice. Eur J Immunol 2015;45: 1855–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 2003; 19: 583–93. [DOI] [PubMed] [Google Scholar]
- 25.Devi S, Wang Y, Chew WK, et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutro-phil homing to the bone marrow. J Exp Med 2013; 210: 2321–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hess U, Ganser A, Schnürch HG, et al. Myelokathexis treated with recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF). Br J Haematol 1992; 80: 254–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.